Abstract
Alcoholic cardiomyopathy represents one of the main clinical complications in chronic alcoholics. This pathology contrasts the seemingly beneficial effect of small doses of alcohol on the cardiovascular system. Studies carried out in liver cells exposed acutely or chronically to varying doses of EtOH indicate that intrahepatic alcohol metabolism results in a major loss of cellular Mg2+. To investigate whether EtOH administration also induced Mg2+ extrusion in cardiac cells, H9C2 cells were exposed to varying doses of EtOH for short- or ling-term periods of time. The results indicate that H9C2 cells exposed to EtOH doses higher than 0.1% (v/v, or 15 mM) extruded Mg2+ into the extracellular medium on a time- and dose-dependent manner. Consistent with the involvement of cyP4502E1 in metabolizing EtOH, administration of chloro-methiazole (CMZ) as an inhibitor of the cytochrome prevented EtOH-induced Mg2+ loss to a large extent. EtOH-induced Mg2+ extrusion was also prevented by the administration of di-thio-treitol (DTT) and n-acetyl-cysteine (NAC), two agents that prevent the negative effects of ROS formation and free radicals generation associated with EtOH metabolism by cyP4502E1.
Taken together, our data indicate that Mg2+ extrusion also occur in cardiac cells exposed to EtOH as a result of alcohol metabolism by cyP4502E1 and associated free radical formation. Interestingly, Mg2+ extrusion only occurs at doses of EtOH higher than 0.1% administered for an extended period of time. The significance of Mg2+ extrusion for the onset of alcoholic cardiomyopathy remains to be elucidated.
Keywords: H9C2 cells, Magnesium, Cardiac, Ethanol, CyP4502E1
Introduction
Magnesium (Mg2+), the second most abundant cation within mammalian cells after potassium [1], is highly represented within nucleus, mitochondria, and endo-sarco-plasmic reticulum [1–3]. Total Mg2+ concentrations ranging between 16–20 mM have been measured within each of these compartments [1–3]. As for the cytoplasm, approximately 4–5 mM Mg2+ is present in the form of a complex with ATP, phosphocreatine and other phospho-nucleotides [2,4], leaving the free Mg2+ concentration ([Mg2+]i) to range between 0.5 and 1 mM [2,3]
A similar distribution has been measured in the majority of mammalian cells including cardiac myocytes [2,3], and in the absence of hormonal or metabolic stimuli, no major changes in cellular Mg2+ concentration are detected. Following stimulation of adrenergic receptors by catecholamine or isoproterenol [5–7] a major and rapid extrusion of Mg2+ across the myocyte cell membrane is observed [2,3], with minimal or no changes in [Mg2+]i [8]. The mechanism responsible for Mg2+ extrusion across the sarcolemma of cardiac cells has been identified with a cAMP-activated Na+/Mg2+ exchanger both in cardiac myocytes [5–7] and sarcolemmal vesicles [9].
Alcohol consumption as one of the main causes of Mg2+ loss from alcohol-responsive tissues including liver [10]. Our laboratory has reported that acute ethanol (EtOH)1 administration to liver cells causes two well distinct effects. By inhibiting anaerobic glycolysis in a dose-dependent manner, EtOH transient decreases cellular ATP [11], reducing its ability to complex cytoplasmic Mg2+. As cytosolic [Mg2+] i increases, Mg2+ is extruded through the Na+/Mg2+ exchanger [11]. In addition, EtOH inhibits Mg2+ entry into the cell for at least 60 min after alcohol removal [12], delaying the restoration of proper cellular Mg2+ homeostasis. Similar effects occur on a more prolonged time period in liver cells of animals chronically exposed to alcohol in the diet [12,13].
Cardiac myocytes are also negatively affected by EtOH administration, and alcoholic cardio-myopathy constitutes one of the major pathological complications in alcoholics [14,15]. This pathology is observed following prolonged exposure to alcohol [14], and contrast the seemingly beneficial, protective effect of acute but moderate EtOH consumption on cardiac cells and the cardiovascular system at large [14]. To explain this discrepancy the dose of alcohol consumed and the frequency of consumption have been implicated. Because cardiac cells lack cytoplasmic alcohol dehydrogenase (EC 1.1.1.1), which rapidly oxidize doses of ethanol smaller than 35–40 mM (≤ 0.25% v/v) attention has focused on the alcohol-inducible cytochrome P450-2E1 (cyP4502E1, EC 1.14.13.n7) located within the cardiac sarcoplasmic reticulum [16]. CyP4502E1, like alcohol dehydrogenase, catalyzes the oxidation of EtOH to acetaldehyde, the moiety to which numerous deleterious effects within the cardiac cell have been attributed [17]. In addition, the reaction catalyzed by the cyP4502E1produces also reactive oxygen species (ROS) and free radicals, which further alter cardiac cell functions and bioenergetics, contributing to the development of alcoholic cardio-myopathy.
