Abstract
Trefoil Factor Family (TFF) plays an essential role in the intestinal epithelial restitution, but the relationship between TFF1 and gastric cancer (GC) is still unclear. The present study aimed to determine the role of TFF1 in repairing gastric mucosa and in the pathogenesis of GC.
The TFF1 expression in different gastric mucosas was measured with immunohistochemistry. Then, siRNA targeting TFF1 or plasmids expressing TFF1 gene were transfected into BGC823 cells, SGC7901 cells and GES-1 cells. The cell proliferation was detected with MTT assay and apoptosis and cell cycle measured by flow cytometry.
From normal gastric mucosa to mucosa with dysplasia and to gastric cancer, the TFF1 expression had a decreasing trend. Down-regulation of TFF1 expression significantly reduced the apoptosis of three cell lines and markedly facilitated their proliferation but had no significant effect on cell cycle. Over-expression of TFF1 could promote apoptosis of three cell lines and inhibit proliferation but had no pronounced effect on cell cycle. TFF1 can inhibit proliferation and induce apoptosis of GC cells in vitro.
KEY WORDS: Trefoil Factor Family 1, gastric cancer, apoptosis, in vitro
INTRODUCTION
Although the incidence of gastric cancer (GC) has declined over the past 30 years worldwide, especially in western countries, it remains the second leading cause of cancer-related death and accounts for 10.4% of cancer deaths globally [1]. About 1150000 GC cases and 738000 cancer deaths are estimated to have occurred in 2008 worldwide [2]. More than one-half of GC patients have lymph node metastases when they are initially diagnosed or operated, which usually results in poor prognosis [3-5]. Therefore, it is important to investigate the pathogenesis of GC, find effective measures to prevent and treat GC. The trefoil factor family (TFF), which comprises gastric peptides pS2/TFF1 and spasmolytic peptide (SP)/TFF2 and intestinal trefoil factor (ITF)/TFF3, plays an essential role in the intestinal epithelial restitution [6]. They are small (7~12 kDa) protease-resistant proteins and are abundantly secreted onto the mucosal surface by mucus-secreting cells in the gastrointestinal tract. The TFFs share an absolutely conserved distinct motif of six cysteine residues that define a so-called “trefoil” domain, which is also known as a “P” domain [7]. At certain physiological conditions, in the presence of a tissue-specific distribution, TFF plays an important role in mucosal protection and wound healing. But in malignant tissues, TFF is highly expressed and correlated strongly with the genesis, metastasis and invasion of tumor cells. These indicate that TFF may be a common mediator of oncogenic responses to different stimuli. The biological functions of TFF involve complex regulatory processes. TFF1 was first discovered in breast cancer cell line MCF-7 in 1982 [8-9]. In normal tissues, the main site of expression of TFF1 is the mucosal epithelial cells of gastric body and antrum in a site-specific fashion. However, under pathological conditions, such as ulceration, the TFF1-expression of site-specific fashion is absent, TFF1 can be identified in any damaged mucosas, and its expression is up-regulated to participate in gastrointestinal epithelial reconstruction and repair process [10]. However, there is lack of TFF1 expression in human GC. To determine the function of TFF1, the mouse TFF1 gene was inactivated. The antral and pyloric gastric mucosa of mpS2-null mice was dysfunctional and exhibited severe hyperplasia and dysplasia. All homozygous mutant mice developed antropyloric adenoma, and 30% developed multifocal intraepithelial or intramucosal carcinomas. These results indicate that TFF1 is essential for the normal differentiation of antral and pyloric gastric mucosa and may function as a gastric-specific tumor suppressor gene [11]. The aim of our study is to determine the role of TFF1 in repairing gastric mucosa and in the pathogenesis of GC. To this end, the TFF1 expression was detected in different gastric mucosas. Then, siRNA targeting TFF1 and plasmids expressing TFF1 were transfected into normal gastric mucosal epithelial cells (GES-1 cells [12]), highly malignant GC cell line (BGC823 cells) and moderately malignant GC cell line (SGC7901 cells), respectively, to investigate the effect of TFF1 on the biological behaviors of gastric mucosal epithelial cells, which provides evidence for further investigation and application of TFF1 target therapy.
