Abstract
The restriction of cell intermingling across boundaries is essential for the establishment of discrete tissues. Eph receptor signaling prevents intermingling at many boundaries. In this issue, Luu et al. (2015. J. Cell Biol. http://dx.doi.org/10.1083/jcb.201409026) report a parallel pathway, mediated by Wnt signaling, Snail1, and paraxial protocadherin (PAPC). This pathway establishes a distinctive organization of cell adhesion and intercellular gaps at the interface between tissues.
The formation and maintenance of organized tissues is challenged by the intermingling of cells, which occurs as a consequence of cell proliferation and morphogenetic cell movements. Mechanisms that prevent intermingling across the boundaries of adjacent tissues thus have essential roles during development. Until recently, the principal mechanism was thought to be differential adhesion, created by tissue-specific expression of distinct cadherins, which mediate stronger homophilic compared with heterophilic cell adhesion (Steinberg, 1970). However, it is now known that at many boundaries, intermingling is prevented by other mechanisms, which are regulated by signaling between the adjacent cell populations (Dahmann et al., 2011; Batlle and Wilkinson, 2012; Fagotto, 2014). Eph receptors and their ephrin ligands have emerged as major mediators of such signaling and may act in boundary formation by modulating cadherin-mediated adhesion, cell repulsion, and cortical tension (Cayuso et al., 2014; Fagotto et al., 2014). Important insights have come from studies of Brachet’s cleft, which forms at the interface of migrating mesoderm cells and ectoderm during early stages of embryogenesis in Xenopus laevis. Brachet’s cleft is an example of a boundary at which cell separation creates a gap between the tissues, within which extracellular matrix becomes deposited. The complementary expression of Eph/ephrin binding partners creates bidirectional Eph receptor activation at the mesoderm–ectoderm interface (Rohani et al., 2011, 2014). Eph activation underlies cycles of adhesion and repulsion, which enables mesoderm cells to migrate on ectoderm while also preventing them from invading (Rohani et al., 2011). The results of altering cadherin levels reveal that a balance is required: with too little adhesion, low level Eph receptor activation within mesoderm causes homotypic repulsion; with too much adhesion, mesoderm cells will invade ectoderm (Rohani et al., 2014).
Previous studies suggested that PAPC and noncanonical Wnt signaling in mesoderm cells act synergistically to promote boundary formation at Brachet’s cleft (Kim et al., 1998; Winklbauer et al., 2001). In this issue, Luu et al. find that cell separation also requires Snail1, a transcription factor that regulates the motility of mesoderm cells (Blanco et al., 2007). The relationships between these components were investigated in an incisive series of gain- and loss-of-function experiments in X. laevis and zebrafish embryos, as well as tissue explant assays of cell separation (Luu et al., 2015). These experiments reveal that a Wnt receptor, XFz7, acts through a noncanonical pathway (Dvl2–RhoA–JNK–c-Jun) to up-regulate Snail1, which in turn enables PAPC to promote cell separation.
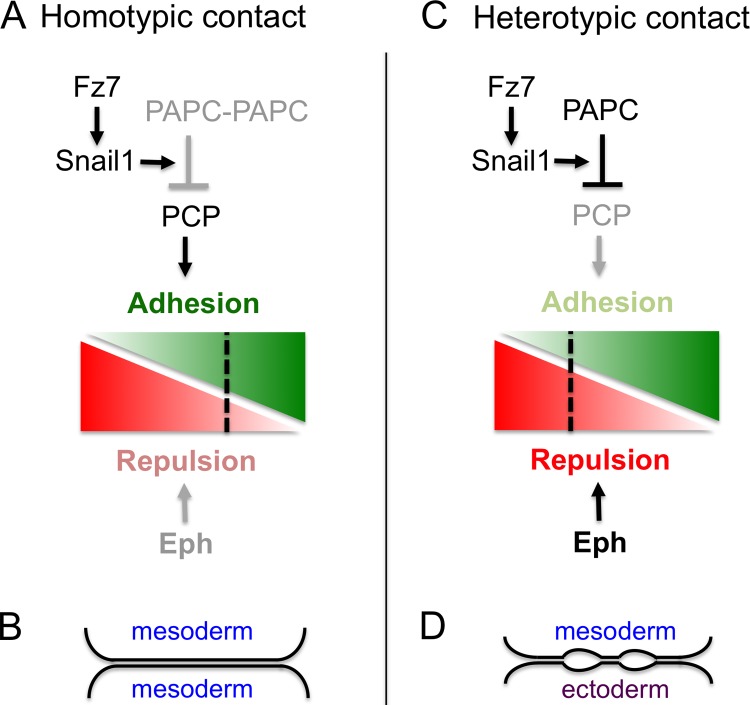
An essential feature of signaling in boundary formation is that it leads to distinct cell responses at heterotypic compared with homotypic contacts of cells. For Eph/ephrin signaling, this is achieved by complementary expression of high affinity binding partners such that strongest Eph receptor activation occurs at the heterotypic interface (Cayuso et al., 2014; Rohani et al., 2014). How then is this achieved for PAPC? Important evidence comes from the finding that overexpression of PAPC in the adjacent tissue prevents Snail1+PAPC from inducing cell separation (Luu et al., 2015). Cell separation thus requires interaction between PAPC-expressing and -nonexpressing cells. Because PAPC can bind to PAPC on adjacent cells, a simple model