Abstract
Vaccination is the best method for the prevention and control of influenza. Vaccination can reduce illness and lessen severity of infection. This review focuses on how currently licensed influenza vaccines are generated in the U.S., why the biology of influenza poses vaccine challenges, and vaccine approaches on the horizon that address these challenges.
Influenza viruses are members of the Orthomyxoviridae family and are comprised of segmented negative-sense single-stranded RNA genomes. Infection with an influenza virus can result in a sudden onset of fever, cough, rhinitis, malaise, headache, and sore throat following an incubation period of 1 to 3 days. There are three genera of influenza viruses (A, B, and C) that are divided based on antigenic differences in the viral nucleoprotein (NP) and matrix protein (M). Both influenza A and B viruses result in annual epidemics, with an attack rate of 5–10% and 20–30% in adults and in children each year, respectively (WHO, 2014). This results in 3 to 5 million infections annually and 250,000 to 500,000 excess deaths worldwide (WHO, 2014). Influenza A viruses from zoonotic sources can also result in occasional pandemics; four have occurred within the past 100 years (Taubenberger and Kash, 2010).
The influenza A and B virus genomes each contain 8 gene segments. Influenza A viruses are further divided based on the antigenic properties of their surface glycoproteins into 16 hemagglutinin (HA) and 9 neuraminidase (NA) subtypes. While only a subset of these have been known to result in human infections, all have been isolated from their natural hosts- waterfowl and shorebirds (Yoon et al., 2014). The genomes of two additional subtypes of HA and NA have recently been sequenced from bats, but these viruses have not yet been isolated (Tong et al., 2012; Tong et al., 2013). Two influenza A subtypes (H1N1 and H3N2) and two antigenically distinct lineages of influenza B viruses currently co-circulate in humans (Grohskopf et al., 2014).
The best method for the prevention and control of influenza is vaccination (WHO, 2014). Vaccination can reduce illness and lessen severity of infection, particularly in groups at risk for complications of influenza, including young children and the elderly. This review is focused on how currently licensed influenza vaccines are generated in the U.S., why the biology of influenza poses vaccine challenges, and vaccine approaches on the horizon that address these challenges.
Currently Licensed Seasonal Influenza Vaccines
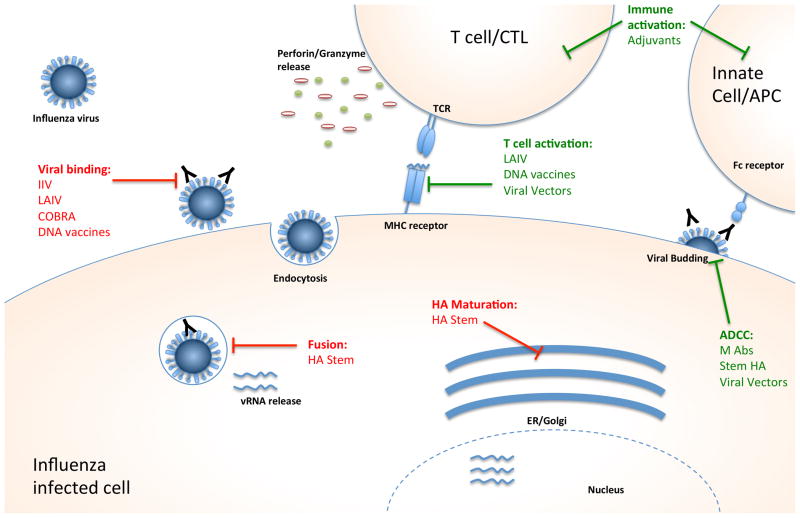
Currently licensed influenza vaccines focus on the production of antibodies against the viral HA protein, which binds host receptors to mediate viral entry. Strain-specific antibodies produced against the HA neutralize the virus and prevent infection (Figure 1). The current seasonal vaccines require annual evaluation and reformulation to keep pace with the antigenic drift of circulating strains. This process is completed twice a year, once each for the northern and southern hemispheres (WHO, 2014). Antigenic drift results from mutations that occur because the error-prone viral RNA-dependent RNA polymerase lacks proofreading function, resulting in mutations in the HA and other viral proteins. Additionally, the HA is under positive selection for antigenic escape from neutralization by pre-existing antibodies. Selection of the vaccine composition for the upcoming season’s vaccine must take place 7 to 8 months in advance of “flu season” to accommodate the steps of vaccine production (WHO, 2014).
Figure 1. Stages of the viral life cycle and vaccine targets.
Many stages of viral infection can be targeted by vaccines; the targets of the vaccines described in this review are indicated. Vaccines that elicit an immune response that blocks a stage in the viral life cycle are shown in red, while vaccines that activate a specific host immune response are shown in green.
