Abstract
Importance
Antiretroviral pre-exposure prophylaxis (PrEP), using tenofovir disoproxil fumarate and combination tenofovir disoproxil fumarate / emtricitabine, is efficacious for prevention of HIV acquisition. PrEP could reduce periconception HIV risk, but the effect on pregnancy outcomes is not well defined.
Objective
To assess pregnancy incidence and outcomes among women using PrEP during the periconception period.
Design
Randomized trial among 1785 HIV serodiscordant heterosexual couples (the Partners PrEP Study) in which the female partner was HIV uninfected that demonstrated that PrEP was efficacious for HIV prevention, conducted between July 2008 and June 2013 at 9 sites in Kenya and Uganda.
Intervention
Daily oral tenofovir disoproxil fumarate (TDF) (n=598), combination tenofovir disoproxil fumarate and emtricitabine (TDF-FTC) (n=566), or placebo (n=621) through July 2011, when PrEP demonstrated efficacy for HIV prevention; thereafter, participants continued receiving active PrEP, without placebo. Pregnancy testing occurred monthly and study medication was discontinued upon pregnancy detection.
Main Outcomes
Pregnancy incidence, birth outcomes (pregnancy loss, preterm birth, congenital anomalies), infant growth.
Results
A total of 431 pregnancies occurred. Pregnancy incidence was 10.0 per 100 person-years among women assigned placebo, 11.9 among those assigned TDF (incidence difference 1.9, 95% confidence interval [CI] −1.1–4.9, p=0.22 versus placebo), and 8.8 among those assigned TDF-FTC (incidence difference −1.3, 95% CI −4.1–1.5, p=0.39 versus placebo). Prior to discontinuation of the placebo treatment group in July 2011, the occurrence of pregnancy loss (96 of 288 pregnancies), was 42.5% for women receiving TDF-FTC compared with 32.3% for those receiving placebo (difference for TDF-FTC versus placebo 10.2%, 95% CI −5.3–25.7, p=0.16) and was 27.7% for those receiving TDF alone (difference versus placebo −4.6%, 95% CI −18.1–8.9, p=0.46). After July 2011, the frequency of pregnancy loss (52 of 143 pregnancies) was 37.5% for TDF-FTC and 36.7% for TDF alone (difference 0.8%, 95% CI −16.8–18.5, p=0.92). Preterm birth and congenital anomalies did not differ significantly for those who received PrEP versus placebo. Infants born to women randomized to PrEP had growth throughout the first year of life not statistically different than placebo and with point estimates that did not suggest growth restriction.
Conclusions and Relevance
Among HIV serodiscordant heterosexual African couples, differences in pregnancy incidence, birth outcomes, and infant growth were not statistically different for women receiving PrEP with TDF alone or combination TDF-FTC compared to placebo at the time of conception. Given that PrEP was discontinued when pregnancy was detected and that confidence intervals for the birth outcomes were wide, definitive statements about safety of PrEP in the periconception period cannot be made. These results should be discussed with HIV uninfected women receiving PrEP who are considering becoming pregnant. (ClinicalTrials.gov number, NCT00557245)
Keywords: pre-exposure prophylaxis, HIV, pregnancy
Introduction
Antiretroviral pre-exposure prophylaxis (PrEP), as daily oral tenofovir disoproxil fumarate and co-formulated emtricitabine/tenofovir disoproxil fumarate, has been demonstrated to be efficacious for the prevention of HIV acquisition in diverse populations.1–3 PrEP could be an important component of safer conception strategies for women at risk for HIV infection, including those in HIV serodiscordant couples (i.e., in which one member is HIV infected and the other uninfected), particularly if the infected partner is not eligible for, willing, or able to take antiretroviral treatment.4, 5 Efforts to implement PrEP as a public health strategy for HIV prevention in heterosexual populations will be accompanied by PrEP exposure during conception and pregnancy, either inadvertently for women with unrecognized early pregnancy or intentionally as part of reducing HIV risk during conception, and thus understanding the safety of PrEP in the periconception period is a priority.
Tenofovir disoproxil fumarate and emtricitabine are pregnancy category B medications, with no evidence of teratogenicity in animal experiments and in observational studies of humans.6 However, as with most medications, few data from controlled human studies in pregnancy are available. Renal and bone toxicity are known potential adverse effects of tenofovir disoproxil fumarate in HIV infected children and adults using tenofovir disoproxil fumarate as part of long-term combination antiretroviral treatment.7–9 Observational studies of HIV infected women using tenofovir disoproxil fumarate compared to other antiretroviral agents during pregnancy have generally indicated safety, although some data suggest slight growth restriction in infants born to women using tenofovir disoproxil fumarate.10–12
To date, PrEP use during conception among HIV uninfected women has not been studied systematically. Within a randomized, placebo-controlled trial of PrEP for HIV prevention among HIV serodiscordant couples, we assessed pregnancy incidence and outcomes for HIV uninfected women and growth and renal function during the first year of life for their infants.
Methods
Study population and procedures
Between July 2008 and November 2010, 4747 heterosexual HIV serodiscordant couples from 9 sites in Kenya and Uganda were enrolled and followed in the Partners PrEP Study, a phase III, randomized, double-blind, placebo-controlled, three-group trial of tenofovir disoproxil fumarate and emtricitabine/tenofovir disoproxil fumarate PrEP. Study sites were selected based on prior experience in similar research, community linkages, and linkages to HIV care providers; the design and primary safety and efficacy outcomes of the trial have been reported.1, 13
Eligible couples were ≥18 years of age, sexually active, and planned to remain in the relationship for the duration of the study. HIV uninfected participants had normal renal, hepatic, and hematologic function and were not infected with hepatitis B. They were randomized in a 1:1:1 fashion to daily oral tenofovir disoproxil fumarate (300 mg), emtricitabine/tenofovir disoproxil fumarate (200 mg/300 mg), or placebo. At monthly follow-up visits for up to 36 months, participants received individualized adherence counseling, HIV testing, and a month’s supply of study medication. At the time of enrollment, HIV infected partners did not meet Kenyan or Ugandan guidelines for initiation of antiretroviral therapy (generally, CD4 counts <350 cells/µL or symptomatic HIV-1 disease) and were not receiving antiretroviral therapy; they were followed quarterly and actively referred for antiretroviral therapy initiation if they became eligible during follow-up. At each study visit participants received a package of HIV prevention services, including risk-reduction counseling, couples counseling, and condoms.
In July 2011, the trial’s independent Data and Safety Monitoring Board recommended discontinuation of the placebo group and public report of the results due to demonstration of the efficacy and safety of PrEP for HIV prevention in the study population. In the primary analysis of HIV prevention efficacy, both tenofovir disoproxil fumarate (hazard ratio [HR] 0.33, 95% confidence interval [CI] 0.19–0.56, p<0.001) and emtricitabine/tenofovir disoproxil fumarate (HR 0.25, 95% CI 0.13–0.45, p<0.001) reduced HIV incidence compared to placebo; the frequency of key safety outcomes did not differ significantly across the study groups.1 Retention and adherence to PrEP were high, and subset analyses demonstrated high PrEP adherence and HIV protection efficacy among women.14 After July 2011, the active PrEP were continued, and subjects originally assigned placebo were offered re-randomization (in a 1:1 ratio) to the active PrEP arms.15 Provision of active PrEP to study population was done to gain additional blinded information on the relative efficacy and safety of PrEP using tenofovir disoproxil fumarate versus emtricitabine/tenofovir disoproxil fumarate while also providing PrEP to participants for a period after the trial, in accordance with international guidance regarding access to effective biomedical prevention interventions against HIV.15–17 Thus, after July 2011, all participants were receiving either tenofovir disoproxil fumarate or emtricitabine/tenofovir disoproxil fumarate, in a blinded fashion, for a period for up to 12 months; follow-up on study product concluded in December 2012, with additional follow-up thereafter of pregnant women.