The present study investigated the effects of short- and long-term effect of EtOH exposure in H9C2 cells, a widely used in-vitro model of cardiac myocytes. The reported results indicate that a dose- and time-dependent magnesium extrusion from these cells, which affects all the major cellular compartments we could reliably assess. The effect of EtOH is reduced by inhibitors of cyP4502E1 metabolism and ROS formation, suggesting that these two mechanisms are essential to mobilize Mg2+ from the cells. Taken together, these data indicate that loss of cellular Mg2+ is an integral component of the effect of EtOH on cardiac cells. The Mg2+ loss affects all main cellular organelles (i.e. cytoplasm, mitochondria, and sarcoplasmic reticulum, with major implications for ATP production and utilization, and Ca2+-dependent cardiac contractility within the heart.
Materials and Methods
Materials
All chemicals were of analytical grade (Sigma, St. Louis). H9C2 cells were from ATCC (Manassas, VA).
H9C2 cell culture
Culture of H9C2 cells (ATCC) were plated at the concentration of 1 × 105 cells/ml in DMEM medium (Gibco), in the presence of 10% FCS, and maintained in 10% CO2 atmosphere. Cells at 80% confluence were used to assess EtOH-induced Mg2+ extrusion. The day of the experiment, cells were removed from the incubator, and their media replaced with a Mg2+ free medium having the following composition (mM): 120 NaCl, 3 KCl, 1.2 CaCl2, 1.2 KH2PO4, 10 NaHCO3, 5 glucose, 10 HEPES, pH 7.2/NaOH, at 37°C [6,7]. Cell plates were placed on a slide warmer set at 37°C, and the cell assessed for Mg2+ extrusion by addition of EtOH in the absence or in the presence of 4-methyl-pyrazole (4-MP, 50 M) or chloromethiazole (CMZ, 100 M). Two aliquots of the medium (0.2 ml) were removed at 2 min interval prior to EtOH addition to establish extracellular Mg2+ baseline. Following EtOH addition, the incubation was continued for 90 minutes, withdrawing 0.2 ml aliquots of the medium at 15 min intervals. The medium aliquots were sedimented at 7,000 rpm × 1 min in microfuge tubes to exclude possible artifacts due to cell lifting. The supernatant was transferred to clean tubes and assessed for Mg2+ content by atomic absorbance spectrophotometry (AAS). At the end of the experiment, the plate was placed on ice, and any residual medium was removed by vacuum aspiration. Cells were rapidly washed (1 ml x 2) with ice-cold 250 mM sucrose, and 0.5 ml 10% HNO3 were used to scrape the cells off the well. The cell pellets were digested overnight in 10% HNO3. Following sedimentation of the denatured protein (8,000 g for 5 min) in microfuge tubes, Mg2+ and Na+ contents of the acid extract were measured by AAS in an Agilent 340 properly calibrated.
Cellular Mg2+ distribution
Total cellular Mg2+ content and distribution among cytoplasm, mitochondria, and other cellular organelles (e.g. sarcoplasmic reticulum and nucleus) was assessed as reported [18]. Briefly, H9C2 cells were washed, and incubated in Mg2+-free medium as described above. Digitonin (50 g/ml final concentration), carbonyl cyanide p-trifluoromethoxyphenylhydrazone (FCCP, 2 g/ml), and A23187 (2 g/ml) were sequentially added to the incubation system at 5 min interval, and aliquots of the incubation medium were withdrawn and sedimented at 5,000 g for 2 min to exclude possible artifacts due to protein or lifted cell. The 5 min interval between agent additions was used because preliminary observation has proven this lapse of time as optimal to mobilize Mg2+ from cytoplasm (digitonin), mitochondria (FCCP) and non-mitochondrial pools (A23187). Magnesium content in the supernatants was measured by AAS. Residual Mg2+ content in cell pellets was measured by AAS after acid digestion performed as reported previously. The Mg2+ content present in the cell pellet and in the extracellular medium prior to the addition of any stimulatory agent were calculated and used as baseline reference to determine the net amount of Mg2+ retained within the cell or released into the incubation medium, respectively.
Oxidative modification of proteins
To determine whether EtOH administration induced Mg2+ extrusion via reactive oxygen species formation and oxidative modification of proteins, H9C2 cells were pretreated with 100 M N-acetyl-cysteine (NAC) or 100 M di-thio-threitol (DTT) for 15 min prior to EtOH administration at 37°C. Following EtOH addition, aliquots of supernatant were removed and assessed for Mg2+ extrusion by AAS as reported previously. At the end of the experimental procedure, residual medium was removed and the cells were washed twice with fresh medium before being resuspended in lysis buffer and prepared for SDS-PAGE. Western Blot analysis was performed with antibodies recognizing HNE/protein adducts [19] to assess for oxidative modification of proteins. Densitometry was performed using Scion Image Program (NIH). Band density of HNE/protein adducts was normalized to β-actin.
