Abstract
Purpose
Pancreatic cancer primary tumor size measurements are often discordant between CT and pathologic specimen after resection. Dimensions of the primary tumor are increasingly relevant in an era of highly conformal radiotherapy.
Methods
We retrospectively evaluated 97 consecutive patients with resected pancreatic cancer at two Boston hospitals. All patients had CT scans prior to surgical resection. Primary endpoints were maximum dimension (in mm) of the primary tumor in any direction as reported by the radiologist on CT and by the pathologist on resected gross fresh specimen. Endoscopic ultrasound (EUS) findings were analyzed if available.
Results
Eighty-seven patients (90%) had pre-operative CT scans available for review, and 46 (47%) had EUS. Among proximal tumors (n = 69), 40 (58%) had pathologic duodenal invasion, which was seen on CT in only 3 cases. Pathologic tumor size was a median of 7 mm larger compared to CT size for the same patient (range: −15–43 mm, p < 0.0001), with 73 patients (84%) having a primary tumor larger on pathology than CT. EUS was somewhat more accurate, with pathologic tumor size being a median of only 5 mm larger compared to EUS size (range, −15–35 mm, p = 0.0003).
Conclusions
CT scans significantly under-represent pancreatic cancer tumor size compared to pathologic specimens in resectable cases. We propose a clinical target volume (CTV) expansion formula for the primary tumor based on our data. The high rate of pathologic duodenal invasion suggests a risk of duodenal under-coverage with highly conformal radiotherapy.
Keywords: Pancreatic cancer, CT, Pathology, Radiation, CTV formula
INTRODUCTION
Pancreatic cancer is an almost uniformly fatal disease, with 5-year survival rates of less than 5% (1). Surgical resection, which offers the best chance at long-term survival, is feasible in fewer than 20% of patients. Locoregional recurrence remains common after resection (2-4), prompting the routine use of adjuvant chemoradiotherapy at least in the United States, based on results from the Gastrointestinal Tumor Study Group trials from the 1980's (5, 6). Up to 40% of patients with pancreatic cancer present with localized but inoperable disease, and these patients are commonly treated with upfront chemoradiotherapy (7-10).
Radiation treatment planning for pancreatic cancer has included two-dimensional approaches still suggested in many textbooks, three-dimensional approaches more commonly used in standard practice and modern clinical trials, and four-dimensional CT planning to account for organ motion. Using 3D treatment planning is associated with reduced toxicity and the ability to escalate radiation doses (8, 11). Given this increased ability to visualize the target, especially in unresected disease, as well as more efficacious systemic chemotherapy, a growing trend is treatment of smaller radiation fields. Despite loco-regional nodal involvement in the majority of pancreatic cancers, elective nodal regions are often not fully covered in modern radiation fields, and in the example of stereotactic body radiotherapy (SBRT) / stereotactic radiosurgery, the planning target volume (PTV) often includes only the gross tumor volume (GTV) plus a 2-3 millimeter margin (12-15).
Given the increasing use of highly conformal radiation fields in pancreatic cancer, there is a growing need to be certain of the volumes being treated. Pancreatic cancer primary tumors often appear as hypoattenuating lesions with ill-defined borders. Modern CT imaging employs a multiple-row detector featuring narrow detector collimation, wide x-ray beam, and rapid table translation, offering thinner image slices and faster acquisition time (16). For pancreatic cancer, multi-detector row CT (MDR-CT) scanners are used with specific contrast administration protocols to optimally visualize the tumor and vascular structures to assess resectability. Pancreatic protocol CT acquisition includes an arterial phase, early venous phase, and delayed venous phase, with thin cuts and coronal re-formats (17, 18).
Prior reports have focused on the ability of MDR-CT scans to determine surgical resectability of pancreatic tumors, but have not assessed their ability to accurately determine primary tumor size. We have anecdotally observed patients with significant discrepancies between the sizes of their pancreatic tumors on pre-operative CT scans, compared to the sizes of their tumors measured by the pathologist on resected gross specimens. This led us to undertake the current study, in which we compared the primary tumor maximum dimension as seen on CT with that measured on pathologic specimen, to see if this clinico-pathologic correlation could yield insights to assist in radiation planning.
METHODS
Patient selection
We retrospectively evaluated consecutive patients with resected pancreatic cancer seen in the Department of Radiation Oncology from 2001-2009 at two hospitals, Massachusetts General Hospital and Dana-Farber Cancer Institute / Brigham and Women's Hospital, both in Boston, MA, after obtaining IRB approval. All patients had pancreatic protocol MDR-CT scans prior to surgical resection. Some patients also underwent endoscopic ultrasound (EUS) pre-operatively, which was included for analysis when available.
Clinico-pathologic primary endpoints
Primary endpoints were maximum dimension of the primary tumor measured in any direction on pre-operative CT as measured by the radiologist on the radiology report, and maximum dimension measured in any direction on the resected gross fresh specimen as reported by the pathologist for that same patient (Figure 1). Three tumor dimensions were reported by the radiologist for each tumor on almost all CT scans, and all pathology reports included three tumor dimensions. Cases in which a primary tumor was not detected on pre-operative CT scan were analyzed separately. Fresh pathologic specimens were analyzed before any fixation, with the common bile duct cannulated in the case of Whipple / pancreaticoduodenectomy, and specimens were bisected along that plane. Primary tumor size was measured in any direction that gave the maximum dimension. For patients who had pre-operative EUS, maximum dimension of the primary tumor noted on the EUS report was recorded.