Pregnancy among HIV uninfected women
The safety of PrEP in HIV uninfected women who became pregnant was defined in the study protocol as a secondary objective of the trial. At the time of enrollment, HIV uninfected women were not pregnant, breastfeeding, or intending to become pregnant. They were counseled on the available safety data for use of emtricitabine and tenofovir disoproxil fumarate in pregnancy and advised to use contraception. Contraceptive counseling was provided at each visit and contraceptives (oral contraceptive pills, injectable depot medroxyprogesterone acetate, intrauterine devices, hormonal implants, as well as condoms) were offered on-site at no cost; however, contraceptive use was not a requirement for trial participation and effective contraception was used at ~55% of follow-up visits.18 Urine β-hCG pregnancy tests were performed at enrollment and at each monthly visit,13 and study medication was discontinued in the event of pregnancy, for the duration of pregnancy and breastfeeding. Given the sensitivity of monthly pregnancy testing, the study team estimated that the duration of study medication exposure in the event of pregnancy would be approximately 6 weeks or less. Pregnant women were referred for antenatal care, were not counseled by study staff about pregnancy viability or provided any inducement for pregnancy termination, and were encouraged to breastfeed infants, in accordance with World Health Organization (WHO) guidelines. Monthly HIV testing continued throughout pregnancy and breastfeeding, and women who seroconverted to HIV during pregnancy or breastfeeding received expedited HIV resistance testing and referral for immediate initiation of antiretroviral therapy.
Pregnancy data were ascertained through standardized case report forms completed through participant report and summarization of medical records, when available. For pregnancies that terminated early, data on timing and nature of pregnancy loss (spontaneous or elective) was recorded. The duration of pregnancy was estimated between the first day of the last menstrual period to the date of delivery or pregnancy loss. Live-born infants were followed over the first year of life, with the initial visit scheduled within the first month and then quarterly. Evaluation of infants included assessment for congenital anomalies, measures of infant growth (weight, length and head circumference), done quarterly, and serum creatinine, which was measured at two visits occurring within one month of birth and at 3 months after birth. Because infants were not delivered by the study team, and were sometimes delivered at home, birth weight was inconsistently recorded and thus was not included as an outcome.
Ethical review
The study protocol was approved by the University of Washington Human Subjects Review Committee and ethics review committees at each of the study sites. All participants provided written informed consent in English or their local language.
Statistical Analysis
Several types of outcomes were defined: incidence of pregnancy, birth outcomes, and infant outcomes. Pregnancy incidence was defined as the number of pregnancies detected over the number of woman-years of follow-up, excluding follow-up time during pregnancy. Birth outcomes included live births, pregnancy losses, and congenital anomalies. Infant outcomes included growth, mortality, and serum creatinine. The 2006 WHO growth standard by age in days was used to calculate sex and age-adjusted z-scores for weight, length, and head circumference during post-natal follow-up for infants born at term;19 for infants born prior to term, preterm growth standards were used and z-scores were adjusted for gestational age.20 Infants’ age of life in days was derived from computing days between date of delivery and date of each study visit.
All analyses were limited to the subset of couples in which the HIV uninfected partner was female. The primary, pre-specified analysis included data collected on incident pregnancy, birth outcomes, and follow-up of infant outcomes for pregnancies detected up through discontinuation of the trial’s placebo group in July 2011; for those pregnancies, the last birth was in March 2012, with last infant follow-up occurring in February 2013. This primary, placebo-controlled analysis compared each active PrEP group (tenofovir disoproxil fumarate and combined emtricitabine/tenofovir disoproxil fumarate) separately to the placebo group. After this primary analysis was completed, an additional, post hoc analysis was conducted, comparing the effect of tenofovir disoproxil fumarate alone versus combined emtricitabine/tenofovir disoproxil fumarate PrEP on pregnancy incidence and birth outcomes. The post hoc analysis was motivated by a suggestion of a higher frequency of pregnancy losses in the emtricitabine/tenofovir disoproxil fumarate group compared to the tenofovir disoproxil fumarate only group in the primary analysis period. The post hoc analysis included all pregnancies identified during the trial period, including those identified after July 2011, both from women initially randomized to the trial’s active groups and from those re-randomized to active PrEP from placebo. The last birth in the post hoc analysis dataset occurred in June 2013.
All analyses were performed following intention-to-treat principles, with the exception that pregnancies occurring after HIV seroconversion were excluded, as women were discontinued from study medication upon acquisition of HIV. For the period covered by the primary, pre-specified analysis, a total of seven pregnancies occurred after HIV seroconversion and were excluded: two among women assigned tenofovir disoproxil fumarate (1 and 18 months after HIV seroconversion) and five among women assigned placebo (3, 6, 6, 12, and 18 months after HIV seroconversion).
Pregnancy incidence was compared using Cox proportional hazards models, stratified by study site; women were removed from the risk set while pregnant and the Andersen-Gill modification was used to account for multiple pregnancies per woman. Logistic regression was used to test for differences between groups for birth outcomes, with generalized estimating equations used to account for multiple pregnancies. Infant mortality was compared using the Fisher’s exact test. To assess differences by group in standardized growth outcomes, two sample t-tests were used; in addition, growth over time by group was compared using linear mixed effects models with time on study, randomization group, and their interaction as fixed effects and participant as a random effect. Missing data were rare and time points with missing data were omitted from analyses.
The design of the clinical trial was end-point driven for the primary HIV protection efficacy endpoint.13 No sample size calculations were conducted prior to the trial specifically for the secondary outcome of pregnancy safety, as the duration of the study was to be determined by the accumulation of HIV endpoints.
Analyses were performed using SAS version 9.3 (SAS Institute Inc., Cary, NC, USA). Statistical testing was two-sided and p-values <0.05 were considered statistically significant.
Results
Population characteristics
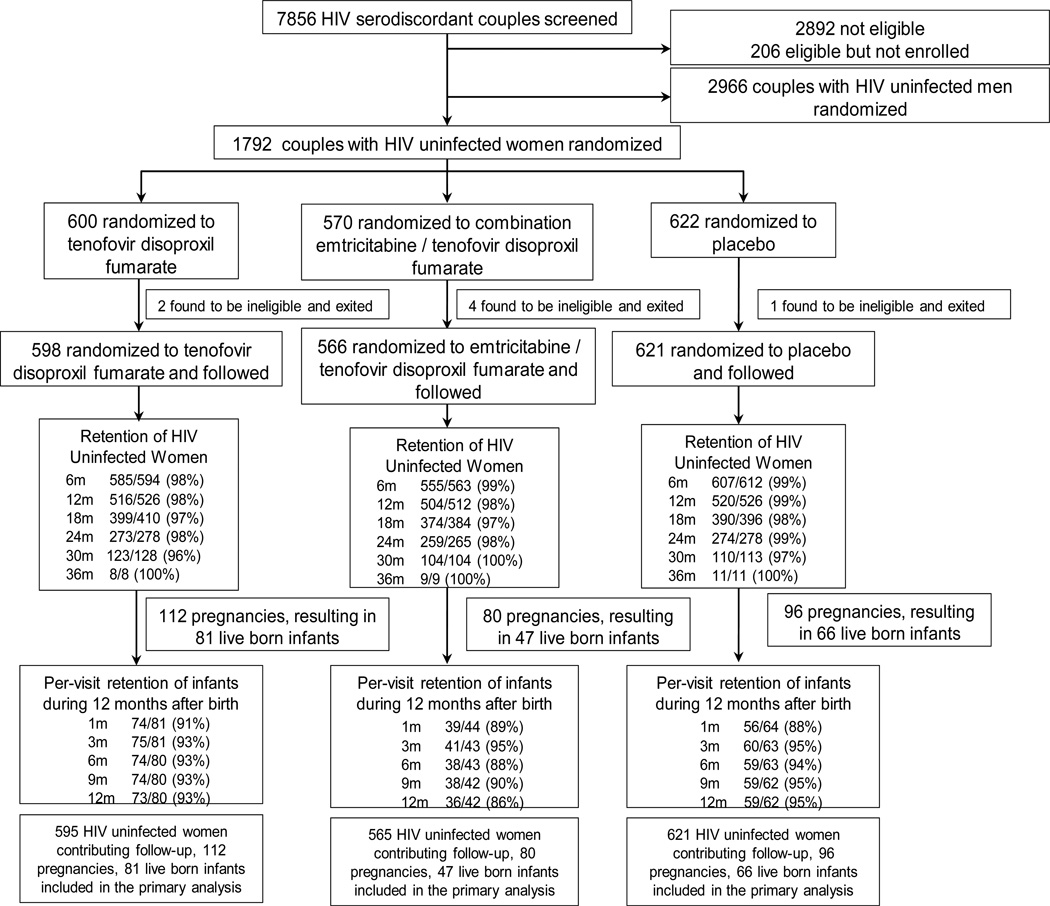
Of the 4747 HIV uninfected participants enrolled and followed in the Partners PrEP Study, 1785 (37.6%) were women, of whom 598 (33.5%), 566 (31.7%) and 621 (34.8%) were randomized to tenofovir disoproxil fumarate, emtricitabine/tenofovir disoproxil fumarate and placebo groups, respectively (Table 1, Figure 1). For these 1785 women, the median age was 33 years (interquartile range [IQR] 28–38), they had a median of 6 years of education (IQR 3–8), and 1704 (95.5%) had ever had a child, although 262 (14.7%) had not had a child with the HIV infected partner with whom they enrolled in the trial. Most (98.7%) were married to their HIV infected study partner, with a median partnership duration of 11.9 years (IQR 6.0–18.5), although the median duration of knowledge of their HIV serodiscordancy was only 0.67 years (0.08–2.08). HIV infected male partners had a median age of 39 years (IQR 33–44) and a median CD4 count of 457 cells/µL (IQR 354–596); 54 (20.2%) initiated antiretroviral therapy during follow-up.
