Abstract
Large scale genetic association meta-analyses showed that neurocan (NCAN) gene polymorphism rs1064395 is susceptibility locus for bipolar disorder. These studies also included patients with bipolar disorder originated from Bosnia and Herzegovina. Followed by theory of shared genetic elements between bipolar disorder and schizophrenia susceptibility, other studies explored several genetic factors with schizophrenia vulnerability as well. In this work, authors investigated the association between previously confirmed bipolar disorder genetic risk factor-neurocan with schizophrenia in a population sample of Bosnia and Herzegovina.
Ethical aspects of this research were assessed by Ethics Committee of Clinical Center University of Sarajevo. Blood samples for DNA extraction were taken from the total of 86 patients and healthy individuals who previously signed informed consent. Genotyping for rs 1064395 was done using direct sequencing method. A case-control analysis of common genetic polymorphism within neurocan gene and schizophrenia status in a consecutively sampled patient cohort have been done using Fisher-exact test with odds-ratio calculation. No statistically significant allele and genotype association with disease status was found (p>0.05).
Our finding supports the fact that large-scale genetic association studies approach need to be employed when detecting the variants with small additive effect in phenotypes with complex ethiology.
Keywords: NCAN (neurocan), genetic association, bipolar disorder, schizophrenia
INTRODUCTION
Classical genetic studies proved that schizophrenia is a highly heritable disease. Genetic make-up of this psychiatric disorder is being thoroughly explored for improvement of diagnostic methods and therapeutic targets. Although significant research and technological efforts were made, no gene was found to be pointed out as a single contributory genetic risk factor for development of disease or specific treatment responses in schizophrenia [1-2]. Underlying reason for that is quite clear from the investigator’s point of view: schizophrenia is a complex polygenic trait with significant influence of non-genetic environmental factors. When genetic component of disease was dissected it became an inevitable fact that no single gene is sufficient either necessary for onset and development of schizophrenia in stipulated polygenic model indicating more than 30-60 loci involved [2].
Extensive research work done globally derived the list of candidate genes for schizophrenia and bipolar disorder (BD) on which most of the genes were actually proved to be the shared genetic factors [3-5]. The most of the identified gene effects could not be replicated in different population samples [3]. For the detection of common variants with small additive effects, generally the strategy of detection based on increased size of population sample and resolution of genomic arrays, followed by functional studies, represent the only rational approach applicable in genetic characterization of schizophrenia and other complex diseases. A recent study [6] found genome-wide significant association between common variation in the gene neurocan (NCAN, rs1064395) and BD. In view of accumulating evidence that BD and schizophrenia partly share genetic risk factors [7]. This single-nucleotide polymorphism was tested for association with schizophrenia in three independent patient–control samples of European ancestry. The rs1064395 A-allele, which confers risk for BD, was significantly over-represented in schizophrenia patients compared to controls (p = 2.28 × 10-3; odds ratio = 1.11). In this study we tested association of same locus (NCAN, rs1064395) with schizophrenia patients originating from Bosnia and Herzegovina.
MATERIALS AND METHODS
All procedures and methods used in this study were approved by Ethics Committee of Clinical Center of University of Sarajevo. Following informed consent process and relevant document signature, all participation of both patients and control individuals was voluntary. Diagnosis of schizophrenia was done based on DSM-IV corresponding structured clinical interview (SCID-I) done by single psychiatrist in all cases [8].
Patients
Healthy individuals free of family history of major psychiatric disorders (schizophrenia, bipolar disorder and depression) were included in this study.
Procedures
Blood specimens were collected from 56 patients and 30 healthy volunteers. Genetic material used for further analysis of NCAN locus and rs1064395 as informative site, was extracted from EDTA anticoagulated blood using salting out procedure [9]. Based on FASTA sequence for 3’UTR of neurocan gene obtained from NCBI, we designed the flanking primers for rs1064395: forward 5’-CATTGCCTGGTGGTCTAGAAA-3’ and reverse 5’-GGAGGAAGGCAAGGTGAGTT-3’. For in vitro amplification we used water solution of 2X PCR premix (REDTaq® ReadyMix™ PCR Reaction Mix, SIGMA-ALDRICH), 100ng of DNA template, 20pmol of each primer template in 25 μl total reaction volume. Reaction conditions were optimized as follows: initial denaturation step at 95°C for 4 minutes was followed by 30 cycles of denaturation at 95°C for 30 s, primer annealing for 30s at 62°C and extension at 72°C for 1 min, final elongation at 72°C for 7 minutes and unlimited hold at 4°C. Genotype detection was done on ABI 310 followed by Big-Dye terminator single primer (5’-CATTGCCTGGTGGTCTAGAAA-3’) extension reaction. Automatically obtained sequences were read manually for exclusion of false homozygotes.
Statistical analysis
Generated genotype data were collected for further statistical analysis using Fisher-exact test with odds-ratio calculation as a part of Powermarker [10] and MedCalc software for Windows, version 9.5.0.0 (MedCalc Software, Mariakerke, Belgium).
