Abstract
Trefoil factors (TFF) are secretory products of mucin producing cells. They play a key role in the maintenance of the surface integrity of oral mucosa and enhance healing of the gastrointestinal mucosa by a process called restitution. TFF comprises the gastric peptides (TFF1), spasmolytic peptide (TFF2), and the intestinal trefoil factor (TFF3). They have an important and necessary role in epithelial restitution within the gastrointestinal tract. Significant amounts of TFF are present in human milk. This study aimed to determine a possible correlation between TFF3 isolated from human breast milk and levels of cytokines (IL8 and IL6) and defensins (hBD2 and hBD4) in intestinal epithelial cells HT-29 treated with trefoil. Samples of human milk were collected within 2-4 weeks postpartum from healthy human mothers (18-30-years-old) by manual breast massage, and TFF3 was purified by ammonium sulfate precipitation, isoelectric precipitation, DEAE-chromatography, and gel filtration. In this work we measured the concentrations and mRNA levels of cytokines and defensins by immunoassay (ELISA) and semiquantitative RT-PCR technique, respectively. Also we measured the peroxidase activity. We present the first evidence of human milk TFF3 purification. Here we show that the presence of TFF3 isolated from milk strongly correlates with downregulation of IL8 and IL6 in human intestinal epithelial cells. On the other hand, TFF3 activated the epithelial cells in culture to produce beta defensins 2 (hBD2) and beta defensins 4 (hBD4). These findings suggest that TFF can activate intestinal epithelial cells and could actively participate in the immune system of breastfed babies by inducing the production of peptides related to innate defence, such as defensins.
Keywords: breast milk, trefoil, cytokines, defensins
INTRODUCTION
Human breast milk is considered the best form of nourishment for infants during the first year of life. It is a dynamic secretion, and its composition varies at different stages of lactation. Breast milk provides nutrients and bioactive factors [1-3] that themselves might modulate maturation and development of the gastrointestinal tract. Human milk, which is usually accepted as the best source to feed infants, constitutes a good source to isolate many bioactive compounds that have been demonstrated to have a beneficial role on gut development, maturation, and immune function [4]. Milk therefore plays a central role in mammalian gut physiology. In colostrum, the concentration of immunoglobulins is particularly high, with IgA being the major immunoglobulin present in human milk. In addition to immunoglobulins, both colostrum and mature milk contain viable cells, including neutrophils, lymphocytes, and macrophages, which secrete a range of immune-related components into milk [5]. These include cytokines, antimicrobial proteins and peptides, such as defensins, and trefoil factors [6]. Increasingly sophisticated proteomics technologies are being applied to identify and characterize the functions of the minor components of human milk that may have an important role in the physiology of the neonate gastrointestinal tract. The trefoil peptide family are polypeptides of fewer than 80 amino acids that are found along the gastrointestinal tract. In human and other mammals the three main trefoil peptide families are the gastric peptides (TFF1), the spasmolytic peptide (TFF2), and the intestinal trefoil factor (TFF3). Individual members of these three families have been implicated in epithelial restitution within the gastrointestinal tract [7]. Trefoil peptides are widely distributed in intestinal epithelial cells and are often present at high concentrations in saliva, meconium, and human breast milk [6, 8]. Trefoil families are protease-resistant peptides that are amply secreted onto the intestinal mucosal surface by specific cells of the human gastrointestinal tract. The TFFs share a completely conserved distinctive motif of six cysteine residues, which form three disulfide bonds and define the “trefoil” domain, which is also known as a “P” domain [9]. In the present work we describe that trefoil 3 isolated from human breast milk can activate intestinal epithelial cells and promote human beta defensin expression and cytokine regulation. Defensins are antimicrobial peptides that act mainly by disrupting the structure of bacterial cell membranes and are found in many compartments of the body [10, 11]. Evidence is accumulating that defensins play a central role in defense against pathogens, and they are considered as a part of the innate immune response [11]. They have generally been considered to contribute to mucosal health; however, it is possible that these peptides can be considered biological factors that can be upregulated by bioactive compounds presents in human breast milk. In this sense, the intestinal production of antimicrobial peptides as hBD2 and hBD4 by trefoil from milk might play an important role on neonate colonization, thereby enhancing the immune response of newborn against pathogens with which they may come in contact. This positive effect would be a new role exercised through breastfeeding on neonatal intestinal maturation. Human milk seems to be defensive against gut inflammation. A study of chemical colitis in rats revealed decreased myeloperoxidase activity in rats fed human milk [7]. Also, other work has demonstrated the modulation of human intestinal epithelial cell IL8 secretion by human milk factors [12, 13]. We obtained evidence for the first time that TFF3 triggers a signal transduction pathway that downregulates proinflammatory cytokines (IL8 and IL6) and induces the expression of important antimicrobial peptides (hBD2 and hBD4). Finally, this article will describe the biological effects of the TFFs on cytokine and antimicrobial peptide regulation and their potential therapeutic use.
MATERIALS AND METHODS
Human milk collection
Samples of human milk were collected within 2-4 weeks postpartum from healthy human mothers (18-30-years-old) by manual breast massage. None of the women had a history of rheumatological, respiratory, cardiovascular, or gastrointestinal diseases. All samples were obtained with informed consent. The study was approved by the Research Ethics Committee of the Instituto Venezolano de Investigaciones Científicas (IVIC).
Cultured cells
The HT-29 colonic cell line (passages 7-17) was obtained from the American Type Culture Collection (ATCC, Manassas, USA) and cultured according to the supplier’s instructions in Dulbecco’s Modified Eagle’s medium (DMEM) containing 2 mM glutamine, 50 IU/ml penicillin, 50 mg/ml streptomycin, and 10% heat inactivated fetal bovine serum as standard medium at 37°C in a water-saturated atmosphere with 5% CO2.