Table 1.
Enrollment characteristics of HIV uninfected women participating in a randomized trial of PrEP for HIV prevention (n=1785)
| Median (interquartile range) or N (%) | |||
|---|---|---|---|
| Tenofovir disoproxil fumarate n=598 |
Emtricitabine / tenofovir disoproxil fumarate n=566 |
Placebo n=621 |
|
| Demographic characteristics | |||
| Age, years (median, IQR) | 32 (27, 37) | 33 (28, 39) | 33 (28, 39) |
| Education, years | 6 (3, 8) | 6 (3, 8) | 6 (3, 8) |
| Clinical characteristics | |||
| Using effective contraception1 | 263 (44.0%) | 275 (48.6%) | 299 (48.1%) |
| Couple characteristics | |||
| Married | 587 (98.2%) | 562 (99.3%) | 612 (98.6%) |
| No children in the partnership | 81 (13.5%) | 88 (15.5%) | 93 (15%) |
| Sexual behavior | |||
| Number of sex acts in prior month | 4 (2, 7) | 4 (2, 7) | 4 (2, 8) |
| Any unprotected sex acts in prior month | 141 (24.1%) | 121 (21.9%) | 144 (23.9%) |
| HIV infected male partner characteristics | |||
| Age, years | 38 (34, 44) | 39 (33, 45) | 38 (33, 43) |
| CD4 count, cells/µL | 453 (349, 598) | 466 (362, 584) | 451 (353, 600) |
| Plasma HIV RNA, log10 copies/mL | 4.06 (3.41, 4.70) | 4.21 (3.46, 4.72) | 4.08 (3.39, 4.72) |
Defined as oral, injectable, or implantable hormonal contraception, an intrauterine device, or surgical sterilization
Figure 1. Randomization and follow-up for the primary analysis cohort (i.e., prior to discontinuation of the trial’s placebo group in July 2011), including retention of HIV uninfected women during follow-up and visit attendance of infants during the first 12 months after birth.
1785 HIV uninfected women were randomized in a 1:1:1 fashion to daily oral tenofovir disoproxil fumarate, combination emtricitabine/tenofovir disoproxil fumarate, or placebo and followed for up to 36 months, through July 2011. Cumulative retention for women is detailed: denominators indicate women eligible for follow-up through different periods up to 36 months from enrollment and numerators note those completing such follow-up. Four women contributed no follow-up: 3 randomized to tenofovir disoproxil fumarate and 1 to emtricitabine/tenofovir disoproxil fumarate. 194 live-born infants were followed with scheduled visits within the first month of life and then quarterly. Per-visit retention is provided, with denominators referring to infants eligible to have attended the visit (i.e., excluding infants who died) and numerators referring to infants who attended the visit.
Follow-up
Among the 1785 women, 1781 (99.8%) completed at least one post-randomization visit, with retention >95% throughout follow-up, and 2805 total person-years of follow-up accrued for assessment of pregnancy incidence (median 17.0 months, IQR 10.1–24.9). Study medication was dispensed at 92.5% of attended visits. Factoring in missed visits, other reasons for non-dispensation of study medication, non-adherence to dispensed study pills (as measured by pill counts of unused study product), and censoring time during pregnancy and breastfeeding, 92.2% of follow-up time was covered by study medication. In the period following discontinuation of the placebo treatment group, an additional 1294 person-years of follow-up for assessment of incident pregnancy were accrued between the two PrEP groups and retention remained >95% (eFigure 1).
Pregnancy incidence and birth outcomes
During the primary, placebo-controlled analysis period, 288 pregnancies occurred among 267 HIV uninfected women, at an overall pregnancy incidence of 10.3 per 100 person-years (Table 2). Pregnancy incidence did not differ significantly by randomization group: 11.9, 8.8 and 10.0 per 100 person-years in the tenofovir disoproxil fumarate, emtricitabine/tenofovir disoproxil fumarate and placebo groups, respectively (incidence difference 1.9, 95% confidence interval [CI] −1.1–4.9, p=0.22 for tenofovir disoproxil fumarate versus placebo and incidence difference −1.3, 95% CI −4.1–1.5, p=0.39 for emtricitabine/tenofovir disoproxil fumarate versus placebo). One pregnancy (in the emtricitabine/tenofovir disoproxil fumarate group) occurred in a woman who had been off study medication for more than 3 months, due to missed visits. The median duration of gestation at the time of pregnancy detection was 35 days (IQR 29–45): 37 (IQR 29–46) for tenofovir disoproxil fumarate (p=0.34 vs. placebo), 35 (IQR 29–42) for emtricitabine/tenofovir disoproxil fumarate (p=0.89 vs. placebo), and 35 (IQR 28–46) for placebo.
Table 2.
Pregnancy incidence and birth outcomes
| Tenofovir disoproxil fumarate | Emtricitabine/tenofovir disoproxil fumarate |
Placebo | Primary analysis, data through July 2011 and discontinuation of the trial’s placebo arm3 |
Post hoc analysis comparison of PrEP groups data through end of trial3 |
||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Before July 2011 |
After July 2011 |
Total | Before July 2011 |
After July 2011 |
Total | Before July 2011 |
tenofovir disoproxil fumarate vs placebo |
emtricitabine/ tenofovir disoproxil fumarate vs placebo |
emtricitabine/ tenofovir disoproxil fumarate vs tenofovir disoproxil fumarate |
|
| Number of women contributing follow- up |
595 | 800 | 832 | 565 | 768 | 802 | 621 | |||
| Person-years of follow-up |
939 | 657 | 1596 | 911 | 637 | 1548 | 955 | |||
| Number of pregnancies |
112 | 62 | 174 | 80 | 81 | 161 | 96 | |||
| Pregnancy incidence per 100 person-years |
11.9 | 9.4 | 10.9 | 8.8 | 12.7 | 10.4 | 10.0 | P=0.22 absolute difference 1.9 (95% CI −1.1–4.9) |
P=0.39 absolute difference −1.3 (95% CI −4.1–1.5) |
P=0.77 absolute difference −0.5 (95% CI −2.8–1.8) |
| Live birth1, n (%) | 81 (72.3%) |
38 (63.3%) |
119 (69.2%) |
46 (57.5%) |
50 (62.5%) |
96 (60.0%) |
65 (67.7%) |
P=0.46 absolute difference ending in pregnancy loss −4.6% (95% CI −18.1%-8.9%) |
P=0.16 absolute difference ending in pregnancy loss 10.2% (95% CI −5.3%-25.7%) |
P=0.09 absolute difference ending in pregnancy loss 9.2% (95% CI −1.7%-20.1%) |
| Pregnancy loss2, n (%) |
31 (27.7%) |
22 (36.7%) |
53 (30.8%) |
34 (42.5%) |
30 (37.5%) |
64 (40.0%) |
31 (32.3%) |
|||
| Preterm birth, n (% of live births) |
2 (2.5%) |
2 (5.3%) |
4 (3.4%) |
4 (8.7%) |
3 (6.0%) |
7 (7.3%) |
5 (7.7%) |
P=0.16 absolute difference −5.2% (95% CI −13.9%-3.5%) |
P=0.85 absolute difference 1.0% (95% CI −11.3%-13.3%) |
P=0.20 absolute difference 3.9% (95% CI −3.1%-11.0%) |
| Any anomaly, n (% of live born infants) |
4 (4.9%) |
2 (5.1%) |
6 (5.0%) |
4 (8.5%) |
2 (3.9 %) |
6 (6.1%) |
5 (7.6%) |
P=0.51 absolute difference −2.6% (95% CI −12.0%-6.7%) |
P=0.86 absolute difference 0.9% (95% CI −11.1%-13.0%) |
P=0.72 absolute difference 1.1% (95% CI −6.0%-8.2%) |
In the primary, placebo-controlled analysis, 192 pregnancies ended in live births resulted in 194 live-born infants (1 set of twins each in the emtricitabine/tenofovir disoproxil fumarate and placebo groups). An additional 88 pregnancies ended in live births (2 set of twins, one per each active PrEP group) occurred after July 2011. Data on pregnancy outcomes were missing for 3 pregnancies, all occurring after July 2011: 2 tenofovir disoproxil fumarate and 1 emtricitabine/tenofovir disoproxil fumarate.
