Abstract
Background
The aim of this study was to investigate the expression level of circulating microRNA-31(miRNA-31) in lung cancer patients and its clinical significance.
Material/Methods
Real-time fluorescent quantitative PCR was utilized to detect the circulating miRNA-31 expression levels in 300 lung cancer patients and 300 health control subjects. The ROC curve was drawn to evaluate the diagnostic value of the circulating miRNA-31 expression levels in lung cancer. The 300 lung cancer patients were divided into a miRNA-31 low-expression group and a miRNA-31 high-expression group. A survival curve was drawn according to the Kaplan-Meier method to evaluate the prognostic value of the circulating microRNA-31 expression levels for lung cancer.
Results
The circulating miRNA-31 expression levels in the lung cancer patients (l.88±0. 67) increased significantly (P<0.001) compared to the healthy controls (0.58±0. 44). The area under the ROC curve drawn according to the circulating miRNA-31 expression levels was 0.785 (95% CI=0.486–0.763). When the critical value was 1.27, the sensitivity and specificity for lung cancer diagnosis according to the circulating miRNA-31 expression levels were 0.769 and 0.745, respectively. The difference in the survival curve between the miRNA-31 low-expression group (123 cases) and high-expression group (177 cases) was statistically significant (P=0.004). Median survival period of the low-expression group (38.44 months) was longer than that of the high-expression group (25.23 months).
Conclusions
miRNA-31 may be a molecular marker for the diagnostic and prognostic evaluation of primary lung cancer.
MeSH Keywords: Blood Circulation, Lung Neoplasms, RNA Isoforms
Background
Lung cancer is the malignant tumor with the highest morbidity and mortality around the world. Its incidence is gradually increasing, and the 5-year survival rate is only 15% [1]. MicroRNAs (miRs) are endogenous non-coding RNAs of ~23-mer, which have important roles in regulation of gene expression. The mature form of miRs silence gene expression by binding to the 3′-UTR of target mRNAs and initiate translational repression or cleavage of cognate mRNAs [2]. miRNA-31 was reported to be associated with human cancers [3]. Overexpression of miRNA -31 inhibits cancer cell proliferation by p53-dependent mechanisms in ovarian cancer cells [4]. miRNA -31 was also reported to inhibit metastasis, and it enhanced primary tumor growth in breast cancer [5]. Furthermore, there is a report suggesting that miRNA -31 inhibits cell proliferation, migration, and invasion in malignant mesothelioma [6]. Contributions of miRNA -31 to the activation of hypoxia-inducible factor for development of head and neck squamous cell cancer were also described [7]. Previous studies [8,9] have found high expression of miRNA-31 in lung cancer tissues, but few studies have focused on its expression level in peripheral blood. We collected peripheral blood from primary lung cancer patients, and detected and analyzed the miRNA-31 expression through real-time fluorescent quantitation PCR method to investigate the potential value of miRNA-31 expression level in peripheral blood for early diagnosis and prognosis of lung cancer.
Material and Methods
Subjects
We enrolled 300 primary lung cancer patients treated with carcinectomy in the Department of Respiratory Medicine, Nanjing Medical University Affiliated Nanjing Hospital from May 2005 to December 2014. All primary lung cancer patients without any chemoradiation before surgery were found for the first time and finally diagnosed by pathology. There were 123 cases ≤60 years old and 177 cases were >60 years old; 192 cases were smokers and 108 cases were not smokers; 114 cases were in primary lesion T1 grade, 105 cases were in T2 grade, and 81 cases were in T3 grade; 54 cases were in clinical stage I, 48 cases were in stage II, 75 cases were in stage III, and 123 cases were in stage IV; 72 cases were well differentiated, 111 cases were moderately differentiated, and 117 cases were poorly differentiated; 126 cases had lymph nodes metastasis and 174 cases were without lymph nodes metastasis. All the patients were treated with standard therapy such as chemotherapy and radiotherapy after the operation. A total of 300 health adults were enrolled as controls.
