Abstract
Interdigitating dendritic cell sarcoma is extremely rare neoplasm that mainly occurs in the lymph nodes. Only 45 cases have been reported in the literature to date.
We report a case of this sarcoma arising from the liver and lung, a previosly unreported site for this neoplasm. An 19-year-old girl deteriorated rapidly after artificial abortion and died 4 weeks later. Autopsy showed markedly enlarged liver and lung with numerous nodules up to 0.5 centimeters in diameter. Microscopically, nodules was composed of large pleomorphic cells that were immunohistochemically positive for proteins S-100 and vimentin, some of them expressed positivity to fascin and CD 68, with a rich small CD3 positive T lymphocytic infiltrateite around them.
Based of these findings, the present case was diagnosed as interdigitating dendritic cell sarcoma, a neoplasm that remains a diagnostic and clinical challenge, because it can mimic a wide variety of other malignant tumors and tumor-like lesions.
KEY WORDS: interdigital dendritic cells, sarcoma, extranodal location
INTRODUCTION
Accessory immune system contains two categories of cells: antigen-presenting (dendritic cells) and antigen- processing cells (macrophages). Dendritic cells are heterogeneous group of cells to which we include: Langerhans cells (skin, cervix, vagina, stomach and esophagus), dermal dendrocytes, follicular dendritic cells (FDS) and interdigital dendritic cells (IDS). IDS are primary located in T cell zone of lymphoid tissue (lymph nodes, thymus and lien). According to the WHO classification of hematopoetic and lymphoid tumor tissues, tumors of dendritic cells are classified as “neoplasm of histiocytes and dendritic cells origin”, which has a distinct category of dendritic cells neoplasm, which also includes sarcoma IDS [1]. Sarcomas of dendritic cells are very rare. The most often are diagnosed FDS sarcomas in lymph nodes [2], while extranodal location is extremely rare and is found in 1/3 of published cases [3-10]. IDS sarcomas (SIDS) are even more rare neoplasm which same as FDS sarcoma appears mostly inside lymph nodes [10]. Up to date literature described so far 45 cases of SIDS [11]. Extranodal localization of neoplasm is recorded in duodenum, spleen, thyroid, breast, palpebra, tibia, salivary glands, testicle [11-19]. In this paper we described clinical and pathological characteristics of a case of extranodal localization of SIDS in liver and lungs, entity that clinically was not recognized and had fast and fatal outcome.
CASE REPORT
In 19-year old women, following abortion due to unplanned pregnancy four times curettage was performed due to metrorrhagia, which was believed to be caused by the residua of placental tissue in uterine cavity. Curetment was not submitted to a pathohistological analysis. Patient complained to slackness, fast lost of body weight and high body temperature which was followed in early stage of disease with jaundice development. Routine laboratory finding established anemia and slightly elevated sedimentation. Day before death and four weeks following abortion patient developed melena, which was diagnosed via gastroscopy as ulcer and treated conservatively. Couple of hours following gastroscopy patient became unconscious, developed coma and in the end died.
Autopsy findings
Liver was extremely enlarged. Lower liver edge reached upper pelvic edge. In liver and in the lungs numerous grayish-white nodules diameter to 5 mm surrounded with hyperemic zone were found. Bilaterally in lungs in lower lobes a single area without air, dark red color, on cut surface triangle shape with the tip of the triangle directed toward hilus and base to the surface of lungs. Below pleura and also in lung parenchyma numerous areas of infiltration with blood. Renal capsule was easy to remove, the surface was smooth. Renal cortex was widened and unclear picture. The brain was extremely swollen. Dark red blood clots were found in uterine cavity. Prominent cyanosis was found in other tissues and organs.