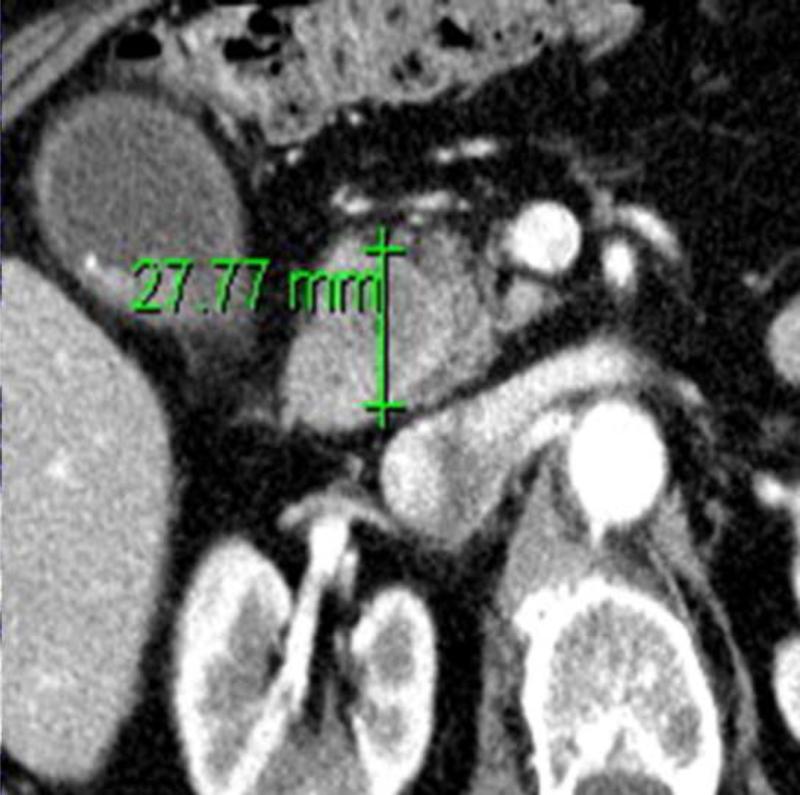
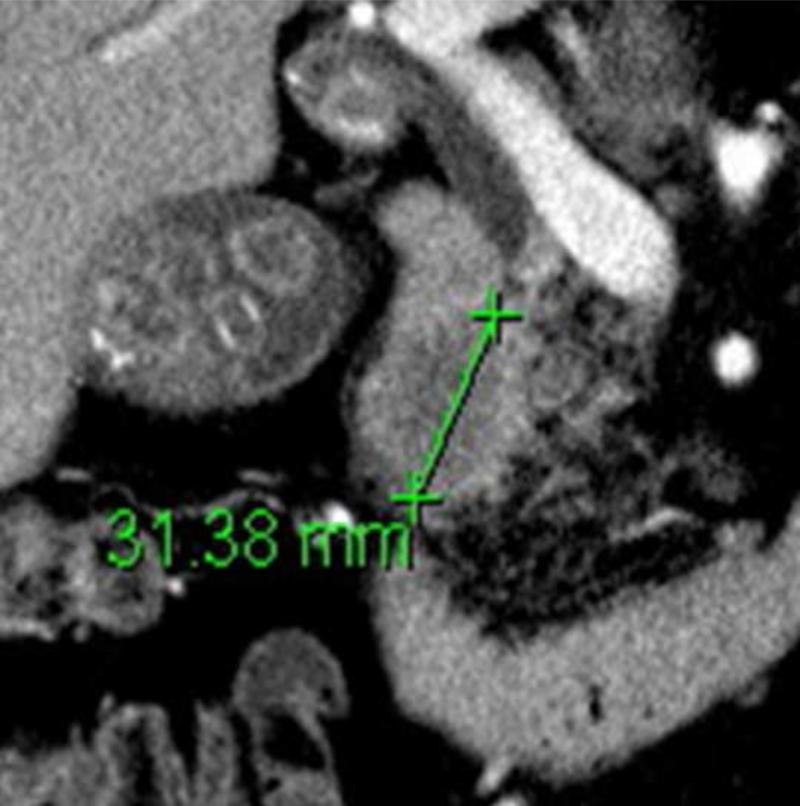
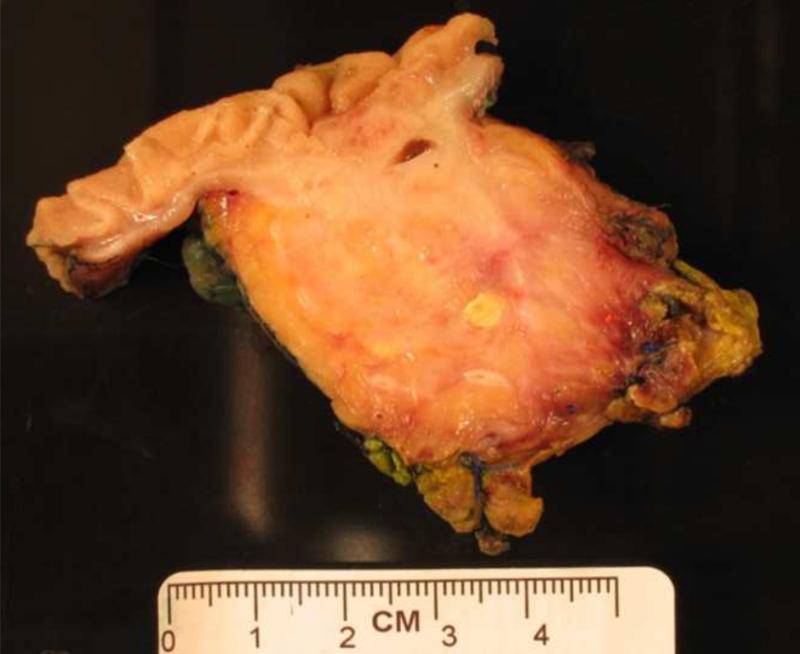
Figure 1.