Additional procedures
Aliquots of the incubation medium were collected at 5 min interval, and LDH activity measured by enzymatic kit (Sigma) sensitive to detect changes in the U/ml range, and expressed as U/L. LDH activity was assessed as a percentage of the total amount of the enzyme releasable from digitonin-permeabilized cells.
Protein content was determined by Lowry assay [20] using bovine serum albumin as a standard.
Statistical analysis
The data are reported as mean ± SE. Data were first analyzed by one-way ANOVA. Multiple means were then compared by Tukey’s multiple comparison test performed with a q-value established for statistical significance of P<0.05.
Results
H9C2 cells were used as an in-vitro model to investigate the effect of short- and long-term exposure to EtOH on cellular Mg2+ homeostasis.
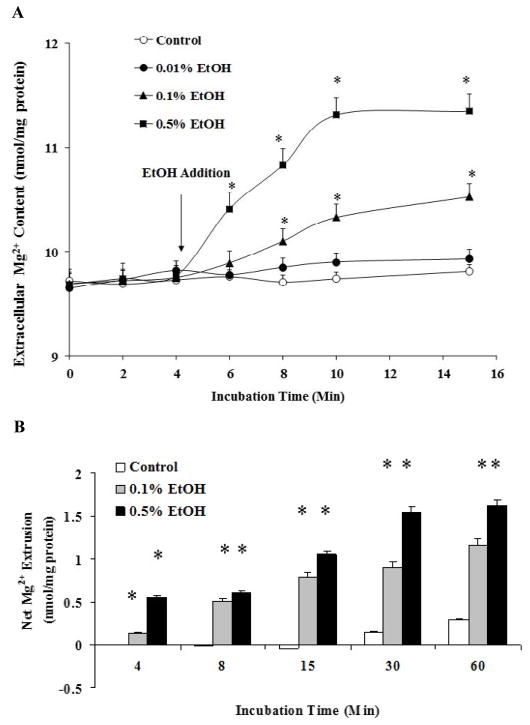
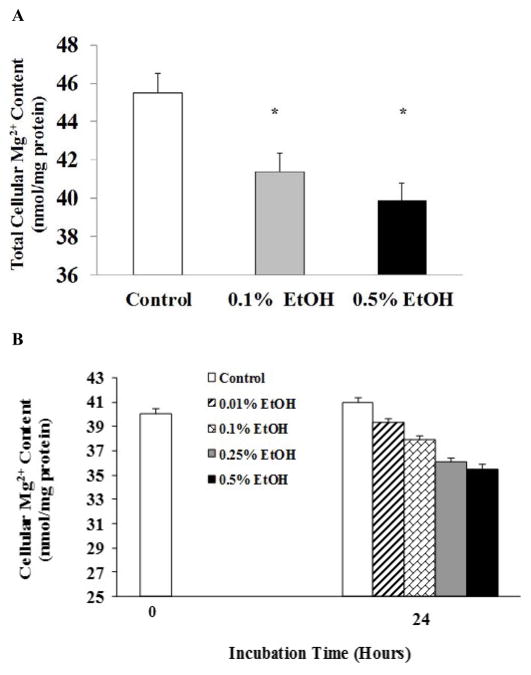
As Figure 1 shows, H9C2 cells exposed to EtOH extruded Mg2+ across the cell membrane into the extracellular medium in a dose- and a time-dependent manner (Figure 1A). The Mg2+ extrusion was observed as net increase in the extracellular medium (Figure 1B), or as a decrease in total cellular Mg2+ content (Figure 2A). Exposure to EtOH for 24 h also resulted in a detectable Mg2+ loss (Figure 2B) that was slightly higher than that observed in cells exposed to EtOH for 60 min (Figure 2A).
Figure 1. Ethanol-induced Mg2+ extrusion in H9C2 cells.
H9C2 cells, plated as indicated under Material and Methods, were stimulated by addition of varying doses of EtOH to the incubation medium. At the time points reported in the figure, aliquots of extracellular medium were withdrawn and Mg2+ content was assessed by AAS. Figure 1A reports a typical Mg2+ extrusion profile for H9C2 cells. Net Mg2+ extrusion is reported in Figure 1B. Data reported in Figure 1A and 1B are means ± S.E. of 5 different cell preparations, each tested in duplicate for all the experimental conditions. *Statistical significant (p<0.01) vs. corresponding time points in control sample and 0.01% stimulated cells.
