Abstract
Background
Tremendous advances have occurred in therapies for peripheral vascular disease (PVD); however, until recently it has not been possible to examine the entire clinical trial portfolio of studies for treatment of PVD (both arterial and venous disease).
Methods and Results
We examined interventional trials registered in ClinicalTrials.gov from October 2007 through September 2010 (n=40,970) and identified 676 (1.7%) PVD trials (n=493 arterial only, n=170 venous only, n=13 both arterial and venous). Most arterial studies investigated lower extremity peripheral artery disease and acute stroke (35% and 24%, respectively), while most venous studies examined deep vein thrombosis/pulmonary embolus prevention (42%) or venous ulceration (25%). A placebo-controlled trial design was used in 27% of the PVD trials, and 4% of the PVD trials excluded patients aged >65 years. Enrollment in at least 1 US site decreased from 51% in 2007 to 41% of trials in 2010. Compared with non-cardiology disciplines, PVD trials were more likely to be double-blinded, investigate use of devices and procedures, and have industry sponsorship and assumed funding source, and less likely to investigate drug and behavioral therapies. Geographic access to PVD clinical trials within the United States is limited to primarily large metropolitan areas.
Conclusions
PVD studies represent a small group of trials registered in ClinicalTrials.gov, despite the high prevalence of vascular disease in the general population. This low number, compounded by the decreasing number of PVD trials in the United States, is concerning and may limit the ability to inform current clinical practice of patients with PVD.
Keywords: peripheral vascular disease, trials, registries, prevention
With the aging US population, we can expect a greater incidence of peripheral vascular disease (PVD) (both arterial and venous disease) and substantial costs associated with its treatment.1,2,3 Accordingly, the Institute of Medicine has listed the comparison of various therapies for treatment of vascular claudication alone among its top 50 comparative effectiveness research topics, and vascular claudication is the only cardiovascular condition besides a trial fibrillation on this list.4
Recent advances in therapies for PVD have provided greater options for patients and clinicians. Several mechanical devices are now available for endovascular treatment of lower extremity peripheral artery disease (PAD),5,6 extra- and intra-cranial cerebrovascular disease, and aortic disease.7 For patients with advanced PAD with development of critical limb ischemia (CLI), there remains promise for treatment with novel biologic compounds.8,9 Furthermore, there has been an evolution in prevention and treatment of deep vein thrombosis (DVT) and pulmonary embolus (PE) with novel anticoagulation therapies, as well as the potential for catheter-directed thrombolysis to prevent post-thrombotic syndrome and improve quality of life.10,11 Similarly, percutaneous ablation of lower extremity venous disease has largely replaced traditional surgical phlebectomy for treatment of venous claudication and venous reflux.12 While the number of PVD therapies has grown rapidly, little is known about the current state of the entire PVD trial portfolio and current trial designs. Using a database developed for analysis of trials registered in ClinicalTrials.gov (CTG), we sought to describe the current state of clinical trials for treatment of PVD.
Methods
ClinicalTrials.gov
The CTG registry comprises over 110,000 clinical research studies conducted in more than 175 countries and allows analysis of studies from various disciplines. CTG was developed to increase transparency and improve the conduct and monitoring of research,13 and now serves as one of the primary repositories for information on clinical studies to be published in all member journals of the International Committee of Medical Journal Editors (ICMJE).14 The Clinical Trials Transformation Initiative (CTTI) is a public-private partnership developed by the US Food and Drug Administration (FDA) and Duke University, and includes more than 60 member organizations from across the clinical trial enterprise, with the mission to identify and promote practices that will increase the quality and efficiency of clinical trials. CTTI has developed a high-quality, downloadable relational database of information contained in CTG.15
Development of the CTG Data Set
A data set of 96,346 clinical trials registered in CTG was downloaded in XML format on September 27, 2010. The data were subsequently captured in a database to facilitate aggregate analysis of data from CTG as described previously.15,16
Creation of the PVD Data Set
Analysis was restricted to 40,970 studies with the “interventional” study type registered with CTG between October 1, 2007, and September 27, 2010. The PVD study data set was developed using disease condition terms (Medical Subject Headings [MeSH] and non-MeSH) provided by the data submitters and additional condition MeSH terms generated by the National Library of Medicine (NLM) algorithm. One primary Duke vascular trained clinical investigator (SS) and 2 secondary vascular trained investigators (MRP and WSJ) reviewed 2797 unique MeSH terms and 1220 frequently used free-text terms from the conditions or NLM-generated condition_browse fields for these studies. The investigators annotated 165 MeSH terms and 55 free-text terms as potentially relevant to PVD. The investigators included any term that might potentially be associated with extra-cardiac arterial or venous disease. These terms identified an initial potential subset of 3175 studies with at least 1 condition term potentially relevant to PVD. The investigators manually reviewed each of these study descriptive entries within CTG. Only studies that examined therapies for PVD and included enrollment of patients with PVD were included in the final data set. We sought to describe the registered trials of extra-cardiac vascular disease commonly cared for by most cardiologists, vascular medicine specialists, vascular surgeons, interventional radiologists, and neuroradiologists. Aneurysmal disease (such as thoracic or abdominal aortic aneurysm) was included. We excluded studies of external/indwelling devices, management of sequelae of vascular disease (i.e., stroke rehabilitation, amputation rehabilitation), brain arterio-venous malformations, arterio-venous grafts/shunts, orthostatic hypotension, vasculitis, hemostatic devices for arterial or venous procedures, and chronic cerebrospinal venous insufficiency. Any study that did not explicitly state whether the therapy was being investigated in patients with established PVD was excluded. This yielded a final data set of 676 PVD trials.
Subgroups of PVD Data Set
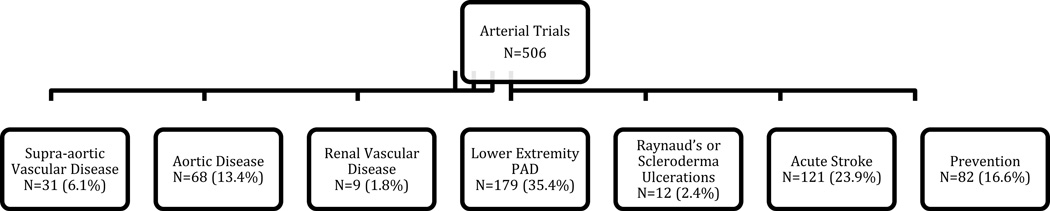
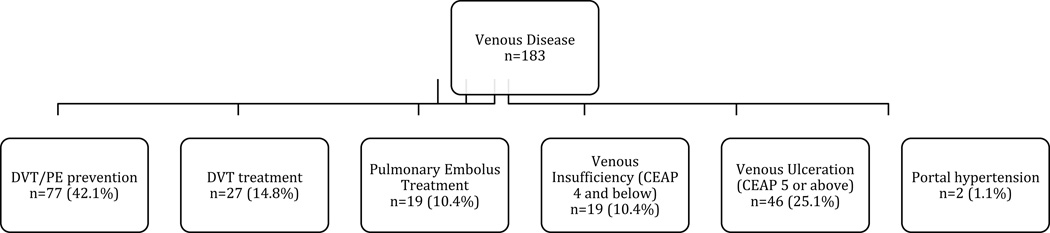
Each of the PVD studies was categorized into trials of arterial disease and/or venous disease (Figure 1), and then further subcategorized (Figure 2). Descriptions and rationale for these subcategories are provided in the online-only Data Supplement.