Abbreviations: IIV: inactivated influenza vaccine; LAIV: live attenuated influenza vaccine; COBRA: computationally optimized broadly reactive antigen; HA: hemagglutinin; ADCC: antibody dependent cell cytotoxicity
There are three classes of licensed seasonal vaccines including inactivated, live attenuated, and recombinant HA vaccines (Grohskopf et al., 2014). All three vaccines are multivalent, with components representing influenza A and B viruses anticipated to circulate in the next influenza season. The inactivated influenza vaccine (IIV) is a split virion or subunit vaccine that contains 15μg of each purified HA protein administered intramuscularly, or 9μg of each purified HA protein administered intradermally (Grohskopf et al., 2014). There is also a higher dose of antigen available for the elderly population aged 65 years and older, in which 60 μg of each HA is administered in order to increase the immunogenicity of the vaccine. The trivalent inactivated vaccine (TIV) contains H1N1 and H3N2 subtypes of influenza A along with the predicted dominant lineage of influenza B. A recently licensed quadrivalent influenza vaccine (QIV) includes two lineages of influenza B along with the H1N1 and H3N2 subtypes of influenza A. The IIV induce a strain-specific serum IgG antibody response and are licensed for individuals aged 6 months and older.
The second licensed vaccine product is the live attenuated influenza vaccine (LAIV). This vaccine also contains a mixture of the same four influenza strains as the QIV, but is administered intranasally as a spray. The LAIV contains live viruses with temperature-sensitive and attenuating mutations (Coelingh et al., 2014). As a result of these mutations, the vaccine virus is restricted in replication at the temperature of the lower respiratory tract, but can replicate at the cooler temperature of the nasal cavity. Vaccination with LAIV results in the production of strain-specific serum IgG as well as mucosal IgA and T cell responses (Coelingh et al., 2014). LAIV is also effective against some antigenically drifted strains of influenza (Coelingh et al., 2014). The LAIV is licensed for healthy individuals between the ages of 2 and 49 years and the CDC recommends that children between the ages of two and eight years receive the LAIV over IIV if available (Grohskopf et al., 2014).
The third licensed product is FluBlok, which is a recombinant HA vaccine with HA proteins that are expressed in insect cells from baculovirus vectors. FluBlok is currently licensed for adults aged 18 to 49 years and can be used in individuals who are allergic to eggs (Grohskopf et al., 2014). The manufacturing process for this vaccine has a shorter timeframe, which would be valuable during a pandemic response.
The safety of seasonal influenza vaccines is well accepted. The most common adverse events reported for IIV involve reactions at the site of injection, including pain, redness, and swelling (Grohskopf et al., 2014). For the LAIV the most common events involve a runny nose and nasal congestion, although fever and sore throat have also been reported in specific age groups (Grohskopf et al., 2014). Current recommendations in the U.S. are for annual vaccination in individuals 6 months and older, with an emphasis on children, persons over 65 years of age, pregnant women, individuals with chronic health conditions, and healthcare workers (Grohskopf et al., 2014; WHO, 2014).
Challenges in Optimizing Influenza Vaccines
Although the currently licensed influenza vaccines are effective in healthy young adults, Table 1 summarizes several challenges that remain. They include the dependence on embryonated eggs for vaccine production, the lengthy timeline for vaccine production, the need for annual vaccination, the emergence of antigenically novel viruses, the need for improved immunogenicity in the elderly, and the need for an improved correlate of protection. Several approaches have been developed to overcome these challenges and improve the immunogenicity and efficacy of influenza vaccines.
Table 1.
Summary of current vaccine approaches against influenza viruses
| Vaccine Format | Viral Targets | Mode of Action | Advantages | Solution to Vaccine Challenge |
|---|---|---|---|---|
| Currently Licensed | ||||
| IIV |
|
|
|
|
| LAIV |
|
|
|
|
|
| ||||
| Emerging Approaches | ||||
| Recombinant DNA |
|
|
|
|
| COBRA |
|
|
|
|
| Stem HA Antibodies |
|
|
|
|
| Viral Vectors |
|
|
|
|
| M2 Antibodies |
|
|
|
|
| Adjuvants |
|
|
|
|
Dependence on embryonated eggs
One disadvantage that is shared by IIV and LAIV is the need for embryonated eggs for production. A pandemic will likely result in a higher demand for vaccine and embryonated eggs may be in short supply if the pandemic virus is pathogenic for poultry (Hannoun, 2013). Several new influenza vaccines have been licensed within recent years that do not rely on production in eggs. Flucelvax is a newly licensed vaccine that is produced in a mammalian cell line and subsequent manufacturing steps are similar to egg-based IIV (Grohskopf et al., 2014). As mentioned previously, the recently licensed recombinant HA vaccine FluBlok is expressed in insect cells. Also, DNA vaccines and virus-like particles (VLPs) are vaccine strategies that are in clinical development and are not manufactured in eggs.
Lengthy timeline for vaccine production
The selection of strains to include in annual influenza vaccines is based on global surveillance of circulating influenza viruses. Predictions are made months ahead of the arrival of “flu season” in order to accommodate all the steps of vaccine production; including the generation of three or four vaccine seed viruses, amplification, inactivation, purification and dispensing into vials for IIV and blending and filling of sprayers for LAIV.