Figure 2. Ethanol-induced Mg2+ loss in H9C2 cells.
H9C2 cells, plated as indicated under Material and Methods, were stimulated by addition of EtOH to the incubation medium. At the end of the experimental protocol, residual medium was removed and cells were digested in 10% HNO3. Cellular Mg2+ content was assessed by AAS in the acid extract upon sedimentation of the denatured protein as indicated under Materials and Methods. Figure 2A reports residual cellular Mg2+ content following 60 min stimulation with the reported doses of EtOH. Figure 2B reports residual cellular Mg2+ content following 24h stimulation with the reported doses of EtOH. Data reported in Figure 2A and 2B are means ± S.E. of 4 different cell preparations, each tested in duplicate for all the experimental conditions. *Statistical significant (p <0.01) vs. corresponding time points in control sample.
To determine whether EtOH-induced Mg2+ extrusion depleted specific cellular compartments, H9C2 cells were sequentially treated with digitonin, FCCP, and A23187 following 60 min or 24 hours exposure to different doses of EtOH. We have successfully used this approach to quantitate Mg2+ from the cytoplasm (digitonin), mitochondria (FCCP) and other, non-mitochondrial, cellular pools (A23187) in other cell models [18,21]. The results reported in Figure 3A indicate that EtOH administration depleted all cellular Mg2+ pools in a dose-dependent manner. A similar ubiquitous depletion was observed in H9C2 cells treated with 0.5% EtOH for 24 hours (Figure 3B). The loss of Mg2+ induced by EtOH was associated with a decrease in total ATP content: 4.32 ± 0.044 nmol/mg protein in control cells vs. 3.98 ± 0.037 nmol/mg protein in 0.1% EtOH treated cells vs. 3.76 ± 0.042 nmol/mg protein in 0.2% EtOH treated cells vs.3.52 ± 0.041 nmol/mg protein in 0.5% EtOH-treated cells. At the two lower EtOH concentrations the decrease in cellular ATP did not achieve statistical significance but the downward trend persisted throughout all the EtOH concentrations tested. For the 0.5% EtOH dose the decrease in phosphonucleotide content was approx. 17% than the corresponding Mg2+ loss (approximately 25%, Figure 3B).
Figure 3. Cellular Mg2+ distribution following EtOH stimulation.
H9C2 cells were harvested and stimulated in vitro with different doses of EtOH. Following 60 min stimulation (Figure 3A), cells were sedimented (800 g x 2 min) and gently resuspended in Mg2+ free-medium (see Materials and Methods). Following a few minutes of equilibration, digitonin, mitochondrial uncoupler (FCCP) and ionophore (A23187) were added sequentially to mobilize Mg2+ from different cellular compartments (Figure 3A, inset). Estimated Mg2+ contents present in cytoplasm, mitochondria, and non-mitochondrial compartments are reported in Figure 3A. For the experiment reported in Figure 3B, H9C2 cells were stimulated in vitro for 24 h with the reported doses of EtOH before being harvested and treated as reported above. Data reported in Figure 3A (including onset) and Figure 3B are means ± S.E. of 4 different cell preparations, each tested in duplicate for all the experimental conditions. *Statistical significant (p<0.01) vs. corresponding time point in control sample.
In cardiac cells, EtOH is oxidized to acetaldehyde by the reticular cytochrome P450-2E1 (CYP2E1, EC 1.14.13.n7), in a reaction coupled with the production of reactive oxygen species, which 0 in turn – lead to free radicals and lipid peroxidation generation within the cell [17]. In liver cells, inhibition of alcohol metabolism prevents ATP loss and Mg2+ extrusion in liver cells [11]. Hence, we assessed the ability of chloromethiazole (CMZ), a specific inhibitor of CYP2E1 to prevent Mg2+ extrusion by blocking ethanol metabolism [22]. For comparison, we used 4-methyl-pyrazole (4-MP). This compound is more effective at inhibiting the alcohol dehydrogenase and inhibits the cyP4502E1 only partially [22]. As Figure 4 shows, administration of CMZ resulted in a larger retention of cellular Mg2+ within the cell following 60 min exposure to 0.1% or 0.5% EtOH while 4-MP pre-treatment was much less effective. Administration of 100 M CMZ inhibited the effect of 0.1% and 0.5% EtOH at all the time points (not shown) and reduced net Mg2+ extrusion at t = 60 min by more than 50% (Figure 4). Administration of 150M CMZ inhibited Mg2+ extrusion by approximately 70% (not shown). No higher concentrations of CMZ were tested. In contrast, administration of 50 M 4-MP to H9C2 cells inhibited 0.1% EtOH-induced Mg2+ extrusion by <25% at all time points (not shown) and reduced net Mg2+ extrusion at t=60 min by ~30% (Figure 4), consistent with the partial inhibition of CYP2E1 activity by this agent [22]. When H9C2 cells were challenged with 0.5% EtOH, the protection provided by 4-MP was ~30% at all time points (not shown) including t=60 min (Figure 4). Administration of 100 M 4-MP did not provide a more effective protection (not shown), and higher concentrations of 4-MP were not tested. Co-addition of 4-MP (50 M) and CMZ (100 M) did not attain higher inhibition than the one observed with CMZ alone (not shown).