Detection of miRNA-31 in peripheral blood
We collected 5 ml of venous blood from each patient, using EDTA- anticoagulation tubes, before the operation, and centrifuged them at 3000 r/m at 4°C for 10 min, then placed the supernatant into DEPC-treated EP tubes, and stored them at −80°C. We treated the total RNA with a reverse transcription kit (Bioteck, Beijing, China) according to the protocol to obtain cDNA and then performed real-time fluorescent quantitation PCR with an ABI 7500 fluorescent quantitation PCR analyzer (ABI, CA, USA). Taking miRNA-16 as a reference, we calculated the mean of 3 multiple wells for each sample. We used 3 multiple wells for each sample to obtain their mean and calculated the miRNA-31 expression levels with 2−ΔCT (ΔCT=CTmiRNA-31-CTmiRNA-16).
Statistical analyses
Data were processed using SPSS 21.0 (Chicago, IL, USA). Two independent-samples t-tests were utilized to compare miRNA-31 expression levels in peripheral blood of lung cancer patients and healthy adults. The χ2 test was used to compare the high-expression rate of miRNA-31 in peripheral blood of patients with different clinical pathology characteristics. We established an ROC curve to evaluate the diagnostic value of miRNA-31 expression level in peripheral blood for primary lung cancer. Taking death as the terminal event after follow-up for 5 years, we drew the survival curve for the miRNA-31 high-expression group and low-expression group using the Kaplan-Meier method and used the log-rank test for the 2 curves with the test size of P<0.05.
Results
Comparison of circulating miRNA-31 expression level in peripheral blood of lung cancer patients and health controls
The miRNA-31 expression level in peripheral blood of lung cancer patients (l.88±0. 67) increased significantly (P<0.001) compared to the health controls (0.58±0. 44).
Comparison of circulating miRNA-31 expression level in patients with different clinical pathology characteristics
Taking 1.88 – the mean of miRNA-31 expression levels in peripheral blood of 300 lung cancer patients – as critical value, we categorized cases whose peripheral blood miRNA-31 were <1.88 into the low-expression group and that ≥1.88 into the high-expression group; therefore, 177 cases were in the high-expression group and 123 cases were in the low-expression group. The comparison of miRNA-31 expression level in peripheral blood of patients with different clinical pathology characteristics is shown in Table 1.
Table 1.
