Ivaska has taken the road less traveled to show how integrin inactivation regulates cell migration and invasion.
Ivaska has taken the road less traveled to show how integrin inactivation regulates cell migration and invasion.
Johanna Ivaska will tell you that she never planned to continue studying integrins, the sticky transmembrane receptors that grab onto a cell’s extracellular environment, after her PhD. But somehow the integrins seemed to be stuck on her.

Johanna Ivaska
PHOTO COURTESY OF VESA-MATTI VÄÄRÄ
As a PhD student at the University of Turku in Finland, Ivaska chose a laboratory investigating collagen-binding integrins as a way to move into cancer research. When choosing a postdoctoral lab, Ivaska wanted to gain some experience in intracellular signaling, so she joined Peter Parker’s group at Cancer Research UK in London working on the protein kinase C (PKC) pathway. She took the lead on an open project to investigate how integrins and PKCs work together in cancer migration—and integrins have become integral to her own line of inquiry ever since.
In 2003, she moved back to Turku and set up her own group at VTT Technical Research Centre of Finland. In yeast two-hybrid studies using the cytoplasmic tail domains of integrin α subunits as bait, her lab pulled out two surprising “fish.” The first was a phosphatase, which her group demonstrated was activated by adhesion and decreased EGF receptor phosphorylation (1). The second α subunit binder was a Rab GTPase that regulated the trafficking of integrins through endocytic vesicles (2). In 2011, her group identified the first ubiquitously expressed β1-integrin inactivator, SHARPIN (3, 4), another α subunit binding partner. Most recently, her laboratory, now based at the University of Turku, has investigated the unconventional myosin-X, a transporter of integrins, and its role in breast cancer metastasis (5).
“It just struck me as weird that there wouldn’t be any inactivators of integrins.”
This month, Ivaska shared with JCB why she can’t seem to escape discoveries about the lesser-studied integrin α subunit and her penchant for outdoor adventures.
GRABBY MOLECULES
How do you describe integrins—some of the most sophisticated of receptor molecules—to students?
I always describe them as the hands of the cell. Or the hands and feet, I suppose. They’re the things that a cell uses to grab its environment and hold on or pull itself forward. Integrins are either holding onto other cells or a matrix protein in the environment.
But that’s only one half of what integrins do, because they then transmit signals from what they’re holding onto inside the cell. One of the most important, unique properties of integrins is that they act as mechanosensors that can signal in both directions. They act like springs and tell the cell about environmental stiffness and composition. That’s called outside-in signaling.
On the other hand, intracellular events can tell the cell to activate integrins. This inside-out signaling tells the cell to grasp harder or migrate faster.
Are both processes important in cancer?
A normal epithelial cell or fibroblast needs to adhere. If it becomes dissociated for whatever reason and shed, it knows that, “OK, I’m an epithelial cell, I’m not supposed to be floating around. I should kill myself.” And it undergoes programmed cell death. But this process is impaired in cancer. Cancer cells have become anchorage-independent. This is an important process in cancer where dysregulation of integrin function is really critical.
Thus far, the field of inside-out signaling has focused predominantly on the regulation of integrin activation in nonadherent cells like platelets and leukocytes. But inside-out signaling also regulates processes like migration, invasion, and metastasis in adherent cells. Maybe not globally in the whole cell, but just in localized areas. This is an exciting area that we don’t quite fully understand yet.
While many integrin researchers study activation and β subunit binding partners, you’ve focused on integrin inactivation and the α subunit. Why?
I suppose it’s a combination of trying to find my niche and being curious. Every field has a gap that, for whatever reason, nobody’s looked into. And you get the idea that surely there has to be some kind of mechanism or regulation in this α subunit domain, too.
Similarly, there were numerous high-impact, impressive publications about how integrins are activated. And very few people were asking, what would be the counterforce? You have a kinase, you have a phosphatase. You have an oncogene, you have a tumor suppressor. It just struck me as weird that there wouldn’t be any inactivators of integrins.
ALPHA TALES
What did you find when you looked for binding partners of the a subunit cytoplasmic tail?
My first graduate student—I recruited her at an extreme hiking competition when she hadn’t slept for three days—found this TCPTP phosphatase, which was specifically recruited to collagen-bound integrins. Adhesion to collagen activated the phosphatase, which turned off a number of receptor tyrosine kinases (RTKs), including the EGF receptor.
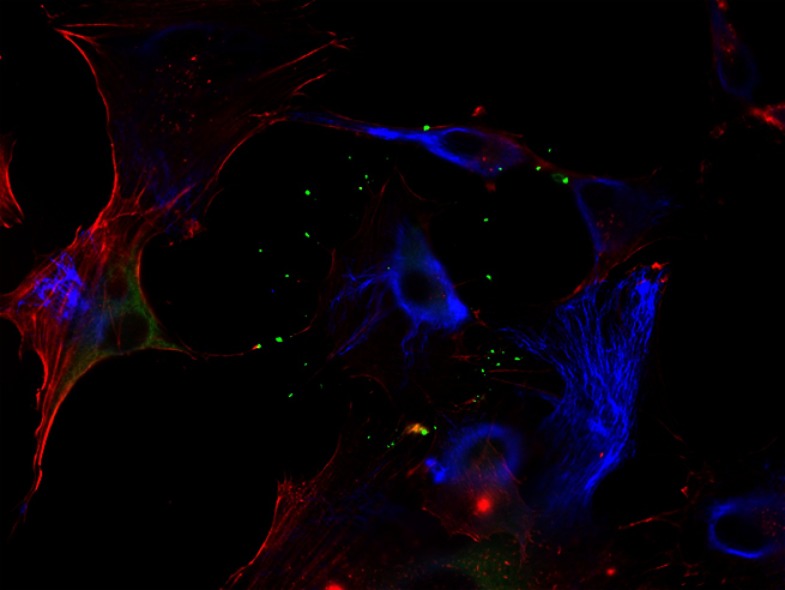
Myosin-X (green) localizes to the tips of filopodia and along the actin fibers (red), but not the intermediate filaments (blue), of human brain epithelial cells.