Of the 96 pregnancy losses, 88 (91.7%) ended at <20 weeks’ gestation: tenofovir disoproxil fumarate 27/31 (87.1%, p=0.61 vs. placebo, Fisher’s exact test), emtricitabine/ tenofovir disoproxil fumarate 31/34 (91.2%, p=0.61 vs. placebo), and placebo 30/31 (96.7%). 19 pregnancies were reported to have ended in induced loss: 8 tenofovir disoproxil fumarate, 3 emtricitabine/tenofovir disoproxil fumarate, 8 placebo. For the 52 pregnancy losses occurring in the data from after July 2011, 50 (96.2%) ended at <20 weeks’ gestation: tenofovir disoproxil fumarate 22/22 (100.0%) and emtricitabine/tenofovir disoproxil fumarate 28/30 (93.3%, p=0.50 vs. tenofovir disoproxil fumarate, Fisher’s exact test); 17 pregnancies were reported to have ended in induced loss: 8 tenofovir disoproxil fumarate and 9 emtricitabine/tenofovir disoproxil fumarate.
Statistical tests used: for comparison of pregnancy incidence = Cox proportional hazards model with Anderson Gill method for multiple pregnancies, for comparison of frequency of pregnancy losses, preterm birth, and congenital anomalies = generalized estimating equations with logistic link to account for multiple pregnancies.
Of the 288 pregnancies, 192 (66.7%) ended in live births and 96 (33.3%) ended in pregnancy losses, including 19 induced losses. Most pregnancy losses (91.7%) occurred before 20 weeks’ gestation. For the live births, 47 (24.5%) were home deliveries and 182 (94.8%) were vaginally delivered. Eleven (5.7%) were born preterm (<37 weeks’ gestation). There was no statistically significant association between those receiving PrEP and those receiving placebo and the occurrence of pregnancy losses (difference of proportions −4.6%, 95% CI −18.1%-8.9%, p=0.46 for tenofovir disoproxil fumarate versus placebo and −10.2%, 95% CI −5.3%-25.7%, p=0.16 for emtricitabine/tenofovir disoproxil fumarate versus placebo) or preterm births (difference of proportions −5.2%, 95% CI −13.9%-3.5%, p=0.16 for tenofovir disoproxil fumarate versus placebo and 1.0%, 95% CI −11.3%-13.3%, p=0.85 for emtricitabine/tenofovir disoproxil fumarate versus placebo).
Thirteen infants (6.7% of live born infants) were born with a total of 17 congenital anomalies, a frequency that was not statistically different across randomization groups: 7.6% in the placebo group, 4.9% in the tenofovir disoproxil fumarate group (difference in proportions −2.6%, 95% CI −12.0–6.7, p=0.51 versus placebo), and 8.5% in the emtricitabine/tenofovir disoproxil fumarate group (difference in proportions 0.9%, 95% CI −11.1–13.0, p=0.86 versus placebo). The details of the types of congenital anomalies are reported in the online supplemental materials.
In the period after the placebo treatment group was discontinued in July 2011 an additional 143 pregnancies were observed in the two active PrEP groups, among 137 women, for an overall pregnancy incidence of 10.9 per 100 person-years in the tenofovir disoproxil fumarate group and 10.4 in the emtricitabine/tenofovir disoproxil fumarate group (incidence difference −0.5 per 100 person-years, 95% CI −2.8–1.8), p=0.77). Of these 143 pregnancies, 88 (61.5 %) ended in live births and 52 (36.4%) ended in pregnancy losses, and data were missing from 3 pregnancies. Prior to July 2011, there was a higher proportion of pregnancy losses in women assigned emtricitabine/tenofovir disoproxil fumarate (42.5%, difference in proportions 14.8%, 95% CI 0.1%-29.5%, p=0.04) compared to those assigned tenofovir disoproxil fumarate (27.7%), but the frequency of pregnancy losses in the two groups was 36.7% and 37.5%, respectively, for pregnancies occurring after July 2011 (difference in proportions 0.8%, 95% CI −16.8–18.5, p=0.92) and the composite data from the entire study period was not statistically significantly different comparing the two PrEP groups (difference of proportions 9.2%, 95% CI −1.7%-20.1%, p=0.09). The overall occurrence of prematurity was not statistically different between the PrEP groups (difference of proportions 3.9%, 95% CI −3.1–11.0%, p=0.20). An additional five congenital anomalies, occurring in four infants (two in each PrEP group), were observed in pregnancies occurring after July 2011.
Overall, for the women who became pregnant in the entire study, the median number of lifetime pregnancies was 5 (interquartile range 3–6). For only 8 women (2.1%) was the pregnancy experienced during this study their first pregnancy; 22 (of 365 women who had had a prior pregnancy, 6.0%) had had a prior preterm birth. Maternal pregnancy-related complications were rare during the pregnancies followed in this study, with three woman experiencing pre-eclampsia (two in the tenofovir disoproxil fumarate group and one in the emtricitabine/tenofovir disoproxil fumarate group) and no reports of pregnancy induced diabetes. For 53 of the 431 pregnancies observed in the study, the HIV-infected male partner had initiated combination antiretroviral therapy at the time of pregnancy in the uninfected female partner: 25 in the tenofovir disoproxil fumarate group (9 before / 16 after July 2011), 22 emtricitabine/tenofovir disoproxil fumarate group (3 before / 19 after July 2011), and 6 in the placebo group.
Infant outcomes
For infants conceived during the primary analysis period, retention in follow-up during the first year of life was high and comparable across the three study groups (Figure 1). There were 10 infant deaths, of which five occurred within the first 7 days of life; four of these five perinatal deaths were associated with out-of-hospital deliveries. Of these 10 deaths, one was born to a mother who had been assigned tenofovir disoproxil fumarate (acute diarrhea, aged 159 days), five were born to mothers assigned emtricitabine/tenofovir disoproxil fumarate (prematurity, aged 0 and 1 days [a set of twins]; septicemia, aged 2 days; bronchopneumonia, aged 22 days; complications of Trisomy 21, aged 275 days), and four were born to mothers assigned placebo (birth asphyxia, aged 0 days; neonatal septicemia, aged 3 days; malaria, aged 80 days and 200 days). Overall infant mortality was 5.2% (10/194): 1.2% (1/81) in the tenofovir disoproxil fumarate group (p=0.17 versus placebo, difference of proportions −4.8%, 95% CI −12.4–2.8), 10.6% (5/47) in the emtricitabine/tenofovir disproxil fumarate group (p=0.49 versus placebo, difference of proportions 4.6%, 95% CI −7.8–16.9), and 6.1% (4/66) in the placebo group.