is that PAPC–PAPC trans-complexes are inactive, whereas free PAPC is present at the heterotypic interface and promotes cell separation. A clue for how PAPC activity regulates cell behavior came from apparently contradictory findings that although Dvl2 is required for Snail1 expression, cell separation still occurs after Dvl2 knockdown (Luu et al., 2015). This paradox was resolved by experiments using Dvl2 puncta as an indicator of Wnt planar cell polarity (PCP) pathway activity: Snail1+PAPC acts to decrease PCP activity at heterotypic but not at homotypic contacts of mesoderm cells. Because the PCP pathway can promote cell adhesion, this local inhibition alters cell behavior selectively at the mesoderm–ectoderm interface (Fig. 1).
Figure 1.
Signaling and responses to cell interactions. (A) At homotypic contacts of mesoderm cells, the PCP pathway can promote adhesion (green) because PAPC complexes form that have low PCP inhibitory activity. Eph receptor activation that promotes repulsion (red) is weak because coexpressed ephrins have low affinity. Consequently, the balance of cell responses favors adhesion (B). (C) At heterotypic contacts, free PAPC inhibits the PCP pathway. This PAPC activity requires Fz7-induced expression of Snail1. Eph receptors are strongly activated by high affinity ephrins expressed in ectoderm. Consequently, there is a balance of repulsion and adhesion that leads to formation of cleft contacts, characterized by interspersed stretches of adhesion and intercellular gaps (D).
In principle, PAPC and Eph/ephrin activation could underlie boundary formation simply by establishing differential adhesion. However, there is strong evidence that this is not the case, for example, because PAPC overexpression does not alter cell adhesion as measured by tissue surface tension. In studies of the properties of the PAPC+–PAPC− interface, Luu et al. (2015) find that the cells form adhesive contacts yet can slide past each other easily. Electron microscopy reveals that the heterotypic interface is comprised of large intercellular gaps interspersed with stretches of membrane apposition consistent with adhesive interactions. This morphological organization—termed cleft contact—may enable adhesive interactions required for cell migration while preventing intermingling between the tissues. One way to think about cleft contacts is that they are a hybrid of cell adhesion and repulsion that requires input from PAPC and Eph receptor signaling to achieve the appropriate balance (Fig. 1).
These exciting findings set the stage for a number of further questions. One important issue is to understand at the biochemical level the activity of PAPC–PAPC complexes versus free PAPC, as well as the relationship between Snail1 and PAPC. Whereas Snail1 is required for full-length PAPC to promote cell separation, truncated PAPC lacking the cytoplasmic domain is sufficient in the absence of Snail1 (Luu et al., 2015). These observations suggest that a regulatory function of the cytoplasmic domain of PAPC is modulated by binding of a transcriptional target of Snail1. Another question is how Eph/ephrin signaling and PAPC synergize. This may involve convergence on Dvl activity because segregation of Eph receptor and ephrin-expressing cells requires interactions with Dvl (Tanaka et al., 2003). Finally, how does extracellular matrix accumulate in the intercellular gaps at cleft-like boundaries? During somitogenesis, Eph/ephrin signaling initiates boundary formation, and then by inside-out integrin activation promotes accumulation of extracellular matrix at the border (Jülich et al., 2009). Extracellular matrix in turn promotes integrin signaling required for boundary maintenance (Jülich et al., 2005; Koshida et al., 2005). It will be interesting to uncover whether there is a similar role of Eph/ephrin signaling in extracellular matrix deposition at Brachet’s cleft and other cleft-like boundaries.
Acknowledgments
Work in the author’s laboratory is supported by the Medical Research Council (U117532048).