Trefoil factor 3 purification
Human breast milk (25 ml) was thawed at 25°C. The samples were separated by centrifugation at 5000g for 1 h at 4°C into an upper fatty layer, a middle aqueous layer containing the trefoil factors, and the cell pellet. The middle layer was recovered and precipitated by addition of saturated ammonium sulfate to a final concentration of 70% at 4°C for 1 h to precipitate unwanted proteins. The unprecipitated proteins were centrifuged at 40,000g at 4°C for 1 h. Then the supernatant fractions underwent isoelectric precipitation with 1 M Tris at pH 5.1 and 4.5, as previously reported with few modifications [8]. The precipitated proteins were dialyzed against buffer (20 mM Tris-HCl, pH 8.2) at 4°C for 6 h, and samples of 5 mg were loaded onto a column containing 100 ml of Sephadex G-75 equilibrated with 20 mM Tris-HCl, pH 8.0. The eluted TFF3 was again concentrated using centrifugal devices with a 5-kDa cutoff. The TFF3 was further purified by DEAE-Sepharose FF column (0.5 x1 cm; Amersham Pharmacia Biotech, USA) equilibrated with 20 mM Tris-HCl, pH 8.0. The column was washed with 10 column volumes of the equilibration buffer. Bound material was eluted with a linear gradient of 0-1 M NaCl in DEAE equilibration buffer with a total volume of 100 ml. After elution, the isolated TFF3 was dialyzed against buffer (20 mM Tris-HCl, pH 8.0) at 4°C for 12 h. Then, the TFF3 was concentrated using centrifugal devices with a 5-kDa molecular weight cutoff (Millipore, USA) and stored at –80°C. The purity of TFF3 protein was assessed by SDS-PAGE and immunoblotting. The degree of purity achieved makes it unlikely that the biological activity on cell culture seen by TFF3 was caused by contaminating proteins.
SDS-PAGE and immunoblotting
Purified TFF3 was subjected to electrophoresis on 15% SDS-PAGE according to the method of Laemmli [14]. After electrophoresis, the gels were either fixed and proteins were visualized with 0.1% Coomassie brilliant blue R-250 (Sigma, USA) in methanol–water–acetic acid (1 : 8 : 1) (Merck, Germany) or they were electroblotted onto nitrocellulose for 4 h at 4°C (8-10 V/cm). The membranes were incubated 12 h at 4°C with blocking solution (5% nonfat dried milk in PBS containing 0.1% Tween 20). After being blocked, the membranes were incubated for 2 h at room temperature with PBS containing 5% dried milk powder and a 1 : 1000 dilution of rabbit anti-human TTF3 (Santa Cruz Biotechnology, USA). The membranes were washed five times in PBS-Tween and incubated with the peroxidase-coupled anti-rabbit secondary antibody (1 : 3000) (Santa Cruz Biotechnology) in PBS-Tween containing 5% nonfat dried milk for 2 h at room temperature. The membranes were washed three times in PBS-Tween, and specific bands were visualized by luminol reagent (Santa Cruz Biotechnology).
Activity of peroxidase
Peroxidase activity was measured in culture supernatants of HT-29 according to the 2-nitrobenzoic acid/thiocyanate (NBS-SCN) assay as previously described [15]. Briefly, the colorimetric change induced by the reaction between the enzyme and the substrate, Dithiobis-2-nitrobensoic acid (DTNB) in the presence of mercaptoethanol was read at a wavelength of 412 nm for 20 sec. One unit of enzyme activity was defined as the level of enzyme activity needed to cleave 1 μmol NBS/min at 22°C, using a molar extinction coefficient of 12,800.
ELISA
HT-29 cells were grown in 6-well plates to 50% confluence and serum-starved for 24 h. The cell number in wells was normalized by seeding equal quantity of HT-29 cells, previously counted and diluted at final concentration of 2 105 cells/ml. Cells were then treated for 48 h with 10 μg/ml trefoil. In some experiments, HT-29 cells were preincubated for 24 h with LPS (1 μg/ml) before treating with trefoil for 48 h. Afterwards, culture supernatants of HT-29 were collected and centrifuged at 1000g for 15 min at 4°C, and proteins were precipitated by trichloroacetic acid (TCA) [16] as follows: the supernatants were mixed with one volume of pre-chilled 25% TCA and incubated on ice-water for 15 min. The mixture was centrifuged for 10 min at 10,000 g at 4°C, the pellet was resuspended in 1 ml of acetone and dissolved using an ultrasonic water bath, and the mixture was centrifuged. Acetone washing was repeated twice, and the final pellet was air-dried and diluted in 0.05 M carbonate buffer, pH 9.6, 4°C, for 12 h. Subsequently, we blocked the wells with 200 μl of 1% bovine serum albumin (BSA) in PBS at room temperature for 2 h. After washing five times with 200 μl PBS containing 1 ml/liter Tween 20, we incubated 100 μl/well with PBS containing 1% BSA and a 1 : 1000 dilution of goat anti-human BD2 (Santa Cruz Biotechnology) or rabbit anti-human BD4, IL6 or IL8 (Santa Cruz Biotechnology) at room temperature for 2 h. The plates were washed five times with PBS containing 1 ml/liter Tween 20, and wells were incubated at room temperature with 100 μl of peroxidase-coupled anti-goat secondary antibody (Santa Cruz Biotechnology) (for BD2) or anti-rabbit secondary antibody (for IL6, IL8, and BD4) diluted to 1 : 5000 in PBS plus 1 ml/liter Tween 20 for 30 min. Plates were washed five times as described above and incubated with 100 μl of substrate (0.2 M Na2HPO4, 0.1 M citric acid, 0.1% H2O2, 15 mg o-phenylenediamine dihydrochloride) to each well in the dark at room temperature for 10 min. Stop solution (100 μl, 0.5 M H2SO4) was added to each well. Absorbance was measured at 405 nm using a Synergy HT microtiter plate spectrophotometer (BioTek Instruments, USA). We quantified hBDs and cytokines by simultaneous ELISA runs using recombinant hBDs and cytokines as calibrators.