Antigenic characterization of circulating viruses is the most critical criterion for the selection of vaccine strains. The antigenic relationship between circulating viruses is determined by hemagglutination inhibition (HAI) assays, in which their reactivity is tested against a panel of ferret antisera generated against reference strains including the previous year’s vaccine virus. Antigenic change among influenza viruses can be visualized by antigenic cartography (Smith et al., 2004), which is a computational tool for the analysis of HAI assay data that provides a mathematical foundation for quantitative analysis of antigenic data (University of Cambridge, 2014). Antigenic cartography is now applied to the selection of strains for influenza vaccines.
The 2009 pandemic revealed the difficulty in producing and distributing a vaccine against a newly emerged virus within a short timeframe (Lee et al., 2014). The 2009 H1N1pdm IIV was not available in time to prevent the second wave of the pandemic (CDC, 2009). One approach to avoid this predicament in the future would be to stockpile vaccine seed viruses against different subtypes that have pandemic potential. This process involves the selection of representative viruses from each subtype prioritized based on epidemiological data, and testing of the candidate vaccines in preclinical studies and clinical trials (Coelingh et al., 2014; Subbarao and Joseph, 2007).
Need for annual vaccination
The decline in vaccine-specific antibodies and the antigenic drift of influenza viruses over time necessitates annual revaccination. Several strategies are being explored to increase the breadth of protection, or cross-reactivity, of influenza vaccines to avoid the need for annual revaccination. These include the use of a computationally designed HA sequence, induction of antibodies directed at the conserved HA stem, immunization with conserved influenza proteins that target T cell responses, incorporation of an adjuvant, and strategies that combine different vaccine platforms in “prime-boost” formats.
One approach aimed to increase the breadth of the antibody response against the HA protein involves a computationally optimized broadly reactive antigen, or COBRA HA presented in a VLP vaccine (Giles and Ross, 2011). The sequence of the COBRA HA represents a consensus sequence from a vast collection of influenza viruses that incorporates the most common amino acid at each position. This retention of conserved regions within the HA results in the generation of cross-reactive antibodies (Giles et al., 2012a). An H5N1 COBRA vaccine has been shown to induce broadly reactive antibodies against multiple clades of H5N1 viruses and results in less pathology following challenge than a non-consensus VLP vaccine in nonhuman primates (Giles et al., 2012b).
Another approach to enhance the breadth of the antibody response is to elicit antibodies directed at the conserved stalk or stem of the HA. A majority of antibodies elicited during infection target the immunodominant HA head domain that contains the receptor binding site and several well-defined antigenic sites that accumulate mutations as the virus drifts under immune pressure. The HA stem domain is much more conserved and antibodies targeting this region are more broadly reactive. Based on phylogenetic analysis HA proteins fall into two groups; group 1 includes subtypes H1, H2, H5, H6, H8, H9, H11, H12, H13, H16, H17, and H18 while group 2 includes H3, H4, H7, H10, H14, and H15 HAs (reviewed in (Krammer and Palese, 2013). HA stem antibodies recognize subtypes within the same group, and a few demonstrate binding across both groups (Krammer and Palese, 2013). These stem antibodies do not block receptor binding and therefore are not detected by HAI assays (Corti and Lanzavecchia, 2013). Stem antibodies are believed to function at several steps of the viral lifecycle. They can inhibit fusion of the viral membrane through steric hindrance, inhibit maturation of the virus if the antibody binds to the uncleaved HA protein, and clear virally infected cells through antibody-dependent cell-mediated cytotoxicity (ADCC) (Krammer and Palese, 2013). Stem antibodies are not easily detected after vaccination with IIV but are found after natural infection in low amounts (Krammer and Palese, 2013). They are more readily produced following infection with an antigenically distinct virus, and were found in higher amounts following infection with the 2009 pandemic virus (Krammer and Palese, 2013; Li et al., 2012; Sangster et al., 2013). Several vaccine approaches have been designed to elicit stem antibodies. Such techniques include “headless HAs” that lack the immunodominant head portion of the protein, sequential immunization with chimeric HAs bearing different head domains on a constant stalk domain to boost stem antibody responses, and vaccines expressing specific sections of the HA- including the conserved alpha-helix of the stem or the fusion peptide (Krammer and Palese, 2013).
An alternative method of vaccination has focused on expressing additional influenza antigens using viral vectors. Viral vectors are replication-defective viruses that can express high, sustained levels of antigens (Tripp and Tompkins, 2014). Viral vectors can target specific cell types, allow delivery through multiple routes, and the vectors themselves can act as adjuvants to improve the immune response. An example of a viral vectored influenza vaccine is a modified vaccinia virus Ankara (MVA) expressing a fusion protein of influenza NP and matrix 1 protein (M1) that induce T cell responses but not neutralizing antibodies. In phase I clinical trials, the MVA-NP+M1 vaccine was immunogenic in healthy older adults aged 50–85 years, both alone and combined with seasonal TIV (Antrobus et al., 2014; Antrobus et al., 2012). Also, a phase 1/2a trial evaluating a MVA-hemagglutinin-based H5N1 vaccine was safe and immunogenic in young adults, with a booster immunization a year later resulting in a substantial boost in antibody titers in all recipients (Kreijtz et al., 2014). Adenoviral vectors have also been utilized to express the influenza matrix 2 protein (M2) and NP proteins that induced strong IgA and T cell responses after a mucosal administration and provided heterosubtypic immunity in mice and ferrets (Price et al., 2010).