Pathological findings
Microscopic examination of liver tissue revealed that tissue structure was impaired with infiltrate of atypical cells places among sinusoids (Fig- ureiA.), while in portal area and acini, tumor cells there are foci in form of larger and bigger aggregates (Figure 1B.). Inside acini tumor aggregates did not demonstrate zonal predilection. Atypical cells were of very polymorphic shape and size, abundant cytoplasm, round and oval nuclei, among them there were multinuclear giant cells and those with eccentric lobu- lated and deeply notched nuclei, with small invisible nucleoli (Figure 1A.). Numeorus pathological mitoses are visible (do 10/10 HPF). Aggregates of neoplastic cells are surrounded with reactive cells, lymphocytes, mono- and multinuclear histiocytes, plasma cells, and scant polymorphonuclear leukocytes. Surrounding hepatocytes demonstrate reactive changes. Liver tissue was distorted in site of nodular aggregates. Hyperemia was observed in lungs with accentuated capillary thrombosis and focally hemorrhagic infiltration of parenchyma (Figure 2A.). In both lower lobes, macroscopically observed area of hemorrhagic infarction was confirmed microscopically. Below pleura and focally in interstitium visible nodular aggregates of atypical cells with identical morphology (Figure 2B.) same as those described in liver. Acute tubular necrosis was found in kidneys. In other tissues besides hyperemia areas of focal hemorrhagic infiltration are found. Lymph nodes are not affected the tumor process. The endometrium has only basal layer which is moderately infiltrated by lymphocytes, histiocytes, plasma cells and eosinophils. Endocervix was chronically inflamed.
FIGURE 1.
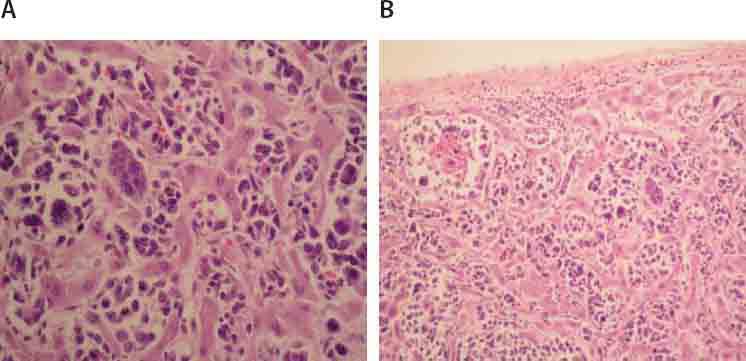
Tumor cells in liver sinusoids (A.; HE, 250X) and in form of aggregates in acini (B; HE, 125X).
FIGURE 2.
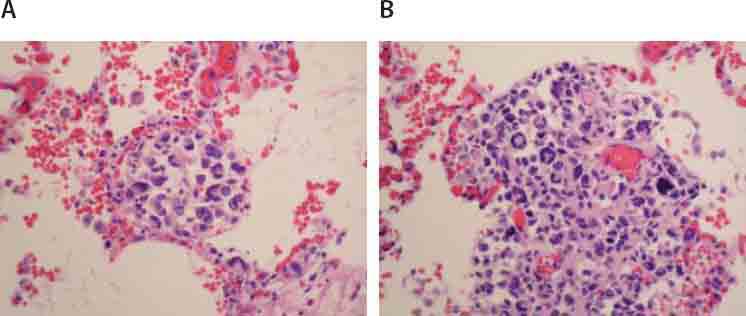
Noticeable hyperemia and focal hemorrhagic infiltration (A; HE,125X), as well as aggregates of tumor cells in lung interstitium (B; 250X).
Immunohistochemical findings
Imunochistochemically, tumor cells demonstrated strong, uniform expression of S-100 protein (Figure 3) andi vimentin (Figure 4.), focal positivity to fascin (Figure 5.) and CD 68 (Figure 6.), while they were negative to CD15, CD30, CD45RO, CD43, CD23, CD21, CK116, PLAP, ALK, HMB45, EMA, CD20, CD79a, Cyclin D1, lysosim, actin and desmin. Surrounding tumor cells there was variable number of small CD3 positive T lymfocites (Figure 7.).
FIGURE 3.