Reverse transcription polymerase chain reaction (RT-PCR)
HT-29 cells were grown to 80% confluence in 6-well plates and serum-starved for 24 h. Cells were then treated for 48 h with 10 μg/ml trefoil. In some experiments, HT-29 cells were preincubated for 24 h with LPS (1 μg/ml) before treating with trefoil for 48 h. RNA was extracted from HT-29 intestinal cells by Trizol™ (Invitrogen, USA) according to the manufacturer’s instructions. RNA concentration and purity were measured using a Synergy HT spectrophotometer (BioTek Instruments). Total RNA (1 μg) was reverse transcribed into cDNA using a commercial kit (Invitrogen Thermo-Script™ RT-PCR System) according to the manufacturer’s instructions. Control reactions to check for DNA contamination were run in parallel with samples processed without reverse transcriptase. PCR was performed in a final volume of 25 μl containing 1 μl of the reverse transcription reaction, 50 μM deoxynucleotide triphosphates, 1.5 mM MgCl2, 50 mM Tris-HCl (pH 8.0), 1 IU Taq DNA polymerase, and 0.2 μM each of sense and antisense of defensin (hBD2 and hBD4) or cytokine (IL8 and IL6) primers (see sequences below). PCR was performed in an Eppendorf Mastercycler STM thermocycler for 35 cycles consisting of denaturation at 94°C (1 min), annealing at 60°C (1 min), and extension at 72°C (1 min). Amplification was terminated by a final extension step at 72°C for 5 min. A negative control without the cDNA template was run with every assay to evaluate the overall specificity. The integrity of the template RNA was checked by confirming expression of β-actin mRNA using β-actin-specific primers and a PCR protocol. The primer sequences were: β-actin sense, CACGCCATCCTGCGTCGGAC; β-actin antisense, CATGCCATCCTGCGTCTGGAC; hBD2 sense, TTCCTGATGCCTCTTCCA; and hBD2 antisense, ATGTCGCACGTCTCTGA; hBD4 sense, GGCAGTCCCATAACCACATATTC; and hBD4 antisense, TGCTGCTATTAGCCGTTTCTCTT; IL8 sense, GGCTCTCTTGGCAGCCTTCCTG; and IL8 antisense, CTTCTCCACAACCGTCTCACCC; IL6 sense, CGAGCCCACCGGGAACGAAA; and IL6 antisense, GCTTCGTCAGCAGGCTGGCAT. Aliquots (10 μl) of the polymerase chain reaction products were electrophoresed on 1.5% agarose gels and stained with SYBR Gold nucleic acid gel stain (Invitrogen; Molecular Probes, USA).
Densitometric analyses were performed using the image analysis software Quantity One (Bio-Rad, USA). Briefly, the digital image was analyzed to determine the pixel intensity of each band. Relative quantities of hBD2, hBD4, IL8, and IL6 mRNA among different preparations were calculated as the ratio of the hBD2/β3-actin, hBD4/β-actin, IL8/β-actin, and IL6/β-actin pixel intensities from three independent RT-PCR experiments. Positive results were based on the presence of DNA bands of the expected size.
RESULTS
Purification of trefoil
Trefoil factor 3 (TFF3) was purified from human breast milk by ammonium sulfate precipitation followed by isoelectric precipitation, gel filtration, and DEAE-Sepharose chromatography to ensure the removal of putative contaminating proteins (Figure 1, A and B). The purity of TFF3 was confirmed by SDS-PAGE (Figure 1C). Our TFF3 preparations contained a band with apparent mass of approximately 7 kDa. This band corresponds to molecular weight reported for TFF3, as indicated by the fact that it is recognized by anti-TFF3 antibody (Fig. 1D). We obtained five main fractions in the gel filtration chromatography (Figure 1a); each fraction was concentrated and underwent western blot analysis, and only the fifth fraction (P5) contained the trefoil factor 3 (data not shown). Then the P5 fraction (Figure 1a) was concentrated and loaded in DEAE-Sepharose chromatography (Figure 1B). After elution with NaCl, the P2 fraction contained the purified trefoil factor 3.
FIGURE 1.