MATERIALS AND METHODS
Sample collection and immunohistochemistry
GC tissues and adjacent normal and atypical hyperplasia gastric mucosas were obtained from 45 patients undergoing gastroscopy at Tongji Hospital of Tongji University. The characteristics of these patients are shown in Table 1. All antibodies used for immunohistochemistry were purchased from Zhongshan Goldenbridge Biotechnology CO., LTD (Beijing, China). All other chemicals and reagents were commercially available and had the highest purity. Tissues were fixed in 10% formaldehyde in phosphate buffered saline (PBS), embedded in paraffin, and cut into 5-μm sections. Sections were heated at 50°C overnight, deparaffinzed with xylene twice, and rinsed in a decreasing ethanol series (100-70%) for 5 min/solution. Samples were treated with 3% H2O2 for 30 min to inactivate endogenous peroxidase. Antigen retrieval was done with 0.01 M Na-citrate buffer (pH 6.0) in a microwave oven for 30 min. Sections were incubated at 4 °C overnight in moist chambers with primary antibody (1:100) and then with biotinylated secondary antibody (Zhongshan Goldenbridge Biotechnology CO., LTD., Beijing, China, SP-Kit) for 30 min at room temperature, followed by incubation with streptavidin peroxidase. Visualization was done with diaminobenziding tetrachloride and counterstaining was performed with haematoxylin. In negative controls, the primary antibody was replaced with PBS. Sections were observed under a light microscope and positive cells had brown granules in cytoplasm. Five fields were randomly selected from each section at high magnification, and 100 cells were counted in each field, followed by calculation of percentage of positive cells. Sections with positive cells of >15% was regarded as positive.
TABLE 1.
Characteristics of 45 patients

Cell culture
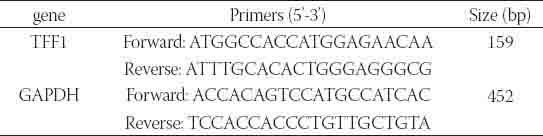
Two gastric adenocarcinoma cell lines (BGC823 cells and SGC7901 cells) (Cell bank of Chinese Academic of Sciences), as well as normal gastric epithelial cell line (GES-1 cells) (Tumor Institute of Beijing Medical University, China) were maintained in RPMI 1640 medium (Gibco BRL, USA) supplemented with 10% fetal bovine serum (FBS; Hangzhou Sijiqing, China) and 100 μg/ml streptomycin and penicillin G (Amresco, USA) at 37 oC under 5% humidified CO2. Passaging was performed every 3 days by trypsinization (Sigma, USA). Synthesis of TFF1-siRNA and Cell Transfection mRNA sequence of TFF1 was obtained from GeneBank. With Invitrogen’s online design software BLOCK-iTTM RNAi Designer, three sites (stealth 115, stealth 144, stealth 268), and they were selected and designed to be three sets of targeting stealth siRNA sequence. The selection and design were based on three principles: avoiding the 5’ and 3’ end non-coding region, selecting the sequences with G/C ratio between 35% and 55% and using BLAST to exclude other coding sequences. Stealth siRNA sequences are shown in Table 2. When the cell confluence reached about 40%~60%, transfection of Stealth siRNAs and stealth RNA negative control was performed with LipofectamineTM2000 according to manufacturer’s instructions (Invitrogen, USA). Cells transfected with Stealth siRNAs was defined as Stealthgroup, those transfect with Stealth negative control as negative control group, and those without transfection as blank control group. Detection of TFF1 mRNA expression by RT-PCR Total RNA was extracted with Trizol reagent (Invitrogen, USA). About 5 μg of RNA were used for reverse transcription with random primers to synthesize first strand cDNA, followed by conventional PCR amplification with 1 μl of cDNA as template. The forward primers, reverse primers and anticipated size of products and GAPDH are shown in Table 3. The volume of reaction system was 25 μl, and reaction conditions were as follows: denaturation at 94 °C for 1 min; annealing at 59 °C for 1 min; extension at 72 °C for 1 min. The products were then subjected to agarose gel electrophoresis, and images were captured to analyze the quality of RNA.