Figure 1.
Subgroups of trials of therapies for (A) arterial and (B) venous studies. PAD indicates peripheral artery disease; DVT, deep vein thrombosis; PE, pulmonary embolus. Studies were allowed to be in more than one subgroup if they enrolled patients categorized within different subgroups. Four arterial studies with other conditions are not included. Seven venous studies were counted in more than one subgroup.
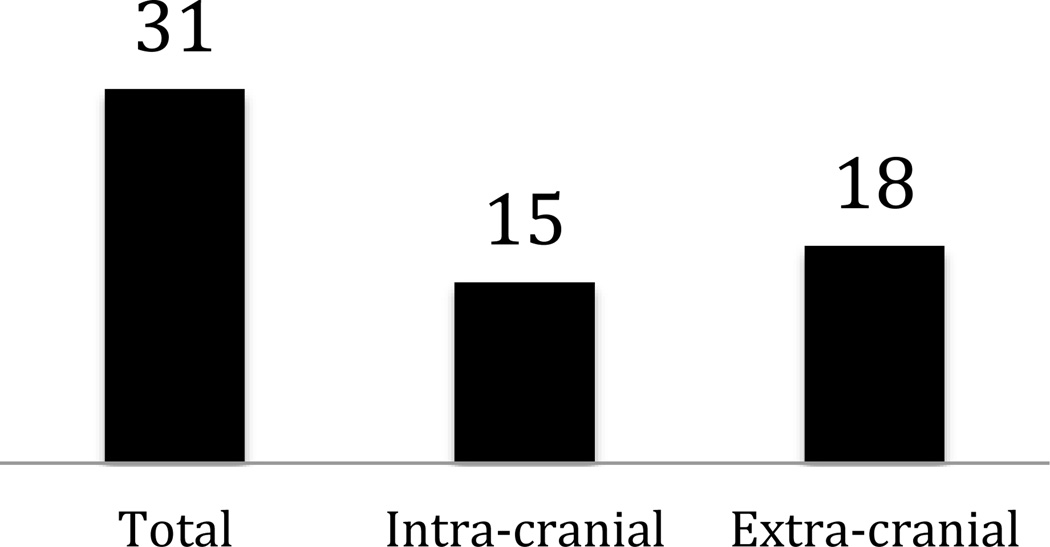
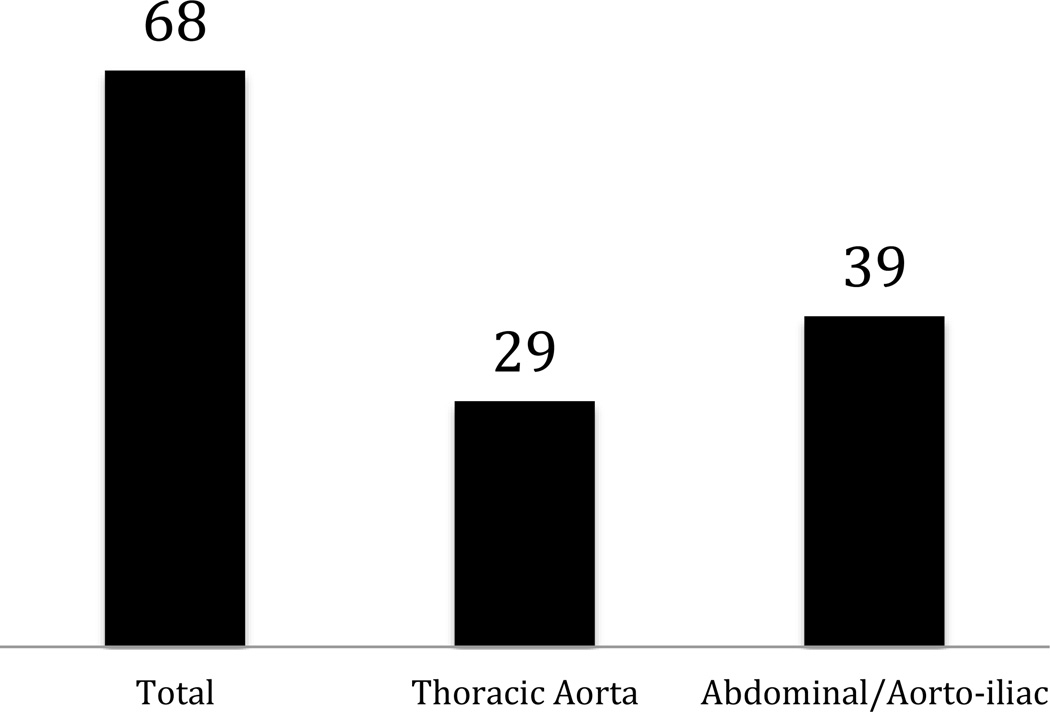
Figure 2.
Subcategorization of (A) supra-aortic, (B) aortic, (C) lower extremity PAD, and (D) acute stroke trials. Studies were allowed to be in more than one subgroup if they enrolled patients categorized within different subgroups.
Analysis
Counts and percentages are provided for categorical trial characteristics, while continuous characteristics are presented as median (first and third quartiles). Missing values were excluded from calculations unless indicated otherwise. The portfolio characteristics included phase of study, study design, enrollment, lead sponsor, collaborators, and study location. Study design attributes included interventions, comparators, masking, allocation, and purpose of intervention. Lead sponsor was defined as the primary organization that oversaw implementation of the study and was responsible for data analysis (e.g., National Institutes of Health or a pharmaceutical company). Collaborators were defined as other organizations (if any) that provided support, including funding, design, implementation, data analysis, and reporting. The assumed funding source was derived using the lead sponsor and collaborators fields, and is described in the online-only Data Supplement. International regional variation was described by location of enrolling sites. We compared the characteristics of the PVD studies with characteristics of cardiology trials (excluding any PVD studies), as well as the entire cohort of studies in CTG. The online-only Data Supplement includes data on the methodology for identification of cardiac trials by cardiology specialists at Duke University; these data have been previously presented17 and published.18
Within the United States, we further described the geographic access to PVD clinical trial sites graphically on a map by locating trial sites at the county level. Study sites were excluded if they did not provide a valid zip code or if the city and state entered in CTG did not match the zip code. Additionally, to illustrate how geographic access to clinical trials compared with geographic prevalence of end-stage disease, we compared the geographic variation of sites with lower extremity PAD trials versus geographic variation in amputation rates per state compared with the national average among Medicare beneficiaries.19 The map of geographic variation was developed by calculating annual rates of amputation from 2000 to 2008 among Medicare beneficiaries and mapping the geographic variation of ratio of amputation in each state compared with the national average.