Comparison of miRNA-31 expression level in peripheral blood of patients with different clinical pathology characteristics.
| Group | n | Low-expression | High-expression | P value |
|---|---|---|---|---|
| Age (years) | 0.157 | |||
| ≤60 | 123 | 48 | 75 | |
| >60 | 177 | 84 | 93 | |
| Smoking | 0.001 | |||
| Yes | 192 | 69 | 123 | |
| No | 108 | 63 | 45 | |
| Grade | 0.633 | |||
| T1 | 114 | 45 | 69 | |
| T2 | 105 | 48 | 57 | |
| T3 | 81 | 39 | 42 | |
| Clinical stages | <0.001 | |||
| I | 54 | 36 | 18 | |
| II | 48 | 33 | 15 | |
| III | 75 | 24 | 51 | |
| IV | 123 | 39 | 84 | |
| Differentiation | 0.444 | |||
| Well | 72 | 27 | 45 | |
| Moderately | 111 | 51 | 60 | |
| Poorly | 117 | 54 | 63 | |
| Lymph node metastasis | <0.001 | |||
| Yes | 126 | 30 | 96 | |
| No | 174 | 102 | 72 |
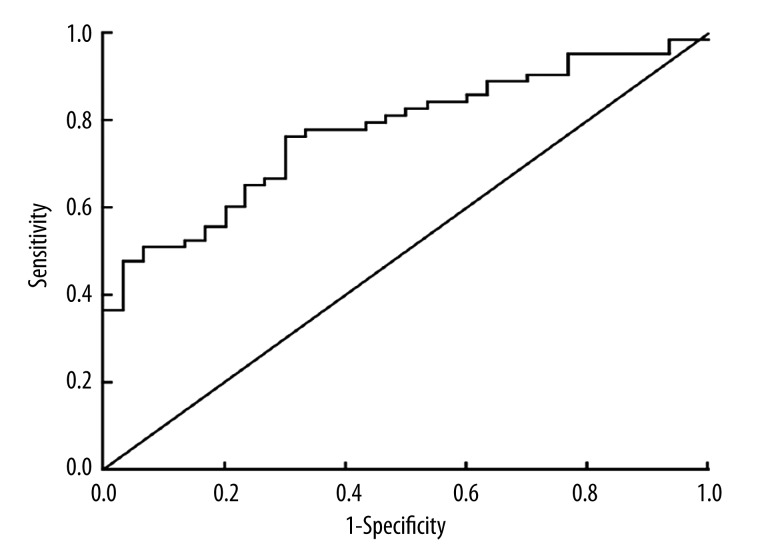
ROC curve for diagnostic value of miRNA-31 expression level in peripheral blood for primary lung cancer
As shown in Figure 1, the area under the ROC curve was 0.785 (95% CI=0.486–0.763). Sensitivity and specificity for primary lung cancer diagnosis according to the miRNA-31 expression levels in peripheral blood were 0.769 and 0.745, respectively, when the critical value was 1.27.
Figure 1.
ROC curve for expression of circulating miRNA-31 levels in lung cancer, with an area under the ROC curve=0.785.
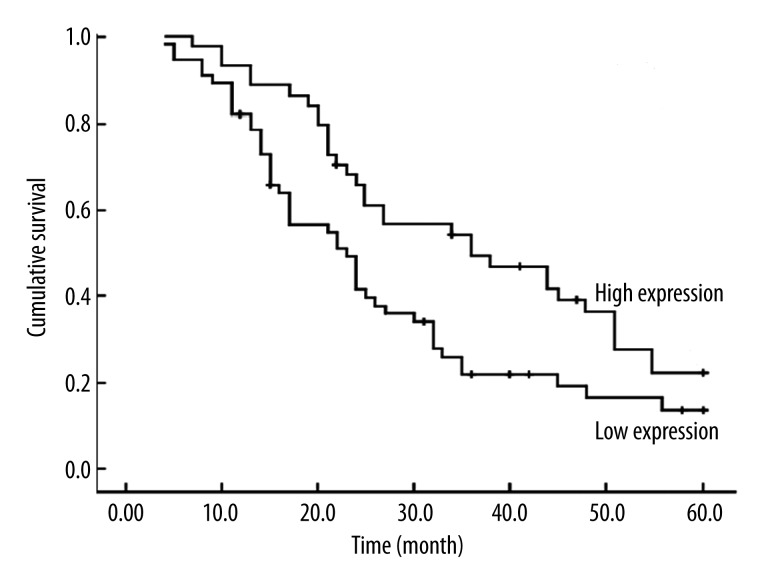
Relationship between miRNA-31 expression level in peripheral blood of lung cancer patients and their prognosis
The 2 survival curves of miRNA-31 high-expression group and low-expression group are shown in Figure 2; their differences were statistical significance (P=0.004). The median survival period of the low-expression group (38.44 months) was longer than that of the high-expression group (25.23 months).
Figure 2.
Kaplan-Meier analysis curve of postoperative survival times of lung cancer patients according to the miRNA-31 relative expression. Patients with high miRNA-31 expression had significantly poorer survival times compared to those with low miRNA-31 expression. P values were calculated by Kaplan-Meier analysis (P<0.001).
Discussion
In the present study, we found the circulating miRNA-31 expression levels in the lung cancer patients increased significantly compared to the health controls. Also, median survival period of the low-expression group was longer than that of the high-expression group. Our results indicated that miRNA-31 is a molecular marker for the diagnostic and prognostic evaluation of primary lung cancer.