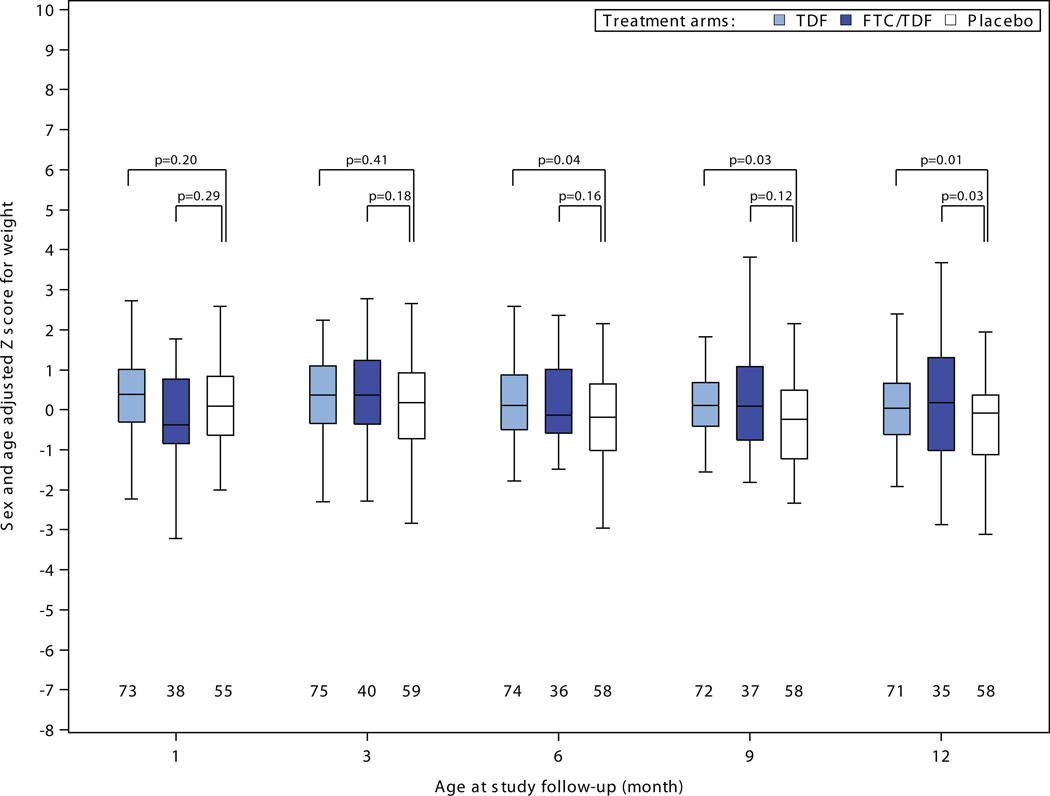
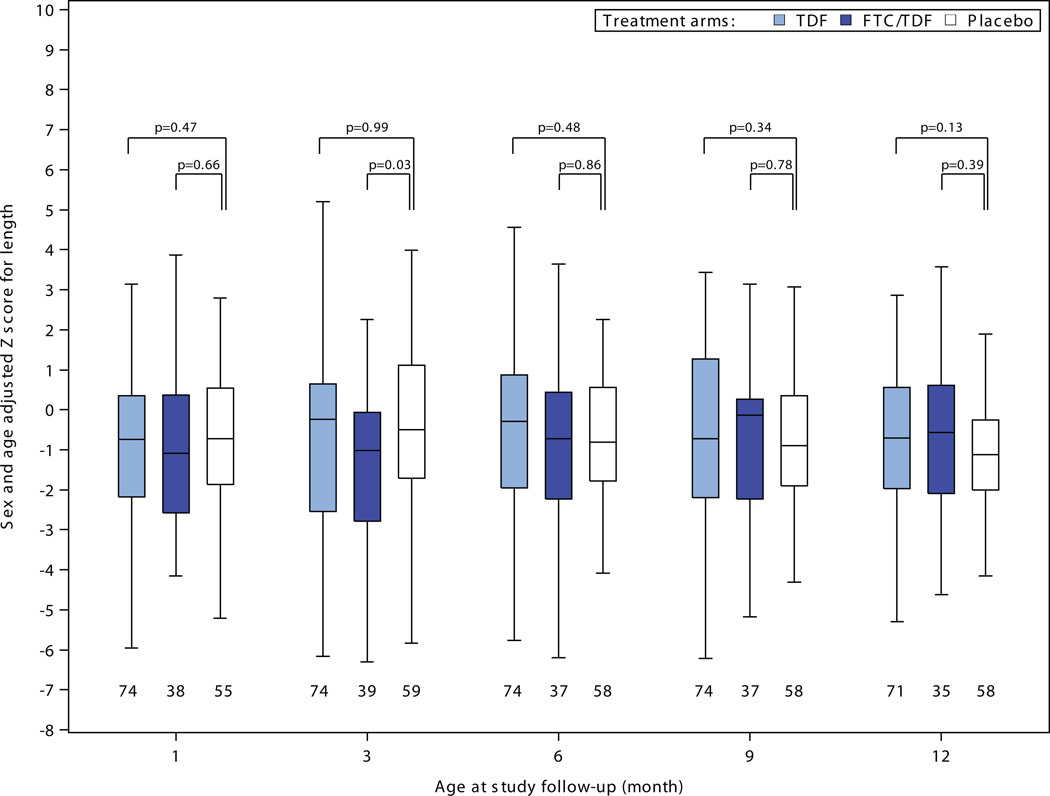
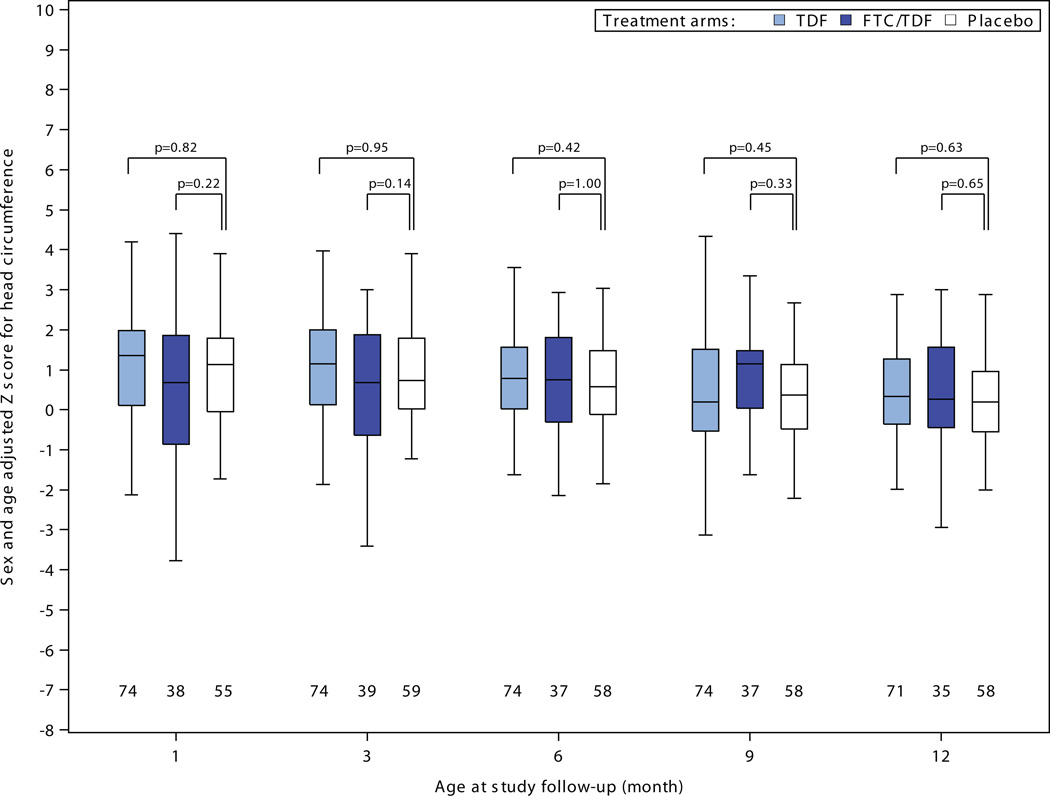
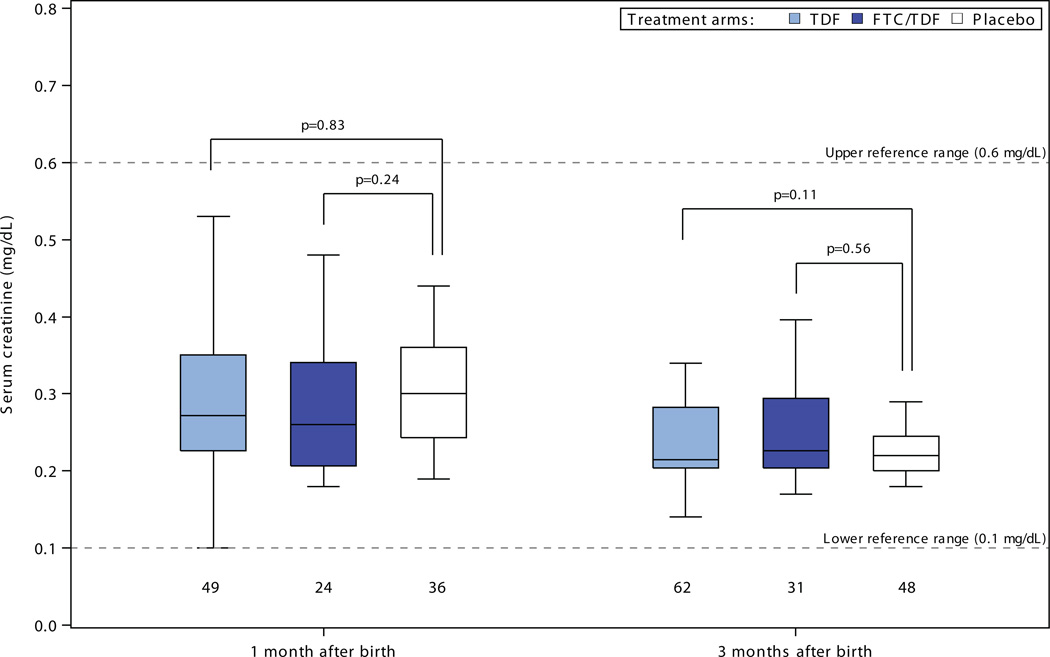
There were no statistically significant differences in head circumference, length, or weight to suggest growth retardation for infants born to women assigned PrEP compared to placebo (Figure 2). Among 30 comparisons, 4 measures reached statistical significance (p<0.05) versus placebo – tenofovir disoproxil fumarate weight-adjusted z-scores at 6, 9, and 12 months and emtrictabine/tenofovir disoproxil fumarate weight-adjusted z-score at 12 months – each of these indicating less growth restriction for the PrEP group compared to the placebo group. In addition, in linear mixed-effects models assessing growth during the entirety of follow-up, differences in slope over time for adjusted z-scores relative to the placebo were: for weight 0.03 (p=0.02) for tenofovir disoproxil fumarate and 0.06 (p<0.001) for emtricitabine/tenofovir disoproxil fumarate ; for length 0.03 (p=0.42) for tenofovir disoproxil fumarate and 0.07 (p=0.08) for emtricitabine/tenofovir disoproxil fumarate; and for head circumference 0.02 (p=0.35) for tenofovir disoproxil fumarate and 0.07 (p=0.008) for emtricitabine/tenofovir disoproxil fumarate; thus, all models indicated no reduced rate of growth for infants born to women in the tenofovir disoproxil fumarate and emtricitabine/tenofovir disoproxil fumarate groups and slightly faster growth in some measures for those groups relative to placebo. There were no statistically significant differences in serum creatinine concentrations for infants born to women assigned PrEP versus placebo (Figure 3).
Figure 2. Infant growth: sex- and age-adjusted Z-scores for A) weight, B) length, and C) head circumference, by randomization group.
Box plots depict median (central line), interquartile range (box), and range (whiskers); numbers below each box indicate the number of subjects who attended the visit and who had growth parameters recorded. P-value comparisons are each active PrEP group versus placebo, using two sample t-tests for the comparisons.
Figure 3. Serum creatinine levels in infants, by randomization group.
Box plots depict median (central line), interquartile range (box), and range (whiskers); numbers below each box indicate the number of subjects. P-value comparisons are each active PrEP group versus placebo, using two sample t-tests for the comparisons.
Discussion
In a randomized, double-blind, placebo-controlled trial of PrEP that demonstrated high HIV protection in the study population of African HIV serodiscordant couples, we assessed the effect of PrEP on pregnancy for HIV uninfected women, finding no statistically significant adverse relationship between each PrEP randomization group of tenofovir disoproxil fumarate or emtricitabine/tenofovir disoproxil fumarate compared to placebo and pregnancy incidence, birth outcomes, or infant growth and renal function. To our knowledge, these results are the first data exploring these outcomes in a randomized trial of daily oral PrEP when used in the periconception period. However, for some outcomes, including pregnancy loss, preterm birth, congenital anomalies, and infant mortality, confidence intervals were wide, including both a null effect and potential harm, and thus definitive statements about safety of PrEP in the periconception period cannot be made.
Other data, including a recent systematic review,21 have suggested that use of tenofovir disoproxil fumarate and emtricitabine during pregnancy appears safe when used by HIV infected women taking combination antiretroviral treatment. Data on teratogenicity related to in utero exposure to tenofovir disoproxil fumarate and emtricitabine has been reassuring, with no increase in congenital anomalies compared to the expected background rate for infants enrolled in the Antiretroviral Pregnancy Registry6 (n=1982 and n=1400 to date with first trimester exposure to tenofovir disoproxil fumarate and emtricitabine, respectively) and in prospective studies of women receiving antiretroviral treatment.11 Small decreases in bone mineral density have been observed with use of tenofovir disoproxil fumarate in animal