Figure 4. Inhibitory effect of CMZ and 4-MP on EtOH -induced Mg2+ extrusion in H9C2 cells.
H9C2 cells, plated as indicated under Material and Methods, were stimulated by addition of 0.1% or 0.5% EtOH to the incubation medium in the presence of chloromethiazole (CMZ, 100 M) or 4-methyl-pyrazole (4-MP, 50 M). At the time points reported in the figure, aliquots of extracellular medium were withdrawn and Mg2+ contents assessed by AAS. For simplicity, net Mg2+ extrusion at t=60 min is reported. Data are means ± S.E. of 5 different cell preparations, each tested in duplicate for all the experimental conditions. *Statistical significant (p<0.01) vs. corresponding time point in EtOH-treated sample in the absence of inhibitors.
To assess the involvement of reactive oxygen species and acetaldehyde formation in EtOH-induced Mg2+ extrusion, H9C2 cells were pre-treated with N-acetyl-cysteine (NAC) or di-thio-threitol (DTT) for 15 min prior to EtOH administration. The results reported in Figure 5A indicate that both DTT and NAC prevented EtOH-induced Mg2+ extrusion to a significant extent (60% to 65%) irrespective of the dose of EtOH administered. Consistent with this protective effect, Western blot analysis indicated a decrease in HNE modified cardiac proteins (Figures 5B and 5C), suggesting a reduced formation of ROS and lipid peroxidation products.
Figure 5. Inhibitory effect of DTT and NAC on EtOH-induced Mg2+ extrusion in H9C2 cells.
H9C2 cells, plated as indicated under Material and Methods, were stimulated by addition of 0.1% or 0.5% EtOH to the incubation medium in the presence of dithio-threitol (DTT, 100 M) or N-acetyl-cysteine (NAC, 50 M). At the time points reported in the figure, aliquots of extracellular medium were withdrawn and Mg2+ contents assessed by AAS. Figure 5A reports net Mg2+ extrusion at t = 60 min for simplicity. Figure 5B reports typical western Blot experiments for the formation of HNE-protein adducts in H9C2 cells treated with EtOH in the absence and in the presence of DTT or NAC. A β-actin western blot is reported for loading comparison purposes. Densitometry of 4 different similar experiments is reported in Figure 5C. Data in Figure 5A and 5C are means ± S.E. of 4 different cell preparations. For the data in Figure 5A, each cell preparation was tested in duplicate for all the experimental conditions. *Statistical significant (p<0.01) vs. corresponding time point in EtOH-treated sample in the absence of inhibitors.
Discussion
Consumption of small doses of EtOH has beneficial effects on the cardiovascular system especially if alcohol consumption is intermittent [14]. In contrast, prolonged consumption of alcohol, especially in high doses, results in the development of alcoholic cardiomyopathy in human subjects [14,15]. The disease has been attributed to the oxidation of EtOH to acetaldehyde by the cytP4502E1 located within the sarcoplasmic reticulum of the cardiac myocyte [16], and the coupled production of reactive oxygen species, free radicals, and lipid peroxidation products, which all react readily with phospholipids, signaling proteins, and enzymes [16]. As cytP450-2E1 activity is induced by high doses of EtOH, its enzymatic activity can reasonably explain the different effects of moderate versus chronic EtOH consumption on cardiac functions. The production of large quantities of acetaldehyde depresses the cardiac contractile function [23] and results in the release of significant amounts of troponin C into the extracellular space [24]. Combined, these processes affect cardiac contractility and cardiac ejection fraction to a significant extent, setting the conditions for the development and progression of alcoholic cardiomyopathy.
While attention has been paid to the effect of acute and chronic EtOH administration on cellular and reticular Ca2+ homeostasis and its impact on myocyte contractility, no information is currently available as to whether EtOH metabolism affects cardiac Mg2+ homeostasis. Magnesium is abundantly present with the cardiac myocyte, and evidence in the literature indicates that cellular and extracellular Mg2+ play major roles in cardiac physiology [1–3] by controlling action potential duration and regulation of Na+ and Ca2+ channels [25]. Conversely, an increased risk of ischemic heart disease [26] and specific forms of arrhythmias including the long QT syndrome have been associated with a less than optimal Mg2+ content within the cardiac tissue [27].
