Abstract
Insulin-like Growth Factor-1 (IGF-1) is one of the significant substances affecting the growth and development of cartilage tissue in the body. The aim of this study is to evaluate the possible histopathological effects of local IGF-1 injection on the viability of rabbit auricular cartilage autografts.
To this end, the single-piece and sliced cartilage tissues obtained from 20 albino rabbits’ auricula were implanted in the subcutaneous pockets created on the back skins of the experimental animals. Every two weeks IGF-1 (10 mg/ml) injections were performed on the autograft implants of one group and normal saline (0.9%) injections were performed on the other group. Experimental animals were sacrificed at the end of the third month. A total of 34 tissue samples obtained after dissection were evaluated and scored histopathologically according to their cartilage viability, environmental reaction, and regenerative activities.
The intergroup evaluation carried out for the single-piece and sliced cartilage grafts revealed that there was statistically more cartilage viability and less foreign-body reaction in the IGF-1 group than the normal saline group (p<0.05).
While there was a statistically significant difference between the groups for single-piece grafts regarding regenerative activity (p<0.05), there was no significant difference for sliced grafts. The IGF-1 group, however, showed more activity.
The results we obtained point out to the fact that IGF-1 increases the tissue viability of the implanted auricular autograft and it suppresses immune modulation effect.
Keywords: auricle, rabbit, cartilage, autograft, IGF-1
INTRODUCTION
Cartilage grafts are frequently needed in cosmetic and reconstructive surgery of the head and neck area. The autogeneous cartilage tissue that is used to this end is typically preferred because they are easily obtainable, shapeable, and they have high histocompatibility [1]. On the other hand, inflammatory tissue response and shortages in tissue nutrition make it hard for the graft tissue to survive in the implanted area. When the fact that the auricular cartilage tissue is structurally thin and labile is added to these effects, it all together causes loss of volume and tissue resistance in the implant [1, 2]. Insulin-like Growth Factor-1 (IGF-1), also known as Somatomedin-C, which is a growth factor having a protein structure and coded to IGF-gene in humans [3,4]. The IGF-1 molecule includes 70 amino acids with a molecular weight of 7649 Dalton. It has 3 disulphide bonds in a single chain [5]. IGF-1 produced by many tissues during embryogenesis starts to be produced by the liver at the end of embryogenesis. The main source of IGF-1 for adults is the liver [6]. The Insulinlike Growth Factors (IGFs) are carried through the binding proteins in blood and form their effects on the tissues by way of Insulin-like Growth Factor (IGF) receptors [7, 8]. IGF-1 is an important factor in the growth and differentiation of many tissues in the body, especially in the growth and differentiation of the skeleton [9]. All the cells responsible for bone modeling (osteoprogenitor cells, osteoblasts, osteocytes, and osteoclasts) can produce IGF [10]. Studies carried out with osteoblast cell cultures demonstrated that IGFs increase the proliferation, differentiation, matrix production, and mineralization functions of the chondroblasts at every stage of development [10, 11]. There are many studies proving the anabolic and chondrogenic effects of IGF-1 on the cartilage tissue by increasing the type 2 collagen mRNA expression and proteoglycan synthesis [12, 13]. Due to its mentioned characteristics, we have therefore, evaluated the local effects of IGF-1 on rabbits’ auricular cartilage autograft viability.
MATERIALS AND METHODS
The study was carried out at Ankara University, Faculty of Veterinary Medicine’s Animal Research Laboratory with the approval of the same university’s animal research ethics board. All the procedures were performed in compliance with the rules set by Ankara University Board of Animal Research Ethics.
Animals
Twenty albino rabbits weighing between 2.5-3 kg were used in the study as experimental animals. The animals were kept in single cages of their own with water and food present all the time at 50% humidity and a temperature of 23°C.