Results
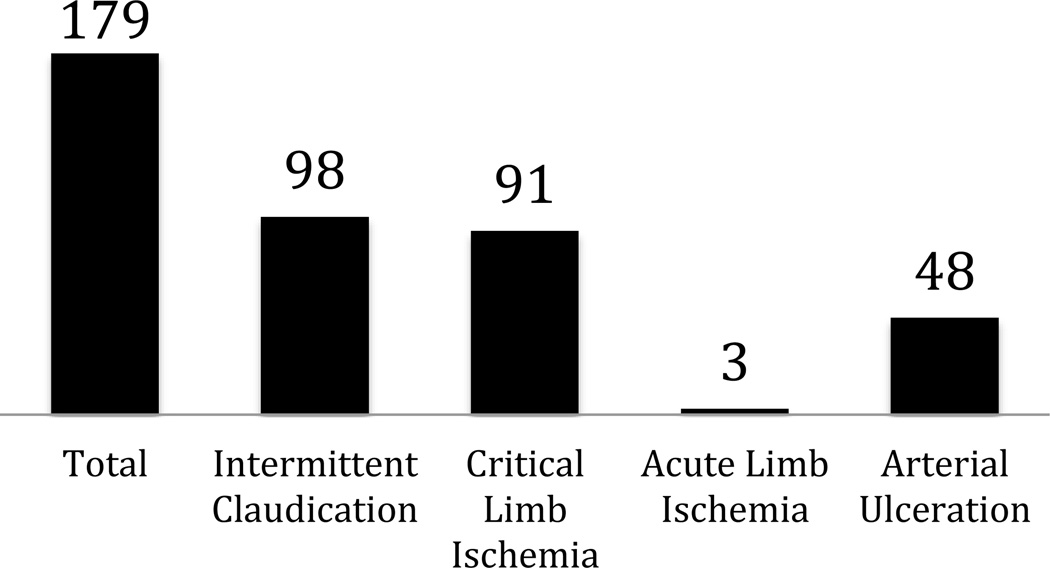
The PVD trial portfolio represents only 1.7% (n=676) of the 40,970 interventional clinical trials registered within CTG from October 1, 2007, through September 27, 2010. Among the PVD trial portfolio, the vast majority of studies focused on therapies for arterial conditions compared with venous disease (n=493 arterial only, n=170 venous only, n=13 both arterial and venous) (Figure 1). Most of the arterial studies sought to investigate treatment for lower extremity PAD and acute stroke (35% and 24%, respectively). Most venous trials sought to investigate therapies for DVT/PE prevention (42%) or venous ulceration (25%) (Figure 1).
The proportion of PVD trials enrolling patients within the United States declined over the study period; among studies starting enrollment in 2007, 51% (36/71) had sites enrolling patients within the United States compared with 49% (77/156) in 2008, 45% (73/163) in 2009, and 41% (44/108) of the trials in 2010.
Comparison of PVD Studies Versus Other Disciplines
PVD trials were much more likely to investigate the use of devices and procedures, and less likely to investigate drug, behavioral, and genetic therapies compared to studies of non-cardiology disciplines. Rates of randomization were similar among PVD and other disease states (71% versus 68%, respectively). However, compared with trials of other disease states, PVD studies were more likely to be double-blinded than the aggregate of studies of other disciplines (Table 1). Similar to cardiology trials, PVD studies were more likely to be later phase and larger compared with studies of other disciplines. Though the use of an active comparator was greater than in non-cardiology disciplines, it was used in less than half the PVD studies (47%). Similar to other disciplines, a placebo comparator was used in roughly one-fourth of the PVD trials. Similar to cardiology trials, few PVD studies (4%) excluded elderly participants (those aged >65 years), compared with 34% of non-cardiology trials. PVD trials were also more likely to have industry sponsorship as well as an assumed funding source compared with cardiology and other studies within CTG.
Table 1.
Overall Trial Characteristics of PVD Trials Versus Cardiology Trials Versus Other Disease Trials Registered in ClinicalTrials.gov From October 2007–September 2010
| PVD N=676 |
Cardiology N=2077 |
Other N=38217 |
|
|---|---|---|---|
| Masking, n/N (%) | |||
| Open | 348/671 (51.9%) | 1074/2051 (52.4%) | 20812/37149 (56.0%) |
| Single blind | 73/671 (10.9%) | 315/2051 (15.4%) | 4069/37149 (11.0%) |
| Double blind | 250/671 (37.3%) | 662/2051 (32.3%) | 12268/37149 (33.0%) |
| Allocation, n/N (%) | |||
| Randomized | 474/669 (70.9%) | 1562/2041 (76.5%) | 24991/36530 (68.4%) |
| Intervention types, n/N (%)* | |||
| Drug | 351/676 (51.9%) | 911/2077 (43.9%) | 23489/38217 (61.5%) |
| Device | 205/676 (30.3%) | 529/2077 (25.5%) | 3065/38217 (8.0%) |
| Procedure | 85/676 (12.6%) | 304/2077 (14.6%) | 3715/38217 (9.7%) |
| Behavioral | 21/676 (3.1%) | 133/2077 (6.4%) | 3153/38217 (8.3%) |
| Genetic or biologic | 31/676 (4.6%) | 59/2077 (2.8%) | 3144/38217 (8.2%) |
| Dietary supplement | 7/676 (1.0%) | 47/2077 (2.3%) | 1549/38217 (4.1%) |
| Phase, n/N (%) | |||
| Not applicable | 179/676 (26.5%) | 725/2077 (34.9%) | 10128/38217 (26.5%) |
| Phase 0 | 7/497 (1.4%) | 14/1352 (1.0%) | 295/28089 (1.1%) |
| Phase 1 | 43/497 (8.7%) | 74/1352 (5.5%) | 6105/28089 (21.7%) |
| Phase 1/Phase 2 | 26/497 (5.2%) | 55/1352 (4.1%) | 2024/28089 (7.2%) |
| Phase 2 | 122/497 (24.5%) | 267/1352 (19.7%) | 8095/28089 (28.8%) |
| Phase 2/Phase 3 | 36/497 (7.2%) | 64/1352 (4.7%) | 955/28089 (3.4%) |
| Phase 3 | 130/497 (26.2%) | 339/1352 (25.1%) | 5728/28089 (20.4%) |
| Phase 4 | 133/497 (26.8%) | 539/1352 (39.9%) | 4887/28089 (17.4%) |
| Number of arms, n/N (%) | |||
| 1 | 185/667 (27.7%) | 522/2031 (25.7%) | 11869/36547 (32.5%) |
| 2 | 383/667 (57.4%) | 1244/2031 (61.3%) | 17578/36547 (48.1%) |
| 3 or more | 99/667 (14.8%) | 265/2031 (13.0%) | 7098/36547 (19.4%) |
| Arm types, n/N (%)† | |||
| Active comparator | 282/600 (47.0%) | 945/1866 (50.6%) | 13587/32629 (41.6%) |
| No intervention arm | 59/600 (9.8%) | 252/1866 (13.5%) | 2745/32629 (8.4%) |
| Experimental arm | 438/600 (73.0%) | 1215/1866 (65.1%) | 25082/32629 (76.9%) |
| Placebo comparator arm | 159/600 (26.5%) | 451/1866 (24.2%) | 8347/32629 (25.6%) |
| Sham comparator arm | 12/600 (2.0%) | 24/1866 (1.3%) | 454/32629 (1.4%) |
| Other arm | 42/600 (7.0%) | 152/1866 (8.1%) | 1806/32629 (5.5%) |
| Median enrollment (Q1, Q3) | 114.5 (49.5, 280.0) | 117.0 (50.0, 316.0) | 60.0 (30.0, 169.0) |
| Size of trial (enrollment), n/N (%) | |||
| 0 | 1/672 (0.1%) | 0/2053 (0.0%) | 85/37641 (0.2%) |
| 1 to 100 | 317/672 (47.2%) | 984/2053 (47.9%) | 24085/37641 (64.0%) |
| 101 to 1000 | 308/672 (45.8%) | 877/2053 (42.7%) | 12241/37641 (32.5%) |
| 1001 to 5000 | 40/672 (6.0%) | 150/2053 (7.3%) | 1045/37641 (2.8%) |
| More than 5000 | 6/672 (0.9%) | 42/2053 (2.0%) | 185/37641 (0.5%) |
| Age eligible for enrollment, n/N (%) | |||
| Study excludes age > 65 years | 27/676 (4.0%) | 84/2077 (4.0%) | 12936/38217 (33.9%) |
| Study excludes age < 65 years | 2/676 (0.3%) | 28/2077 (1.4%) | 442/38217 (1.2%) |
| Study site locations, n/N (%)‡ | |||
| Africa | 21/628 (3.3%) | 29/1928 (1.5%) | 767/34964 (2.2%) |
| Central & South America | 38/628 (6.1%) | 104/1928 (5.4%) | 1651/34964 (4.7%) |
| Europe | 249/628 (39.6%) | 829/1928 (43.0%) | 10233/34964 (29.3%) |
| North America | 315/628 (50.2%) | 840/1928 (43.6%) | 20426/34964 (58.4%) |
| Asia/Middle East | 146/628 (23.2%) | 411/1928 (21.3%) | 6362/34964 (18.2%) |
| Lead sponsor, n/N (%) | |||
| Industry | 298/676 (44.1%) | 612/2077 (29.5%) | 14338/38217 (37.5%) |
| NIH | 8/676 (1.2%) | 42/2077 (2.0%) | 1056/38217 (2.8%) |
| Assumed funding source, n/N (%)§ | |||
| Industry | 359/676 (53.1%) | 839/2077 (40.4%) | 17639/38217 (46.2%) |
| NIH | 30/676 (4.4%) | 86/2077 (4.1%) | 3422/38217 (9.0%) |
PVD indicates peripheral vascular disease; Q1, first quartile; Q3, third quartile; NIH, National Institutes of Health.