Another technique to broaden the cross-reactivity of influenza vaccines is to target T cell responses. The T cell response to influenza is targeted primarily against the internal proteins of the virus, including the NP and M1 proteins (Lee et al., 2014). These viral proteins are highly conserved; therefore they induce cytotoxic T lymphocyte (CTL) responses that are more cross-reactive than antibody responses directed at the HA. T cell immunity does not prevent infection, but can reduce the severity and duration of infection (Lee et al., 2014). The role of T cell immunity in ameliorating the severity of disease was demonstrated during the 2009 H1N1 pandemic, where the magnitude of the pre-existing CTL response inversely correlated with disease severity in individuals without detectable neutralizing antibody (Sridhar et al., 2013). Of the already licensed vaccines, LAIV results in higher CTL responses than IIV (He et al., 2006; Lee et al., 2014).
Including adjuvants to boost and broaden the immune response is an additional approach to broaden the immune response of vaccines (Lee et al., 2014). Adjuvants can also result in dose sparing of antigen. Several adjuvants are approved for use in human vaccines in other countries, but adjuvanted influenza vaccines are not yet approved in the U.S. (Even-Or et al., 2013). Oil-in-water emulsions that include the sterol squalene, such as AS03 and MF59 (Lee et al., 2014) activate cells near the injection site to produce cytokines and chemokines and recruit immune cells to the area. Inclusion of MF59 in H1N1 and H5N1 IIVs resulted in increased magnitude and altered quality of the antibody response (Khurana et al., 2010; Khurana et al., 2011).
A number of recent studies have also demonstrated the benefit of prime/boost schedules on the efficacy and strength of the immune response. Some strategies include sequential use of different vaccine platforms. In mice and ferrets, priming with a NP and M2 DNA vaccine and boosting with an antigen-matched mucosal adenoviral vector led to robust IgA and T cell responses in the lungs, and protected the animals from challenge with several subtypes of influenza A viruses (Price et al., 2009). In phase I clinical trials priming with an H5 HA DNA vaccine, adenovirus-vectored vaccine or pLAIV and boosting with a matched inactivated vaccine resulted in enhanced immunogenicity with cross-reactive antibodies (Babu TM, 2014; Gurwith et al., 2013; Ledgerwood et al., 2011; Lee et al., 2014; Talaat et al., 2014).
Emergence of novel viruses
Although currently available vaccines are effective against seasonal influenza viruses, strain-specific immunity fails to protect against drifted seasonal influenza viruses or from antigenically novel pandemic viruses. Within the last century there have been four influenza pandemics associated with high infection and mortality rates- in 1918, 1957, 1968, and most recently in 2009 (Taubenberger and Kash, 2010) caused by viruses that were antigenically distinct from the circulating seasonal strains of the period. Antigenic shift can result in a pandemic when novel influenza A viruses infect the human population and have the capacity for human-to-human transmission. Pigs and domestic poultry have served as zoonotic sources for influenza viruses of novel antigenicity entering into the human population (Taubenberger and Kash, 2010). Several other subtypes of influenza A virus (including H5N1, H7N9, H9N2 among others) have also resulted in sporadic human infections, but have lacked the ability for sustained human-to-human transmission, and therefore have not led to a pandemic.
Both the IIV and LAIV platforms have been utilized in the development of pandemic (p) influenza vaccines for use in the event of emergence of novel subtypes from zoonotic sources. The pIIV vaccines have typically displayed low immunogenicity and required high antigen doses, multiple vaccinations, or the inclusion of adjuvants to achieve serum antibody responses that are predicted to be protective (Baz et al., 2013; Nicholson et al., 2001; Treanor et al., 2001). On initial evaluation, pLAIV were found to be variably immunogenic in phase I clinical trials (Coelingh et al., 2014). However, recent data demonstrate that H5N1 and H7N7 pLAIV established a robust long-term B cell memory (Babu TM, 2014; Talaat et al., 2010; Talaat et al., 2014). Nonetheless, pLAIV cannot be used until a pandemic is imminent in order to avoid reassortment of the vaccine virus with circulating influenza viruses (Jin and Subbarao, 2015).