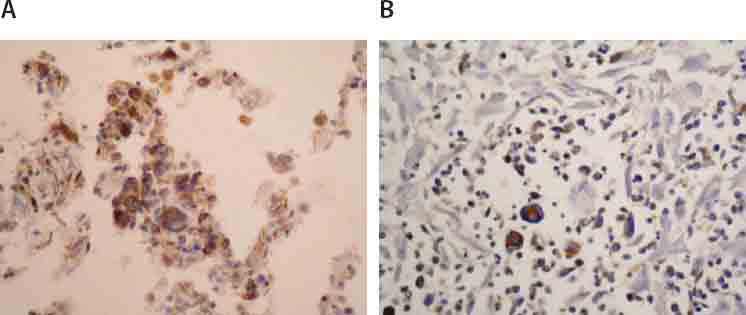
Immunohistochemical expression of protein S-100 in tumor cells of lung (A;125X) and liver (B;125X).
FIGURE 4.
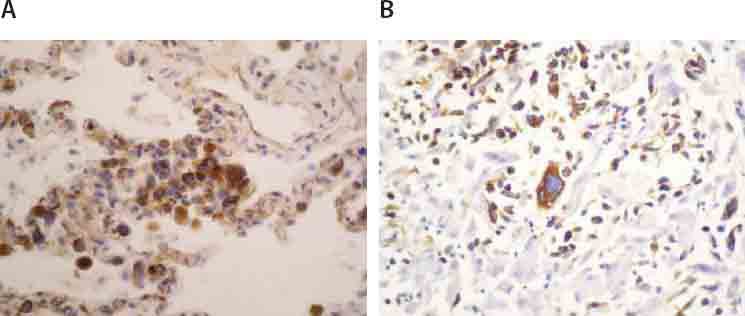
Immunohistochemical expression of vimentin in tumor cells of lung (A; 125X) and liver (B; 125X).
FIGURE 5.
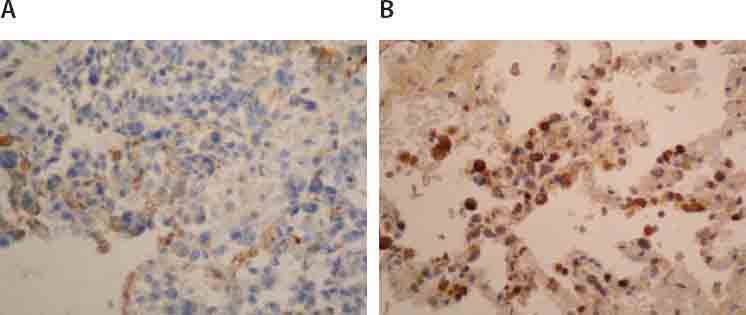
Immunohistochemical expression of fascin in tumor cells of liver (A; 125X) and lung (B.; 125X).
FIGURE 6.
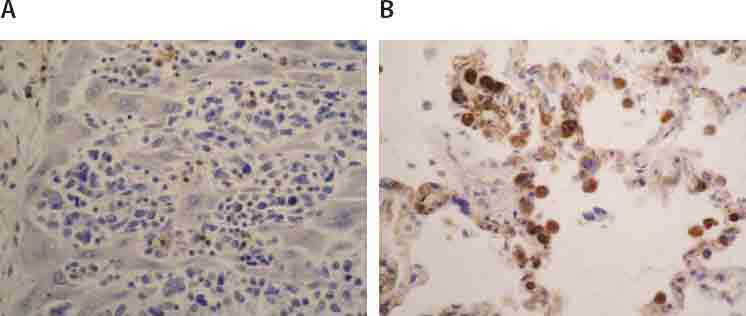
Immunohistochemical expression of CD 68 in tumor cells of liver (A;125X). and lung (B; 125X).
FIGURE 7.
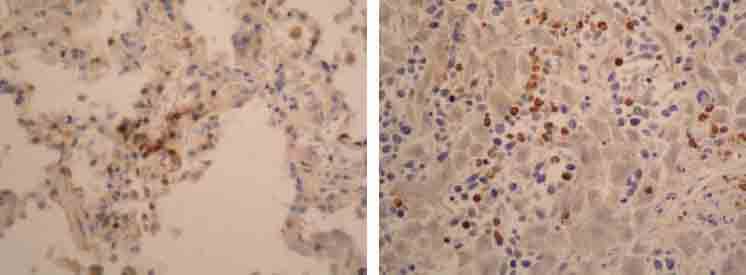
CD3 positive T lymphocytes in background of tumor cells in lung (A; IH, 125X) and liver (B; IH, 125X).