(a) Axial and (b) coronal slices of an example patient's pancreatic primary tumor on pre-operative CT scan. (c) Pathologic gross fresh specimen from that same patient. Maximum dimension in any direction on CT scan was 31 mm, and on pathologic specimen was 46 mm.
Clinico-pathologic secondary endpoints
Other features recorded from the CT scan reports include date of the scan in relation to date of surgery, and presence of adjacent organ invasion. Pathologic features recorded included histology, grade, background histology (e.g. pancreatic intraepithelial neoplasia [PanIN]), T stage, number of lymph nodes resected and number positive for malignancy, margin status, closest or involved margin, lymphatic invasion, vascular invasion, perineural invasion, major vessel invasion, and duodenal or other adjacent organ invasion.
Tumor size on CT scan was independently re-measured by the radiation oncology investigators, blinded to the radiology and pathology reports. Maximum dimension of the primary tumor was recorded in any direction on the axial or coronal reformatted images. In tumors with ill-defined borders, maximum dimension was measured to reflect what would be contoured as GTV on a radiation planning CT scan.
Statistics
Primary tumor sizes were log-transformed for analyses. Log-normal distribution of tumor sizes was confirmed with the Shapiro-Wilk W test, and variance in tumor size was found to be similar between CT, EUS, and pathologic specimen using a variance ratio test. Median differences were calculated between CT scan measurements and pathologic specimen measurements, with paired t tests used since we were able to compare radiographic size and pathologic size for the same patient. Logistic regression analyses with bootstrap standard error were used to calculate odds ratios associated with tumors appearing undetectable on CT scan. We used linear regression analysis, 95% confidence intervals for prediction, and standard error of prediction to develop a formula for a clinical target volume (CTV) expansion off the primary tumor.
RESULTS
Patient characteristics
We evaluated 97 consecutive patients with resected pancreatic cancer, evenly divided between two hospitals. Patient and imaging characteristics are reported in Table 1. One patient received neoadjuvant chemotherapy (gemcitabine plus cisplatin), but no patients received radiotherapy prior to surgery. The patient who received chemotherapy completed this treatment before having the pre-operative CT scan performed. Eighty-seven patients (90%) had pre-operative MDR-CT scans available for review, with the remaining scans being archived remotely and unavailable for direct measurements. CT scans were performed a median of 19 days before surgery (range, 0–90 days). Forty-six patients (47%) had pre-operative EUS performed with data available for review.
Table 1.
Patient characteristics (N = 97).
| Patient characteristics | Data |
|---|---|
| Treating institution (no. of patients) | |
| Massachusetts General Hospital | 49 |
| Dana-Farber Cancer Institute / Brigham and Women's Hospital | 48 |
| Neoadjuvant chemotherapy* | 1 |
| Neoadjuvant radiotherapy | 0 |
| Pre-operative MDR-CT scan available | 87 (90%) |
| Pre-operative EUS available | 46 (47%) |
| Median days between MDR-CT and surgery | 19 (range, 0-90) |
| Median age at surgery (y) | 63 |
| Year of surgery | 2001-2009 |
Abbreviations: MDR-CT = multi-detector row computed tomography; EUS = endoscopic ultrasound.
Patient received neoadjuvant gemcitabine plus cisplatin.
Data are presented as no. of patients (%) unless otherwise indicated.
Pathologic features
Pathologic features are reported in Table 2. Sixty-nine patients (71%) had proximal tumors located in the pancreatic head, neck, or uncinate process, and 70 patients (72%) had pathologic T3 tumors signifying extra-pancreatic spread of tumor. With regard to histology, 86 tumors (89%) were adenocarcinoma, with 74 of those tumors (86%) being grade 2 or 3. Over half of the tumors had lymphatic invasion (55%) or vascular invasion (59%), with only 6 patients (6%) having major vessel invasion. Over one third of patients (36%) had an involved margin, with the retroperitoneal margin representing the most commonly involved site. Seventy-seven patients (79%) had involved lymph nodes.
Table 2.
Pathologic characteristics (N = 97).
| Pathologic characteristics | Data |
|---|---|
| Tumor location | |
| Head / neck / uncinate | 69 (71%) |
| Body / tail | 28 (29%) |
| Pathologic T stage | |
| T1 | 2 (2%) |
| T2 | 14 (14%) |
| T3 | 70 (72%) |
| Not recorded | 11 (11%) |
| Histology | |
| Adenocarcinoma | 86 (89%) |
| PNET | 5 (5%) |
| Acinar cell carcinoma | 3 (3%) |
| Other | 3 (3%) |
| Adenocarcinoma grade | |
| 1 | 5 (6%) |
| 2 | 38 (44%) |
| 3 | 36 (42%) |
| Not recorded | 7 (8%) |
| Adenocarcinoma background histology | |
| PanIN | 46 (53%) |
| IPMN | 7 (8%) |
| Chronic pancreatitis | 2 (2%) |
| Not recorded | 31 (36%) |
| Lymphatic invasion | 53 (55%) |
| Vascular invasion | 57 (59%) |
| Major vessel invasion | 6 (6%) |
| Perineural invasion | 75 (77%) |
| Margin involved | 35 (36%) |
| Location of closest or involved margin | |
| Transection margin | 15 (15%) |
| Retroperitoneal margin | 34 (35%) |
| Uncinate margin | 13 (13%) |
| Common bile duct margin | 2 (2%) |
| Celiac margin | 1 (1%) |
| Radial margin | 3 (3%) |
| Not recorded | 29 (30%) |
| Lymph node positive | 77 (79%) |
Abbreviations: PNET = pancreatic neuroendocrine tumor; PanIN = pancreatic intraepithelial neoplasia; IPMN = intraductal papillary mucinous neoplasm.
Data are presented as no. of patients (%) unless otherwise indicated.