Early detection and early treatment are effective ways to improve the recovery rate and the prognosis of lung cancer patients [10,11]. Therefore, it is very important to search for markers for early diagnosis and prognosis evaluation. The correlations between miRNA expression and tumor generation, development, and prognosis have been widely studied with the development of miRNA expression spectrum research [12–16]. The initial evidence of the relationship between miRNA and tumor was from the study of Calin et al. in 2002 [17], who found that expression of miR-15a and miR-16 are decreased or deleted in most chronic lymphocytic leukemia patients. miRNA stability is a prerequisite for potential tumor markers [18]. A recent meta-analysis indicates that miR-21 detection has a prognostic value in patients with gastric cancer [19]. Another recent study suggested that the expression of miR-101 is down-regulated in bladder transitional cell carcinoma (BTCC) and may play an important role as a diagnostic and prognostic marker in BTCC [20]. Utilizing miRNA expression level in peripheral blood to diagnose tumors early is effective and deserves to be explored further because miRNA is very stable in blood plasma and serum. Liu et al. [8] found that miRNA-31 is highly expressed in lung cancer tissue. Xi et al. [9] confirmed that miRNA-31 expression in non-small-cell lung carcinoma tissue was 4.13-fold that in normal lung tissue. We have detected the expression of miRNA-31 in peripheral blood of 300 cases of primary lung cancer and 300 healthy adults. Our results show that miRNA-31 expression level in peripheral blood of lung cancer patients is significantly higher than that of health adults. miRNA-31 expression level in peripheral blood of lung cancer patients who are smokers with high clinical stage and lymph node metastasis are significantly higher than that of patients who are not smokers and who have low clinical stage and are without lymph node metastasis.
MiRNA-31 was first identified in HeLa cells [21] and is located on chromosome 9p21.3. Mounting evidence shows that MiRNA-31 has different expression patterns in different cancers: it is up-regulated in colorectal cancer (CRC) [22], head and neck squamous cell carcinoma (HNSCC) [23], hepatocellular carcinoma [24], squamous cell carcinoma of the tongue [25], and lung cancer [26]; but it is down-regulated in invasive urothelial carcinoma of the bladder [27], prostate cancer [28], gastric cancer [29], breast cancer [30], and serous ovarian cancer [31]. Although there is growing evidence that miR-31 level varies among cancer types, functional roles for miR-31 have yet to be defined. However, the circulating miR-31 level may act as a clinical biomarker for cancer. In the present study, we drew the ROC curve according to miRNA-31 expression level in peripheral blood, and the area under curve reached 0.785 (95% CI=0.486–0.763). Survival analysis shows that median survival period of the miRNA low-expression group was longer than that of the miRNA high-expression group, suggesting that miRNA-31 expression level in peripheral blood has good diagnostic value and can be utilized to evaluate patient prognosis.
Conclusions
Our study has confirmed the existence of miRNA-31 in peripheral blood of lung cancer patients, showing that the expression level of miRNA-31 in peripheral blood can be utilized in clinics as a molecular marker for evaluation of lung cancer diagnosis and prognosis.