DISCUSSION
Our case presents primary localization of SIDS in lungs and liver, form that so far has not been presented in literature. The disease developed in 19 female patient developed without any symptoms and they appeared for the first time during her last month of life and overlapping with period following abortion. SIDS is diagnosed post mortem, following autopsy, based on morphological and immunohistochemical finding of tumor tissue. Interdigitating dendritic cells sarcoma is a very rare neoplasm of antigen presenting cells. So far 45 cases of SIDS has been published worldwide. Mostly it was a nodal localization of the tumor in cervical lymph nodes with secondary spread to other tissues and organs (bone marrow, bones, lien, liver, lings, ovary and skin). The disease was rarely accompanied with appearance of systemic symptoms appearance of systemic symptoms (loss of body weight, increased body temperature and/or anemia). Most often it develops asymptomatically. Most of the patients were adults (age range was 6-87), more often male gender (M: F=1,5:1), average survival period to 15 months following diagnosis [12]. The disease was of unknown etiology [10]. There were couple of cases of close sarcoma of follicular dendritic cells there was and infections with EBV or HHV- 8 [10]. Extranodal localization of SIDS was described in 1/3 of cases [12]. The optimal therapy for this malignant disease (radiotherapy or standard chemotherapy) still has not been determined due to small number of diagnosed cases and on the other hand to relatively fast lethal outcome which does not leave enough time for diagnosis and study of the disease.
CONCLUSION
Above described case is interesting for three reasons. First of all, SIDS is a very rare neoplasm. Second one, coincidence of abortion and primary disease. And in the end, according to our knowledge this is the first published case of primary localization of the SIDS in lungs and liver.
DECLARATION OF INTEREST
The authors declare no conflict of interest.
REFERENCES
- [1].Lim MS. Commentary on the WHO 2008 classification of neoplasms arising from histiocytic and other accessory cells. J Hematop. 2009;2(2):75–76. doi: 10.1007/s12308-009-0035-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Back W, Grobholz R, Riedel F. Follicular dendritic cell tumour: a rare but characteristic neoplasia. Int J Pathol. 2004;3:1. [Google Scholar]
- [3].Hollowood K, Stamp G, Zouvani I, Fleteher CD. Extranodal follicular dendritic cell gastrointestinal tract. Morphologic, immunohistochemical and utrastructural analysis. Am J Clin Pathol. 1995;103:90–97. doi: 10.1093/ajcp/103.1.90. [DOI] [PubMed] [Google Scholar]
- [4].Shek TW, Liu CL, Peh WC, Fan ST, Ng IO. Intra-abdominal follicular dendritic cel tumor in need of recognation. Histopathology. 1998;33:465–470. doi: 10.1046/j.1365-2559.1998.00547.x. [DOI] [PubMed] [Google Scholar]
- [5].Chang KC, Jin YT, Chen FF, Su IJ. Follicular dendritic cell sarcoma of the colon. Histopathology. 2001;38:25–29. doi: 10.1046/j.1365-2559.2001.01035.x. [DOI] [PubMed] [Google Scholar]
- [6].Dominwu-Malagón H, Cano-Valdez AM, Mosqueda-Taylor A, Hes O. Folliculardendritic cell sarcoma of the pharyngeal region: histologic, cytologic, immunohistochemical, and ultrastructural study of three cases. Ann Diagn Pathol. 2004;8(6):325–332. doi: 10.1053/j.anndiagpath.2004.08.001. [DOI] [PubMed] [Google Scholar]