A study may have more than 1 intervention type.
A study may have more than 1 arm type.
Regions are defined as at http://www.clinicaltrials.gov/ct2/search/browse?brwse=locn_cat. A study may have sites in more than 1 region. Information about location of sites was missing in 8% of studies.
Derived from lead sponsor and collaborator fields.
Roughly 45% of trials with a device intervention reported using randomization, compared with ~82% of PVD trials without a device intervention (data not shown). With respect to blinding, roughly 75% of PVD trials with a device intervention were open label, compared to ~42% of PVD trials without a device intervention, and approximately 13% of trials with device intervention were double blinded compared with 48% of trials without device intervention (data not shown).
Arterial and Venous Disease Trials
Most trials of supra-aortic, aortic, and lower extremity PAD sought to investigate device therapies for treatment: For (a) supra-aortic disease, 67% of intra-cranial studies were examining device therapies, and 67% of extra-cranial trials investigated devices; for (b) aortic disease, 62% of thoracic and 67% of abdominal/aorto-iliac studies were investigating devices; and for (c) lower extremity PAD, 53% of intermittent claudication studies, 50% of CLI studies, and 67% of acute limb ischemia studies investigated devices (online-only Data Supplement Table 1). Behavior modification was investigated in 10% of the intermittent claudication trials. For CLI, 17% of trials investigated genetic or biologic therapies. While intermittent claudication trials tended to be later phase (87% Phases 2–4), CLI trials included earlier phase therapies (33% Phase 1 or 1/2). Meanwhile, for acute stroke, most trials were examining use of drug therapies (66% of trials for ischemic stroke and 74% for hemorrhagic stroke).
Most venous disease trials were examining either treatment or prevention of DVT or PE (57% [104/183]), with 8 of these studies of treatment using vena cava filters. A large percentage of registered venous trials examined drug treatments: 70% of trials for DVT treatment were drug trials versus 15% device trials; for DVT/PE prevention studies, 82% were drug trials versus 13% device trials; for venous insufficiency (CEAP ≤4), 42% were drug trials versus 21% device trials; for PE treatment, 95% were drug trials versus 11% device trials. None of the trials for venous insufficiency used compression therapy as a comparator; 1 study compared duration of compression therapy after venous ablation. However, for treatment of venous ulceration (CEAP ≥5), most trials investigated use of devices (50%) compared with drugs (26%). Other important trial characteristics of venous trials are provided in online-only Data Supplement Table 2.
Geographic Variation
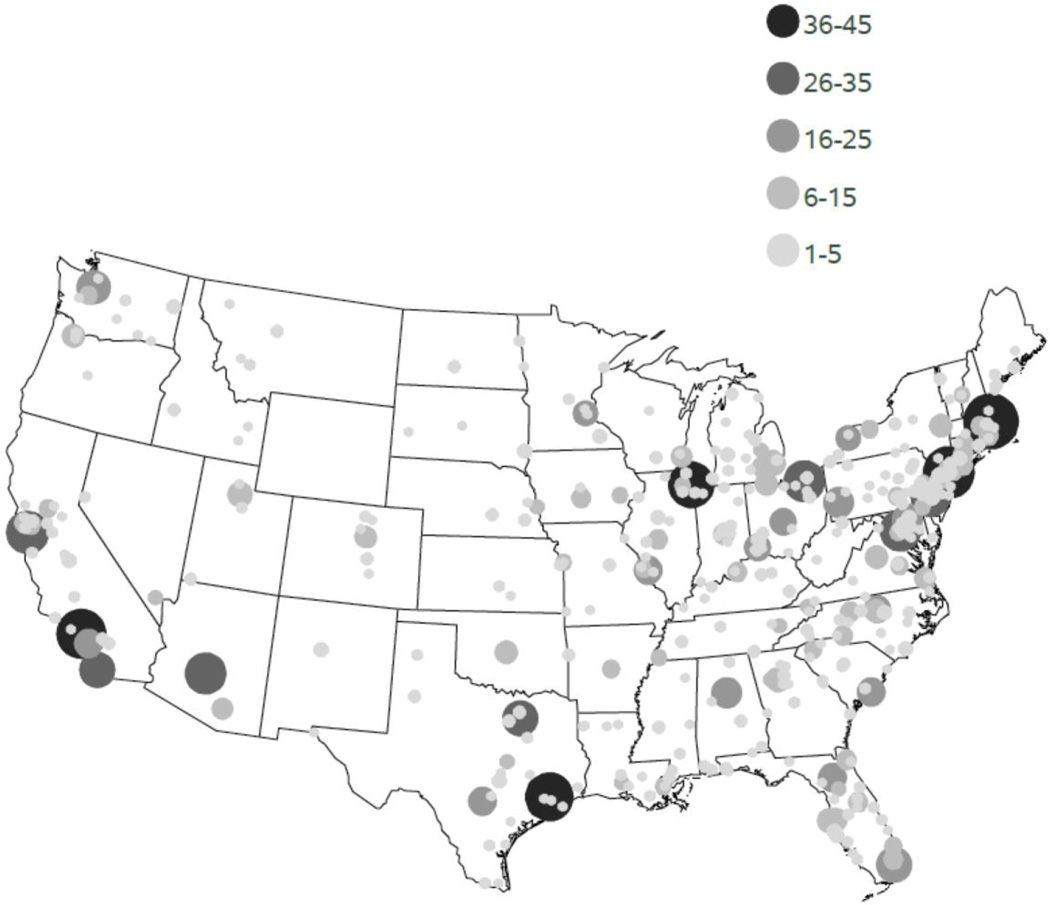
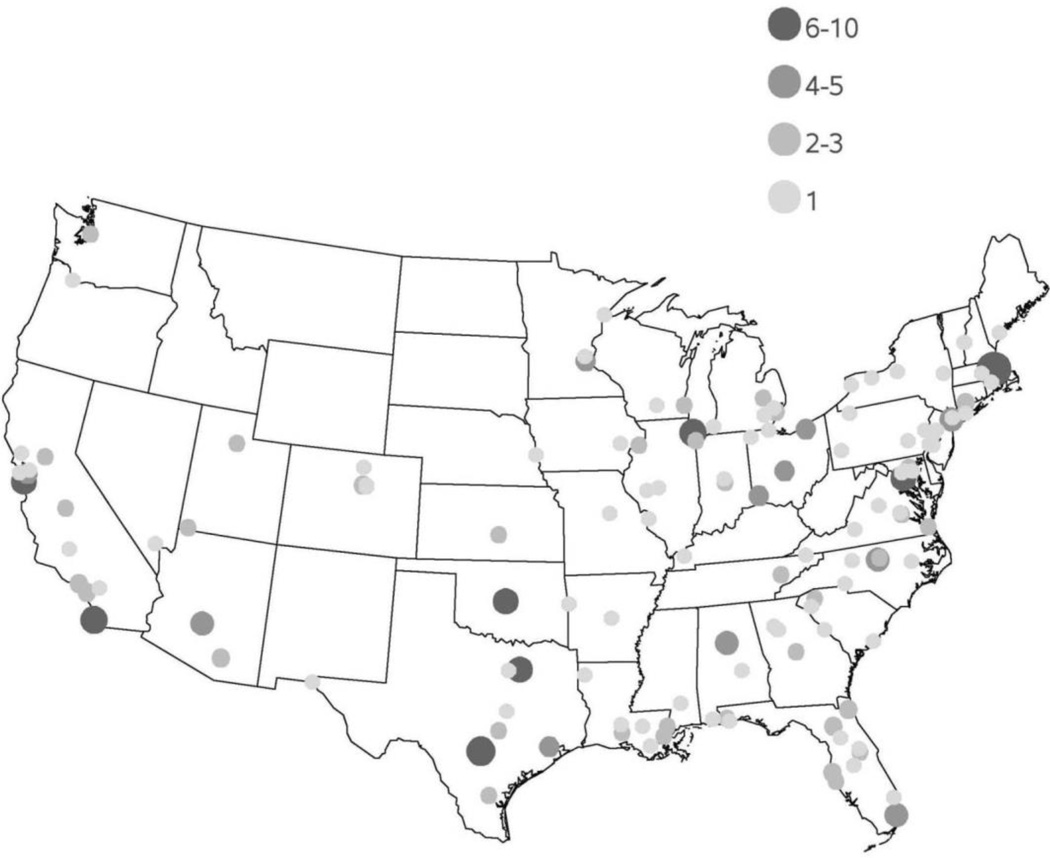