Procedures
The surgical procedures performed on the animals were performed under general anaesthesia in sterile operation room conditions. Intraperitoneal ketamine (30mg/kg) (KE-TALAR, Pfizer-Pharmaceutical Company) and Xylazine (5mg/kg) (Rompun, Bayer) were used for general anaesthesia. The hair on the ear and dorsal areas of all the animals were shaved with a shaving machine under anesthesia. The exposed skin tissues were cleansed with an iodinated disinfectant solution. One auricle of each animal was excised with an electrocautery leaving some cartilage tissue around the external ear canal. After peeling the skin layer of the excised auricular tissue, the auricular cartilage was cut into 2×2 cm square shaped parts protecting the pericondrium on the cartilage tissues. One of these parts was further cut into smaller parts measuring 2-3 mm by a scalpel. Two cm skin incisions with 4-5 cm distance among them were performed on the rabbits’ dorsal area. The skin and the subcutaneous tissues were cut and pockets were formed with some elevation laterally from the incision line in this area. Utmost care was paid to prevent bleeding and surrounding tissue damage. The cartilage tissues were placed in these pockets and the incision line was sutured with 3.0 prolene suture material. The skin covering the implantation area was marked with drawing ink. IGF-1 (Octreotide), 0.1 mg/ml (Sandostatin LAR Vial No-vartis) for the experiment group, and 0.9% normal saline/ml (Adeka ampoule) for the control group were subcutaneously injected into the pocket where the implant was placed, the first being injected following the implantation. The same procedure was repeated every two weeks for a total of 3 months. The hair on the application area was shaved and cleansed with 70% alcohol before each injection when necessary. Three animals died during the study due to various reasons. The remaining 17 experimental animals were sacrificed at the end of three months by administrating a high dose of ketamine and intramuscular muscle relaxant. The skin and the subcutaneous tissue were penetrated with an incision on the implantation area. The implant and the surrounding soft tissue were taken out in a single piece. A total of 34 tissue samples were obtained having taken cartilage graft samples 2 from each group (1 sliced, 1 single-pieced) from 8 animals from the IGF-1 group and 9 from the control group. Each group was further categorized into subgroups of single-pieced and sliced samples. The obtained tissue samples were fixed in 10% buffered formaldehyde in separate storage boxes and they were labelled and enumerated.
Histopathological evaluation
The histopathological evaluation of the obtained samples was carried out at Ankara Social Security Administration Education and Research Hospital’s Pathology Clinic Laboratory. All the tissue samples were stained in hematoxylin eosin after taking sections of 5 microns thickness following the routine tissue follow-up stages and the stained preparations were analyzed with Olympus B×50 model light microscope. The following pathological evaluation criteria were formed according to the observed tissue changes. Cartilage viability, foreign body reaction (environment reaction), regenerative activity and cytoplasmic changes were scored as 0, 1, 2, 3. (Figure 1-3), (Table 1).
FIGURE 1.
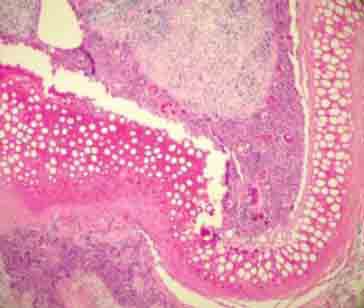
Totally empty lacunas and definite foreign body (environmental reaction) in sliced cartilage group (H&E ×100).
FIGURE 3.
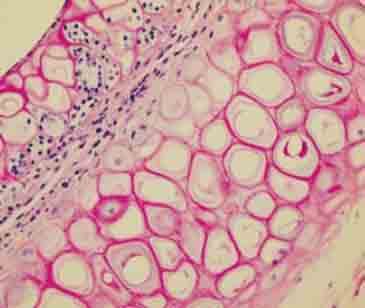
Cytoplasmic vacuolization and regenerative activity findings observed on the mid-line in sliced cartilage group (H&E ×400).
TABLE 1.
Histopathological evaluation criterias
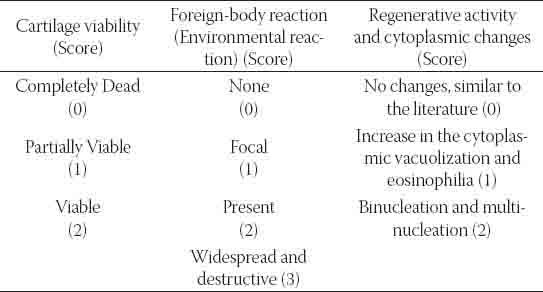
FIGURE 2.