TABLE 2.
Sequences of stealth siRNAs

TABLE 3.
Primers for RT-PCR
Determination of cell proliferation with MTT assay
Cell viability was determined by MTT assay. Different cell lines were seeded at 105/ml into 96-well plates, and then divided into blank control group, blank transfection group, stealth_115 group, stealth_144 group and stealth_268 group. At 24 h after transfection, the cell proliferation was determined by MTT assay every other 24 h for 5 days. In brief, at designed time points, MTT was added into each well at a final concentration of 0.5 mg/ml followed by incubation for 4 h at 37 °C. The formazan was dissolved by addition of dimethylsulfoxide (DMSO) and absorbance (A) was measured with microplate reader (Bio-Rad, USA) at 570 nm. The inhibition rate (IR) was calculated formulas follow: IR% = (1-Aexperiment /Acontrol)×100%.
Detection of cell cycle by flow cytometry
At 72 h after transfection, cells (1×106 cells) were harvested and washed twice in cold PBS. Cells were fixed in 70% ethanol and washed in cold PBS. Then, the cells were suspended in 1 ml of propidium iodide (PI, Sigma, USA) solution containing 40 μg/ml PI, 100 μg/ml RNAase (Sigma, USA), 0.1% (w/v) sodium citrate and 0.1% (v/v) Triton X. Cells were incubated at room temperature in dark for at least 30 min, and analyzed by flow cytometry (Beckman, USA).
Detection of cell apoptosis by flow cytometry
At 72 h after transfection, 5×105 cells were harvested. According to the instructions in Annexin V-FITC kit (Nanjing Keygen Biotech. Co. Ltd., China), cells were suspended in 500 μl of binding buffer and 5 μl of Annexin V-FITC followed by addition of 5 μl of PI and subsequent incubation for 15 min at room temperature in dark. Apoptosis was detected by flow cytometry.
Construction of plasmid TFF1-pcDNA3.1 and cell transfection
Human plasmid TFF1-pcDNA3.1 was constructed and identified by Shanghai Shuiyuan Biotechnology Company. Cells were divided into TFF1-pcDNA3.1 transfection group, pcDNA3.1 negative control group and blank control group. Cells were seeded into 24-well plates. When cell confluence reached about 50%, transfection was performed with LipofectamineTM2000 according to manufacturer’s instructions (Invitrogen, USA).
Detection of TFF1 protein expression by western blot
Total protein was extracted and 30 μg of proteins were subjected to polyacrylamide gel electrophoresis. Then, the proteins were transferred onto PVDF membrane, which were blocked for 2 h at 4 °C in 5% non-fat milk and then incubated with mouse anti-human TFF1 or GAPDH monoclonal antibody (Santa Cruz, USA; 1:2000) for 1.5 h at room temperature. After washing in PBST thrice (10 min for each), the membrane was incubated with HRP-conjugated goat anti-mouse secondary antibody (Santa Cruz, USA; 1:2000) at room temperature for 1.5 h. Following rinsing in PBS, visualization was done and images were captured with a Touching gel imaging system. In negative control group, primary antibody was replaced with PBS.
Determination of cell proliferation with MTT assay
Different cell lines were inoculated at 105/ml into 96-well plates, and then divided into blank control group, TFF1-pcDNA3.1-transfection group and pcDNA3.1 transfection group. At 24 h after transfection, cell proliferation was measured with MTT assay every other 24 h for 5 days. The procedures of MTT assay were abovementioned.
Detection of cell cycle by flow cytometry
At 48 h after transfection, cells (1×106 cells) were harvested and processed with above procedures. Cell cycle was measured by flow cytometry.