Our inability to predict the subtype that will cause the next influenza pandemic and the delay in delivery of the 2009 pandemic vaccine has increased interest in a “universal vaccine” that will produce more broadly cross-reactive immunity and will not require annual updates (Subbarao and Matsuoka, 2013). The two leading candidates for universal vaccines include the highly conserved stem of the HA and the M2 protein. The HA stem approach was discussed earlier (Krammer and Palese, 2013). The M2 protein is displayed on the surface of the virion, and acts as an ion channel that is critical for uncoating of the virus upon entry. During natural infection, antibodies are elicited against all of the surface viral proteins, including HA, NA, and M2 (Li et al., 2013). Antibodies directed against M2 do not neutralize virus infectivity, but can reduce the severity of infection by clearing infected cells through antibody-dependent cell-mediated cytotoxicity (ADCC). Although M2 antibodies induced by natural infection are rare and short-lived, they have been shown to provide broad protection against a range of influenza A viruses in animal models, and were immunogenic in phase I clinical trials (Lee et al., 2014). Vaccines focusing on M2 protein typically incorporate the protein into a VLP or express the protein in a recombinant vaccine by fusing the gene encoding M2 or tandem repeats of the ectodomain of M2 (M2e) to a carrier protein or molecule (Lee et al., 2014).
Immunosenescence in the elderly
The elderly are most vulnerable to severe complications from influenza, but the effectiveness of standard vaccines in this age group is very poor. The progressive decline in systemic immunity with increasing age is referred to as immunosenescence (Haq and McElhaney, 2014). Two strategies to enhance immunogenicity of IIV in the elderly are to increase the antigen dose or include an adjuvant. A high-dose IIV, with four times the normal amount of HA antigen has been introduced for use in the elderly (Grohskopf et al., 2014). Inclusion of oil-in-water adjuvants MF59 or AS03 also enhances the immunogenicity of IIV in the elderly (Haq and McElhaney, 2014).
Systems biology studies define the components of the immune response that result in effective vaccine protection and can lead to improvements in vaccines (Lambert et al., 2012). Such studies have uncovered early immune signatures of vaccines that are predictive of immunogenicity. Studies of IIV found the calmodulin-dependent protein kinase IV (CaMKIV) gene, thought to be involved in calcium-mediated signaling, was inversely correlated with the final serum antibody titers in vaccinees (Nakaya et al., 2011). By comparing the responses between healthy young adults and older adults, these studies can identify which components of the immune response are affected by immunosenescence (Lambert et al., 2012).
An accurate correlate of protection
The HAI antibody titer induced by vaccination is currently accepted as the correlate of protection against influenza. An HAI titer of ≥ 1:40 in healthy adults is the titer at which approximately 50% of individuals are protected from infection. However, some studies have indicated that a higher HAI titer may be required in children, and that T cells may be a better indicator for protection in the elderly (Black et al., 2011; Haq and McElhaney, 2014). In addition, serum HAI antibody titer is not a reliable correlate of protection for seasonal and pandemic LAIV vaccines. LAIV has been shown to be effective in the absence of a robust serum antibody response (Coelingh et al., 2014). The type and magnitude of the immune response that will provide optimal protection against pandemic influenza viruses is unknown. Furthermore, although safety and immunogenicity of pandemic vaccines are assessed in clinical trials, efficacy is inferred from studies in animal models or by extrapolation from experience with human influenza virus vaccines (Subbarao and Joseph, 2007). The HAI antibody titer also fails to take into account other aspects of immune memory against the virus, including the contribution of non-neutralizing antibodies and T cell responses to protection. A more comprehensive correlate of protection is needed to better interpret influenza vaccine efficacy.
Conclusions and Future Prospects
Licensed seasonal TIV and LAIV displayed a mean efficacy of 60% in healthy adults and 83% in children, respectively in recent meta-analyses (Ohmit et al., 2013; Osterholm et al., 2012). However, when the match between the vaccine strain and circulating epidemic strain is poor, or when a new pandemic virus emerges, these vaccines fail to provide optimal protection. The IIV does not induce robust immunity in the elderly and LAIV is only licensed for individuals up to the age of 49 years, leaving the most vulnerable section of the population poorly protected (Grohskopf et al., 2014). Influenza vaccines must protect all age groups, particularly those most vulnerable to complications of severe influenza. Ideally, new vaccines should increase the breadth of the immune response to include antigenically distinct viruses within the same subtype and viruses of other subtypes, should not be manufactured in eggs, and should require less time to manufacture than currently licensed technologies. The ultimate goal of a universal influenza vaccine is to protect against all influenza A viruses, obviating the need for annual revaccination. Several promising approaches are under development to improve or overcome the drawbacks of the currently licensed vaccines and to induce broad immunity against other subtypes of influenza with pandemic potential.