More specifically for the chronic ethanol administration, Mg2+ supplementation has been reported to ameliorate the myocardial dysfunction associated with alcoholic cardiomyopathy, renormalizing heart size, isometric force and isotonic shortening [15]. How exactly Mg2+ elicits these effects has not been investigated. Because Mg2+ acts as a natural Ca2+-channel blocker, it is possible that cardiac force development and cardiac cell shortening depend on the restoration of normal cytosolic Ca2+ levels, especially in diastoles, when the effect of abnormally elevated resting Ca2+ levels directly impact on contractile myofilaments’ function. Less clear is whether restoring physiological Ca2+ levels within the cardiac myocyte attenuates Ca2+-mediated signaling leading to hypertrophy [28].
The present study investigated the effect of EtOH administration on Mg2+ homeostasis in an in-vitro model of cardiac myocytes. The results suggest that EtOH metabolism via cyP4502E1 promotes Mg2+ loss from cytoplasm, sarcoplasmic reticulum, and mitochondria (albeit to a lesser extent). Evidence for the occurrence of such a loss is that: 1) total cellular and subcellular Mg2+ decrease in a time-dependent manner that directly correlates to the dose of EtOH administered, and 2) inhibition of EtOH metabolism prevents the Mg2+ loss from cardiac cells. Evidence that Mg2+ loss from cardiac cells depends on alcohol oxidation and related processes is provided by the protective effect of CMZ, an inhibitor of cytP4502E1 activity, DTT and NAC, two anti-oxidant agents.
Cytoplasm, mitochondria, and sarcoplasmic reticulum are the three main Mg2+ compartments within nucleated mammalian cells including cardiac myocytes [1–3], and Mg2+ plays a significant role in each of them by controlling ATP production, cardiac bioenergetics, and Ca2+ release and cycling, respectively [1–3]. Thus, loss of Mg2+ from these compartments can affect cellular bioenergetics and metabolic processes to a varying extent. Adenosine triphosphate is the main agent forming a complex with Mg2+ within the cytoplasm and the mitochondrial matrix. Hence, loss of ATP as a result of EtOH administration [11] limits the ability of the cell to retain Mg2+ in cytoplasm and mitochondria. In turn, mitochondria depend on proper Mg2+ homeostasis and Ca2+/Mg2+ ratio for proper dehydrogenases activity and to maintain an optimal ATP level for the cardiac myocyte [29]. A decrease in matrix Mg2+ level has been associated with structural and functional alteration of mitochondrial complexes and dehydrogenases [29] and with a marked decrease in mitochondrial respiratory rate [29]. The decreased utilization of oxygen by the mitochondrial electron chain has been related to the increased production of reactive oxygen species (ROS) [30]. This mitochondrial mechanism would be in addition to ROS production as a byproduct of cytP4502E1 activity. Hence, the increase in ROS production and associated lipid peroxidation observed following EtOH administration can be largely explained through these mechanisms. In turn, loss of Mg2+ from the various cellular organelles can depend on changes in membrane integrity following EtOH metabolism-related ROS and free radicals formation and associated lipid peroxidation.
It could be argued that the loss of Mg2+ observed in this in vitro model (i.e. about 2 nmol Mg2+/mg protein over 60 min, or ~5 nmol Mg2+/mg protein over 24h) is too small to be significant in absolute terms and of relevance for the development of alcohol related cardiomyopathy. It has to be considered, however, that when re-calculated for the cell volume, a loss of 5–6 nmol Mg2+/mg protein actually represents a loss equivalent to approximately 10% of the total cellular Mg2+ content. The average rat cardiac myocytes volume is estimated at ~30–35 pL [31] while initial estimates for the cardiac myocytes cell line used in our study suggest a cell volume of ~25 pl (Romani, unpublished observation). When taking into account these average cell volumes the Mg2+ losses observed under our experimental conditions accounts for a ~1.5–2 mM decrease in total Mg2+ content (out of a total cardiac myocytes concentration of ~16–20 mM [1,6,8,10]). When reported to the volume of organelles such as virtual cytoplasm (i.e. cytoplasm devoid of contractile filaments), mitochondria and sarcoplasmic reticulum, in which they were observed to occur, these losses can reflect higher decreases in Mg2+ concentration, and have a much stronger impact on the physiological operation of specific enzymes or proteins located within these compartments.
To our knowledge, this study is the first to investigate EtOH-induced changes in Mg2+ homeostasis in an in-vitro model of cardiac cells largely used to study cardiac hypertrophy. The results of this study unveils interesting new lines of research to elucidate the mechanisms responsible for Mg2+ loss in cardiac cells, and the potential relevance for cell bioenergetics, contractile function, and cardiac hypertrophy.