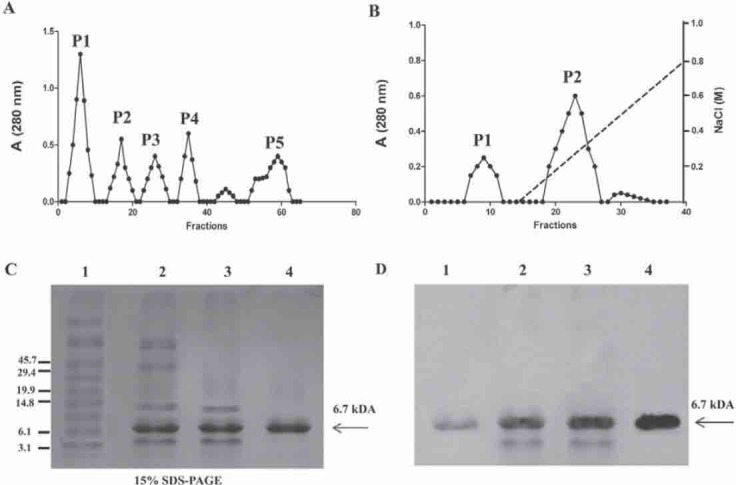
Purification of secreted TFF3. Frozen human milk samples (25 ml) were thawed at 37°C and TFF3 was purified by ammonium sulphate precipitation followed by isoelectric precipitation, gel filtration, and DEAE-Sepharose chromatography. A) Gel-filtration chromatography. Precipitated proteins by isoelectric precipitation were loaded onto a column containing 100 ml of Sephadex G-75 equilibrated with 20 mM Tris-HCl, pH 8.0. Five peaks are present, with the last peak 5 (P5) corresponding to an estimated molecular size of 7 kDa of TFF3. The P5 was again concentrated using centrifugal devices with a 5-kDa cutoff. B) The TFF3 (P5) was further purified by DEAE-Sepharose FF column equilibrated with 20 mM Tris-HCl, pH 8.0. The column was washed with 10 column volumes of the equilibration buffer. Bound material was eluted with a linear gradient of 0-1 M NaCl in DEAE equilibration buffer with a total volume of 100 ml. Two peaks are present, with the large peak 2 (P2) corresponding to purified TFF3. C) The purity of TFF3 was assessed by nondenaturing 15% SDS-PAGE. Total homogenate (lane 1), isoelectric precipitation (lane 2), gel-filtration chromatography (P5) (lane 3), DEAE chromatography (P2) (lane 4). D) Immunoblotting with anti-TFF3.
Trefoil factor 3 isolated from human breast milk downregulates IL6 and IL8 in HT-29 cells
ELISA was used to determine whether TFF3 induce a decrease of pro-inflammatory cytokines (IL6 and IL8) in intestinal epithelial cell culture supernatants from HT-29 cells col lected after LPS treatment. HT-29 cells were grown in 6-well plates to 50% confluence and serum-starved for 24 h. Cells were preincubated for 24 h with LPS (1 μg/ml), then treated for 48 h with 10 μg/ml trefoil. Afterwards, culture supernatants of HT-29 were collected and centrifuged at 1000g for 15 min at 4°C, the proteins were precipitated by TCA, and cytokine (IL6 and IL8) levels were measured by ELISA. TCA precipitation concentrates proteins contained in cell culture supernatants and prevents nonspecific reactions in the immunoassay [16]. As shown in Figure 2, the levels of IL6 (Figure 2a) and IL8 (Figure 2b) after treatment with LPS was significantly higher than in untreated cells. The treatment of HT-29 with TFF3 for 48 h after LPS treatment led to decrease of IL6 and IL8 similar to the basal levels. The treatment with vehicle (20 mM Tris-HCl, pH 8.0) for 48 h after LPS incubation did not modify the cytokine levels. Finally, the treatment with TFF3 was similar to the basal levels in untreated cells. Additionally, semiquantitative RT-PCR was used to determine whether trefoil factor 3 decreases intestinal epithelial cell (HT-29) expression of IL6 and IL8 mRNA. As shown in Figure 3, the treatment of HT-29 with TFF3 for 48 h after LPS treatment decreased IL6 and IL8 mRNA similar to the basal levels. Together, the results demonstrate that IL6 and IL8 were downregulated in HT-29 cells treated with TFF3.
FIGURE 2.

Cytokine (IL6 and IL8) levels in cell culture supernatants. HT-29 cells were grown in 6-well plates to 50% confluence and serum-starved for 24 h. Cells were then treated for 48 h with trefoil (10 μg/ml). In some experiments, HT-29 cells were preincubated for 24 h with LPS (1 μg/ml) before treating with trefoil for 48 h. Afterwards, culture supernatants of HT-29 were collected and centrifuged at 1000g for 15 min at 4°C, and proteins were precipitated by TCA. Concentrations of cytokines were determined by ELISA using anti-IL6 or anti-IL8 as primary antibody as indicated in “Materials and Methods”. The figure shows the measured concentrations of IL6 (A) and IL8 (B) expressed as μg/ml. Each assay was carried out in three independent experiments, and the results are reported as mean ± S.D.
FIGURE 3.

Quantification of differentially-expressed cytokines mRNAs by RT-PCR. A) Specific primers and annealing temperatures employed. B) RT-PCR for IL6, IL8, and β-actin were carried out from cell culture samples divided in five groups: basal (lane 1); cells treated with LPS 1 μg/ml for 24 h (lane 2); cells were preincubated for 24 h with LPS (1 μg/ml) before treating with vehicle (lane 3) or trefoil (lane 4) for 48 h; and cells treated only with TFF3 (lane 5). The PCR products were run on 2% agarose gel electrophoresis. Control reactions without reverse transcriptase were carried out. PCR was performed in a final volume of 25 μl containing 1 μl of the reverse transcription reaction, 50 μM of dNTPs, 1.5 mM MgCl2, 50 mM Tris-HCl (pH 8.0), 1 IU Taq polymerase, and 0.2 μM each of sense and antisense primers. Specific PCR for a constitutively expressed gene (β-actin) was carried out as a positive control. The relative amount of product was quantified by densitometry analysis of DNA bands (C). Cytokine mRNA expression levels are shown normalized to β-actin. Results are mean ± SEM of three independent experiments.