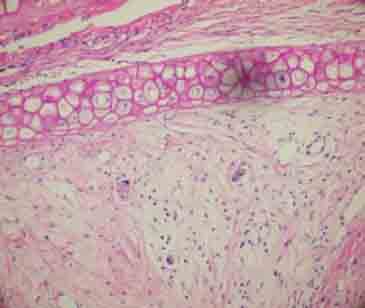
Viable cartilage tissue and minimal environmental reaction in single-piece cartilage group (H&E ×200).
RESULTS
The histopathological scoring results according to cartilage viability, environmental reaction, cytoplasmic changes, and regenerative activities of 17 single-piece and 17 sliced samples of all 34 cartilage tissue samples, 16 of which belonged to the IGF-1 group and 18 of which belonged to the control group were evaluated. The scoring results of the study were evaluated with Shapiro-Wilk’s normality test. The variables were seen to have a normal range. Intergroup differences were statistically analyzed by using the Mann-Whitney U test. The intergroup evaluation of all the cartilages revealed that the IGF-1 group had more cartilage viability and cytoplasmic activity while it expressed less environmental reaction than the control group. The differences between these values were found to be statistically significant (p<0.05), (Table 2, Graphs 1, 2)
TABLE 2.
The mean ± standard deviation and p values of histopathological evaluation. Scores for all groups.
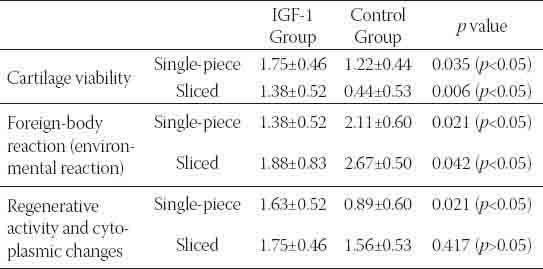
GRAPH 1.
Comparison of histopathologic evaluation scores of the sliced cartilage groups.
GRAPH 2.
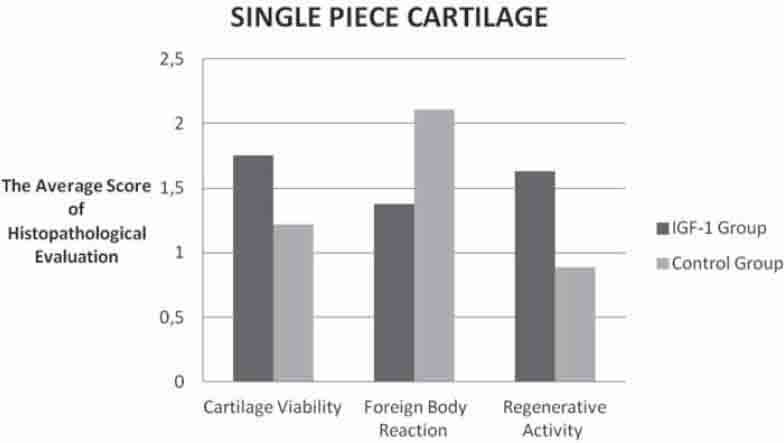
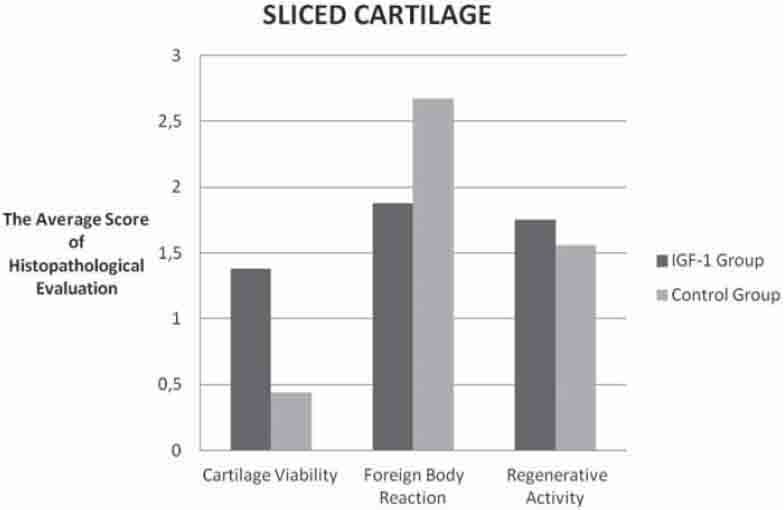
Comparison of the histopathologic evaluation scores belongs to the one pieced cartilage groups.