Acknowledgments
Antibody recognizing HNE/protein adducts were a kind gift of Dr. L. Szweda (Oklahoma Medical Center). This study was supported by NIAAA-11593.
Abbreviations
- 4-MP
4-Methyl-Pyrazole
- cyP4502E1
Cytochrome P450-2E1
- CMZ
Chloromethiazole
- EtOH
Ethanol
- DTT
Dithiothreitol
- FCCP
Carbonyl Cyanide p-trifluoromethoxyphenylhydrazone
- [Mg2+] i
Cytoplasmic Free Magnesium Concentration
- NAC
N-Acetyl-Cysteine
References
- 1.Romani A, Scarpa A. Regulation of cell magnesium. Arch Biochem Biophys. 1992;298:1–12. doi: 10.1016/0003-9861(92)90086-c. [DOI] [PubMed] [Google Scholar]
- 2.Wolf FI, Torsello A, Fasanella S, Cittadini A. Cell physiology of magnesium. Mol Aspects Med. 2003;24:11–26. doi: 10.1016/s0098-2997(02)00088-2. [DOI] [PubMed] [Google Scholar]
- 3.Gunther T. Functional compartmentation of intracellular magnesium. Magnesium. 1986;5:53–59. [PubMed] [Google Scholar]
- 4.Scarpa A, Brinley FJ. In situ measurements of free cytosolic magnesium ions. Fed Proc. 1981;40:2646–2652. [PubMed] [Google Scholar]
- 5.Vormann J, Gunther T. Amiloride sensitive net Mg2+ efflux from isolated perfused rat hearts. Magnesium. 1987;6:220–224. [PubMed] [Google Scholar]
- 6.Romani A, Scarpa A. Hormonal control of Mg2+ in the heart. Nature. 1990;346:841–844. doi: 10.1038/346841a0. [DOI] [PubMed] [Google Scholar]
- 7.Romani A, Marfella C, Scarpa A. Regulation of magnesium uptake and release in the heart and in isolated ventricular myocytes. Circ Res. 1993;72:1139–1148. doi: 10.1161/01.res.72.6.1139. [DOI] [PubMed] [Google Scholar]
- 8.Fatholahi M, La Noue K, Romani A, Scarpa A. Relationship between total and free cellular Mg2+ during metabolic stimulation of rat cardiac myocytes and perfused hearts. Arch Biochem Biophys. 2000;374:395–401. doi: 10.1006/abbi.1999.1619. [DOI] [PubMed] [Google Scholar]
- 9.Cefaratti C, Romani AMP. Functional characterization of two distinct Mg2+ extrusion mechanisms in cardiac sarcolemmal vesicles. Mol Cell Biochem. 2007;303:63–72. doi: 10.1007/s11010-007-9456-z. [DOI] [PubMed] [Google Scholar]
- 10.Romani AMP. Magnesium homeostasis and alcohol consumption. Magnes Res. 2008;21:197–204. [PubMed] [Google Scholar]
- 11.Tessman PA, Romani A. Acute effect of EtOH on Mg2+ homeostasis in liver cells: evidence for the activation of an Na+/Mg2+ exchanger. Am J Physiol. 1998;275:G1106–G1116. doi: 10.1152/ajpgi.1998.275.5.G1106. [DOI] [PubMed] [Google Scholar]
- 12.Torres LM, Konopnika B, Berti-Mattera LN, Liedtke C, Romani A. Defective translocation of PKC in EtOH-induced inhibition of Mg2+ accumulation in rat hepatocytes. Alcohol Clin Exp Res. 2010;34:1659–1669. doi: 10.1111/j.1530-0277.2010.01252.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Young A, Cefaratti C, Romani A. Chronic EtOH administration alters liverMg2+ homeostasis. Am J Physiol. 2003;284:G57–G67. doi: 10.1152/ajpgi.00153.2002. [DOI] [PubMed] [Google Scholar]
- 14.Lucas DL, Brown RA, Wassef M, Giles TD. Alcohol and the cardiovascular system: Research Challenges and opportunities. J Am Coll Cardiol. 2005;45:1916–1924. doi: 10.1016/j.jacc.2005.02.075. [DOI] [PubMed] [Google Scholar]
- 15.Brown RA, Crawford M, Natavio M, Petrowski P, Ren J. Dietary magnesium supplementation attenuates ethanol-induced myocardial dysfunction. Alcohol Clin Exp Res. 1998;22:2062–2072. [PubMed] [Google Scholar]
- 16.Schurmann Tolstrup J, Nordestgaard BG, Rasmussen S, Tybjærg-Hansen A, Grønbæk M. Alcoholism and alcohol drinking habits predicted from alcohol dehydrogenase genes. Pharmacogenomics J. 2008;8:220–227. doi: 10.1038/sj.tpj.6500471. [DOI] [PubMed] [Google Scholar]