Trefoil factor 3 isolated from human breast milk upregulates hBD2 and hBD4 in HT-29 cells
To evaluate the effect of TFF3 on expression of defensins, we directly measured the levels of these antimicrobial peptides in cell culture supernatants after 48 h incubation with TFF3 isolated from human breast milk. HT-29 cells with either LPS or TFF3 for 48 h led to increase of hBD2 and hBD4 concentrations to levels 2-fold higher than in untreated cells (Figure 4). We tested five TFF3 concentrations from 1 to 50 μg/ml. Treatment with 10, 20, and 50 μg/ml produces upregulation of hBD2 and hBD4, although 10 μg/ml of TFF3 did not produce an increase of hBD4. The treatment with vehicle (20mM Tris-HCl, pH 8.0) for 48 h did not modify the defensin levels. Also, semiquantitative RT-PCR was used to determine whether TFF3 produces an increase in epithelial cell expression of hBD2 and hBD4 mRNA. As shown in Figure 5, in HT-29 cells treated with TFF3 there was a significantly higher expression of hBD2 and hBD4 compared with defensin levels in untreated cells. Together, the results demonstrate that hBD2 and hBD4 were upregulated in cells treated with TFF3.
FIGURE 4.
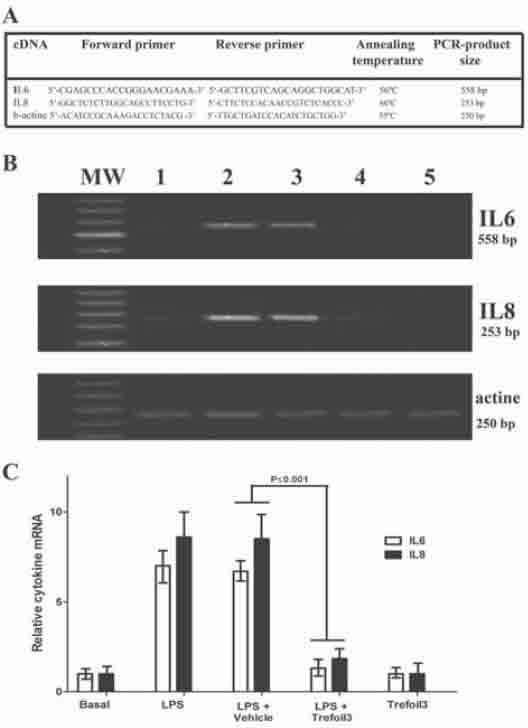
Defensin (hBD2 and hBD4) levels in cell culture supernatants. HT-29 cells were grown in 6-well plates to 50% confluence and serum-starved for 24 h. Cells were then treated for 24 h with LPS (1 μg/ml) or TFF3 at concentrations from 1 to 50 μg/ml for 48 h. Afterwards, culture supernatants of HT-29 were collected and centrifuged at 1000g for 15 min at 4°C, and proteins were precipitated by TCA. Concentrations of defensins were determined by ELISA using anti-hBD2 or anti-hBD4 as primary antibody as indicated in “Materials and Methods”. The figure shows the measured concentrations of hBD2 (A) and hBD4 (B) expressed as μg/ml. Each assay was carried out in three independent experiments, and results are reported as mean ± S.D.
FIGURE 5.
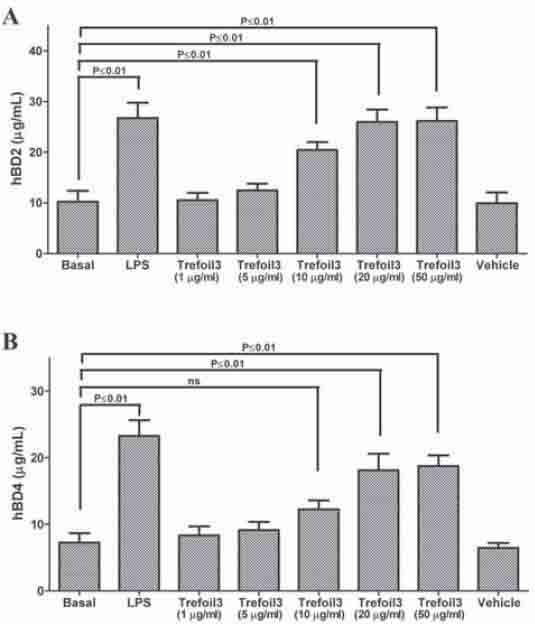
Quantification of differentially expressed defensin mRNAs by RT-PCR. A) Specific primers and annealing temperatures employed. B) RT-PCR for hBD2, hBD4, and β-actin were carried out from cell culture samples divided in five groups: basal (lane 1); cells treated with 1 μg/ml LPS for 24 h (lane 2); cells treated with TFF3 at 10 μg/ml (lane 3’) or 50 μg/ml (lane 4) for 48 h; and cells treated with vehicle (lane 5). The PCR-products were run on 2% agarose gel electrophoresis. Control reactions without reverse transcriptase were carried out. PCR was performed in a final volume of 25 μl containing 1 μl of the reverse transcription reaction, 50 μM of dNTPs, 1.5 mM MgCl2, 50 mM Tris-HCl (pH 8.0), 1 IU Taq polymerase, and 0.2 μM each of sense and antisense primers. Specific PCR for a constitutively expressed gene (β-actin) was carried out as a positive control. The relative amount of product was quantified by densitometric analysis of DNA bands (C). Defensin mRNA expression levels are shown normalized to β-actin. Results are mean ± SEM of three independent experiments.