The intergroup evaluation of the sliced cartilages revealed that the IGF-1 group had more cartilage viability while it showed less environmental reaction than the control group (Graph 1). The differences between these values were found to be statistically significant (p<0.05). There was, however, no statistically significant difference between the groups regarding cytoplasmic activity (p<0.05), but there was more activity in the IGF-1 group (Table 2), (Graph 1). There was no significant difference regarding all three histopathological parameters for the sliced and single-piece cartilage groups of the IGF-1 group (p>0.05), (Graph 3).
GRAPH 3.
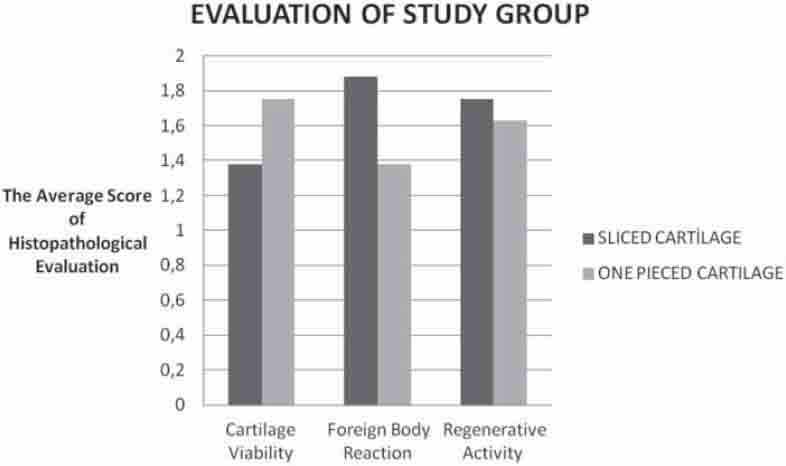
Comparison of the histopathologic evaluation scores in all cartilage groups.
DISCUSSION AND CONCLUSION
One of the substances used in order to reproduce and augment cartilage tissue is IGF-1. An ample amount of studies have been conducted until now evaluating the effects of this factor on cartilage tissue have either been done only with IGF-1 or in addition to that in combination with different growth factors. One of the first studies advocating that IGF-1 has positive effects on cartilage tissue was conducted by Jennische et al. who demonstrated that perichondrial cells were expressed a maximum level of hypertrophy at week two by using human IGF-1 following freeze-thaw injury in rats’ ears [14]. Van Osch et al. [15] compare the chondrogenic capacity of auricular and nasal perichondrium from young and adult rabbits, using serum containing IGF-1 (10 ng/mL) plus transforming growth factor-beta2 (TGF-beta2) (10 ng/mL) and serum-free culture conditions. When serum was replaced, however, by IGF-1 plus TGF-beta2 an increased glycosaminoglycan production and induction of collagen type II was observed, especially in cells isolated from ear perichondrium. Cells derived from perichondrium of young rabbits showed larger chondrogenic potential than cells from perichondrium of adult rabbits. Moreover, stimulation of both glycosaminoglycan synthesis and collagen type II production was about five times higher in cells isolated from the ear perichondrium of young rabbits than of adult rabbits. In a study by Bos et al. [16] using a rabbit auricular cartilage wound model, immunohistochemical staining of IGF-1 was used to define growth factor expression at the cartilage wound sites. The authors ascertained that this increase was related to the regeneration capacity and that the expression reached maximum limits in 7 days but then decreased gradually. Another study holds that IGF-I possessed promotional effects on proliferation, while the combination of fibroblast growth factor 2 (FGF-2) with insulin or IGF-I synergistically enhanced auricular chondrocyte proliferation [17]. To summarize, all the above mentioned studies have clearly demonstrated that IGF-1 plays a crucial role in cartilage tissue regeneration and the proliferation of chondrocytes. Following these results, scientists began to conduct studies on how to obtain implantable cartilage tissue using in vitro tissue engineering techniques and/or by way of in vivo reproduction of graft material. Studies using IGF-1 in order to achieve this did not get the same successful results. Within the framework of a study by Kaplan et al. [18] conducted for this purpose, a portion of the chondrocytes obtained from a 3-4 week old rabbit was implanted to the back of a donor rabbit after having been in vitro exposed to IGF-1 (50 ng/mL) in a tissue culture. This cartilage tissue was evaluated regarding its histological and biomechanical characteristics 8 weeks later and no difference was found between the study group and the control group. Another study by Westreich et al. [19] dealt