RESULTS
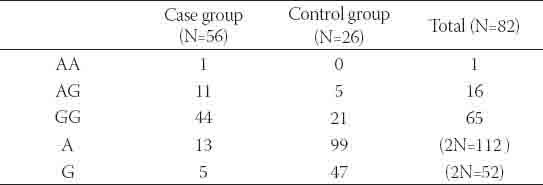
Total of 86 individuals (56 patients and 30 healthy volunteers) were genotyped for rs1064395 using direct sequencing method. All three expected genotypes: GG, AG and AA were observed (Figure 1). Four genotype data from control group could not be used for further statistical analysis due to low quality of DNA electropherograms. Hardy-Weinberg Equilibrium (HWE) test showed that case group data subset was in the range of expected frequency distribution (p=0.5419) supporting the quality of procedures of sampling and genotyping. In case group, among 112 observed genomes, thirteen minor allele variants (A) were detected (11.6 %) as for in control group out of 52 genomes five minor alleles were found (9.6 %). All expected genotypes (AA, AG and GG) were observed in all cases (Table 1) with relative frequencies 1.8 %, 19.6 % and 78.6 % respectively in case and 0 %, 19.2 % and 80.8 % in control group. A case-control analysis of common genetic polymorphism within NCAN gene and schizophrenia status has been done using Fisher-exact test with odds-ratio calculation. No statistically significant allele association with disease status was found (Exact p value=0.5854, OR=1.23 with p=0.7). Association analysis for genotype frequencies distributions between two analyzed groups was also negative (Exact p-value >0.5). Re-sampling with 1000 iterations showed no difference between artificially generated and actual sample.
FIGURE 1.
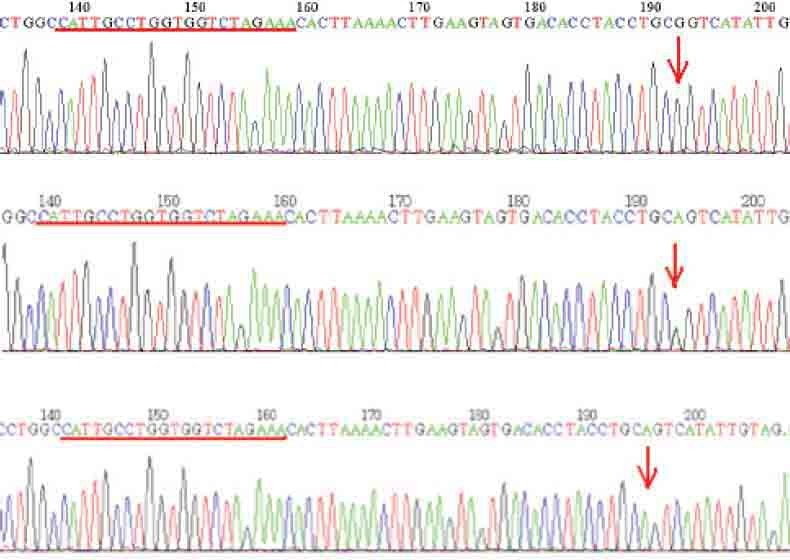
Presentation of DNA electropherogram for threeobserved genotypes: G/G – top, G/A (middle) and A/A (down). Red line shows the priming region of sequencing primer.
TABLE 1.
Observed allele and genotype frequencies
DISCUSSION
A risk conferring allele A at locus rs1064395 of NCAN was analysed in terms of its engagement in pathophysiology of bipolar disorder in a sample comprised of several subsets of European descent subpopulations including Bosnian and Herzegovinian with rs1064395 A allele overrepresentation of 21% in cases versus 18% in controls and OR =1.2 and p<0.05. Meta-analysis of pooled population data undoubtedly confirmed genetic association of NCAN structure variation with actual disease state [6]. Driven by previously and scientifically supported hypothesis of schizophrenia and bipolar disorder shared pathophysiology in rather large population datasets of more than 5000 patients and 9500 controls [7] confirmed the genetic association of minor A-ellele at locus rs1064395 with OR of at least 1.07 and p=0.0239. Both findings implicate that person who carries at least one copy of A allele has an increased risk for development of disease, namely 1.07 times more in schizophrenia and 1.17 times more in bipolar disorder in relation to major allele carriers. In our study of genetic association of rs1064395 we observed a difference in distribution of minor allele frequencies between patients and control individuals that was not statistically significant (p>0.05). Risk allele is overrepresented in a case group (11.6 %) versus control group (9.6 %) indicating the significance of this variant in a genetic make-up of a disease (OR=1.23 with p=0.7). The statistical insignificance of observed differences could be largely due to investigated population size but lack of association results in our sample cannot be completely overruled for this reason since re-sampling statistical procedures suggest that sample size of investigated groups possibly had no effect on final results of genetic association. As common risk variant NCAN gene has small effect size in polygenic model of schizophrenia unable to reach statistical significance in some association studies. This is the main reason why in some independent studies described before [7] lack of association was observed. However in meta-analysis of the same independent schizophrenia samples statistical significance was reached at p=0.023 [7] but not even close as for bipolar disorder p=2.14×10-9 [6]. Therefore, findings related to BP [6] and schizophrenia samples from Bosnia and Herzegovina as well as in other populations [7, 11, 12] implicate that allele A at neurocan locus rs1064395 is more frequently represented in etiopatology of bipolar disorder than of schizophrenia.