Footnotes
Source of support: The present study was supported by the Nanjing Science and Technology Development Project: (201201074)
References
- 1.Sheel AR, McShane J, Poullis MP. Survival of patients with or without symptoms undergoing potentially curative resections for primary lung cancer. Ann Thorac Surg. 2013;95(1):276–84. doi: 10.1016/j.athoracsur.2012.09.051. [DOI] [PubMed] [Google Scholar]
- 2.Del Vescovo V, Grasso M, Barbareschi M, Denti MA. MicroRNAs as lung cancer biomarkers. World J Clin Oncol. 2014;5(4):604–20. doi: 10.5306/wjco.v5.i4.604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Mitamura T, Watari H, Wang L, et al. Downregulation of miRNA-31 induces taxane resistance in ovarian cancer cells through increase of receptor tyrosine kinase MET. Oncogenesis. 2013;2:e40. doi: 10.1038/oncsis.2013.3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Creighton CJ, Fountain MD, Yu Z, et al. Molecular profiling uncovers a p53-associated role for microRNA-31 in inhibiting the proliferation of serous ovarian carcinomas and other cancers. Cancer Res. 2010;70:1906–15. doi: 10.1158/0008-5472.CAN-09-3875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Valastyan S, Reinhardt F, Benaich N, et al. A pleiotropically acting microRNA, miR-31, inhibits breast cancer metastasis. Cell. 2009;137:1032–46. doi: 10.1016/j.cell.2009.03.047. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 6.Ivanov SV, Goparaju CM, Lopez P, et al. Pro-tumorigenic effects of miR-31 loss in mesothelioma. J Biol Chem. 2010;285:22809–17. doi: 10.1074/jbc.M110.100354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Liu CJ, Tsai MM, Hung PS, et al. miR-31 ablates expression of the HIF regulatory factor FIH to activate the HIF pathway in head and neck carcinoma. Cancer Res. 2010;70:1635–44. doi: 10.1158/0008-5472.CAN-09-2291. [DOI] [PubMed] [Google Scholar]
- 8.Liu X, Sempere LF, Ouyang H, et al. MicroRNA-31 functions as an oncogenic microRNA in mouse and human lung cancer cells by repressing specific tumor suppressors. J Clin Invest. 2010;120(4):1298–309. doi: 10.1172/JCI39566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Xi S, Yang M, Tao Y, et al. Cigarette smoke induces C/EBP-β-mediated activation of miR-31 in normal human respiratory epithelia and lung cancer cells. PLoS One. 2010;5(10):e13764. doi: 10.1371/journal.pone.0013764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Zhang XC, Lu S, Zhang L, et al. Consensus on dignosis for ALK positive non-small cell lung cancer in China, the 2013 version. Zhonghua Bing Li Xue Za Zhi. 2013;42(6):402–6. doi: 10.3760/cma.j.issn.0529-5807.2013.06.012. [DOI] [PubMed] [Google Scholar]
- 11.Zhang G, Cao M, Wang Z, Li Q. Characteristics and clinical significance of diffusing capacity in the patients with lung cancer. Zhongguo Fei Ai Za Zhi. 2002;5(3):207–10. doi: 10.3779/j.issn.1009-3419.2002.03.15. [DOI] [PubMed] [Google Scholar]
- 12.Xia L, Ren Y, Fang X, et al. Prognostic Role of Common MicroRNA Polymorphisms in Cancers: Evidence from a Meta-Analysis. PLoS One. 2014;9(10):e106799. doi: 10.1371/journal.pone.0106799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Li J, Zheng Y, Sun G, Xiong S. Restoration of miR-7 expression suppresses the growth of Lewis lung cancer cells by modulating epidermal growth factor receptor signaling. Oncol Rep. 2014;32(6):2511–16. doi: 10.3892/or.2014.3519. [DOI] [PubMed] [Google Scholar]
- 14.Joshi P, Middleton J, Jeon YJ, Garofalo M. MicroRNAs in lung cancer. World J Methodol. 2014;4(2):59–72. doi: 10.5662/wjm.v4.i2.59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Dong Z, Ren L, Lin L, et al. Effect of microRNA-21 on multidrug resistance reversal in A549/DDP human lung cancer cells. Mol Med Rep. 2015;11(1):682–90. doi: 10.3892/mmr.2014.2662. [DOI] [PubMed] [Google Scholar]