TFF3 reduces the peroxidase activity in HT-29 cells
We found that the treatment of HT-29 with TFF3 for 48 h after LPS treatment led to decrease of IL6 and IL8 similar to the basal levels. In this sense, we tested the antiinflam-matory effects of TFF3 in HT-29 cells measuring the peroxidase activity in cells culture supernatants as described in “Materials and Methods”. HT-29 cells were grown in 6-well plates to 50% confluence and serum-starved for 24 h. Cells were preincubated for 24 h with LPS (1 μg/ml), and then treated by 48 h with 10 μg/ml trefoil. As shown in Figure 6, the peroxidase activity after treatment with LPS was significantly higher than in untreated cells. Then, the treatment of HT-29 with TFF3 for 48 h after LPS treatment led to decrease of peroxidase activity similar to the basal levels. The treatment with vehicle (20 mM Tris-HCl, pH 8.0) for 48 h after LPS incubation did not modified the enzyme activity.
FIGURE 6.
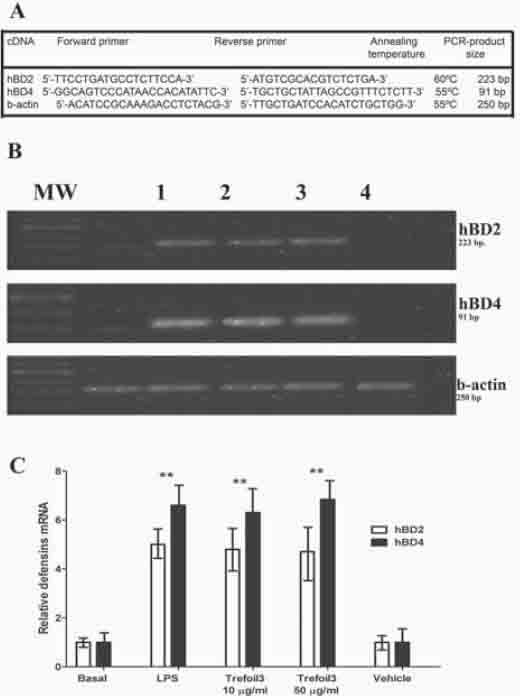
Peroxidase activity in cells treated with TFF3. Peroxidase activity was measured according to the 2-nitrobenzoic acid-thiocyanate (NBS-SCN) assay as previously described [15]. HT-29 cells were grown in 6-well plates to 50% confluence and serum-starved for 24 h. Cells were then treated for 48 h with 10 μg/ml trefoil. In some experiments HT-29 cells were preincubated for 24 h with LPS (1 μg/ml) before treating with trefoil for 48 h. Afterwards, culture supernatants of HT-29 were collected, centrifuged at 1000g for 15 min at 4°C, and peroxidase activity was measured.
DISCUSSION
Human breast milk constituents are potential candidates as biological factors influencing gastrointestinal tract physiology. Many breast milk protein components, such as glycoprotein, immunoglobulins, agglutinin, lactoferrin, defensins, and trefoil factors are thought to have many roles in the gut. Trefoil peptides (literally referring to its spatial conformation) are members of a family of peptides synthesized and secreted by epithelial cells, those that have claimed a major impact on the repair of mucosal lesions and that intervene in the processes of inflammation [17]. In this work, TFF3 was purified by ammonium sulphate precipitation, isoelectric precipitation, DEAE chromatography, and gel filtration. According to Yu et al. [8], 70% w/v of ammonium sulphate is insufficient to precipitate trefoil. It is for this reason that protein precipitation was a useful tool to precipitate unwanted proteins. In the literature there are conflicting with respect to pI values of TFF3. According to Kou et al. [18] the isoelectric point for the recombinant TFF3 was 4.5, and we also determined the isoelectric point based in protein sequence analysis by bioinformatics using ProtParam Tool, ExPASy Proteomics server (http://expasy.org/cgi-bin/protparam) and we obtained a value of 5.1. In the same way, Yu et al. [8] reported 4.9 of pI based on the calculation with protein sequence analysis software Antheprot (http://antheprot-pbil.ibcp.fr). The presence of pI values in this range was the reason for our two-step isoelectric precipitation process in this pH interval (4.5 and 5.1). There are three types of trefoil factors reported. According to Vestergaard et al. [6], human breast milk contains high amounts of TFF3, less TFF1, and virtually no TFF2. In this work, our purification method leads only to TFF3 isolation. There are three possible explanations for this: (i) TFF3 is the main trefoil factor present in human breast milk, (ii) the isoelectric precipitation isolates mainly TFF3 because its pI, (iii) the pI of TFF1 is 4.29 (http://expasy.org/cgi-bin/ protparam). In the same way, we tested by immunoblot our TFF3 preparations with an anti-human TFF1 (Santa Cruz Biotechnology) confirming that TFF3 purified from human breast milk do not contains TFF1 (data not shown). Intestinal epithelial cells have been reported to participate in the innate host defense by expressing immune effectors, such as proinflammatory cytokines and defensin in response to inflammatory stimuli such as LPS [19, 20]. In this work we found that trefoil factor 3 isolated from human breast milk downregulated IL6 and IL8 in HT-29 cells treated with LPS. In the same way, previous studies have shown that human milk is protective against necrotizing enterocolitis (NEC) via an unknown mechanism [21]. We speculate that human breast milk contains bioactive compounds that would decrease stimulated proinflammatory cytokine secretion in intestinal epithelial cells. In this study we have shown that treatment with TFF3 reduces inflammatory cytokines and peroxidase activity. Ulcerative colitis and Crohn’s disease exemplifies a group of chronic illnesses characterized by inflammation of the gastrointestinal tract. Inflammatory bowel disease seems to involve a multifaceted interplay between certain environmental, genetic, and immunological factors. Previous studies suggests TFF3 as therapeutic agents in inflammatory bowel disease [17]; however, the molecular role of TFF3 peptides in the gastrointestinal tract is not entirely understood. In this sense, we showed that TFF3 isolated from human breast milk reduces proinflammatory cytokines and may have therapeutic potential in the treatment of inflammatory bowel disease. It is therefore