with chondrocytes obtained from New Zealand white rabbits’ ears treated with b-FGF and IGF-1 in order to enable elasticity in cartilage tissues obtained in vitro and to prevent volume losses in cartilage tissues obtained in vivo. The cells were injected to the subcutaneous rabbit tissue with tissue seal fibrin adhesive material and were left in the implanted area for 3 months. The results showed that IGF-1 decreases the efficiency of the chondrocytes. In another study by Liu et al. [20] conducted on New Zealand rabbits, a pedunculated flap was formed from the perichondrium in rabbits’ ears and the demineralized ZMK bone matrix tissue was buried there. The 3rd day after the operation, the rabbits were injected with growth factors [TGF-β1, IGF-1 and Bone Morphogenic Protein-2 (BMP-2)] every 3 days. Grafts were collected in the 3rd and 6th week after the ZMK bone matrix implantation, and the silvers made from them were stained for the presence of collagen II, collagen I, and macrophages, and analyzed morphometrically. It was found that the application of growth factors only slightly intensified the synthesis of collagen II, and had no effect on the degree of macrophage infiltration or collagen I contents, while the numerous injections exerted a negative impact on the architecture of the newly-formed tissue and contributed to an increased number of complications (haematomas, infections). The results of this study which are in contradiction with our information about the effects of IGF-1 on the preservation of viability of the cartilage tissue and its reproduction directed us to conduct our own study on the subject. In our study, the auricular cartilage tissue, which was subcutaneously treated for three months with IGF-1 (0.1 mg/1 ml), was evaluated at the end of three months. The cartilage tissue was evaluated regarding the cytoplasmic changes in the chondrocytes, regenerative activity, cartilage viability, and foreign-body reaction and was histopathologically scored. These evaluations were carried out by the pathologist who had done the analysis during the microscopic analysis of the graft tissue samples based on the changes that he thought was related to tissue viability. The reason why the evaluation was carried out in this manner was the fact that the changes that would happen in the cartilage graft and the surrounding tissues was not known beforehand and that there was no known standard histopathological evaluation method on the subject. Single-piece cartilage graft intergroup comparison revealed that there was statistically more cartilage viability and cytoplasmic activity, and statistically less foreign-body reaction in the IGF-1 group than in the control group. Sliced cartilage intergroup evaluation, too, revealed that there was statistically more cartilage viability and statistically less foreign-body reaction in the IGF-1 group than in the control group. There was, however, no statistically significant difference between the groups, but the IGF-1 group showed more cytoplasmic activity. These results demonstrated that IGF-1 increased tissue viability and repressed the former foreign-body reaction independent of (not effected by) the fact that whether the implanted auricular cartilage tissue was in a single-piece or sliced. The results of our study support other studies which argue that IGF-1 has positive effects on the viability and development of cartilage tissue [14-17]. On the other hand, it is clear that the results are in contradiction with the views that IGF-1 has no positive effects on the implanted cartilage tissue [18] and further it decreases the efficiency of the chondrocytes [19]. We believe that it is of utmost importance to cleanse the hair on the skin thoroughly