Detection of cell apoptosis by flow cytometry
At 48 h after transfection, 5×105 cells were harvested and processed with above procedures. Cell apoptosis was detected by flow cytometry.
Statistical analysis
SPSS version 12.0.1 statistical software was employed for statistical analysis and data were expressed as means ± standard deviation (¯ ± s). Independent sample t-test was used to compare data between two groups and one-way ANOVA to compare date among multiple groups. If there were significant differences, a further least significant difference method would be used for pairwise comparison. A value of p <0.05 was considered statistically significant.
RESULTS
TFF1 Expressionin different mucosal tissues
TFF1 were mainly expressed in the cytoplasm of gastric mucosal cells. Perinuclear accumulation was the most obvious and positive cells were stained brown. The closer to the cell membrane is, the deeper the color is. The positive expression rate of TFF1 in normal gastric mucosa was 100% (15/15). In mucosas with dysplasia, the TFF1 expression was slightly reduced and the positive expression rate was 80.0% (16/20). From normal gastric mucosa, dysplasic mucosa to GC, the TFF1 expression had a gradually decreasing trend, and significant difference in TFF1 expression was noted among groups (Table 4, Figure 1). The positive expression rate of TFF1 was 82.1% in males (23/28) and 77.8% in females (21/27) showing no significant difference. The positive expression rate of TFF1 was 77.4% in patients aged ≥ 60 years (24/31) and 83.3% in those aged <60 years (20/24) showing no marked difference. These results suggest that TFF1 expression was independent of both age and gender (Table 4).
TABLE 4.
TFF1 protein expression in different patients

FIGURE 1.

Immunohistochemistry for TFF1 in different gastric mucosas. A: normal gastric mucosa. B: dysplasic gastric mucosa. C: GC.
Stealth siRNA inhibited mRNA expression of TFF1
Results from RT-PCT showed stealth siRNA significantly inhibited TFF1 expression in a time dependent manner. At 24 h after transfection, the inhibition of TFF1 expression was present, and then reached a maximal level at 48 h after transfection but became to reduce at 72 h after transfection. Different stealth siRNAs inhibited the TFF1 expression when compared with control group and blank transfection group. The inhibitory effect of stealth_144 was the most obvious and, at 48 h after transfection, the inhibition rate of TFF1 expression in GES-1 cells, BGC823 cells and SGC7901 cells was 69.0%, 55.6% and 70.5%, respectively. The inhibitory effect was comparable among 3 different cell lines. Although TFF1 expression in negative control group was slightly lower than that in control group, there was no significant difference (p>0.05) (Figure 2).
FIGURE 2.

TFF1 mRNA expression in stealth_144 group at 48 h after transfection. M: DNA Marker; 1: GES-1 cells with transfection; 2: control GES-1 cells; 3: BGC-823 cells with transfection; 4: control BGC-823 cells; 5: SGC7901 cells with transfection; 6: control SGC7901 cells
Down-regulation of TFF1 reduced apoptosis rate and promoted cell proliferation but had no effect on cell cycle
MTT assay showed that the proliferation of three cell lines undergoing transfection with stealth siRNAs increased significantly at 48 h and 72 h after transfection (p<0.05), and the increase in steath_144 group was the most obvious and reached a peak level at 72 h after transfection. The proliferation remained comparable among cells at 24 h, 96 h and 120 h after transfection (p>0.05) (Table 5, Figure 3). The apoptosis rate of three cell lines was significantly reduced at 72 h after TFF1 stealth siRNA transfection when compared with control group (p<0.05). The reduction of apoptosis rate in stealth_144 group was most obvious and the apoptosis rate in BGC823 cells, SGC7901 cells and GES-1 cells was reduced by 41.4%, 45.4% and 45.3%, respectively. However, there was no marked difference in apoptosis among cells transfected with different stealth siRNAs. There was no significant alteration in cell cycle among stealth_115 group, stealth_144 group and stealth_268 group at 72 h after transfection (p>0.05) (Table 6).