models and among adults taking tenofovir disoproxil fumarate-containing HIV treatment regimens, but several studies have not shown increased risk of growth or bone abnormalities in infants born to HIV-infected women receiving tenofovir disoproxil fumarate,21 with one study showing slightly smaller infant length and head circumference at one year, of uncertain significance.12 In a study of antiretroviral treatment conducted among HIV infected women in Uganda and Zimbabwe (the DART study), infants exposed to tenofovir disoproxil fumarate in pregnancy, compared to infants exposed to non-tenofovir disoproxil fumarate containing treatment regimens, had similar neonatal morbidity, mortality or infant growth within 2 years of follow up.11 In agreement with this body of information, WHO and US guidelines for the treatment of HIV infection in pregnant women recommend tenofovir disoproxil fumarate- and emtricitabine-containing regimens as first-line therapy.22, 23
Few data have been available to assess the safety of tenofovir disoproxil fumarate and emtricitabine in pregnant women without HIV infection; the recent systematic review of tenofovir disoproxil fumarate safety in pregnancy included data from only eleven HIV uninfected women, exposed to tenofovir disoproxil fumarate as part of treatment for hepatitis B infection.24 While tenofovir disoproxil fumarate has been associated with renal abnormalities, including elevations in serum creatinine and proximal renal tubular dysfunction in a minority of adults receiving tenofovir disoproxil fumarate-containing treatment regimens,9 most with pre-existing renal compromise or other risk factors for renal disease, to our knowledge, no data have been published on the effect of tenofovir disoproxil fumarate exposure in utero on infant renal function. Our results, which characterized pregnancy incidence, birth outcomes and infant growth and renal function in a randomized comparison of HIV uninfected women who became pregnant while on daily oral tenofovir disoproxil fumarate, emtricitabine/tenofovir disoproxil fumarate, or placebo, thus substantially add to the available data regarding the use of tenofovir disoproxil fumarate and emtricitabine in early pregnancy.
The absolute frequency of pregnancy loss was higher for women receiving emtricitabine/tenofovir disoproxil fumarate than those assigned tenofovir disoproxil fumarate alone or placebo. Although the differences were not statistically significant, the 95% CIs compared to placebo were wide and ranged from −5.3% (protective) to 25.7% (harmful). The difference in the frequency of pregnancy loss between tenofovir disoproxil fumarate alone and emtricitabine/tenofovir disoproxil fumarate was attenuated in the data accumulated after July 2011. In post hoc analysis of the composite data for the entire study period, pregnancy loss was higher among women receiving emtricitabine/tenofovir disoproxil fumarate compared to those receiving tenofovir disoproxil fumarate alone, but the difference was not statistically significant (difference of proportions 9.2%, 95% CI −1.7%-20.1%, p=0.09). Additional studies including outcomes of pregnancy in women using PrEP during the peri-conception period are warranted. For more rare outcomes (premature birth, congenital anomalies) only very large datasets would have substantial statistical power, particularly for specific anomalies. The Antiretroviral Pregnancy Registry has open collection of data on pregnancies with exposure to antiretroviral agents,6 including when used as PrEP, and the manufacturer of FTC/TDF is conducting a prospective observational study of women who become pregnant while using PrEP.25
One-third of pregnancies detected in our study ended in pregnancy losses; this rate may be related to monthly pregnancy testing using sensitive urine β-hCG assays, which was performed to detect pregnancies before clinical recognition to limit fetal exposure to PrEP in the clinical trial. Prior studies of sensitive pregnancy monitoring have demonstrated that ~30% of pregnancies are lost early, most without clinical recognition (sometimes referred to as “chemical pregnancies”).26 The average duration of in utero PrEP exposure in our study was approximately 5 weeks.