Acknowledgments
The authors are supported by the Intramural Research Program of the NIH and NIAID.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Antrobus RD, Berthoud TK, Mullarkey CE, Hoschler K, Coughlan L, Zambon M, Hill AV, Gilbert SC. Coadministration of seasonal influenza vaccine and MVA-NP+M1 simultaneously achieves potent humoral and cell-mediated responses. Molecular therapy : the journal of the American Society of Gene Therapy. 2014;22:233–238. doi: 10.1038/mt.2013.162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Antrobus RD, Lillie PJ, Berthoud TK, Spencer AJ, McLaren JE, Ladell K, Lambe T, Milicic A, Price DA, Hill AV, et al. A T cell-inducing influenza vaccine for the elderly: safety and immunogenicity of MVA-NP+M1 in adults aged over 50 years. PloS one. 2012;7:e48322. doi: 10.1371/journal.pone.0048322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Babu TM, LM, Fitzgerald T, Luke C, Sangster MY, Jin H, Topham D, Katz J, Treanor J, Subbarao K. Live attenuated H7N7 influenza vaccine primes for a vigorous antibody response to inactivated H7N7 influenza vaccine. Vaccine. 2014 doi: 10.1016/j.vaccine.2014.09.070. In Press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baz M, Luke CJ, Cheng X, Jin H, Subbarao K. H5N1 vaccines in humans. Virus Res. 2013;178:78–98. doi: 10.1016/j.virusres.2013.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Black S, Nicolay U, Vesikari T, Knuf M, Del Giudice G, Della Cioppa G, Tsai T, Clemens R, Rappuoli R. Hemagglutination inhibition antibody titers as a correlate of protection for inactivated influenza vaccines in children. Pediatr Infect Dis J. 2011;30:1081–1085. doi: 10.1097/INF.0b013e3182367662. [DOI] [PubMed] [Google Scholar]
- CDC. Use of influenza A (H1N1) 2009 monovalent vaccine: recommendations of the Advisory Committee on Immunization Practices (ACIP), 2009. MMWR Recomm Rep. 2009;58:1–8. [PubMed] [Google Scholar]
- Coelingh KL, Luke CJ, Jin H, Talaat KR. Development of live attenuated influenza vaccines against pandemic influenza strains. Expert review of vaccines. 2014;13:855–871. doi: 10.1586/14760584.2014.922417. [DOI] [PubMed] [Google Scholar]
- Corti D, Lanzavecchia A. Broadly neutralizing antiviral antibodies. Annu Rev Immunol. 2013;31:705–742. doi: 10.1146/annurev-immunol-032712-095916. [DOI] [PubMed] [Google Scholar]
- Even-Or O, Samira S, Ellis R, Kedar E, Barenholz Y. Adjuvanted influenza vaccines. Expert review of vaccines. 2013;12:1095–1108. doi: 10.1586/14760584.2013.825445. [DOI] [PubMed] [Google Scholar]
- Giles BM, Bissel SJ, Dealmeida DR, Wiley CA, Ross TM. Antibody breadth and protective efficacy are increased by vaccination with computationally optimized hemagglutinin but not with polyvalent hemagglutinin-based H5N1 virus-like particle vaccines. Clinical and vaccine immunology : CVI. 2012a;19:128–139. doi: 10.1128/CVI.05533-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giles BM, Crevar CJ, Carter DM, Bissel SJ, Schultz-Cherry S, Wiley CA, Ross TM. A computationally optimized hemagglutinin virus-like particle vaccine elicits broadly reactive antibodies that protect nonhuman primates from H5N1 infection. J Infect Dis. 2012b;205:1562–1570. doi: 10.1093/infdis/jis232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giles BM, Ross TM. A computationally optimized broadly reactive antigen (COBRA) based H5N1 VLP vaccine elicits broadly reactive antibodies in mice and ferrets. Vaccine. 2011;29:3043–3054. doi: 10.1016/j.vaccine.2011.01.100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grohskopf LA, Olsen SJ, Sokolow LZ, Bresee JS, Cox NJ, Broder KR, Karron RA, Walter EB Centers for Disease C and Prevention. Prevention and control of seasonal influenza with vaccines: recommendations of the Advisory Committee on Immunization Practices (ACIP) -- United States, 2014–15 influenza season. MMWR Morbidity and mortality weekly report. 2014;63:691–697. [PMC free article] [PubMed] [Google Scholar]
- Gurwith M, Lock M, Taylor EM, Ishioka G, Alexander J, Mayall T, Ervin JE, Greenberg RN, Strout C, Treanor JJ, et al. Safety and immunogenicity of an oral, replicating adenovirus serotype 4 vector vaccine for H5N1 influenza: a randomised, double-blind, placebo-controlled, phase 1 study. Lancet Infect Dis. 2013;13:238–250. doi: 10.1016/S1473-3099(12)70345-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hannoun C. The evolving history of influenza viruses and influenza vaccines. Expert review of vaccines. 2013;12:1085–1094. doi: 10.1586/14760584.2013.824709. [DOI] [PubMed] [Google Scholar]