TABLE 5.
Proliferation of BGC823 cells, SGC7901 cells and GES-1 cells at 72 h after stealth siRNA transfection

FIGURE 3.

Stealth siRNA promotes cell proliferation at 72 h after transfection (×200) A: control SGC7901 cells. B: SGC7901 transfected with stealth_144. C: control BGC823 cells. D: BGC823 transfected with stealth_144. E: control GES-1 cells. F: GES-1 transfected with stealth_144.
TABLE 6.
Cell cycle and apoptosis rate of BGC823 cells at 72 h after stealth siRNA transfection

TFF1 protein expression after transfection with TFF1-pcD-NA3.1
When compared with TFF1 expression before transfection, the TFF1 protein expression in GES-1 cells, BGC823 cells and SGC7901 cells was markedly increases (p<0.05) in a time dependent manner. Increase of TFF1 expression was noted as early as 24 h after transfection, but the TFF1 expression was similar to that in control group (p>0.05). TFF1 expression reached a peak level at 48 h after transfection but began to reduce at 72 h after transfection (Figure 4)
FIGURE 4.

TFF1 protein expression at 48 h after transfection with TFF1-pcDNA3.1. 1: GES-1 cells with transfection; 2: control GES-1 cells; 3: BGC-823 cells with transfection; 4: control BGC-823 cells; 5: SGC7901 cells with transfection; 6: control SGC7901 cells
Over-expression of TFF1 promoted apoptosis, inhibited cell proliferation but had no effect on cell cycle
MTT assay showed that cell proliferation reduced after TFF1-pcDNA3.1 transfection,. The IR was the most obvious at 48 h after transfection (p<0.05). The IRs of GES-1 cells, BGC823 cells and SGC7901 cells were 23.8%, 32.5% and 29.8%, respectively. At 72 h and 96 h after transfection, the IR was also dramatically reduced when compared with control group (p<0.05). At 24 h and 120 h after transfection, there was no significant alteration in the IR (p>0.05) (Table 7, Figure 5). The apoptosis rate of TFF1-pcDNA3.1 transfected cells markedly increased at 48 h after transfection when compared with control group (p<0.05). The apoptosis rate increased from 6.28 ± 0.33 to 18.46 ± 1.23 in GES-1 cells, from 4.83 ± 0.56 to 14.38 ± 0.89 in BGC823 cells, and from 7.23 ± 0.48 to 19.85 ± 0.74 in SGC7901 cells (Table 8). After TFF1-pcDNA3.1 transfection, the proportion of cells in G1 and G2/M phase markedly decreased, while that in S-phase significantly increased, indicating that cells were arrested in S phase. The cell cycle distribution was markedly different from that in control group (p<0.05) (Table 8).
TABLE 7.
Effect of pcDNA3.1 transfection on proliferation of BGC823 cells, SGC7901 cells and GES-1 cells
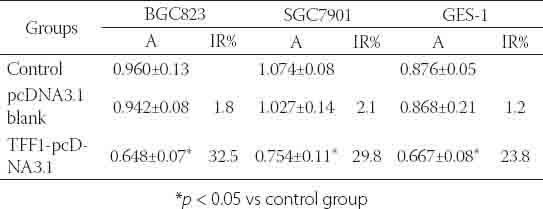
FIGURE 5.