Duodenal invasion
We assessed adjacent organ invasion among pathologic T3 tumors (n = 70), with results shown in Table 3. Among proximal tumors in the pancreatic head, neck, or uncinate regions, 40 of 57 patients (70%) had pathologic invasion of the duodenum. Only 3 of these 57 patients (5%) had evidence of duodenal invasion on their pre-operative CT scans, and no patients had evidence of invasion noted on pre-operative EUS. For all proximal tumors regardless of T stage, 40 of 69 patients (58%) had pathologic duodenal invasion. Among proximal T3 tumors, 9 patients (16%) had pathologic invasion of the common bile duct and 5 patients (9%) had invasion of the ampulla of Vater, none of which was visualized on pre-operative CT scan. Among distal tumors in the body or tail, 1 of 13 patients (8%) had pathologic invasion of the spleen and 1 patient had invasion of the stomach; neither case was seen pre-operatively on CT.
Table 3.
Pathologic organ invasion among T3 tumors (N = 70).
| Tumor location | Pathologic organ invasion | Data |
|---|---|---|
| Proximal* (n = 57) | Duodenum | 40 (70%) |
| Common bile duct | 9 (16%) | |
| Ampulla of Vater | 5 (9%) | |
| Distal† (n = 13) | Stomach | 1 (8%) |
| Spleen | 1 (8%) | |
Tumor located in pancreatic head, neck, or uncinate process.
Tumor located in pancreatic body or tail.
Data are presented as no. of patients (%) unless otherwise indicated.
Tumors not detected on CT scan
Among the 87 patients with pre-operative MDR-CT scans available, 16 patients (18%) had no pancreatic primary tumor detected on CT. The median pathologic size (maximum dimension) of these tumors not seen by CT was 26 mm (range, 5–55 mm). Logistic regression analysis demonstrated that the only factor associated with a primary tumor being undetectable on CT scan was tumor location in the pancreatic head, with an odds ratio of 6.9 (95% confidence interval, 1.8–26.9; p = 0.005). Likewise, we found that tumors located in the pancreatic head were significantly smaller than in other locations, with a mean difference of 11.2 mm smaller for pancreatic head locations (p = 0.002). Among the 46 patients who had both pre-operative CT scan and EUS, CT failed to detect 9 tumors, whereas EUS missed only 1 tumor, which was also missed by CT.
Clinico-pathologic comparison
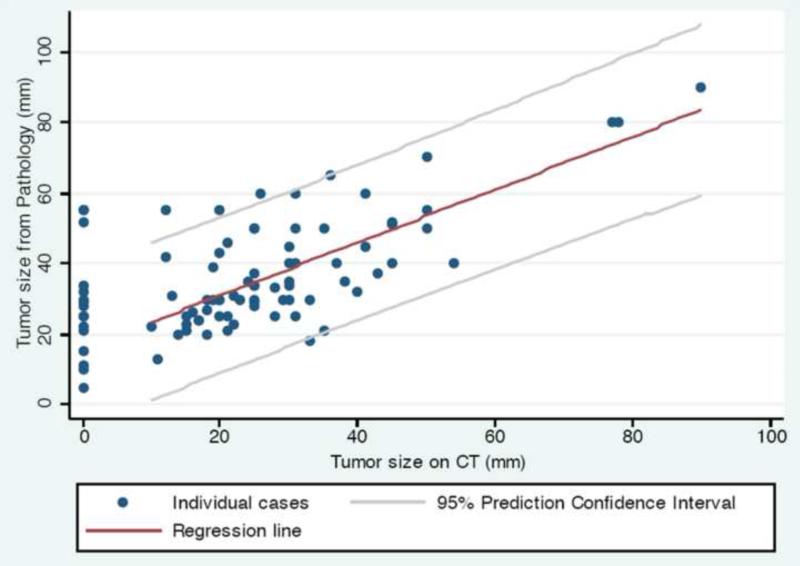
CT scan vs. pathology tumor size comparisons are reported in Table 4. Median primary tumor size on pre-operative CT scan was 25 mm (range, 10–90 mm), while the median tumor size on pathologic specimen was 34 mm (range, 13–90 mm). Pathologic specimen tumor size was a median of 7 mm larger when compared to the CT scan measurement on that same patient (range: −15–43 mm, p < 0.0001). For the group overall, 73 of 87 patients (84%) had a primary tumor that was larger on pathology than on pre-operative CT scan. These findings were similar between patients treated at both hospitals in our study, when examined separately. We plotted tumor size on CT scan vs. tumor size on pathology (Figure 2), and found that the larger the tumor size on CT scan, the smaller the discrepancy with the eventual pathologic specimen (p = 0.005). In other words, the larger the tumor appeared on pre-operative CT, the closer it matched up with the pathologic specimen tumor size.
Table 4.
Primary tumor size on CT vs. pathology.
| Primary tumor size (median) | |
| CT (detectable cases) | 25 mm |
| Pathologic specimen | 34 mm |
| Median difference, CT vs. pathology | 7 mm larger on pathology (range, −15-43 mm) p < 0.0001 |
| Primary tumor larger on pathology (no. of patients) | 73 (84%) |
Figure 2.
Primary tumor size on pre-operative CT scan vs. pathologic specimen (n = 87).