In implementation of PrEP as an HIV prevention strategy for heterosexual populations, pregnancies will occur; indeed, pregnancy rates >10% per year are common in women enrolled into clinical trials of novel HIV prevention strategies, even when counseled to avoid pregnancy during the study period.18, 27–31 In sub-Saharan Africa, young women are the population at greatest risk for HIV acquisition and the season for highest HIV risk overlaps with periods of greatest fertility. For example, in Kenya, 65% of HIV infections in women occur before the age of 35, the peak period for child-bearing.32 Safe and effective HIV prevention options for women that do not require negotiations for safe sex and do not interfere with conception and pregnancy outcomes are a priority. For known, mutually-disclosed HIV serodiscordant couples, such as those enrolled in this trial, becoming pregnant risks HIV transmission, and most couples worldwide do not have access to assisted reproduction options to reduce HIV risk. The desire for pregnancy among serodiscordant couples is often great and can override fear of HIV transmission associated with conception attempts.31, 33–35 Our findings provide additional evidence to support the option of periconception administration of antiretroviral PrEP for HIV-uninfected women in both high and low income populations, along with other strategies such as antiretroviral treatment of their HIV-infected partners and limiting unprotected sex to peak fertility periods to reduce the risk of sexual transmission of HIV.36
Our study had several important strengths. A key strength was its randomized, placebo-controlled design. Similar data are rarely available to assess medication risks when used in early pregnancy, and recent analyses of tenofovir disoproxil fumarate use in pregnancy have called for randomized evidence.21 Additional strengths include the large sample size, high retention (including of infants followed for a year after birth), and high adherence to the study medication during the periconception period, as we recently reported.18
However, our study also had several limitations. First, our findings are limited to periconception exposure of tenofovir disoproxil fumarate and emtricitabine with short duration of in utero exposure after conception. In a non-research setting, women would likely have a longer exposure after achieving pregnancy; reassuringly, longer durations of in utero exposure in observational cohorts of HIV infected women suggest safety of these medications when used during pregnancy. Observational studies have suggested that pregnant women face increased risk of HIV acquisition,37 and additional data are needed on the safety of continuation of tenofovir disoproxil fumarate-based PrEP throughout pregnancy, including maternal and infant bone density safety after extended exposure. The US Food and Drug Administration registered a formal label indication for emtricitabine/tenofovir disoproxil fumarate as the first agent for the prevention of sexual transmission of HIV in 2012;25 the approved label includes consideration for continuing emtricitabine/tenofovir disoproxil fumarate PrEP in pregnant women with ongoing HIV risk. For women who breastfeed after pregnancy, limited data are available regarding excretion into breastmilk and absorption by infants and additional studies are needed. Recent comprehensive PrEP guidelines from the US Centers for Disease Control and Prevention also address use during peri-conception periods and during pregnancy, as well as additional considerations for persons with other comoribidities (e.g., chronic active hepatitis B infection, renal impairment, and other factors).38 Second, for some rare outcomes, such as preterm births and congenital anomalies, our results had wide confidence intervals. Third, considering that the confidence intervals for pregnancy loss were wide, overlapping both null effects and potential harm, and that PrEP was discontinued when pregnancy was detected, definitive conclusions about harms and safety, and possible differences between emtricitabine/tenofovir disoproxil fumarate compared to tenofovir disoproxil fumarate alone, cannot be made and will require further investigation to fully characterize the safety of PrEP in pregnancy.
Conclusions
Among HIV serodiscordant heterosexual African couples, differences in pregnancy incidence, birth outcomes, and infant growth were not statistically different for women receiving PrEP with tenofovir disoproxil fumarate or combination emtricitabine/tenofovir disoproxil fumarate compared to placebo. However, given that confidence intervals for the birth outcomes were wide, definitive statements about safety of PrEP in the periconception period cannot be made. These results should be discussed with HIV uninfected women receiving PrEP who are considering becoming pregnant.
Supplementary Material
Acknowledgements
We thank the site teams, particularly the site clinicians who conducted the assessments of women and their infants for this analysis, and the couples who participated in this study. The study team acknowledges the Director KEMRI for support. Dr. Baeten had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis. The funder of the study had no role in the design and conduct of the study; collection, management, analysis, and interpretation of the data; and preparation, review, or approval of the manuscript; and decision to submit the manuscript for publication.
Appendix
Partners PrEP Study Team
University of Washington Coordinating Center and Central Laboratories: Connie Celum (principal investigator, protocol co-chair), Jared M. Baeten (medical director, protocol co-chair), Deborah Donnell (protocol statistician), Robert W. Coombs, Lisa Frenkel, Craig W. Hendrix, Jairam Lingappa, M. Juliana McElrath.
Study sites and site principal investigators:
Eldoret, Kenya (Moi University, Indiana University): Kenneth Fife, Edwin Were; Kabwohe, Uganda (Kabwohe Clinical Research Center): Elioda Tumwesigye; Jinja, Uganda (Makerere University, University of Washington): Patrick Ndase, Elly Katabira; Kampala, Uganda (Makerere University): Elly Katabira, Allan Ronald; Kisumu, Kenya (Kenya Medical Research Institute, University of California San Francisco): Elizabeth Bukusi, Craig Cohen; Mbale, Uganda (The AIDS Support Organization, CDC-Uganda): Jonathan Wangisi, James Campbell, Jordan Tappero; Nairobi, Kenya (University of Nairobi, University of Washington): James Kiarie, Carey Farquhar, Grace John-Stewart; Thika, Kenya (University of Nairobi, University of Washington): Nelly Rwamba Mugo; Tororo, Uganda (CDC-Uganda, The AIDS Support Organization): James Campbell, Jordan Tappero, Jonathan Wangisi.
Data management was provided by DF/Net Research, Inc. (Seattle, USA) and site laboratory oversight was provided by Contract Laboratory Services (University of the Witwatersrand, Johannesburg, South Africa).
The Partners PrEP Study was funded by The Bill and Melinda Gates Foundation (grant OPP47674).