without damaging the skin in order to harvest and implant the cartilage tissue under sterile conditions, to use sterile disposable injectors with appropriate needle diameter during the subcutaneous injections, to clean the skin with a disinfectant agent, and to have a careful skin care process following the implantation in order to reduce the risk of infections and tissue rejection. Further, we also think that in order to prevent haematoma, infection, granulation tissue, and tissue rejection, it is again of utmost importance to dissect the skin in a way that will not upset the tissue plans and integrity of the area where the tissue is implanted during the implantation and performing a subcutaneous injection in an appropriate plan without giving way to tissue destruction. We are of the opinion that such complications seen in other studies [20] might be related to such application mistakes and that it is not right to connect these effects to IGF-1. The results of our study demonstrate that when local subcutaneous IGF-1 injections are carried out under appropriate conditions using appropriate surgical techniques, they increase the viability of the auricular cartilage in the implanted area and reduce the risk of tissue rejection. On the other hand, the fact that there is a limited number of in vivo studies and that the results of these studies are in contradiction, necessitates novel studies to be conducted on the subject. We believe that comprehensive studies that will be conducted especially to elucidate the dose, duration, and combinations with other agents of IGF-1 applications to gain maximum benefit, will contribute to clear the confusion on the subject. The positive results that might be obtained by these studies can give us the chance to have a practical method to sustain the viability, to extend the lifespan, and even to increase the tissue volumes of the cartilage grafts in the area where they were implanted.
DECLARATION OF INTEREST
There is no financial support received for this present study for all authors, no financial involvement of any kind or affiliation with any organization whose financial interests may be affected by material in the manuscript or which may potentially bias it.
REFERENCES
- [1].van Osch GJ, van der Veen SW, Verwoerd-Verhoef HL. In vitro redifferentiation of culture-expanded rabbit and human auricular chondrocytes for cartilage reconstruction. Plast Reconstr Surg. 2001;107(2):433–440. doi: 10.1097/00006534-200102000-00020. [DOI] [PubMed] [Google Scholar]
- [2].Westreich R, Kaufman M, Gannon P, Lawson W. Validating the subcutaneous model of injectable autologous cartilage using a fibrin glue scaffold. Laryngoscope. 2004;114:2154–2160. doi: 10.1097/10.mlg.0000149449.37640.0d. [DOI] [PubMed] [Google Scholar]
- [3].Hoppener JW, de Pagter-Holthuizen P, Geurts van Kessel AH, Jansen M, Kittur SD, Antonarakis SE, Lips CJ, Sussenbach JS. The human gene encoding insulin-like growth factor I is located on chromosome 12. Hum Genet. 1985;69(2):157–160. doi: 10.1007/BF00293288. [DOI] [PubMed] [Google Scholar]
- [4].Jansen M, Van Schaik FM, Ricker AT, Bullock B, Woods DE, Gabbay KH, et al. Sequence of cDNA encoding human insulin-like growth factor I precursor. Nature. 1983;306 (5943):609–611. doi: 10.1038/306609a0. [DOI] [PubMed] [Google Scholar]
- [5].Allan GJ, Flint DJ, Patel K. Insulin-like growth factor axis during embryonic development. Reproduction. 2001;122(1):31–39. doi: 10.1530/rep.0.1220031. [DOI] [PubMed] [Google Scholar]
- [6].Stratikopoulos E, Szabolcs M, Dragatsis I, Klinakis A, Efstratiadis A. The hormonal action of IGF1 in postnatal mouse growth. Proc Natl Acad Sci U S A. 2008;105(49):19378–19383. doi: 10.1073/pnas.0809223105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Florini JR, Ewton DZ, Coolican SA. Growth hormone and the insulin-like growth factor system in myogenesis. Endocr Rev. 1996;17(5):481–517. doi: 10.1210/edrv-17-5-481. [DOI] [PubMed] [Google Scholar]