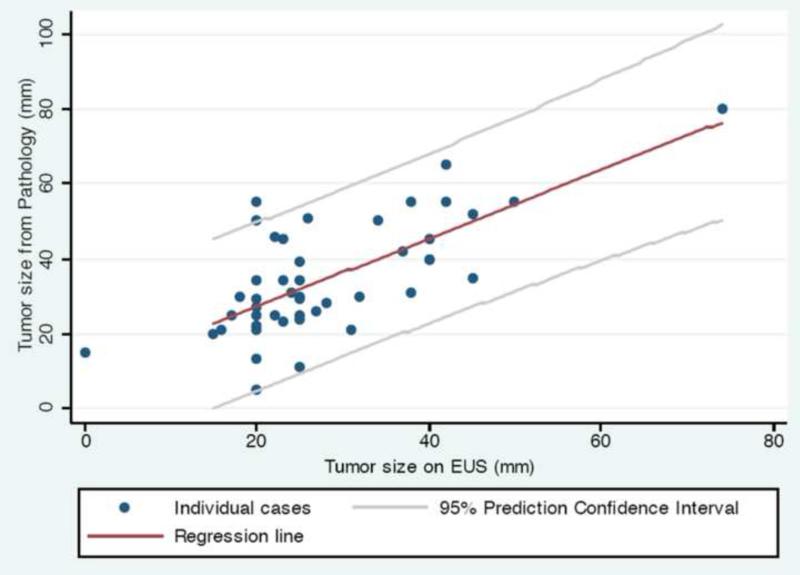
We performed a similar analysis for pre-operative EUS. Median primary tumor size on pre-operative EUS was 25 mm (range, 15–74 mm). Pathologic specimen tumor size was a median of 5 mm larger when compared to pre-operative EUS measurement on that same patient (range, −15–35 mm, p = 0.0003). We plotted tumor size on EUS vs. tumor size on pathology (Figure 3), and noted a trend for EUS having higher accuracy vs. CT for smaller tumors, when compared to the eventual pathologic specimen (p = 0.1).
Figure 3.
Primary tumor size on pre-operative EUS vs. pathologic specimen (n = 46). Abbreviation: EUS = endoscopic ultrasound.
Maximum tumor size (in any direction) on CT scan was independently re-measured by the radiation oncology investigators, blinded to the radiology and pathology reports. Median primary tumor size on pre-operative CT on re-measurement was 24 mm (range, 12–92 mm). Primary tumor size on radiology report was a median of 1 mm larger than our re-measurement (range, 15 mm smaller to 6 mm larger on radiology report, p = NS).
CTV primary expansion formula
A linear regression analysis was performed to relate primary tumor size on pre-operative CT scan to size on pathologic specimen. This yielded the equation:
This represented the systematic under-representation of the primary tumor size on CT compared to pathology, with the magnitude of the discrepancy dependent on the size seen on CT scan. By definition of a regression formula, 50% of pathologic specimens would be expected to be larger than the equation output and 50% would be expected to be smaller. We thus modified the formula by incorporating the standard error of prediction for our dataset, and calculated 95% confidence intervals for prediction. This would allow us to eventually generate a formula for a clinical target volume (CTV) expansion from the primary tumor as seen on CT, that would cover 97.5% of pathologic tumors (95% confidence interval plus the lower bound 2.5%, which would already be included in the GTV). The average standard error of prediction for our dataset (n = 87) was 11.1 mm. The equation for inclusion of 97.5% of pathologic tumors was:
The above formula predicts inclusion of 97.5% of pathologic tumors. In order to translate this into an actual CTV primary tumor expansion formula as a circumferential margin in all directions, we made the following modifications:
Therefore the following represents the CTV primary expansion formula for CT-based planning, which covers 97.5% of ‘actual’/pathologic tumors as a circumferential margin/expansion in all directions off the primary tumor GTV as seen on CT scan:
We also performed a similar linear regression analysis to relate primary tumor size on pre-operative EUS to size on pathologic specimen. After going through the same steps above, the analogous CTV primary expansion formula for EUS is:
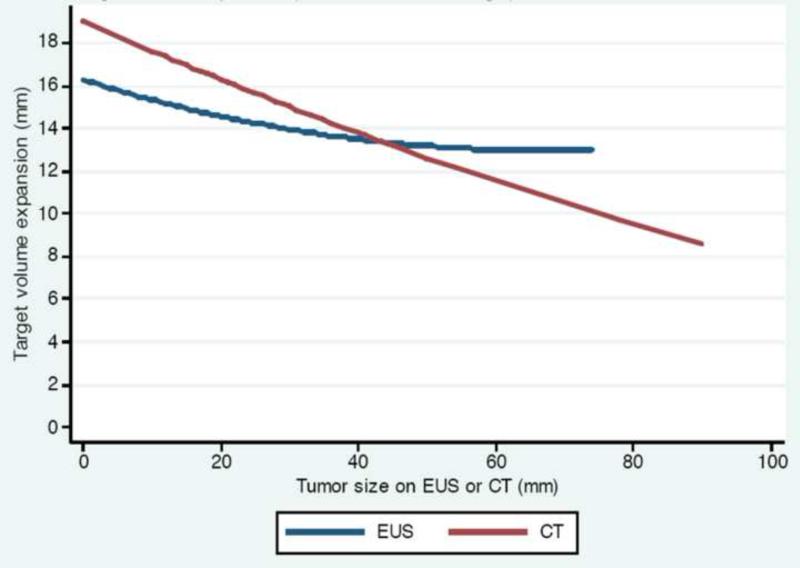
We plotted tumor size on CT or EUS against the CTV primary expansion formula output for both CT and EUS (Figure 4). The decreasing slope with increasing tumor size reflects our observation that the larger the tumor size on imaging, the smaller the discrepancy with the eventual pathologic specimen, and hence the smaller the expansion margin required.
Figure 4.
CTV primary expansion formula for margin required to cover 97.5% of pathologic tumors vs. tumor size on CT or EUS.
Abbreviation: CTV = clinical target volume.