References
- 1.Baeten JM, Donnell D, Ndase P, et al. Antiretroviral prophylaxis for HIV prevention in heterosexual men and women. N Engl J Med. 2012;367(5):399–410. doi: 10.1056/NEJMoa1108524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Grant RM, Lama JR, Anderson PL, et al. Preexposure chemoprophylaxis for HIV prevention in men who have sex with men. N Engl J Med. 2010;363(27):2587–2599. doi: 10.1056/NEJMoa1011205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Choopanya K, Martin M, Suntharasamai P, et al. Antiretroviral prophylaxis for HIV infection in injecting drug users in Bangkok, Thailand (the Bangkok Tenofovir Study): a randomised, double-blind, placebo-controlled phase 3 trial. Lancet. 2013;381(9883):2083–2090. doi: 10.1016/S0140-6736(13)61127-7. [DOI] [PubMed] [Google Scholar]
- 4.Cohen MS, Chen YQ, McCauley M, et al. Prevention of HIV-1 infection with early antiretroviral therapy. N Engl J Med. 2011;365(6):493–505. doi: 10.1056/NEJMoa1105243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Mujugira A, Heffron R, Celum C, Mugo N, Nakku-Joloba E, Baeten JM. Fertility intentions and interest in early antiretroviral therapy among East African HIV-1-infected individuals in serodiscordant partnerships. J Acquir Immune Defic Syndr. 2013;63(1):e33–e35. doi: 10.1097/QAI.0b013e318288bb32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Antiretroviral Pregnancy Registry Interim Report, 1 January 1989 through 31 July 2013. [Accessed 25 January 2014];2013 http://www.apregistry.com/forms/interim_report.pdf. [Google Scholar]
- 7.Stellbrink HJ, Orkin C, Arribas JR, et al. Comparison of changes in bone density and turnover with abacavir-lamivudine versus tenofovir-emtricitabine in HIV-infected adults: 48-week results from the ASSERT study. Clin Infect Dis. 2010;51(8):963–972. doi: 10.1086/656417. [DOI] [PubMed] [Google Scholar]
- 8.McComsey GA, Tebas P, Shane E, et al. Bone disease in HIV infection: a practical review and recommendations for HIV care providers. Clin Infect Dis. 2010;51(8):937–946. doi: 10.1086/656412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cooper RD, Wiebe N, Smith N, Keiser P, Naicker S, Tonelli M. Systematic review and meta-analysis: renal safety of tenofovir disoproxil fumarate in HIV-infected patients. Clin Infect Dis. 2010;51(5):496–505. doi: 10.1086/655681. [DOI] [PubMed] [Google Scholar]
- 10.Ransom CE, Huo Y, Patel K, et al. Infant growth outcomes after maternal tenofovir disoproxil fumarate use during pregnancy. J Acquir Immune Defic Syndr. 2013;64(4):374–381. doi: 10.1097/QAI.0b013e3182a7adb2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gibb DM, Kizito H, Russell EC, et al. Pregnancy and infant outcomes among HIV-infected women taking long-term ART with and without tenofovir in the DART trial. PLOS Med. 2012;9(5):e1001217. doi: 10.1371/journal.pmed.1001217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Siberry GK, Williams PL, Mendez H, et al. Safety of tenofovir use during pregnancy: early growth outcomes in HIV-exposed uninfected infants. AIDS. 2012;26(9):1151–1159. doi: 10.1097/QAD.0b013e328352d135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Mujugira A, Baeten JM, Donnell D, et al. Characteristics of HIV-1 serodiscordant couples enrolled in a clinical trial of antiretroviral pre-exposure prophylaxis for HIV-1 prevention. PLOS ONE. 2011;6(10):e25828. doi: 10.1371/journal.pone.0025828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Murnane PM, Celum C, Mugo N, et al. Efficacy of preexposure prophylaxis for HIV-1 prevention among high-risk heterosexuals: subgroup analyses from a randomized trial. AIDS. 2013;27(13):2155–2160. doi: 10.1097/QAD.0b013e3283629037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ndase P, Celum C, Campbell J, et al. Successful discontinuation of the placebo arm and provision of an effective HIV prevention product after a positive interim efficacy result: the Partners PrEP Study experience. J Acquir Immune Defic Syndr. 2014;66(2):206–212. doi: 10.1097/QAI.0000000000000141. [DOI] [PubMed] [Google Scholar]
- 16.Joint United National Programme on HIV/AIDS (UNAIDS), AVAC. Good participatory practice: guidelines for biomedical HIV prevention trials 2011. Geneva: Switzerland; 2011. [Google Scholar]
- 17.UNAIDS/WHO. Ethical considerations in biomedical HIV prevention trials. Geneva: Joint United Nations Programme on HIV/AIDS; 2007. [Google Scholar]
- 18.Matthews LT, Heffron R, Mugo NR, et al. High medication adherence during periconception periods among HIV-1-uninfected women in a clinical trial of antiretroviral pre-exposure prophylaxis. J Acquir Immune Defic Syndr. doi: 10.1097/QAI.0000000000000246. Electronic published ahead of print: http://journals.lww.com/jaids/Abstract/publishahead/High_medication_adherence_during_periconception.97879.aspx. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.World Health Organization. [Accessed 25 January 2014];The WHO Child Growth Standards. 2006 http://www.who.int/childgrowth/en/
- 20.Fenton TR. A new growth chart for preterm babies: Babson and Benda's chart updated with recent data and a new format. BMC Pediatr. 2003;3(13):13. doi: 10.1186/1471-2431-3-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wang L, Kourtis AP, Ellington S, Legardy-Williams J, Bulterys M. Safety of tenofovir during pregnancy for the mother and fetus: a systematic review. Clin Infect Dis. 2013;57(12):1773–1781. doi: 10.1093/cid/cit601. [DOI] [PubMed] [Google Scholar]
- 22.World Health Organization. Consolidated guidelines on the use of antiretroviral drugs for treating and preventing HIV infection: recommendations for a public health approach. Geneva: World Health Organization; 2013. [PubMed] [Google Scholar]
- 23.HHS Panel on Treatment of HIV-Infected Pregnant Women and Prevention of Perinatal Transmission. [Accessed 26 January 2014];Recommendations for use of antiretroviral drugs in pregnant HIV-1-infected women for maternal health and interventions to reduce perinatal HIV transmission in the United States. 2012 http://aidsinfo.nih.gov/contentfiles/lvguidelines/PerinatalGL.pdf.
- 24.Pan CQ, Mi LJ, Bunchorntavakul C, et al. Tenofovir disoproxil fumarate for prevention of vertical transmission of hepatitis B virus infection by highly viremic pregnant women: a case series. Dig Dis Sci. 2012;57(9):2423–2429. doi: 10.1007/s10620-012-2187-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.U.S. Food and Drug Administration. [Accessed 25 January 2014];FDA approves first drug for reducing the risk of sexually acquired HIV infection. 2012 http://www.fda.gov/NewsEvents/Newsroom/PressAnnouncements/ucm312210.htm.
- 26.Wilcox AJ, Weinberg CR, O'Connor JF, et al. Incidence of early loss of pregnancy. N Engl J Med. 1988;319(4):189–194. doi: 10.1056/NEJM198807283190401. [DOI] [PubMed] [Google Scholar]
- 27.Ngure K, Heffron R, Mugo NR, et al. Contraceptive method and pregnancy incidence among women in HIV-1-serodiscordant partnerships. AIDS. 2012;26(4):513–518. doi: 10.1097/QAD.0b013e32834f981c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Odutola A, Baisley K, Hayes RJ, et al. Pregnancy and contraceptive use among women participating in an HIV prevention trial in Tanzania. Sex Transm Infect. 2012;88(6):436–443. doi: 10.1136/sextrans-2011-050412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Reid SE, Dai JY, Wang J, et al. Pregnancy, contraceptive use, and HIV acquisition in HPTN 039: relevance for HIV prevention trials among African women. J Acquir Immune Defic Syndr. 2010;53(5):606–613. doi: 10.1097/QAI.0b013e3181bc4869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Skoler-Karpoff S, Ramjee G, Ahmed K, et al. Efficacy of Carraguard for prevention of HIV infection in women in South Africa: a randomised, double-blind, placebo-controlled trial. Lancet. 2008;372(9654):1977–1987. doi: 10.1016/S0140-6736(08)61842-5. [DOI] [PubMed] [Google Scholar]
- 31.Ngure K, Mugo N, Celum C, et al. A qualitative study of barriers to consistent condom use among HIV-1 serodiscordant couples in Kenya. AIDS Care. 2012;24(4):509–516. doi: 10.1080/09540121.2011.613911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Cherutich P, Kaiser R, Galbraith J, et al. Lack of knowledge of HIV status a major barrier to HIV prevention, care and treatment efforts in Kenya: results from a nationally representative study. PLOS ONE. 2012;7(5):e36797. doi: 10.1371/journal.pone.0036797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Beyeza-Kashesya J, Kaharuza F, Mirembe F, Neema S, Ekstrom AM, Kulane A. The dilemma of safe sex and having children: challenges facing HIV sero-discordant couples in Uganda. Afr Health Sci. 2009;9(1):2–12. [PMC free article] [PubMed] [Google Scholar]
- 34.Beyeza-Kashesya J, Ekstrom AM, Kaharuza F, Mirembe F, Neema S, Kulane A. My partner wants a child: a cross-sectional study of the determinants of the desire for children among mutually disclosed sero-discordant couples receiving care in Uganda. BMC Public Health. 2010;10(247):247. doi: 10.1186/1471-2458-10-247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kakaire O, Osinde MO, Kaye DK. Factors that predict fertility desires for people living with HIV infection at a support and treatment centre in Kabale, Uganda. Reprod Health. 2010;7(27):27. doi: 10.1186/1742-4755-7-27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Matthews LT, Baeten JM, Celum C, Bangsberg DR. Periconception pre-exposure prophylaxis to prevent HIV transmission: benefits, risks, and challenges to implementation. AIDS. 2010;24(13):1975–1982. doi: 10.1097/QAD.0b013e32833bedeb. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Mugo NR, Heffron R, Donnell D, et al. Increased risk of HIV-1 transmission in pregnancy: a prospective study among African HIV-1 serodiscordant couples. AIDS. 2011;25(15):1887–1895. doi: 10.1097/QAD.0b013e32834a9338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Centers for Disease Control and Prevention. Preexposure prophylaxis for the prevention of HIV infection in the United States - 2014: a clinical practice guideline. 2014 [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.