Geographic access to PVD clinical trials within the United States is pictured in Figure 3. Most trials were concentrated in metropolitan areas with greatest population density, with the New England region having the most access to PVD trials. The counties with the greatest access to PVD trials were Suffolk County, MA (n=43), New York County, NY (n=41), Los Angeles County, CA (n=39), Harris County, TX (n=37), and Cook County, IL (n=36).
Figure 3.
Geographic variation in access to clinical trials in peripheral vascular disease (PVD) by county level within the continental United States. Size and color of dot represents number of PVD trials with a site at each county location. Alaska and Hawaii each had 1 PVD trial and are not shown on the map.
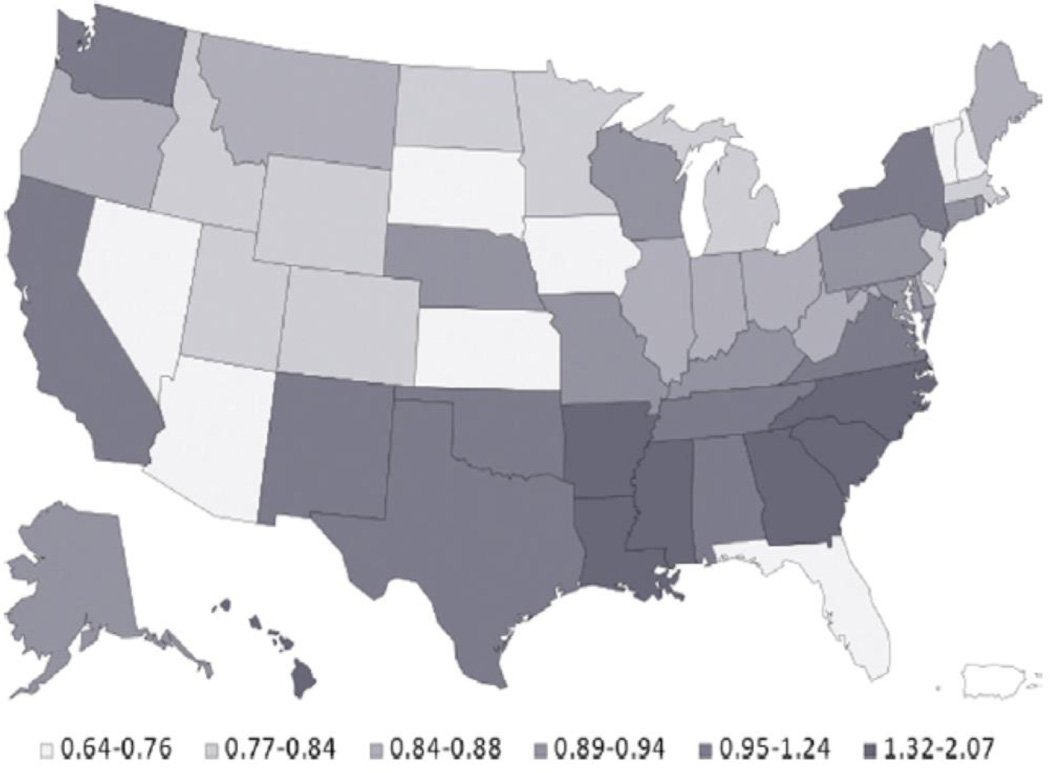
To demonstrate how geographic access to a trial can differ from the geographic distribution of disease, Figure 4 describes the regional distribution of amputation rates (terminal state for progression of PAD), whereas Figure 5 shows the geographic variation in access to lower extremity PAD trials. Though the Southeast has the greatest rates of amputation, there remains relatively limited access to clinical trials in this region of the United States.
Figure 4.
Geographic prevalence of lower extremity amputation per state compared with the national average among Medicare beneficiaries. Reproduced with permission from the Journal of the American College of Cardiology.19
Figure 5.
Geographic variation in access to clinical trials in lower extremity PAD by county level within the continental United States. Size and color of dot represents number of lower extremity PAD trials with a site at each county location.
Discussion
The present analysis is the first characterization of the current clinical trial portfolio of studies seeking treatment of PVD. This research provides a foundation for discussion and policy on ways to optimize the PVD trial portfolio to best inform clinical practice. Despite a large percentage of the population having vascular disease, invasive trials of PVD represent a small fraction of the studies registered in CTG. Compared with other disease states, PVD trials are more likely to be later phase (Phases 2–4), have greater enrollment rates, to investigate devices, and to be industry-sponsored and funded, and less likely to investigate behavioral interventions. PVD trials also allow enrollment of older patients (age >65 years) in a vast majority of cases (96%), which correctly represents the older age population typically representative of vascular disease. The proportion of studies being registered with US enrollment was noted to decrease over time such that 40% of PVD studies starting enrollment during 2010 included US enrollment. Additionally, there remains geographic variation in access to trials within the United States.