The author declares no competing financial interests.
References
- Batlle E., and Wilkinson D.G.. 2012. Molecular mechanisms of cell segregation and boundary formation in development and tumorigenesis. Cold Spring Harb. Perspect. Biol. 4:a008227 10.1101/cshperspect.a008227 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blanco M.J., Barrallo-Gimeno A., Acloque H., Reyes A.E., Tada M., Allende M.L., Mayor R., and Nieto M.A.. 2007. Snail1a and Snail1b cooperate in the anterior migration of the axial mesendoderm in the zebrafish embryo. Development. 134:4073–4081 10.1242/dev.006858 [DOI] [PubMed] [Google Scholar]
- Cayuso J., Xu Q., and Wilkinson D.G.. 2014. Mechanisms of boundary formation by Eph receptor and ephrin signaling. Dev. Biol. In press 10.1016/j.ydbio.2014.11.013 [DOI] [PubMed] [Google Scholar]
- Dahmann C., Oates A.C., and Brand M.. 2011. Boundary formation and maintenance in tissue development. Nat. Rev. Genet. 12:43–55 10.1038/nrg2902 [DOI] [PubMed] [Google Scholar]
- Fagotto F.2014. The cellular basis of tissue separation. Development. 141:3303–3318 10.1242/dev.090332 [DOI] [PubMed] [Google Scholar]
- Fagotto F., Winklbauer R., and Rohani N.. 2014. Ephrin-Eph signaling in embryonic tissue separation. Cell Adhes. Migr. 8:308–326 10.4161/19336918.2014.970028 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jülich D., Geisler R., and Holley S.A.. Tübingen 2000 Screen Consortium. 2005. Integrinα5 and Delta/Notch signaling have complementary spatiotemporal requirements during zebrafish somitogenesis. Dev. Cell. 8:575–586 10.1016/j.devcel.2005.01.016 [DOI] [PubMed] [Google Scholar]
- Jülich D., Mould A.P., Koper E., and Holley S.A.. 2009. Control of extracellular matrix assembly along tissue boundaries via Integrin and Eph/Ephrin signaling. Development. 136:2913–2921 10.1242/dev.038935 [DOI] [PubMed] [Google Scholar]
- Kim S.H., Yamamoto A., Bouwmeester T., Agius E., and Robertis E.M.. 1998. The role of paraxial protocadherin in selective adhesion and cell movements of the mesoderm during Xenopus gastrulation. Development. 125:4681–4690. [DOI] [PubMed] [Google Scholar]
- Koshida S., Kishimoto Y., Ustumi H., Shimizu T., Furutani-Seiki M., Kondoh H., and Takada S.. 2005. Integrinα5-dependent fibronectin accumulation for maintenance of somite boundaries in zebrafish embryos. Dev. Cell. 8:587–598 10.1016/j.devcel.2005.03.006 [DOI] [PubMed] [Google Scholar]
- Luu O., Damm E.W., Parent S., Barua D., Smith T.H.L., Wen J.W.H., Lepage S.E., Nagel M., Ibrahim-Gawel H., Huang Y., et al. 2015. PAPC mediates self/non-self distinction during Snail-dependent tissue separation. J. Cell Biol. 208:839–856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rohani N., Canty L., Luu O., Fagotto F., and Winklbauer R.. 2011. EphrinB/EphB signaling controls embryonic germ layer separation by contact-induced cell detachment. PLoS Biol. 9:e1000597 10.1371/journal.pbio.1000597 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rohani N., Parmeggiani A., Winklbauer R., and Fagotto F.. 2014. Variable combinations of specific ephrin ligand/Eph receptor pairs control embryonic tissue separation. PLoS Biol. 12:e1001955 10.1371/journal.pbio.1001955 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steinberg M.S.1970. Does differential adhesion govern self-assembly processes in histogenesis? Equilibrium configurations and the emergence of a hierarchy among populations of embryonic cells. J. Exp. Zool. 173:395–433 10.1002/jez.1401730406 [DOI] [PubMed] [Google Scholar]
- Tanaka M., Kamo T., Ota S., and Sugimura H.. 2003. Association of Dishevelled with Eph tyrosine kinase receptor and ephrin mediates cell repulsion. EMBO J. 22:847–858 10.1093/emboj/cdg088 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winklbauer R., Medina A., Swain R.K., and Steinbeisser H.. 2001. Frizzled-7 signalling controls tissue separation during Xenopus gastrulation. Nature. 413:856–860 10.1038/35101621 [DOI] [PubMed] [Google Scholar]