- 16.Wei J, Ma Z, Li Y, et al. miR-143 inhibits cell proliferation by targeting autophagy-related 2B in non-small cell lung cancer H1299 cells. Mol Med Rep. 2015;11(1):571–76. doi: 10.3892/mmr.2014.2675. [DOI] [PubMed] [Google Scholar]
- 17.Calin GA, Dumitru CD, Shimizu M, et al. Frequent deletions and down-regulation of micro- RNA genes miR15 and miR16 at 13q14 in chronic lymphocytic leukemia. Proc Natl Acad Sci USA. 2002;99(24):15524–29. doi: 10.1073/pnas.242606799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Mitchell PS, Parkin RK, Kroh EM, et al. Circulating microRNAs as stable blood-based markers for cancer detection. Proc Natl Acad Sci USA. 2008;105(30):10513–18. doi: 10.1073/pnas.0804549105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wang Z, Cai Q, Jiang Z, et al. Prognostic role of MicroRNA-21 in gastric cancer: a meta-analysis. Med Sci Monit. 2014;20:1668–74. doi: 10.12659/MSM.892096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Zhang H, Qi F, Cao Y, et al. Down-regulated microRNA-101 in bladder transitional cell carcinoma is associated with poor prognosis. Med Sci Monit. 2014;20:812–17. doi: 10.12659/MSM.890300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lagos-Quintana M, Rauhut R, Lendeckel W, TuschI T. Identification of novel genes coding for small expressed RNAs. Science. 2001;294:853–58. doi: 10.1126/science.1064921. [DOI] [PubMed] [Google Scholar]
- 22.Wang CJ, Zhou ZG, Wang L, et al. Clinicopathological significance of microRNA-31, -143 and -145 expression in colorectal cancer. Dis Markers. 2009;26:27–34. doi: 10.3233/DMA-2009-0601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Liu CJ, Tsai MM, Hung PS, et al. miR-31 ablates expression of the HIF regulatory factor FIH to activate the HIF pathway in head and neck carcinoma. Cancer Res. 2010;70:1635–44. doi: 10.1158/0008-5472.CAN-09-2291. [DOI] [PubMed] [Google Scholar]
- 24.Wong QW, Lung RW, Law PT, et al. MicroRNA-223 is commonly repressed in hepatocellular carcinoma and potentiates expression of Stathmin1. Gastroenterology. 2008;135:257–69. doi: 10.1053/j.gastro.2008.04.003. [DOI] [PubMed] [Google Scholar]
- 25.Wong TS, Liu XB, Wong BY, et al. Mature miR-184 as Potential Oncogenic microRNA of Squamous Cell Carcinoma of Tongue. Clin Cancer Res. 2008;14:2588–92. doi: 10.1158/1078-0432.CCR-07-0666. [DOI] [PubMed] [Google Scholar]
- 26.Liu X, Sempere LF, Ouyang H, et al. MicroRNA-31 functions as an oncogenic microRNA in mouse and human lung cancer cells by repressing specific tumor suppressors. J Clin Invest. 2010;120:1298–309. doi: 10.1172/JCI39566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Wszolek MF, Rieger-Christ KM, Kenney PA, et al. A microRNA expression profile defining the invasive bladder tumor phenotype. Urol Oncol. 2011;29(6):794–801.e1. doi: 10.1016/j.urolonc.2009.08.024. [DOI] [PubMed] [Google Scholar]
- 28.Schaefer A, Jung M, Mollenkopf HJ, et al. Diagnostic and prognostic implications of microRNA profiling in prostate carcinoma. Int J Cancer. 2010;26:1166–76. doi: 10.1002/ijc.24827. [DOI] [PubMed] [Google Scholar]
- 29.Zhang Y, Guo J, Li D, et al. Down-regulation of miR-31 expression in gastric cancer tissues and its clinical significance. Med Oncol. 2010;27:685–89. doi: 10.1007/s12032-009-9269-x. [DOI] [PubMed] [Google Scholar]
- 30.Valastyan S, Reinhardt F, Benaich N, et al. A pleiotropically acting microRNA, miR-31, inhibits breast cancer metastasis. Cell. 2009;137:1032–46. doi: 10.1016/j.cell.2009.03.047. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 31.Creighton CJ, Fountain MD, Yu Z, et al. Molecular profiling uncovers a p53-associated role for microRNA-31 in inhibiting the proliferation of serous ovarian carcinomas and other cancers. Cancer Res. 2010;70:1906–15. doi: 10.1158/0008-5472.CAN-09-3875. [DOI] [PMC free article] [PubMed] [Google Scholar]