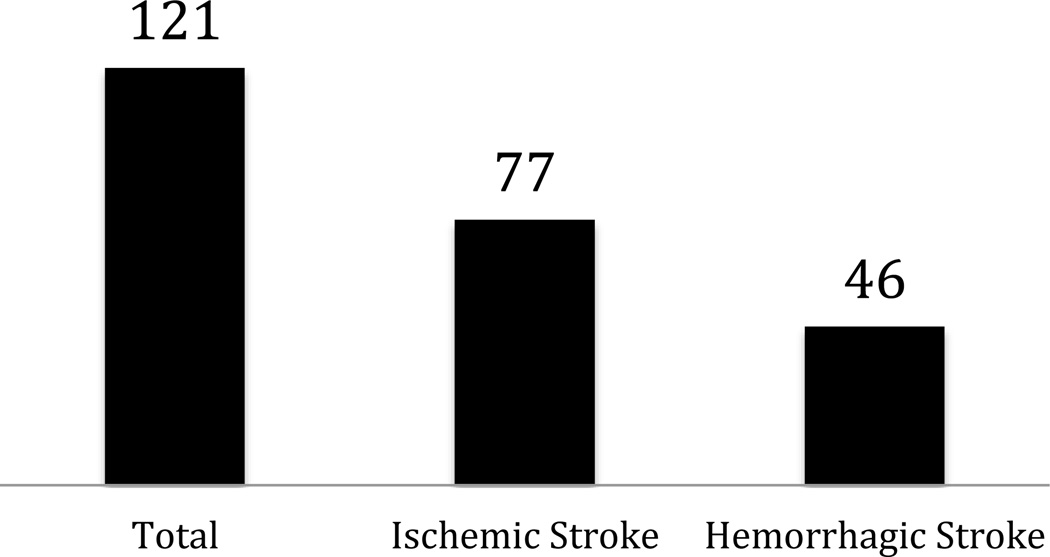
One major theme echoed from the analysis of the PVD trials is the discordance between the current trial portfolio for arterial disease and the current need for data to inform clinical practice. The 121 investigational trials for treatment of acute stroke or transient ischemic attack represent a small fragment (0.3%) of the entire CTG portfolio of investigational trials. Such a number is strikingly low given that stroke was the fourth leading cause of death in the United States in 2010 (though it must be noted that we excluded analysis of trials dealing with sequelae of stroke [i.e., stroke rehabilitation studies]). Given the large morbidity and mortality, trials are desperately needed to improve outcomes among the stroke population.
With regard to lower extremity PAD studies, there is also discordance between the large percentage of registered trials in the current portfolio examining device therapies for PAD as opposed to behavioral modification/interventional therapies (trials that include the following type of interventions: psychotherapy, lifestyle counseling/modification [walking/exercise, educational workshops or educational printed materials], and physical therapy). For instance, we found that only 10% of studies for intermittent claudication included investigation of behavioral intervention (compared with 53% investigating device therapies). Though some trial data demonstrate efficacy and guidelines support use of lifestyle modification, including smoking cessation and exercise,20–22 the optimal behavior modification therapies remain unknown. Additionally, behavior modification trials may be less common because of the potential difficulty getting subjects to comply with these interventions. The greater rate of device trials potentially reflects the need for regulatory approval with devices compared with behavior modifications. This also parallels current trends in use of minimally invasive therapies (compared with surgical treatment) of PAD seen nationally.23 Additionally, as most studies are industry sponsored, it is not surprising that behavioral24 therapies are under-investigated given the lack of a business case and sponsor for studies. Consequently, the burden to investigate efficacy of behavioral modification compared with more invasive therapies will likely be placed on governmental funding. On a similar note, studies investigating devices for treatment of venous disease (venous ulceration and DVT treatment) should include conservative therapies (such as compression and exercise) as an active comparator. Of note, behavior therapies have the potential to be underrepresented in the current data set as they are not covered by the Food and Drug Administration Amendments Act (FDAAA) of 2007 study registration mandate, though they do need to be included in a registry to satisfy ICMJE requirements. With respect to DVT trials investigating use of vena cava filters, none of the registered trials address longer term safety of these devices. Given current FDA concern regarding fracture, migration, and perforation of such devices,25 future trials will need to have longer longitudinal follow-up.
There seems also to be discordance at the patient level with access to trials for PVD. Within the United States, access to PVD trials is largely limited to major metropolitan areas, which tend to be the usual locations of larger medical centers and universities. However, the geographic variation of amputations (the end result of advanced PVD) among Medicare beneficiaries (Figure 4) raises the question of equitable access to the latest technology and therapies for those areas that have the most prevalence and would have the most subsequent proportionate benefit. This is chiefly evident in the southeastern United States as well as in California, Texas, Oklahoma, and New Mexico, which suffer from higher relative amputation rates. Though improved processes of care are necessary for such regions, it can be argued that these patients may also benefit from access to trials given the increased severity of disease burden in the areas.
Compounding this situation is the finding that the number of trials enrolling patients in the United States is decreasing despite the increasing number of trials for PVD. To ensure a broad representation of enrolled patients, future PVD trials should consider a wider geographic catchment area representative of the entire US population with the disease. Additional efforts to enroll patients at such locations may potentially help with recruitment in trials given higher prevalence of disease in these regions. Future efforts may need to target policy to define and ensure appropriate representation of US patients within trials, given that therapies may have different efficacy in different populations.
The present analysis provides several lessons regarding the current PVD trials portfolio. Importantly, there is a limited footprint of non-industry funding sources in the PAD arena. This suggests that the majority of trials are done by industry to support registration and marketing. Though such trials may promote science and care of patients, a much healthier balance of investigator-initiated, multicenter trials and comparative effectiveness studies is needed. Such trials will need to address and compare the effectiveness of non-interventional (medical, exercise) and interventional therapies (endovascular and surgical) for treatment of claudication and effectiveness of surgery versus angioplasty/stenting as revascularization strategies for intermittent claudication or CLI treatment. Within non-interventional therapies themselves, there remain questions as to the optimal strategy for behavioral modification for PVD in general as well as identification of optimal medical therapy for secondary prevention of atrial disease (including use of antiplatelet or antithrombotic therapies). On a similar note, given rising health care costs, a cost effectiveness approach that includes quality of life assessment of novel therapies (primarily devices and genetics) will be required to justify their costs. Though we did not analyze symptomatic status of patients proposed for trials in this data set, future trials should consider differential treatment effect according to symptomatic status, given that PVD tends to present across a spectrum of disease.