DISCUSSION
We observed a significant clinico-pathologic discrepancy between the size of a patient's pancreatic primary tumor as seen on modern CT imaging compared to that same patient's pathologic specimen. Pathologic gross fresh specimens, the gold standard for determining tumor size, indicate that most pancreatic tumors are substantially larger than pre-operative CT scans suggest. Thus at least for resectable pancreatic cancer, CT scans significantly under-represent primary tumor size / GTV compared to pathology. This information is potentially useful for radiation planning in locally advanced, unresectable pancreatic cancer, as well as in the adjuvant setting when considering the extent of the primary tumor bed based on pre-operative imaging. Since 10-30% of tumors appearing resectable on CT are found to be unresectable at the time of surgery (19-21), our findings may actually under-represent the CT vs. pathology discrepancy, given that we only had resectable cases to evaluate.
Based on our findings, we have proposed an expansion formula for margin to add beyond the visible primary GTV on the planning CT scan, to account for the significant likelihood that the CT scan will not detect the full extent of gross tumor. In accordance with the International Commission on Radiation Units & Measurements (ICRU) definitions of GTV and CTV, we have defined our formula as a CTV expansion as opposed to GTV expansion, as it accounts for tumor that is not visible on the planning CT scan, even though our data suggest there is likely gross tumor located in aspects of the CTV expansion.
Our findings also suggest that EUS may play an adjunctive role in radiation planning of pancreatic cancers. EUS was somewhat more accurate than CT for defining primary tumor size in general, though pathologic specimen was still significantly larger than EUS size. EUS trended toward higher accuracy than CT for determining size of smaller tumors in particular, which is compatible with earlier reports demonstrating that EUS is better at detecting small tumors (19-21). To our knowledge, EUS has not been utilized previously as a component of radiation planning for pancreatic cancers. With current techniques, we cannot anatomically fuse EUS images with planning CT scans, and further studies are required to attempt integration of EUS images into radiation planning. However given our findings, EUS measurements could be used in conjunction with CT by comparing maximum tumor size on each modality, and selecting the CTV primary expansion according to Figure 4, knowing that EUS may be more accurate for smaller tumors.
The clinico-pathologic discrepancies we report have potentially important implications for highly conformal radiation approaches to pancreatic cancer. In an attempt to improve local control of unresectable disease, some physicians have dose-escalated radiation therapy by shrinking radiation fields off of elective lymph node regions (22), or minimized the treatment volume to include the GTV only plus 2-3 mm margin for PTV setup error in a stereotactic radiosurgical approach (12-15). 4D-CT scans may help account for organ motion due to breathing, though tumor motion on planning 4D-CT scans has not been shown to correlate with intra-fraction tumor motion during treatment (23). More recent approaches to account for tumor motion include implanted transponders that are tracked during treatment (24). In stereotactic radiosurgical approaches, 25 Gray are typically delivered in a single fraction, prescribed to the isodose line completely covering 95% of the PTV. Given our findings of significant under-representation of the primary tumor / GTV on CT scan, and minimal PTV expansions adding only 2-3 mm to the GTV, it is possible that these approaches miss gross tumor outside the PTV in some cases. Using 4D-CT scans or implanted transponders to increase precision of tumor localization during treatment still does not account for pathologic gross extension of tumor not visualized on imaging, if very small margins are used to generate the PTV. Most randomized trials with locally advanced pancreatic cancer show local failure as a component of disease progression in 30-60% of patients despite local treatment (7, 8, 10, 25). High-dose, hypofractionated stereotactic radiation for locally advanced disease is associated with an improvement in local control and time to local progression, but local failure as a component of disease progression is still on the order of 20% with median follow-up of only 6 months (14, 15). It is unclear whether an expanded target volume to account for the clinico-pathologic discrepancy we observed is feasible with hypofractionated schedules, given the potential toxicities of expanded volumes, and it is similarly unclear if increased coverage could further improve local control rates.
We found that nearly 60% of all tumors in the proximal pancreas (head, neck, or uncinate process) had pathologic invasion into the duodenum, despite rare evidence of duodenal invasion on pre-operative CT and no cases seen on EUS. This is notable given that highly conformal, dose-escalated radiation approaches for pancreatic cancer are often limited by the potential for duodenal toxicity, which is often more pronounced in the late rather than acute setting. Late grade 3-4 duodenal toxicity including severe mucositis, ulceration, perforation, GI bleeding, and stricture / obstruction, has been consistently observed in at least 10-20% of patients receiving treatment-intensified local therapies, including stereotactic radiosurgery, hypofractionated radiotherapy, and sometimes radiotherapy with concurrent full-dose gemcitabine, with more data forthcoming (14, 15, 26-28). Thus while duodenal sparing remains a goal when designing treatment fields to reduce this significant risk of toxicity, our data suggest that among proximal tumors the adjacent duodenum is usually involved by cancer, and therefore should not be spared coverage from an oncologic standpoint. We favor of inclusion of the adjacent portion of the duodenum within the CTV during 3D-conformal radiotherapy (3D-CRT) or intensity-modulated radiotherapy (IMRT) planning for proximal tumors, due to the high risk of duodenal invasion we observed, and based on elective coverage of pancreatico-duodenal lymph nodes at risk. There appears to be low/acceptable risk associated with adjacent duodenal coverage for conventionally fractionated 3D-CRT or IMRT. Dose-escalated or stereotactic radiation approaches for pancreatic cancer appear to face these competing risks between duodenal injury and duodenal under-coverage. Longer follow-up of these techniques is required to better assess duodenal toxicity, though follow-up may be limited by relatively short median survival and challenges with accurately imaging this region. Based on our findings, we recommend caution with stereotactic or dose-escalated approaches from the standpoint of potential under-coverage of duodenal invasion, and the emerging risks of duodenal toxicity even with modest duodenal coverage.