- [7].Pruneri G, Masullo M, Renne G, Taccagni G, Manzotti M, Luini A, et al. Follicular dendritic cell sarcoma of the breast. Virchows Arch. 2002;441(2):194–199. doi: 10.1007/s00428-002-0660-7. [DOI] [PubMed] [Google Scholar]
- [8].Youene KY, Waugh MS. Extranodal follicular cell sarcoma. Arch Pathol Lab Med. 2008;132(10):1683–1687. doi: 10.5858/2008-132-1683-EFDCS. [DOI] [PubMed] [Google Scholar]
- [9].Boddle DA, Ro JY, Yoon GS, Yong YWH, Ayala AGA, Ordonez NG. Extranodal follicular dendritic cell sarcoma of the head and neck region: three new cases, with review of the literature. Mod Pathol. 2002;15(1):50–58. doi: 10.1038/modpathol.3880489. [DOI] [PubMed] [Google Scholar]
- [10].Bai LY, Kwang WK, Chiang IP, Chen PM. Follicular dendritic cell tumor of the liver associated with Epstein_Barr virus. Jap J Clin Onc. 2006;36(4):249–253. doi: 10.1093/jjco/hyl001. [DOI] [PubMed] [Google Scholar]
- [11].Gaertner EM, Tsokos M, Derringer GA, Neuhauser TS, Arciero C, Andriko JA. Interdigitating dendritic cell sarcoma: a report of four cases and review of the literature. J Clin Pathol. 2001;115(4):589–597. doi: 10.1309/M95G-7DQ2-TLQL-7Q11. [DOI] [PubMed] [Google Scholar]
- [12].Boldin I, Brix-Grunvald G, Scarpatteti MM, Beham-Schmid C, Klein A. Interdigitating dendritic cell sarcoma of the eyelid with a rapidly fatal course. Arch Ophtalmol. 2008;126(5):738–740. doi: 10.1001/archopht.126.5.738. [DOI] [PubMed] [Google Scholar]
- [13].Kanaan H, Al-Maghrabi J, Linjawi A, Al-Abbassi A, Dandan A, Haider AR. Interdigitating dendritic cell sarcoma of the duodenum with rapidly fatal course: a case report and review of the literature. Arch Pathol Lab Med. 2006;130(2):205–208. doi: 10.5858/2006-130-205-IDCSOT. [DOI] [PubMed] [Google Scholar]
- [14].Kawachi K, Nakatani Y, Inayama Y, Kawano N, Toda N, Misugi K. Interdigitating dendritic cell sarcoma of the spleen: report of a case with a review of the literature. Am J Surg Pathol. 2002;26(4):530–537. doi: 10.1097/00000478-200204000-00018. [DOI] [PubMed] [Google Scholar]
- [15].Sharma M, Ahsan F, Ah-See KW, McKean ME, Kain R, Chapman AD. Interdigitating dendritic cell sarcoma of the parotid gland. J Laryngol Otol. 2006;120(3):244–6. doi: 10.1017/S0022215105003634. [DOI] [PubMed] [Google Scholar]
- [16].Uluoğlu O, Akyürek N, Uner A, Coşkun U, Ozdemir A, Gökçora N. Interdigitating dendritic cell tumor with breast and cervical lymphnode involvement: a case report and review of the literature. Virchows Arch. 2005;446(5):546–554. doi: 10.1007/s00428-005-1209-3. [DOI] [PubMed] [Google Scholar]
- [17].Adam Z, Pour L, Veselý K, Krejcí M, Fakan F, Hofstädter F, et al. Interdigitating dendritic cell sarcoma of the leg. Onkologie. 2009;32:364–465. doi: 10.1159/000217796. [DOI] [PubMed] [Google Scholar]
- [18].Barwell N, Howatson R, Jackson R, Johnson A, Jarrett RF, Cook G. Interdigitating dendritic cell sarcoma of salivary gland associated lymphoid tissue not associated with HHV-8 of EBV infection. J Clin Pathol. 2004;57:87–89. doi: 10.1136/jcp.57.1.87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Luk IS, Shek TW, Tang VW, et al. Interdigitating dendritic cell tumor of the testis: a ovel testicular spindle cell neoplasma. Am J Surg Pathol. 1999;23:1141–1148. doi: 10.1097/00000478-199909000-00020. [DOI] [PubMed] [Google Scholar]