- 17.Zhang X, Li S-Y, Brown RA, Ren J. Ethanol and acetaldehyde in alcoholic cardiomyopathy: from bad to ugly en route to oxidative stress. Alcohol. 2004;32:175–186. doi: 10.1016/j.alcohol.2004.01.005. [DOI] [PubMed] [Google Scholar]
- 18.Fagan TE, Cefaratti C, Romani A. Streptozotocin-induced diabetes impairs Mg2+ homeostasis and uptake in rat liver cells. Am J Physiol Endocrinol Metab. 2004;286:E184–E193. doi: 10.1152/ajpendo.00200.2003. [DOI] [PubMed] [Google Scholar]
- 19.Lashin O, Szweda PA, Szweda LI, Romani AM. Decreased complex II respiration and HNE-modified SDH subunit in diabetic heart. Free Rad Biol Med. 2006;40:886–896. doi: 10.1016/j.freeradbiomed.2005.10.040. [DOI] [PubMed] [Google Scholar]
- 20.Lowry OH, Rosebrough NJ, Farr AL, Randall RJ. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951;193:265–275. [PubMed] [Google Scholar]
- 21.Young A, Berti-Mattera L, Romani A. Effect of repeated doses of ethanol on hepatic Mg2+ homeostasis and mobilization. Alcohol Clin Exp Res. 2007;31:1240–1251. doi: 10.1111/j.1530-0277.2007.00408.x. [DOI] [PubMed] [Google Scholar]
- 22.Feierman DE, Cederbaum AI. Interaction of Pyrazole and 4-Methylpyrazole with Hepatic Microsomes: Effect on cytochrome P-450 content, microsomal oxidation of alcohols, and binding spectra. Alcohol Clin Exp Res. 1985;9:421–428. doi: 10.1111/j.1530-0277.1985.tb05576.x. [DOI] [PubMed] [Google Scholar]
- 23.Oba T, Maeno Y, Nagao M, Sakuma N, Murayama T. Cellular redox state protects acetaldehyde-induced alteration in cardiomyocyte function by modifying Ca2+ release from sarcoplasmic reticulum. Am J Physiol Heart Circ Phisiol. 2008;294:H121–H133. doi: 10.1152/ajpheart.00520.2007. [DOI] [PubMed] [Google Scholar]
- 24.Vary TC, Deiter G. Long-term alcohol administration inhibits synthesis of both myofibrillar and sarcoplasmic proteins in heart. Metabolism. 2005;54:212–219. doi: 10.1016/j.metabol.2004.08.015. [DOI] [PubMed] [Google Scholar]
- 25.Howarth FC, Waring J, Hustler BI, Singh J. Effects of extracellular magnesium and beta adrenergic stimulation on contractile force and magnesium mobilization in the isolated rat heart. Magnes Res. 1994;7:187–197. [PubMed] [Google Scholar]
- 26.Swaminathan R. Magnesium metabolism and its disorders. Clin Biochem Rev. 2003;24:47–66. [PMC free article] [PubMed] [Google Scholar]
- 27.Martin KJ, Gonzalez EA, Slatopolsky E. Clinical Consequences and Management of Hypomagnesemia. J Am Soc Nephrol. 2009;20:2291–2295. doi: 10.1681/ASN.2007111194. [DOI] [PubMed] [Google Scholar]
- 28.Molkentin JD. Calcineurin–NFAT signaling regulates the cardiac hypertrophic response in coordination with the MAPKs. Cardiovasc Res. 2004;63:467–475. doi: 10.1016/j.cardiores.2004.01.021. [DOI] [PubMed] [Google Scholar]
- 29.Panov A, Scarpa A. Mg2+ control of respiration in isolated rat liver mitochondria. Biochemistry. 1996;35:12849–12856. doi: 10.1021/bi960139f. [DOI] [PubMed] [Google Scholar]
- 30.Lashin O, Romani A. Mitochondria respiration and susceptibility to ischemia-reperfusion injury in diabetic hearts. Arch Biochem Biophys. 2003;420:298–304. doi: 10.1016/j.abb.2003.09.024. [DOI] [PubMed] [Google Scholar]
- 31.Cheng Y, Yu Z, Hoshijima M, Holst MJ, McCulloch AD, et al. Numerical analysis of Ca2+ signaling in rat ventricular myocytes with realistic transverse-axial tubular geometry and inhibited sarcoplasmic reticulum. PLoS Comput Biol. 2010;6:1–16. doi: 10.1371/journal.pcbi.1000972. [DOI] [PMC free article] [PubMed] [Google Scholar]