Given the high cost of development and subsequent trials, particularly among devices and biologics, novel approaches to trial designs will be needed. One potential solution to these gaps includes partnering academia with industry to design new registries or leverage existing registries to answer questions of efficacy at lower operational costs than with traditional trials. Such registries could be linked to administrative registries (such as the CMS Medicare data set) to provide insight into longer term outcomes, as well as to determine which patients benefit most from interventional therapies. Additionally, trial methodologies other than traditional time-to-event analysis may be considered to provide greater assessment of efficacy and safety of PVD therapies.26
There are several limitations to the CTG data set. Primarily, there is no obligation to register Phase 1 trials, or studies that that do not involve a drug, biologic, or device intervention. Also, trials performed outside the United States do not need to be registered unless they are conducted under an Investigational New Drug or Investigational Device Exemption. However, to increase transparency and improve the conduct and monitoring of research,13 the ICMJE now mandates that trials be registered in a repository (such as CTG) before being published in all member journals. Consequently, it is likely that most trials are registered within CTG given the desire and necessity of scientists and industry sponsors to make their research known to the medical community. Additionally, we relied on data about trials that were entered by someone from the sponsor or funding agency. Therefore, we were limited by the appropriate categorization and medical knowledge of the person entering the data. The locations for site enrollment fields are required by NLM and are flagged as “may be required to comply with FDAAA.” However, there is no consistent manner to monitor this, and it is possible that not all enrollment sites were entered or that sites may have been removed from the study record after enrollment was completed. Another major limitation of the PVD data set was that we included trials in early phase development only if they actually enrolled patients with established extra-cardiac vascular disease; those trials of early phase therapies with a potential target for treatment of PVD that are tested in normal subjects (those without PVD) were excluded in the present analysis. Additionally, our search strategy included condition terms (those focused on disease conditions) and not specific interventions. Given that our main goal was to identify trials seeking treatment of various disease states within PVD, this was a reasonable approach.
In conclusion, the present analysis demonstrates that PVD studies represent a small proportion of trials registered in CTG despite high prevalence of vascular disease in the general population. Most studies investigate therapies for arterial disease and specifically lower extremity PAD and acute stroke. Compared with other trials within CTG, PVD trials tend to be later phase, more likely to investigate devices, more likely to be industry-sponsored and funded, and less likely to investigate behavioral interventions. There remains geographic variation in access to PVD trials for patients in the United States. In aggregate, the present analysis is the first step in understanding the clinical trial portfolio of PVD studies. It will serve as a basis for future discussions and policy aimed at changing the portfolio to reflect those questions of most clinical importance in the field.
Supplementary Material
Acknowledgments
Funding Sources: Financial support for this project was provided by cooperative agreement U19 FD003800 from the US Food and Drug Administration awarded to Duke University for the Clinical Trials Transformation Initiative. Ms. Tidemann-Miller’s traineeship was funded by National Institutes of Health Grant T32HL079896.
Dr Subherwal was supported by National Institutes of Health grant T32HL69749-9. Dr Patel has received research grants to his institution from Johnson and Johnson, Astra Zeneca, Pleuristem, and the National Heart, Blood, and Lung Institute; and has been an advisory board member for Jansen, Bayer, Astra Zeneca, and Genzyme. Dr Conte has served as a consultant to Cook Medical and Medtronic, and serves on a data and safety monitoring board for Talecris Biotherapeutics. Dr White reports no conflicts. Dr Bhatt discloses the following relationships: serving on an advisory board for Medscape Cardiology; serving on the board of directors for Boston VA Research Institute, Society of Chest Pain Centers; serving as chair of the American Heart Association Get With The Guidelines Science Subcommittee; receipt of honoraria from American College of Cardiology (Editor, Clinical Trials, Cardiosource), Duke Clinical Research Institute (clinical trial steering committees), Slack Publications (Chief Medical Editor, Cardiology Today Intervention), WebMD (CME steering committees); receipt of research grants from Amarin, AstraZeneca, Bristol-Myers Squibb, Eisai, Ethicon, Medtronic, Sanofi Aventis, The Medicines Company; Unfunded Research: Flow Co, PLx Pharma, Takeda. Dr Hiatt has received grant funding support to CPC Clinical Research from Aastrom, AstraZeneca, DNAVEC, Osiris, Pluristem, Theravasc. Dr Laird is a consultant and advisory board member for Abbott Vascular, Bard Peripheral Vascular, Boston Scientific, Covidien, and Medtronic, and receives research grant support from WL Gore.
Footnotes
Conflict of Interest Disclosures: Financial disclosure information for Dr. Patel is also publicly available at https://www.dcri.org/about-us/conflict-of-interest. Dr Chiswell reports no conflicts. Ms Tidemann-Miller reports no conflicts. Dr Jones reports no conflicts. Dr Califf's financial disclosure information is available at www.dcri.org/about-us/conflict-of-interest.
References
- 1.Mahoney EM, Wang K, Keo HH, Duval S, Smolderen KG, Cohen DJ, Steg G, Bhatt DL, Hirsch AT Reduction of Atherothrombosis for Continued Health (REACH) Registry Investigators. Vascular hospitalization rates and costs in patients with peripheral artery disease in the United States. Circ Cardiovasc Qual Outcomes. 2010;3:642–651. doi: 10.1161/CIRCOUTCOMES.109.930735. [DOI] [PubMed] [Google Scholar]
- 2.Dormandy J, Rutherford RB. Management of peripheral arterial disease (PAD). TASC working group. TransAtlantic Inter-Society Consensus (TASC) J Vasc Surg. 2000;31:S1–S296. [PubMed] [Google Scholar]
- 3.Naess IA, Christiansen SC, Romundstad P, Cannegieter SC, Rosendaal FR, Hammerstrom J. Incidence and mortality of venous thrombosis: a population-based study. J Thromb Haemost. 2007;5:692–699. doi: 10.1111/j.1538-7836.2007.02450.x. [DOI] [PubMed] [Google Scholar]
- 4.Initial National Priorities for Comparative Effectiveness Research. Washington, DC: The National Academies Press; 2009. Institute of Medicine. [Google Scholar]
- 5.Tepe G, Zeller T, Albrecht T, Heller S, Schwarzwälder U, Beregi JP, Claussen CD, Oldenburg A, Scheller B, Speck U. Local delivery of paclitaxel to inhibit restenosis during angioplasty of the leg. N Engl J Med. 2008;358:689–699. doi: 10.1056/NEJMoa0706356. [DOI] [PubMed] [Google Scholar]
- 6.Rogers JH, Laird JR. Overview of new technologies for lower extremity revascularization. Circulation. 2007;116:2072–2085. doi: 10.1161/CIRCULATIONAHA.107.715433. [DOI] [PubMed] [Google Scholar]