Our study has certain limitations. We only report the maximum dimension of the primary tumor in any direction, and we do not know the orientation of the maximum dimension on CT compared to pathologic specimen, as there was no verifiable orientation on the pathology reports with regard to size. We considered calculating tumor volumes based on the three dimensions reported by the pathologist and comparing these to CT volumes, but this approach was disregarded given the heterogeneous shapes of primary tumors and the potential systematic bias that artificially-calculated pathologic volumes could introduce. The pathology reports contained no measurement of potential microscopic extension of primary tumor beyond the gross tumor dimensions, and as such there is likely some element of microscopic disease not accounted for in our CTV primary formula, which was a limitation of our retrospective review of the pathology reports. Passage of time between pre-operative CT scan and the date of surgery could have biased findings in favor of a larger pathologic specimen due to interval tumor growth, yet with a median time of less than 3 weeks between CT and surgery, we feel that significant pancreatic adenocarcinoma tumor growth is unlikely. We only included patients who had undergone resection because of the need to have a pathologic specimen, and as mentioned this might underestimate the CT vs. pathology discrepancy, given that up to 10-30% of tumors that appear resectable on CT are found to be unresectable at time of surgery. Finally, it is possible that the radiologists’ measurements of the primary tumor dimensions were either arbitrary or imprecise on the CT reports, given that their primary goal is to accurately determine potential tumor resectability / vascular involvement, with less focus on exact tumor size dimensions. This potential limitation is real, yet we were reassured after finding high concordance with the radiology reports after conducting our own blinded re-measurement / determination of the maximum tumor dimension on every patient's CT scan.
Further research is warranted to investigate other potential clinical, radiographic, or pathologic features of pancreatic cancer that may be associated with a size discrepancy between imaging and pathology. As an example, recent data have demonstrated that DPC4 gene immunolabeling status is correlated with eventual metastatic progression but not with local progression (29); this information could potentially be used to select patients upfront for locally aggressive therapy. It is also possible that size discrepancy could be associated with other outcomes after adjuvant radiotherapy, such as survival, which correlated with pancreatic tumor size in at least one study (30). Ongoing study will continue to refine optimal radiotherapy techniques for pancreatic cancer and individualize patient selection for treatment.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Presented orally in abstract form at the 2009 ASTRO Annual Meeting in Chicago, IL
CONFLICTS OF INTEREST NOTIFICATION
None of the authors has any conflicts of interest to disclose.
REFERENCES
- 1.Jemal A, Siegel R, Ward E, et al. Cancer statistics, 2009. CA Cancer J Clin. 2009;59:225. doi: 10.3322/caac.20006. [DOI] [PubMed] [Google Scholar]
- 2.Griffin JF, Smalley SR, Jewell W, et al. Patterns of failure after curative resection of pancreatic carcinoma. Cancer. 1990;66:56–61. doi: 10.1002/1097-0142(19900701)66:1<56::aid-cncr2820660112>3.0.co;2-6. [DOI] [PubMed] [Google Scholar]
- 3.Hattangadi J, Hong TS, Yeap BY, et al. Results and patterns of failure in patients treated with adjuvant combined chemoradiation therapy for resected pancreatic adenocarcinoma. Cancer. 2009;115:3640–3650. doi: 10.1002/cncr.24410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Van den Broeck A, Sergeant G, Ectors N, et al. Patterns of recurrence after curative resection of pancreatic ductal adenocarcinoma. Eur J Surg Oncol. 2009;35:600–604. doi: 10.1016/j.ejso.2008.12.006. [DOI] [PubMed] [Google Scholar]
- 5.Kalser MH, Ellenberg SS. Pancreatic cancer. Adjuvant combined radiation and chemotherapy following curative resection. Arch Surg. 1985;120:899–903. doi: 10.1001/archsurg.1985.01390320023003. [DOI] [PubMed] [Google Scholar]
- 6.Gastrointestinal Tumor Study Group Further evidence of effective adjuvant combined radiation and chemotherapy following curative resection of pancreatic cancer. Cancer. 1987;59:2006–2010. doi: 10.1002/1097-0142(19870615)59:12<2006::aid-cncr2820591206>3.0.co;2-b. [DOI] [PubMed] [Google Scholar]
- 7.Willett CG, Czito BG, Bendell JC, et al. Locally advanced pancreatic cancer. J Clin Oncol. 2005;23:4538–4544. doi: 10.1200/JCO.2005.23.911. [DOI] [PubMed] [Google Scholar]
- 8.Ben-Josef E, Shields AF, Vaishampayan U, et al. Intensity-modulated radiotherapy (IMRT) and concurrent capecitabine for pancreatic cancer. Int J Radiat Oncol Biol Phys. 2004;59:454–459. doi: 10.1016/j.ijrobp.2003.11.019. [DOI] [PubMed] [Google Scholar]
- 9.Okusaka T, Ito Y, Ueno H, et al. Phase II study of radiotherapy combined with gemcitabine for locally advanced pancreatic cancer. Br J Cancer. 2004;91:673–677. doi: 10.1038/sj.bjc.6602001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Shinchi H, Takao S, Noma H, et al. Length and quality of survival after external-beam radiotherapy with concurrent continuous 5-fluorouracil infusion for locally unresectable pancreatic cancer. Int J Radiat Oncol Biol Phys. 2002;53:146–150. doi: 10.1016/s0360-3016(01)02806-1. [DOI] [PubMed] [Google Scholar]