thinkable that TFF3 would decrease the symptoms of inflammatory bowel disease by several mechanisms, both in accelerating tissue repair as previously reported [22], and perhaps by inhibiting inflammatory cytokine secretion. An additional possibility is that the reduction in general inflammation follows as a consequence of the re-establishment of intestinal mucosal integrity. The notable antiinflammatory response of intestinal epithelial cells by TFF3 was confirmed by the significant decrease in peroxidase activity. It is known that inflammatory bowel diseases (Crohn’s disease and ulcerative colitis) exhibit increase in peroxidase activity. Peroxidase is considered to be a marker of the activation and degranulation of polymorphonuclear cells [23]. In inflammatory disease, activated polymorphonuclear cells produce reactive oxygen species (ROS), and peroxidase is involved in this process. We have shown that cells treated with TFF3 have a significantly lower peroxidase activity. It is therefore possible that TFF3 could reduce the symptoms of human inflammatory bowel disease. The expression of defensins in the gastrointestinal track suggests that they may have a central role in protecting intestinal mucosa. Several reasons for this proposal are as follows: (i) defensins have broad antimicrobial activity; (ii) they stimulate the acquired immune system and could function to enhance IgA production as well as IgG production; (iii) these defensins may function to keep overall bacteria in check. Thus, intestinal defensins may provide a natural antibiotic barrier. There are several new findings of this study. First, we present the head evidence of human milk TFF3 purification (Figure 1). Second, the treatment of intestinal cells (HT-29) with TFF3 upregulates antimicrobial peptide (hBD2 and hBD4) (Figures. 4 and 5). Third, the defensin levels found in culture supernatants are in the range of effective antimicrobial function, especially considering the synergistic action of the peptides. Fourth, the correlation of cationic defensins with TFF3 suggests an additional possible protective effect TFF3 in the gastrointestinal track. Conversely, low levels of defensins may result in increased susceptibility to bacterial and viral infections in the gut. In this sense, defensin production by the intestinal cells has been shown to play a role on the colonizing intestinal microbiota, and because some microbes may be more sensitive than others, the relative composition of the microbial population could be shaped by these effector peptides. Finally, this study shows the simultaneous expression of human hBD2 and hBD4 in cell culture HT-29 detected by semiquantitative RT-PCR. Previous studies demonstrated constitutive expression of hBD2 in intestinal tissues [24]. Our analysis of gene expression in cells treated with or without TFF3 showed differential transcriptional levels for the defensins. In cells treated with TFF3, hBD2, and hBD4 expression was at a higher level than control cells. The lower expression of hBD2 and hBD4 in control cells could explain the lower concentration of these antimicrobial peptides in culture supernatants of HT-29 measured by ELISA (Figure 4). The intestinal track, which is colonized by numerous microorganisms, contains a wide selection of antibacterial peptides that play an important role in maintaining its complex ecological homeostasis [25]. We have shown that cells treated with TFF3 have a significantly higher expression and levels of defensins (hBD2 and hBD4) based on both the RT-PCR and ELISA (Figures 4 and 5). The gastrointestinal tract is constantly exposed to microorganisms. Although the normal host relationship with the resident luminal bacteria is often mutually beneficial, the host also requires protection against these microorganisms. Human breast milk and intestinal epithelial cells play a critical role in mediating these protective responses, and there is increasing appreciation of the likely importance of cytokines and antimicrobial peptides that they express. This work supports the hypothesis that during lactation TFF3 secreted in human milk may activate intestinal epithelial cells, which in turn induces hBD2 and hBD4 expression. Our data links together TFF3, the activation of epithelial cells, and the expression of diverse antimicrobial peptides and cytokines, suggesting that it may contribute to regulation of adaptive immune responses in neonate or play an important role in inflammatory bowel disease.
DECLARATION OF INTEREST
The authors report no conflict of interest.
REFERENCES
- [1].Dórea JG, Fenton SE, LaKind JS, Berlin CM., Jr Researching chemicals in human milk can be conducted without discouraging breastfeeding. Bosn J Basic Med Sci. 2012;12(2):137–138. doi: 10.17305/bjbms.2012.2515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Semenov DV, Kanyshkova TG, Kit YY, Khlimankov DY, Akimzhanov AM, Gorbunov DA, et al. Human breast milk immunoglobulins G hydrolyze nucleotides. Biochemistry (Moscow) 1998;63:935–943. [PubMed] [Google Scholar]
- [3].Kit YY, Shipitsin MV, Semenov DV, Richter VA, Nevinsky GA. Phosphorylation of lipids tightly bound to secretory immunoglobulin A in antibody fractions from human breast milk possessing protein kinase activity. Biochemistry (Moscow) 1998;63:719–724. [PubMed] [Google Scholar]