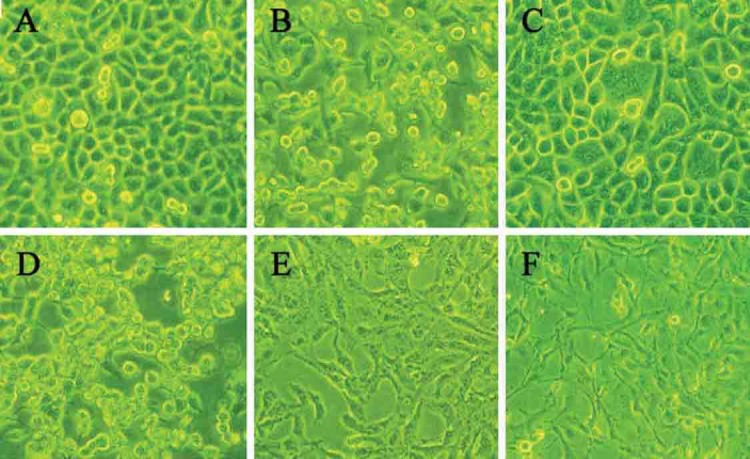
TFF1-pcDNA3.1 transfection inhibited cell proliferation (48 h; ×200) A:control SGC7901 cells; SGC7901 cells with TFF1-pcDNA3.1 transfection; C: control BGC823 cells; D: BGC823 cells with TFF1-pcDNA3.1 transfection; E:control GES-1 cells; F: GES-1 cells with TFF1-pcDNA3.1 transfection
TABLE 8.
Changes in cell cycle and apoptosis rate of BGC823 cells at 48 h after TFF1-pcDNA3.1 transfection (%)

DISCUSSION
Under physioligical conditions, human TFF1 is mainly expressed in epithelial cells of gastrointestinal tract. TFF1 expression has regional and cellular selectivity, and, in normal tissues, TFF1 is expressed in the gastric antrum, the crypt of gastric body and mucosa. Low TFF1 expression is also noted in the small intestine, colon and breast epiderm. However, under pathological conditions, the specificity in TFF1 expression is absent. When the gastrointestinal mucosa is injured, TFF1 may be expressed in the injured mucosa of gastrointestinal tract, and its expression is rapidly up-regulated to participate in the reconstruction and repair process of epithelial cells in gastrointestinal tract. Our study found that, in normal gastric mucosa, the TFF1 expression was mainly found in gastric epithelial cells, gastric pits and glands, which was consistent with previous study. In addition, TFF1 is now considered as a tumor suppressor, and may be a stomach-specific suppressor factor. In humans, about 50% of GC patients show loss of human pS2 expression. It is generally believed that GC is preceded by a precancerous progress with the following well-recognized steps: normal gastric mucosa, chronic active inflammation, multifocal atrophy (gland loss), intestinal metaplasia, complete type-intestinal metaplasia, incomplete type-dysplasia [13, 14]. The accumulation of many genetic and molecular changes is closely related to the evolution [15]. Taupin et al. [16] investigated the expressions of pS2 and ITF in different gastric mucosal injury models. Results showed that, in the gastric mucosa metaplasia-dysplasia-gastric cancer process, the TFF1 expression was gradually reduced, and TFF1 expression was lost earlier than the differentiation of gastric epithelial metaplasia, suggesting that loss of TFF1 expression is an early event in the occurrence of GC. Our results confirmed that TFF1 expression had a decreasing trend from normal gastric mucosa, gastric dysplasia to GC, consistent with previous findings. In recent years, studies have shown that TFF1 is relevant with cell proliferation and apoptosis to some extents. Rodrigues et al. [17] found that, in colon cancer cells undergoing transfection of TFF1, cell dispersion was promoted possibly through cyclooxygenase (COX) and thromboxane A2 (TXA-2) receptor dependent mechanism, enhancing the ability of cell infiltration. Another study [18] indicated that TFF1 played a dual role on the gastrointestinal cells: on one hand, TFF1 can block the transition from G1 phase to S phase, which interrupts the gastrointestinal cell differentiation and reduces cell proliferation; on the other hand, TFF1 prevents chemical factor-induced apoptosis. These reveal TFF1 is a regulatory factor of gastrointestinal cell differentiation. To reduce cell proliferation and induce differentiation are functional characteristics of tumor suppressors. Apoptosis refers to the programmed cell death in the growth, development and differentiation of cells and pathological environments. It is a type of cell death different from necrosis. Recent studies have found