- 11.Crane CH, Wolff RA, Abbruzzese JL, et al. Combining gemcitabine with radiation in pancreatic cancer: understanding important variables influencing the therapeutic index. Semin Oncol. 2001;28:25–33. doi: 10.1053/sonc.2001.22536. [DOI] [PubMed] [Google Scholar]
- 12.Koong AC, Le QT, Ho A, et al. Phase I study of stereotactic radiosurgery in patients with locally advanced pancreatic cancer. Int J Radiat Oncol Biol Phys. 2004;58:1017–1021. doi: 10.1016/j.ijrobp.2003.11.004. [DOI] [PubMed] [Google Scholar]
- 13.Koong AC, Christofferson E, Le QT, et al. Phase II study to assess the efficacy of conventionally fractionated radiotherapy followed by a stereotactic radiosurgery boost in patients with locally advanced pancreatic cancer. Int J Radiat Oncol Biol Phys. 2005;63:320–323. doi: 10.1016/j.ijrobp.2005.07.002. [DOI] [PubMed] [Google Scholar]
- 14.Schellenberg D, Goodman KA, Lee F, et al. Gemcitabine chemotherapy and single-fraction stereotactic body radiotherapy for locally advanced pancreatic cancer. Int J Radiat Oncol Biol Phys. 2008;72:678–686. doi: 10.1016/j.ijrobp.2008.01.051. [DOI] [PubMed] [Google Scholar]
- 15.Chang DT, Schellenberg D, Shen J, et al. Stereotactic radiotherapy for unresectable adenocarcinoma of the pancreas. Cancer. 2009;115:665–672. doi: 10.1002/cncr.24059. [DOI] [PubMed] [Google Scholar]
- 16.Horton KM. Multidetector CT and three-dimensional imaging of the pancreas: state of the art. J Gastrointest Surg. 2002;6:126–128. doi: 10.1016/s1091-255x(01)00055-5. [DOI] [PubMed] [Google Scholar]
- 17.Wong JC, Lu DS. Staging of pancreatic adenocarcinoma by imaging studies. Clin Gastroenterol Hepatol. 2008;6:1301–1308. doi: 10.1016/j.cgh.2008.09.014. [DOI] [PubMed] [Google Scholar]
- 18.Boland GW, O'Malley ME, Saez M, et al. Pancreatic-phase versus portal vein-phase helical CT of the pancreas: optimal temporal window for evaluation of pancreatic adenocarcinoma. AJR Am J Roentgenol. 1999;172:605–608. doi: 10.2214/ajr.172.3.10063844. [DOI] [PubMed] [Google Scholar]
- 19.DeWitt J, Devereaux B, Chriswell M, et al. Comparison of endoscopic ultrasonography and multidetector computed tomography for detecting and staging pancreatic cancer. Ann Intern Med. 2004;141:753–763. doi: 10.7326/0003-4819-141-10-200411160-00006. [DOI] [PubMed] [Google Scholar]
- 20.Muller MF, Meyenberger C, Bertschinger P, et al. Pancreatic tumors: evaluation with endoscopic US, CT, and MR imaging. Radiology. 1994;190:745–751. doi: 10.1148/radiology.190.3.8115622. [DOI] [PubMed] [Google Scholar]
- 21.Legmann P, Vignaux O, Dousset B, et al. Pancreatic tumors: comparison of dual-phase helical CT and endoscopic sonography. AJR Am J Roentgenol. 1998;170:1315–1322. doi: 10.2214/ajr.170.5.9574609. [DOI] [PubMed] [Google Scholar]
- 22.Muler JH, McGinn CJ, Normolle D, et al. Phase I trial using a time-to-event continual reassessment strategy for dose escalation of cisplatin combined with gemcitabine and radiation therapy in pancreatic cancer. J Clin Oncol. 2004;22:238–243. doi: 10.1200/JCO.2004.03.129. [DOI] [PubMed] [Google Scholar]
- 23.Minn AY, Schellenberg D, Maxim P, et al. Pancreatic tumor motion on a single planning 4D-CT does not correlate with intrafraction tumor motion during treatment. Am J Clin Oncol. 2009;32:364–368. doi: 10.1097/COC.0b013e31818da9e0. [DOI] [PubMed] [Google Scholar]
- 24.Metz JM, Kassaee A, Ingram M, et al. First report of real-time tumor tracking in the treatment of pancreatic cancer using the Calypso system [Abstract]. Int J Radiat Oncol Biol Phys. 2009;75:S54–55. [Google Scholar]
- 25.Brade A, Brierley J, Oza A, et al. Concurrent gemcitabine and radiotherapy with and without neoadjuvant gemcitabine for locally advanced unresectable or resected pancreatic cancer: a phase I-II study. Int J Radiat Oncol Biol Phys. 2007;67:1027–1036. doi: 10.1016/j.ijrobp.2006.10.015. [DOI] [PubMed] [Google Scholar]
- 26.Hoyer M, Roed H, Sengelov L, et al. Phase-II study on stereotactic radiotherapy of locally advanced pancreatic carcinoma. Radiother Oncol. 2005;76:48–53. doi: 10.1016/j.radonc.2004.12.022. [DOI] [PubMed] [Google Scholar]
- 27.Murphy JD, Adusumilli S, Griffith KA, et al. Full-dose gemcitabine and concurrent radiotherapy for unresectable pancreatic cancer. Int J Radiat Oncol Biol Phys. 2007;68:801–808. doi: 10.1016/j.ijrobp.2006.12.053. [DOI] [PubMed] [Google Scholar]
- 28.Murphy JD, Dieterich S, Chang DT, et al. Duodenal toxicity in single-fraction stereotactic body radiotherapy [Abstract]. Int J Radiat Oncol Biol Phys. 2009;75:S29–30. doi: 10.1016/j.ijrobp.2009.09.075. [DOI] [PubMed] [Google Scholar]
- 29.Iacobuzio-Donahue CA, Fu B, Yachida S, et al. DPC4 gene status of the primary carcinoma correlates with patterns of failure in patients with pancreatic cancer. J Clin Oncol. 2009;27:1806–1813. doi: 10.1200/JCO.2008.17.7188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Agarwal B, Correa AM, Ho L. Survival in pancreatic carcinoma based on tumor size. Pancreas. 2008;36:e15–20. doi: 10.1097/mpa.0b013e31814de421. [DOI] [PubMed] [Google Scholar]