CONCLUSIONS
In this study we found no genetic association between risk allele A for NCAN locus rs1064395 and schizophrenia. Tests for HWE showed that both population subsets are in equilibrium which contributes to the quality of sampling and technical procedures during the study. Any disequilibrium that could drive to false positive association findings was eliminated. Independent studies worldwide that investigated genetic relatedness of neurocan with schizophrenia had indecisive outputs. Suggestive overrepresentation of risk alleles was found but no observable statistical significance with standard statistical methods for genetic association analysis was reached. Only in large meta-analyses, small additive effect of NCAN could be detected. Our finding supports the fact that large-scale genetic association studies approach need to be employed when detecting the variants with small additive effect in phenotypes with complex etiology.
ACKNOWLEDGEMENTS
This work is realized by funding of Ministry of Science, Education and Youth of Sarajevo Canton and Ministry of Education and Science of Federation Bosnia and Herzegovina.
DECLARATION OF INTEREST
Authors state that there is no conflict of financial or any other interest related to this research and publication.
REFERENCES
- [1].DeRosse P, Lencz T, Burdick KE, Siris SG, Kane JM, Malhotra AK. The genetics of symptom-based phenotypes: toward a molecular classification of schizophrenia. Schizophr Bull. 2008;34(6):1047–1053. doi: 10.1093/schbul/sbn076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Sullivan PF, Daly MJ, O’Donovan M. Genetic architectures of psychiatric disorders: the emerging picture and its implications. Nat Rev Genet. 2012;13(8):537–551. doi: 10.1038/nrg3240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Yu K, Cheung C, Leung M, Li Q, Chua S, McAlonan G. Are bipolar disorder and schizophrenia neuroanatomically distinct? An anatomical likelihood meta-analysis. Front Hum Neurosci. 2010;4:189. doi: 10.3389/fnhum.2010.00189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Lichtenstein P, Yip BH, Björk C, Pawitan Y, Cannon TD, Sullivan PF, et al. Common genetic determinants of schizophrenia and bipolar disorder in Swedish families: a population-based study. Lancet. 2009;373(9659):234–239. doi: 10.1016/S0140-6736(09)60072-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Maier W, Zobel A, Wagner M. Schizophrenia and bipolar disorder: differences and overlaps. Curr Opin Psychiatry. 2006;19(2):16.5–170. doi: 10.1097/01.yco.0000214342.52249.82. [DOI] [PubMed] [Google Scholar]
- [6].Cichon S, Mühleisen TW, Degenhardt FA, Mattheisen M, Miró X, Strohmaier J, et al. Genome-wide association study identifies genetic variation in neurocan as a susceptibility factor for bipolar disorder. Am J Hum Genet. 2011;88(3):372–381. doi: 10.1016/j.ajhg.2011.01.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Mühleisen TW, Mattheisen M, Strohmaier J, Degenhardt F, Priebe L, Schultz CC, et al. Association between schizophrenia and common variation in neurocan (NCAN), a genetic risk factor for bipolar disorder. Schizophr Res. 2012;138(1):69–73. doi: 10.1016/j.schres.2012.03.007. [DOI] [PubMed] [Google Scholar]
- [8].First MB, Spitzer RL, Gibbon M, Williams JBW. Washington, DC: American Psychiatric Press; Structured Clinical Interview for DSM-IV Axis I Disorders (SCID I): Clinician Version. [Google Scholar]
- [9].Miller SA, Dykes DD, Polesky HF. A simple salting out procedure for extracting DNA from nucleated cells. Nucleic Acids Res. 1988;16:1215. doi: 10.1093/nar/16.3.1215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Liu K, Muse S. POWERMARKER: Integrated analysis environment for genetic marker data. Bioinformatics. 2005;21(9):2128–2129. doi: 10.1093/bioinformatics/bti282. [DOI] [PubMed] [Google Scholar]
- [11].Rietschel M, Mattheisen M, Degenhardt F, Kahn RS, et al. GROUP Investigators; Genetic Risk and Outcome in Psychosis (GROUP Investigators) Association between genetic variation in a region on chromosome 11 and schizophrenia in large samples from Europe. Mol Psychiatry. 2012;17(9):906–917. doi: 10.1038/mp.2011.80. [DOI] [PubMed] [Google Scholar]
- [12].Ripke S, Sanders AR, Kendler KS, Levinson DF, Sklar P, Holmans PA, et al. Schizophrenia Psychiatric Genome-Wide Association Study (GWAS) Consortium. Genome-wide association study identifies five new schizophrenia loci. Nat Genet. 2011;43(10):969–976. doi: 10.1038/ng.940. [DOI] [PMC free article] [PubMed] [Google Scholar]