- 7.Katzen BT, Dake MD, MacLean AA, Wang DS. Endovascular repair of abdominal and thoracic aortic aneurysms. Circulation. 2005;112:1663–1675. doi: 10.1161/CIRCULATIONAHA.105.541284. [DOI] [PubMed] [Google Scholar]
- 8.Tateishi-Yuyama E, Matsubara H, Murohara T, Ikeda U, Shintani S, Masaki H, Amano K, Kishimoto Y, Yoshimoto K, Akashi H, Shimada K, Iwasaka T, Imaizumi T Therapeutic Angiogenesis using Cell Transplantation (TACT) Study Investigators. Therapeutic angiogenesis for patients with limb ischaemia by autologous transplantation of bone-marrow cells: a pilot study and a randomised controlled trial. Lancet. 2002;360:427–435. doi: 10.1016/S0140-6736(02)09670-8. [DOI] [PubMed] [Google Scholar]
- 9.Matoba S, Tatsumi T, Murohara T, Imaizumi T, Katsuda Y, Ito M, Saito Y, Uemura S, Suzuki H, Fukumoto S, Yamamoto Y, Onodera R, Teramukai S, Fukushima M, Matsubara H TACT Follow-up Study Investigators. Long-term clinical outcome after intramuscular implantation of bone marrow mononuclear cells (Therapeutic Angiogenesis by Cell Transplantation [TACT] trial) in patients with chronic limb ischemia. Am Heart J. 2008;156:1010–1018. doi: 10.1016/j.ahj.2008.06.025. [DOI] [PubMed] [Google Scholar]
- 10.Buller HR, Cohen AT, Davidson B, Decousus H, Gallus AS, Gent M, Pillion G, Piovella F, Prins MH, Raskob GE van Gogh Investigators. Idraparinux versus standard therapy for venous thromboembolic disease. N Engl J Med. 2007;357:1094–1104. doi: 10.1056/NEJMoa064247. [DOI] [PubMed] [Google Scholar]
- 11.Bauersachs R, Berkowitz SD, Brenner B, Buller HR, Decousus H, Gallus AS, Lensing AW, Misselwitz F, Prins MH, Raskob GE, Segers A, Verhamme P, Wells P, Agnelli G, Bounameaux H, Cohen A, Davidson BL, Piovella F, Schellong S EINSTEIN Investigators. Oral rivaroxaban for symptomatic venous thromboembolism. N Engl J Med. 2010;363:2499–2510. doi: 10.1056/NEJMoa1007903. [DOI] [PubMed] [Google Scholar]
- 12.Eberhardt RT, Raffetto JD. Chronic venous insufficiency. Circulation. 2005;111:2398–2409. doi: 10.1161/01.CIR.0000164199.72440.08. [DOI] [PubMed] [Google Scholar]
- 13.McCray AT, Ide NC. Design and implementation of a national clinical trials registry. J Am Med Inform Assoc. 2000;7:313–323. doi: 10.1136/jamia.2000.0070313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.De Angelis C, Drazen JM, Frizelle FA, Haug C, Hoey J, Horton R, Kotzin S, Laine C, Marusic A, Overbeke AJ, Schroeder TV, Sox HC, Van Der Weyden MB International Committee of Medical Journal Editors. Clinical trial registration: a statement from the International Committee of Medical Journal Editors. N Engl J Med. 2004;351:1250–1251. doi: 10.1056/NEJMe048225. [DOI] [PubMed] [Google Scholar]
- 15.Tasneem A, Aberle L, Ananth H, Chakraborty S, Chiswell K, McCourt BJ, Pietrobon R. The database for aggregate analysis of ClinicalTrials.gov (AACT) and subsequent regrouping by clinical specialty. PLoS ONE. 2012;7:e33677. doi: 10.1371/journal.pone.0033677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Califf RM, Zarin DA, Kramer JM, Sherman RE, Aberle LH, Tasneem A. Characteristics of clinical trials registered in ClinicalTrials.gov, 2007–2010. JAMA. 2012;307:1838–1847. doi: 10.1001/jama.2012.3424. [DOI] [PubMed] [Google Scholar]
- 17.Kong DF. Contemporary clinical research in adult cardiovascular medicine: a perspective from ClinicalTrials.gov. Poster presented at Society for Clinical Trials 33rd Annual Meeting; May 20–23, 2012; Miami, FL. [Accessed February 1, 2014]. Retrieved from http://wwwctti-clinicaltrialsorg/files/documents/StateOfClinicalTrials-Poster_AdultCardiopdf. [Google Scholar]
- 18.Alexander KP, Kong DF, Starr AZ, Kramer J, Chiswell K, Tasneem A, Califf RM. Portfolio of clinical research in adult cardiovascular disease as reflected in ClinicalTrials.gov. J Am Heart Assoc. 2013;2:e000009. doi: 10.1161/JAHA.113.000009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Jones WS, Patel MR, Dai D, Subherwal S, Stafford J, Calhoun S, Peterson ED. Temporal trends and geographic variation of lower-extremity amputation in patients with peripheral artery disease: results from US Medicare 2000–2008. J Am Coll Cardiol. 2012;60:2230–2236. doi: 10.1016/j.jacc.2012.08.983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hirsch AT, Haskal ZJ, Hertzer NR, Bakal CW, Creager MA, Halperin JL, Hiratzka LF, Murphy WR, Olin JW, Puschett JB, Rosenfield KA, Sacks D, Stanley JC, Taylor LM, Jr, White CJ, White J, White RA, Antman EM, Smith SC, Jr, Adams CD, Anderson JL, Faxon DP, Fuster V, Gibbons RJ, Hunt SA, Jacobs AK, Nishimura R, Ornato JP, Page RL, Riegel B. ACC/AHA 2005 Practice Guidelines for the management of patients with peripheral arterial disease (lower extremity, renal, mesenteric, and abdominal aortic): a collaborative report from the American Association for Vascular Surgery/Society for Vascular Surgery, Society for Cardiovascular Angiography and Interventions, Society for Vascular Medicine and Biology, Society of Interventional Radiology, and the ACC/AHA Task Force on Practice Guidelines (Writing Committee to Develop Guidelines for the Management of Patients With Peripheral Arterial Disease): endorsed by the American Association of Cardiovascular and Pulmonary Rehabilitation; National Heart, Lung, and Blood Institute; Society for Vascular Nursing; TransAtlantic Inter-Society Consensus; and Vascular Disease Foundation. Circulation. 2006;113:e463–e654. doi: 10.1161/CIRCULATIONAHA.106.174526. [DOI] [PubMed] [Google Scholar]
- 21.Rooke TW, Hirsch AT, Misra S, Sidawy AN, Beckman JA, Findeiss LK, Golzarian J, Gornik HL, Halperin JL, Jaff MR, Moneta GL, Olin JW, Stanley JC, White CJ, White JV, Zierler RE. Society for Cardiovascular Angiography and Interventions; Society of Interventional Radiology; Society for Vascular Medicine; Society for Vascular Surgery. 2011 ACCF/AHA Focused Update of the Guideline for the Management of Patients With Peripheral Artery Disease (Updating the 2005 Guideline): a report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines. Circulation. 2011;124:2020–2045. [Google Scholar]
- 22.Watson L, Ellis B, Leng GC. Exercise for intermittent claudication. Cochrane Database Syst Rev. 2008;(4) doi: 10.1002/14651858.CD000990.pub2. CD000990. [DOI] [PubMed] [Google Scholar]
- 23.Goodney PP, Beck AW, Nagle J, Welch HG, Zwolak RM. National trends in lower extremity bypass surgery, endovascular interventions, and major amputations. J Vasc Surg. 2009;50:54–60. doi: 10.1016/j.jvs.2009.01.035. [DOI] [PubMed] [Google Scholar]
- 24.Cohen AT, Davidson BL, Gallus AS, Lassen MR, Prins MH, Tomkowski W, Turpie AG, Egberts JF, Lensing AW ARTEMIS Investigators. Efficacy and safety of fondaparinux for the prevention of venous thromboembolism in older acute medical patients: randomised placebo controlled trial. BMJ. 2006;332:325–329. doi: 10.1136/bmj.38733.466748.7C. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.US Food and Drug Administration. Medical devices: removing retrievable inferior vena cava filters: initial communication. [Accessed September 6, 2013]; http://www.fda.gov/MedicalDevices/Safety/AlertsandNotices/ucm221676.htm. Issued August 09, 2010. Updated August 22, 2013.
- 26.Subherwal S, Anstrom KJ, Jones WS, Felker MG, Misra S, Conte MS, Hiatt WR, Patel MR. Use of alternative methodologies for evaluation of composite end points in trials of therapies for critical limb ischemia. Am Heart J. 2012;164:277–284. doi: 10.1016/j.ahj.2012.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.