that apoptosis plays a unique role in the renewing of gastrointestinal mucosa and in the pathological processes of some digestive diseases. TFF1 exerts its anti-tumor effect through regulating the balance between cell proliferation and apoptosis. Taupin et al. [19] investigated the influence of TFF3 and its mutants on apoptosis and results showed that TFF3 trefoil domain mutant and alteration could promote cell migration, and increase the resistance to death during the process of migration. The mutations of trefoil domain were also detectable in TFF1, a protein with similar structure to TFF3, and this region is critical for the anti-tumor effect of TFF1 [20]. In our study, the TFF1 mRNA expression was inhibited by Stealth siRNA transfection, and the TFF1 was over-expressed followed transfection of plasmids expressing TFF1 gene. After inhibition of TFF1 expression by siRNA targeting TFF1, the proliferation rate of human GC cells and normal human gastric epithelial cells significantly increased and apoptosis decreased, but cell cycle was not significantly altered. After transfection with plasmids expressing TFF1, results revealed the proliferation rate of human GC cells and normal human gastric epithelial cells significantly decreased, apoptosis increased, and cells in G1 and G2/M phase decreased, while cells in S-phase increased and cells were arrested in S phase, which suggest that TFF1 can exert important effects on cell proliferation and apoptosis. It is speculated that the inhibition of GC by TFF1 may be attributed to that it can regulate the balance between cell proliferation and apoptosis. Cell proliferation is promoted and apoptosis rate reduced after silencing TFF1 expression which breaks the balance of TFF1 expression. The biological characteristics of a tumor are infinite proliferation and de-differentiation. The reduced TFF1 expression may promote the GC development. This speculation is needed to be further confirmed. As a whole, despite a growing number of studies have confirmed TFF1 plays a crucial role in the occurrence and development of GC, the exact mechanism of anti-tumor effect of TFF1 is still poorly understood and more studies are required.
CONCLUSION
In our study, we find that TFF1 can inhibit proliferation and induce apoptosis of GC cells in vitro.
DECLARATION OF INTEREST
The authors report no conflicts of interest.
REFERENCES
- [1].Jemal A, Siegel R, Ward E, Hao Y, Xu J, Murray T, et al. Cancer statistics 2008. CA Cancer J Clin. 2008;58:71–96. doi: 10.3322/CA.2007.0010. [DOI] [PubMed] [Google Scholar]
- [2].Jemal A, Bray F, Center MM, Ferlay J, Ward E, Forman D. Global Cancer Statistics. CA Cancer J Clin. 2011;61(2):69–90. doi: 10.3322/caac.20107. [DOI] [PubMed] [Google Scholar]
- [3].Abe N, Watanabe T, Suzuki K, Machida H, Toda H, Nakaya Y, et al. Risk factors predictive of lymph node metastasis in depressed early gastric cancer. Am J Surg. 2002;183:168–72. doi: 10.1016/s0002-9610(01)00860-1. [DOI] [PubMed] [Google Scholar]
- [4].Yamaguchi T, Sano T, Katai H, Sasako M, Maruyama K. Nodepositive mucosal gastric cancer: a follow-up study. Jpn J Clin Oncol. 2001;31:153–6. doi: 10.1093/jjco/hye035. [DOI] [PubMed] [Google Scholar]
- [5].Chen CY, Wu CW, Lo SS, Hsieh MC, Lui WY, Shen KH. Peritoneal carcinomatosis and lymph node metastasis are prognostic indicators in patients with Borrmann type IV gastriccarcinoma. Hepatogastroenterology. 2002;49:874–7. [PubMed] [Google Scholar]