- Haq K, McElhaney JE. Immunosenescence: Influenza vaccination and the elderly. Current opinion in immunology. 2014;29:38–42. doi: 10.1016/j.coi.2014.03.008. [DOI] [PubMed] [Google Scholar]
- He XS, Holmes TH, Zhang C, Mahmood K, Kemble GW, Lewis DB, Dekker CL, Greenberg HB, Arvin AM. Cellular immune responses in children and adults receiving inactivated or live attenuated influenza vaccines. Journal of virology. 2006;80:11756–11766. doi: 10.1128/JVI.01460-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jin H, Subbarao K. Live attenuated influenza vaccine. Current topics in microbiology and immunology. 2015;386:181–204. doi: 10.1007/82_2014_410. [DOI] [PubMed] [Google Scholar]
- Khurana S, Chearwae W, Castellino F, Manischewitz J, King LR, Honorkiewicz A, Rock MT, Edwards KM, Del Giudice G, Rappuoli R, et al. Vaccines with MF59 adjuvant expand the antibody repertoire to target protective sites of pandemic avian H5N1 influenza virus. Science translational medicine. 2010;2:15ra15. doi: 10.1126/scitranslmed.3000624. [DOI] [PubMed] [Google Scholar]
- Khurana S, Verma N, Yewdell JW, Hilbert AK, Castellino F, Lattanzi M, Del Giudice G, Rappuoli R, Golding H. MF59 adjuvant enhances diversity and affinity of antibody-mediated immune response to pandemic influenza vaccines. Science translational medicine. 2011;3:85ra48. doi: 10.1126/scitranslmed.3002336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krammer F, Palese P. Influenza virus hemagglutinin stalk-based antibodies and vaccines. Curr Opin Virol. 2013;3:521–530. doi: 10.1016/j.coviro.2013.07.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kreijtz J, Goeijenbier M, Moesker F, van den Dries L, Goeijenbier S, De Gruyter H, Lehmann M, de Mutsert G, van de Vijver D, Volz A, et al. Safety and immunogenicity of a modified-vaccinia-virus-Ankara-based influenza A H5N1 vaccine: a randomised, double-blind phase 1/2a clinical trial. Lancet Infect Dis. 2014 doi: 10.1016/S1473-3099(14)70963-6. In Press. [DOI] [PubMed] [Google Scholar]
- Lambert ND, Ovsyannikova IG, Pankratz VS, Jacobson RM, Poland GA. Understanding the immune response to seasonal influenza vaccination in older adults: a systems biology approach. Expert review of vaccines. 2012;11:985–994. doi: 10.1586/erv.12.61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ledgerwood JE, Wei CJ, Hu Z, Gordon IJ, Enama ME, Hendel CS, McTamney PM, Pearce MB, Yassine HM, Boyington JC, et al. DNA priming and influenza vaccine immunogenicity: two phase 1 open label randomised clinical trials. Lancet Infect Dis. 2011;11:916–924. doi: 10.1016/S1473-3099(11)70240-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee YT, Kim KH, Ko EJ, Lee YN, Kim MC, Kwon YM, Tang Y, Cho MK, Lee YJ, Kang SM. New vaccines against influenza virus. Clin Exp Vaccine Res. 2014;3:12–28. doi: 10.7774/cevr.2014.3.1.12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li CK, Rappuoli R, Xu XN. Correlates of protection against influenza infection in humans--on the path to a universal vaccine? Current opinion in immunology. 2013;25:470–476. doi: 10.1016/j.coi.2013.07.005. [DOI] [PubMed] [Google Scholar]
- Li GM, Chiu C, Wrammert J, McCausland M, Andrews SF, Zheng NY, Lee JH, Huang M, Qu X, Edupuganti S, et al. Pandemic H1N1 influenza vaccine induces a recall response in humans that favors broadly cross-reactive memory B cells. Proc Natl Acad Sci U S A. 2012;109:9047–9052. doi: 10.1073/pnas.1118979109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakaya HI, Wrammert J, Lee EK, Racioppi L, Marie-Kunze S, Haining WN, Means AR, Kasturi SP, Khan N, Li GM, et al. Systems biology of vaccination for seasonal influenza in humans. Nature immunology. 2011;12:786–795. doi: 10.1038/ni.2067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nicholson KG, Colegate AE, Podda A, Stephenson I, Wood J, Ypma E, Zambon MC. Safety and antigenicity of non-adjuvanted and MF59-adjuvanted influenza A/Duck/Singapore/97 (H5N3) vaccine: a randomised trial of two potential vaccines against H5N1 influenza. Lancet. 2001;357:1937–1943. doi: 10.1016/S0140-6736(00)05066-2. [DOI] [PubMed] [Google Scholar]
- Ohmit SE, Petrie JG, Malosh RE, Cowling BJ, Thompson MG, Shay DK, Monto AS. Influenza vaccine effectiveness in the community and the household. Clin Infect Dis. 2013;56:1363–1369. doi: 10.1093/cid/cit060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Osterholm MT, Kelley NS, Sommer A, Belongia EA. Efficacy and effectiveness of influenza vaccines: a systematic review and meta-analysis. Lancet Infect Dis. 2012;12:36–44. doi: 10.1016/S1473-3099(11)70295-X. [DOI] [PubMed] [Google Scholar]