- [4].Hettinga K, van Valenberg H, de Vries S, Boeren S, van Hooijdonk T, van Arendonk J, et al. The host defense proteome of human and bovine milk. PLoS One. 2011;6(4):e19433. doi: 10.1371/journal.pone.0019433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Jarvinen KM, Suomalainen H. Leucocytes in human milk and lymphocyte subsets in cow's milk-allergic infants. Pediatr. Allergy Immunol. 2002;13:243–254. doi: 10.1034/j.1399-3038.2002.00087.x. [DOI] [PubMed] [Google Scholar]
- [6].Vestergaard EM, Nexo E, Wendt A, Guthmann F. Trefoil factors in human milk. Early Hum. Dev. 2008;84:631–635. doi: 10.1016/j.earlhumdev.2008.04.001. [DOI] [PubMed] [Google Scholar]
- [7].Tran CP, Cook GA, Yeomans ND, Thim L, Giraud AS. Trefoil peptide TFF2 (spasmolytic polypeptide) potently accelerates healing and reduces inflammation in a rat model of colitis. Gut. 1999;44:636–642. doi: 10.1136/gut.44.5.636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Yu K, Jiang SF, Lin MF, Wu JB, Lin J. Extraction and purification of biologically active intestinal trefoil factor from human meconium. Lab. Invest. 2004;84:390–392. doi: 10.1038/labinvest.3700042. [DOI] [PubMed] [Google Scholar]
- [9].Polshakov VI, Williams MA, Gargaro AR, Frenkiel TA, Westley BR, Chadwick MP, et al. High-resolution solution structure of human pNR-2/pS2: a single trefoil motif protein. J. Mol. Biol. 1997;267:418–432. doi: 10.1006/jmbi.1997.0896. [DOI] [PubMed] [Google Scholar]
- [10].Chen H, Xu Z, Peng L, Fang X, Yin X, Xu N, et al. Recent advances in the research and development of human defensins. Peptides. 2006;27:931–940. doi: 10.1016/j.peptides.2005.08.018. [DOI] [PubMed] [Google Scholar]
- [11].Ganz T. The role of antimicrobial peptides in innate immunity. Integr. Comp. Biol. 2003;43:300–304. doi: 10.1093/icb/43.2.300. [DOI] [PubMed] [Google Scholar]
- [12].Claud EC, Savidge T, Walker WA. Modulation of human intestinal epithelial cell IL-8 secretion by human milk factors. Pediatr. Res. 2003;53:419–425. doi: 10.1203/01.PDR.0000050141.73528.AD. [DOI] [PubMed] [Google Scholar]
- [13].Wang YS, Li XJ, Zhao WO. TREM-1 is a positive regulator of TNF-a and IL-8 production in U937 foam cells. Bosn J Basic Med Sci. 2012;12:94–101. doi: 10.17305/bjbms.2012.2503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Laemmli UK. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970;227:680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- [15].Reznick AZ, Klein I, Eiserich JP, Cross CE, Nagler RM. Inhibition of oral peroxidase activity by cigarette smoke: in vivo and in vitro studies. Free Radic. Biol. Med. 2003;34:377–384. doi: 10.1016/s0891-5849(02)01297-2. [DOI] [PubMed] [Google Scholar]
- [16].De Jonge N, Fillie YE, Deelder AM. A simple and rapid treatment (trichloroacetic acid precipitation) of serum samples to prevent non-specific reactions in the immunoassay of a proteoglycan. J. Immunol. Methods. 1987;99:195–197. doi: 10.1016/0022-1759(87)90127-x. [DOI] [PubMed] [Google Scholar]
- [17].Teng X, Xu LF, Zhou P, Sun HW, Sun M. Effects of trefoil peptide 3 on expression of TNF-alpha, TLR4, and NF-kappaB in trinitro-benzene sulphonic acid induced colitis mice. Inflammation. 2009;32:120–129. doi: 10.1007/s10753-009-9110-x. [DOI] [PubMed] [Google Scholar]
- [18].Kou RQ, Wang W, Li LY, Ru B. Chemico-physical properties and spectra of recombinant human intestinal trefoil factor. Chin J Biochem Mol Biol. 1999;15(5):787–791. in Chinese. [Google Scholar]
- [19].Angrisano T, Pero R, Peluso S, Keller S, Sacchetti S, Bruni CB, et al. LPS-induced IL-8 activation in human intestinal epithelial cells is accompanied by specific histone H3 acetylation and methylation changes. BMC Microbiol. 2010;10:172–179. doi: 10.1186/1471-2180-10-172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Donnarumma G, Paoletti I, Buommino E, Iovene MR, Tudisco L, Cozza V, et al. Anti-inflammatory effects of moxifloxacin and human beta-defensin 2 association in human lung epithelial cell line (A549) stimulated with lipopolysaccharide. Peptides. 2007;28:2286–2292. doi: 10.1016/j.peptides.2007.09.009. [DOI] [PubMed] [Google Scholar]
- [21].Lucas A, Cole TJ. Breast milk and neonatal necrotising enterocolitis. Lancet. 1990;336:1519–1523. doi: 10.1016/0140-6736(90)93304-8. [DOI] [PubMed] [Google Scholar]
- [22].Mashimo H, Wu DC, Podolsky DK, Fishman MC. Impaired defence of intestinal mucosa in mice lacking intestinal trefoil factor. Science. 1996;274:262–265. doi: 10.1126/science.274.5285.262. [DOI] [PubMed] [Google Scholar]
- [23].Biasucci L M, D’Onofrio G, Liuzzo G, Zini G, Monaco C, Caligiuri G, et al. Intracellular neutrophil myeloperoxidase is reduced in unstable angina and acute myocardial infarction, but its reduction is not related to ischemia. J Am Coll Cardiol. 1996;27:611–616. doi: 10.1016/0735-1097(95)00524-2. [DOI] [PubMed] [Google Scholar]
- [24].Dhaliwal W, Bajaj-Elliott M, Kelly P. Intestinal defensin gene expression in human populations. Mol. Immunol. 2003;40:469–475. doi: 10.1016/s0161-5890(03)00156-1. [DOI] [PubMed] [Google Scholar]
- [25].Salzman NH. Paneth cell defensins and the regulation of the microbiome: détente at mucosal surfaces. Gut Microbes. 2010;1:401–406. doi: 10.4161/gmic.1.6.14076. [DOI] [PMC free article] [PubMed] [Google Scholar]