IMAGE COURTESY OF ANTTI ARJONEN
There are many reports of how integrin-mediated adhesion supports RTK function. So it was interesting that this particular integrin could actually counteract that, suggesting that not all adhesion is equivalent. What this work showed is that a signal in a localized area of the cell can have a dramatic effect. Having the phosphatase activated specifically at the sites of adhesion produces a much more efficient inhibition of the EGF receptor.
How did your group discover the integrin inhibitor SHARPIN?
Here, we went on to do RNAi screens and we thought we had left the α subunit–specific stuff behind. But we pulled out an integrin inactivator that binds to the α subunit, whereas all the other inhibitors have been found to bind to the β subunit. It seems I’m not very good at leaving things behind!
“Scientific talks usually get much better feedback on a remote Baltic island.”
The screen system developed by engineers working with cell biologists at VTT Technical Research Centre is a miniaturized system. You can do up to 12,000 siRNA experiments on a single microscope slide. In every spot on the array you have between 60 and 120 cells that become transfected with the spot’s specific siRNA because they endocytose it from the matrix.
We used a library of about 900 siRNAs corresponding mostly to kinases and phosphatases to screen PC3 prostate cancer cells. But SHARPIN is not a kinase or a phosphatase it turns out—it was misannotated in the library. Lucky for us.
So what is SHARPIN?
SHARPIN is kind of a counterforce. We see that the integrin activator talin is less recruited to the integrin if SHARPIN is there, even though we still don’t quite understand the mechanism. Talin binds the β subunit cytoplasmic tail and SHARPIN binds the α subunit tail. They seem to be mutually exclusive—you either have one or the other, active or inactive.
Another discovery starring the α subunit?
Yes. But with SHARPIN we started with an unbiased approach.
The α subunits are the forgotten half of integrins, though, truly. They must have important functions and therefore their cytoplasmic tails—even if they are a sad, short piece of protein—are important.
There’s been identification of many more cytoplasmic regulators of integrin function, both for the β and the α subunits. One of the challenges we now have in the field is figuring out how they are all coordinated. The tails are very short cytoplasmic domains, between 10 and 30 amino acids. And now there are tons of proteins known to bind to overlapping sequences. Is there some kind of hierarchy? How do the proteins known to bind to the β subunit influence the things that are binding to the α subunit?
How is the unconventional myosin-X connected to integrin and metastasis?
Myosin-X is known to bind integrin via the β-cytoplasmic tail and transport it to the tips of filopodia to provide anchorage. My student, putting together a list of filopodia-regulating genes for a review, noticed that myosin-X is highly up-regulated in basal-like breast cancer—the most invasive, most metastatic type of breast cancer.
We decided to look at whether myosin-X played a role in breast cancer. When it’s massively up-regulated, the filopodia become an invasion engine. It turns out that in patients, mutant p53 puts myosin-X on overdrive, which results in an increasing number of filopodia. These are probably acting as the first protrusions when the cell invades.
PRODUCTIVE GETAWAYS
What have you been up to lately?
I just finished a four-month visiting professorship at the Curie Institute in Paris working with cell biologists and biophysicists. I was so happy to be in the lab as “one of the postdocs.” The general theme was to learn new techniques—micropatterning, traction force microscopy, reconstitution of transmembrane proteins in lipid vesicles—to address the role of integrin activity regulation in adhesion, traction, and signaling.
The Ivaska lab before they sailed a Swan yacht from Turku to Kasnäs, Finland, as part of their annual lab retreat in 2013.
Your lab does an annual retreat—why is it important to get outside the lab together?
Some years the natives teach the foreigners how to cross-country ski, bake sweet buns on an open fire, and ice fish in winter. Recently, we have opted for a few days around midsummer as the white nights allow all-night swimming and sauna sessions.
Getting people away from the lab is good for bonding and a sense of being part of a team. In addition, scientific talks usually get much better feedback on a remote Baltic island than in the institutional seminar room.
Are you an outdoor enthusiast?
Scandinavian people in general are outdoor enthusiasts. We have 35,000 islands near Turku and plenty of wilderness close to the city, so getting out is easy and fun. My hobby is marathon running—as the distance increases and I am all alone with no music or any other gadgets, my brain starts working and I usually get the best work-related ideas.
References
- 1.Mattila E., et al. . 2005. Nat. Cell Biol. 7:78–85. [DOI] [PubMed] [Google Scholar]
- 2.Pellinen T., et al. . 2006. J. Cell Biol. 173:767–780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Rantala J.K., et al. . 2011. Nat. Cell Biol. 13:1315–1324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bouvard D., et al. . 2013. Nat. Rev. Mol. Cell Biol. 14:430–442. [DOI] [PubMed] [Google Scholar]
- 5.Arjonen A., et al. . 2014. J. Clin. Invest. 124:1069–1082. [DOI] [PMC free article] [PubMed] [Google Scholar]