- Price GE, Soboleski MR, Lo CY, Misplon JA, Pappas C, Houser KV, Tumpey TM, Epstein SL. Vaccination focusing immunity on conserved antigens protects mice and ferrets against virulent H1N1 and H5N1 influenza A viruses. Vaccine. 2009;27:6512–6521. doi: 10.1016/j.vaccine.2009.08.053. [DOI] [PubMed] [Google Scholar]
- Price GE, Soboleski MR, Lo CY, Misplon JA, Quirion MR, Houser KV, Pearce MB, Pappas C, Tumpey TM, Epstein SL. Single-dose mucosal immunization with a candidate universal influenza vaccine provides rapid protection from virulent H5N1, H3N2 and H1N1 viruses. PloS one. 2010;5:e13162. doi: 10.1371/journal.pone.0013162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sangster MY, Baer J, Santiago FW, Fitzgerald T, Ilyushina NA, Sundararajan A, Henn AD, Krammer F, Yang H, Luke CJ, et al. B cell response and hemagglutinin stalk-reactive antibody production in different age cohorts following 2009 H1N1 influenza virus vaccination. Clinical and vaccine immunology : CVI. 2013;20:867–876. doi: 10.1128/CVI.00735-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith DJ, Lapedes AS, de Jong JC, Bestebroer TM, Rimmelzwaan GF, Osterhaus AD, Fouchier RA. Mapping the antigenic and genetic evolution of influenza virus. Science. 2004;305:371–376. doi: 10.1126/science.1097211. [DOI] [PubMed] [Google Scholar]
- Sridhar S, Begom S, Bermingham A, Hoschler K, Adamson W, Carman W, Bean T, Barclay W, Deeks JJ, Lalvani A. Cellular immune correlates of protection against symptomatic pandemic influenza. Nature medicine. 2013;19:1305–1312. doi: 10.1038/nm.3350. [DOI] [PubMed] [Google Scholar]
- Subbarao K, Joseph T. Scientific barriers to developing vaccines against avian influenza viruses. Nature reviews Immunology. 2007;7:267–278. doi: 10.1038/nri2054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Subbarao K, Matsuoka Y. The prospects and challenges of universal vaccines for influenza. Trends in microbiology. 2013;21:350–358. doi: 10.1016/j.tim.2013.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Talaat KR, Greenberg ME, Lai MH, Hartel GF, Wichems CH, Rockman S, Jeanfreau RJ, Ghosh MR, Kabongo ML, Gittleson C, et al. A single dose of unadjuvanted novel 2009 H1N1 vaccine is immunogenic and well tolerated in young and elderly adults. J Infect Dis. 2010;202:1327–1337. doi: 10.1086/656601. [DOI] [PubMed] [Google Scholar]
- Talaat KR, Luke CJ, Khurana S, Manischewitz J, King LR, McMahon BA, Karron RA, Lewis KD, Qin J, Follmann DA, et al. A live attenuated influenza A(H5N1) vaccine induces long-term immunity in the absence of a primary antibody response. J Infect Dis. 2014;209:1860–1869. doi: 10.1093/infdis/jiu123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taubenberger JK, Kash JC. Influenza virus evolution, host adaptation, and pandemic formation. Cell host & microbe. 2010;7:440–451. doi: 10.1016/j.chom.2010.05.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tong S, Li Y, Rivailler P, Conrardy C, Castillo DA, Chen LM, Recuenco S, Ellison JA, Davis CT, York IA, et al. A distinct lineage of influenza A virus from bats. Proc Natl Acad Sci U S A. 2012;109:4269–4274. doi: 10.1073/pnas.1116200109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tong S, Zhu X, Li Y, Shi M, Zhang J, Bourgeois M, Yang H, Chen X, Recuenco S, Gomez J, et al. New world bats harbor diverse influenza A viruses. PLoS pathogens. 2013;9:e1003657. doi: 10.1371/journal.ppat.1003657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Treanor JJ, Wilkinson BE, Masseoud F, Hu-Primmer J, Battaglia R, O’Brien D, Wolff M, Rabinovich G, Blackwelder W, Katz JM. Safety and immunogenicity of a recombinant hemagglutinin vaccine for H5 influenza in humans. Vaccine. 2001;19:1732–1737. doi: 10.1016/s0264-410x(00)00395-9. [DOI] [PubMed] [Google Scholar]
- Tripp RA, Tompkins SM. Virus-vectored influenza virus vaccines. Viruses. 2014;6:3055–3079. doi: 10.3390/v6083055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- University of Cambridge, W.C.C. Mapping the Evolution of Pathogens. 2014. [Google Scholar]
- WHO. Influenza (Seasonal) 2014. [Google Scholar]
- Yoon SW, Webby RJ, Webster RG. Evolution and ecology of influenza a viruses. Current topics in microbiology and immunology. 2014;385:359–375. doi: 10.1007/82_2014_396. [DOI] [PubMed] [Google Scholar]