- [6].Kjellev S. The trefoil factor family - small peptides with multiple functionalities. Cell Mol Life Sci. 2009;66(8):1350–69. doi: 10.1007/s00018-008-8646-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Wong WM, Poulsom R, Wright NA. Trefoil peptides. Gut. 1999;44(6):890–895. doi: 10.1136/gut.44.6.890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Masiakowski P, Breathnach R, Bloch J, Gannon F, Krust A, Chambon P. Cloning of cDNA sequences of hormone-regulated genes from the MCF-7 human breast cancer cell line. Nucleic Acids Res. 1982;10(24):7895–903. doi: 10.1093/nar/10.24.7895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Prud’homme JF, Fridlansky F, Le Cunff M, Atger M, Mercier-Bodart C, Pichon MF, et al. Cloning of a gene expressed in human breast cancer and regulated by estrogen in MCF-7 cells. DNA. 1985;4:11–21. doi: 10.1089/dna.1985.4.11. [DOI] [PubMed] [Google Scholar]
- [10].Pera M, Heppell J, Poulsom R, Teixeira FV, Williams J. Ulcer associated cell lineage glands expressing trefoil peptide genes are induced by chronic ulceration in ileal pouch mucosa. Gut. 2001;48:792–796. doi: 10.1136/gut.48.6.792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Lefebvre O, Chenard MP, Masson R, Linares J, Dierich A, LeMeur M, et al. Gastric mucosa abnormalities and tumorigenesis in mice lacking the pS2 trefoil protein. Science. 1996;274(5285):259–62. doi: 10.1126/science.274.5285.259. [DOI] [PubMed] [Google Scholar]
- [12].Ke Y, Ning T, Wang B. Establishment and characterization of a SV40 transformed human fetal gastric epithelial cell line-GES-1. Zhonghua Zhong Liu Za Zhi. 1994;1:7–10. [PubMed] [Google Scholar]
- [13].Correa P, Haenszel W, Cuello C, Tannenbaum S, Archer M. A model for gastric cancer epidemiology. Lancet. 1975;2:58–60. doi: 10.1016/s0140-6736(75)90498-5. [DOI] [PubMed] [Google Scholar]
- [14].Correa P. Human gastric carcinogenesis: a multistep and multifactorial process-First American Cancer Society Award Lecture on Cancer Epidemiology and Prevention. Cancer Res. 1992;52:6735–6740. [PubMed] [Google Scholar]
- [15].Tahara E. Genetic pathways of two types of gastric cancer. IARC Sci Publ. 2004;157:327–349. [PubMed] [Google Scholar]
- [16].Taupin D, Pedersen J, Familari M, Cook G, Yeomans N, Giraud AS. Augmented Intestinal Trefoil Factor (TFF3) and Loss of pS2 (TFFi) Expression Precedes Metaplastic Differentiation of Gastric Epithelium. Lab Invest. 2001;81(3):397–408. doi: 10.1038/labinvest.3780247. [DOI] [PubMed] [Google Scholar]
- [17].Rodrigues S, Van Aken E, Van Bocxlaer S, Attoub S, Nguyen QD, Bruyneel E, et al. refoil peptide as proangiogenic factor in vivo and in vitro: implication of cyclooxygenase-2 and EGF receptor signaling. FASEB J. 2003;17(1):7–16. doi: 10.1096/fj.02-0201com. [DOI] [PubMed] [Google Scholar]
- [18].Bossenmeyer-Pourié C, Kannan R, Ribieras S, Wendling C, Stoll I, Thim L, et al. The trefoil factor i participates in gastrointestinal cell differentiation by delaying Gi-S phase transition and reducing apoptosis. J Cell Biol. 2002;157(5):761–770. doi: 10.1083/jcb200108056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Taupin DR, Kinoshita K, Podolsky DK. Intestinal Trefoil factor confers colonic epithelial resistance to apoptosis. Proc Natl Acad Sci USA. 2000;97(2):799–804. doi: 10.1073/pnas.97.2.799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Yio X, Diamond M, Zhang JY, Weinstein H, Wang LH, Werther L, et al. Trefoil factor family 1 mutations enhance gastric cancer cell invasion through distinct signaling pathways. Gastroenterology. 2006;130(6):1696–706. doi: 10.1053/j.gastro.2006.01.040. [DOI] [PubMed] [Google Scholar]