- [8].Fu Z, Kubo T, Noguchi T, Kato H. Developmental changes in the mRNA levels of IGF-I and its related genes in the reproductive organs of Japanese quail (Coturnix coturnix japonica) Growth Horm IGF Res. 2001;11(1):24–33. doi: 10.1054/ghir.2000.0186. [DOI] [PubMed] [Google Scholar]
- [9].Rosen CJ. Insulin-like growth factor-1 and calcium balance: evolving concepts of an volutionary process. Endocrinology. 2003;144(11):4679–4681. doi: 10.1210/en.2003-1038. [DOI] [PubMed] [Google Scholar]
- [10].Kawai M, Rosen CJ. The IGF-I regulatory system and its impact on skeletal and energy homeostasis. J Cell Biochem. 2010;111(1):14–19. doi: 10.1002/jcb.22678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Jonsson KB, Wiberg K, Ljunghall S, Ljunggren O. Insulin-like growth factor I does not stimulate bone resorption in cultured neonatal mouse calvarial bones. Calcif Tissue Int. 1996;59(5):366–370. doi: 10.1007/s002239900141. [DOI] [PubMed] [Google Scholar]
- [12].Tsukazaki T, Usa T, Matsumoto T, Enomoto H, Ohtsuru A, Namba H, et al. Effect of transforming growth factor-beta on the insulin-like growth factor-1 autocrine / paracrine axis in cultured rat articular chondrocytes. Exp Cell Res. 1994;215(1):9–16. doi: 10.1006/excr.1994.1307. [DOI] [PubMed] [Google Scholar]
- [13].Sah RL, Chen AC, Grodzinsky AJ, Trippel SB. Differential effects of bFGF and IGF-I on matrix metabolism in calf and adult bovine cartilage explants. Arch Biochem Biophys. 1994;308(1):137–147. doi: 10.1006/abbi.1994.1020. [DOI] [PubMed] [Google Scholar]
- [14].Jennische E, Skottner A, Hansson HA. Dynamic changes in insulinlike growth factor I immunoreactivity correlate to repair events in rat ear after freeze-thaw injury. Exp Mol Pathol. 1987;47(2):193–201. doi: 10.1016/0014-4800(87)90074-8. [DOI] [PubMed] [Google Scholar]
- [15].van Osch GJ, van der Veen SW, Burger EH, Verwoerd-Verhoef HL. Chondrogenic potential of in vitro multiplied rabbit perichondrium cells cultured in alginate beads in defined medium. Tissue Eng. 2000;6(4):321–330. doi: 10.1089/107632700418047. [DOI] [PubMed] [Google Scholar]
- [16].Bos PK, van Osch GJ, Frenz DA, Verhaar JA, Verwoerd-Verhoef HL. Growth factor expression in cartilage wound healing: temporal and spatial immunolocalization in a rabbit auricular cartilage wound model. Osteoarthritis Cartilage. 2001;9(4):382–389. doi: 10.1053/joca.2000.0399. [DOI] [PubMed] [Google Scholar]
- [17].Takahashi T, Ogasawara T, Kishimoto J, Liu G, Asato H, Nakatsuka T, et al. Synergistic effects of FGF-2 with insulin or IGF-I on the proliferation of human auricular chondrocytes. Cell Transplant. 2005;14(9):683–693. doi: 10.3727/000000005783982675. [DOI] [PubMed] [Google Scholar]
- [18].Kaplan BA, Gorman CR, Gupta AK, Taylor SR, Iezzoni JC, Park SS. Effects of transforming growth factor Beta and insulinlike growth factor 1 on the biomechanical and histologic properties of tissueengineered cartilage. Arch Facial Plast Surg. 2003;5(1):96–101. doi: 10.1001/archfaci.5.1.96. [DOI] [PubMed] [Google Scholar]
- [19].Westreich R, Kaufman M, Gannon P, Lawson W. Validating the subcutaneous model of injectable autologous cartilage using a fibrin glue scaffold. Laryngoscope. 2004;114(12):2154–2160. doi: 10.1097/10.mlg.0000149449.37640.0d. [DOI] [PubMed] [Google Scholar]
- [20].Liu X, Sun H, Yan D, Zhang L, Lv X, Liu T, et al. In vivo ectopic chondrogenesis of BMSCs directed by mature chondrocytes. Biomaterials. 2010;31(36):9406–9414. doi: 10.1016/j.biomaterials.2010.08.052. [DOI] [PubMed] [Google Scholar]


