Abstract
The literature describing the use of low-intensity ultrasound in four major areas of cancer therapy was reviewed - sonodynamic therapy, ultrasound mediated chemotherapy, ultrasound mediated gene delivery and antivascular ultrasound therapy. Each technique consistently resulted in the death of cancer cells and the bioeffects of ultrasound were primarily attributed to thermal actions and inertial cavitation. In each therapeutic modality, theranostic contrast agents composed of microbubbles played a role in both therapy and vascular imaging. The development of these agents is important as it establishes a therapeutic-diagnostic platform which can monitor the success of anti-cancer therapy. Little attention, however, has been given to either the direct assessment of the underlying mechanisms of the observed bioeffects or to the viability of these therapies in naturally occurring cancers in larger mammals; if such investigations provided encouraging data there could be a prompt application of a therapy technique in treating cancer patients.
Keywords: Low-intensity ultrasound, Cancer therapy, Sonodynamic therapy, Ultrasound mediated chemotherapy, Antivascular ultrasound, Ultrasound bioeffects, Microbubble contrast agent
INTRODUCTION
Low-intensity ultrasound has been used in a variety of therapeutic applications. Together with sensitizing molecules it has been used to affect cancer cells (sonodynamic therapy); it has enhanced the activity of chemotherapeutic molecules in cancer therapy (ultrasound mediated chemotherapy); it has been used to affect cells and their components directly (sonoporation); it has been used for gene delivery or transfection, and to promote bone and tissue heating/healing and for its antivascular actions on tumor neovasculature. This appraisal of the literature focuses on the role of low-intensity ultrasound in cancer therapy. The published studies have included in vitro observations of cancer cell suspensions and cultures, and the treatment of an extensive range of implanted tumors in small laboratory animals. This review covers four of the major areas in which low-intensity ultrasound has been utilized for cancer therapy studies - sonodynamic therapy, ultrasound mediated chemotherapy, ultrasound mediated gene delivery and antivascular ultrasound therapy.
To date there is no widely accepted definition of low-intensity ultrasound but this review has centered on investigations where cancer cells or tumors have generally been insonated with an intensity less than 5.0 W.cm−2, corresponding to a root-mean-square pressure amplitude of about 0.3 MPa. In the literature many variable sonication conditions have been used for the studies making it difficult to make accurate comparisons between the reports. To aid the comparisons in this review, pressure-intensity conversions were made using the formula I = p2/ρc, where I = intensity, p =root mean square pressure amplitude, ρ = density and c = sound speed (Preston 1991).
In general terms, insonation of neoplasms with low-intensity ultrasound is easy to perform as it does not require a focused beam (that must be accurately located), the apparatus is relatively inexpensive, the bioeffects in adjacent normal tissues are commonly believed to be minimal, and it is possible to easily target sensitizing or chemotherapeutic molecules and microbubbles located within the lumens of the tumor neovasculature. Treatment times are, however, prolonged in comparison to those used in high intensity focused ultrasound, but repeated treatments or dose fractionation are easily performed.
SONODYNAMIC THERAPY
The term sonodynamic therapy derives from photodynamic therapy. However, unlike photodynamic therapy where photosensitizers are excited directly by light to produce reactive oxygen species, sonodynamic therapy is mediated via ultrasound induced cavitation and sonosensitizers to produce free radicals that kill nearby rapidly dividing cancer cells. An attraction of sonodynamic therapy, where continuous, low-intensity ultrasound at diagnostic ultrasound frequencies is utilized, is its ability to treat deeply located tumors. On the other hand, photodynamic therapy utilizes visible light, which attenuates rapidly in tissues and has limited penetration, and can only be employed superficially or intra-operatively. When comparing the efficacy of the two methods, Jin et al (2000) treated a subcutaneously located murine squamous cell carcinoma and found that sonodynamic therapy inhibited tumor growth by 77%, compared with 27% for photodynamic therapy. The latter was not as effective a therapy in the deeper regions of the tumor.
Sonodynamic therapy initially utilized the same light-sensitive agents, hematoporphyrin and its derivatives, that had been developed for photodynamic therapy. An ideal sensitizing agent should be preferentially uptaken and retained in the tumor so that the therapy damages cancer cells but has minimal bioeffects in the surrounding normal tissues; the agent should also be relatively non-toxic in normal mammalian tissues. To improve the efficacy of treating solid tumors, it is important that the sonosensitizer is injected intravenously prior to insonation, rather than directly into the tumor, so that it is more fully and evenly distributed throughout the neoplasm (Ninomiya et al 2012).
Overviews of the sonosensitizers utilized in the therapy have been published (Kuroki et al 2007; Feril et al 2011; Shibaguchi et al 2011; Chen et al 2014). In sonodynamic therapy the sonication parameters (usually 1.0-2.0 MHz at an intensity of 0.5 to 3.0 W.cm−2; Tables 1 and 2) have been selected to produce inertial cavitation in a cell culture or tumor, where a bubble in a liquid rapidly collapses, producing a shock wave which produces free radicals and a cascade of molecular events that activate the sonosensitizer and in turn damage the cancer cells (Misik and Riesz 2000; Rosenthal et al 2004; Yu et al 2004[c]). Although the production of reactive oxygen species appear important in the anti-tumor affect, Wang et al 2011[a] have stated that thermal effects cannot be excluded. In addition to these direct cytotoxic effects on the neoplastic cells, it is also important to consider other possible effects on the growing tumor including its vascular supply. Gao et al (2013) reported that sonodynamic therapy also had an antivascular effect and inhibited tumor neovascularization. Another approach has been to utilize a chemotherapeutic agent as the sonosensitizer. In in vitro studies of adriamycin (Gao et al 2010), cisplatin (Bernard et al 2011; 2012 [0.4 ± 0.02 MPa]) and doxorubicin (Liang et al 2013) it was found that these agents were cytostatic and apoptosis was further enhanced when they were used in combination with chlorine e6 (Gao et al 2010) or an hematoporphyrin (Liang et al 2013).
Table 1.
Sonodynamic therapy of cell cultures and suspensions
| Cancer cell culture/suspension |
Sonosensitizer | Insonation parameters | Reference | |
|---|---|---|---|---|
| MHz | W.cm−2 | |||
| (i) murine sarcoma 180 | hematoporphyrin | 1.92 | 1.27-3.18 | Yumita et al.1989 |
| II | 1.9 | 1.8 | Umemura et al 1990 | |
| II | 1.75 | 1.4 | Tang et al 2008[a] | |
| II | 1.8 | 2.1 | Tang et al 2008[c] | |
| II | 1.6 | 1.0-6.0 | Wang et al 2008[a] | |
| pheobromide-a | 1.92 | 4.5 | Umemura et al 1996[b] | |
| ATX-S10 | 1.92 | 4.5 | Yumita et al 2000[a] | |
| photofrin II | 1.93 | 5.9 | Yumita et al 2000[c] | |
| protoporphyrin IX | 1.0 | 5.0 | Umemura et al 1996[a] | |
| II | 2.2 | 1.0-7.0 | Liu et al 2006[b] | |
| II | 2.2 | 3.0 | Wang et al 2008[c] | |
| II | 2.2 | 3.0 | Wang et al 2008[b] | |
| II | 1.1 | 0.64-2.1 | Wang et al 2010 | |
| rose Bengal derivative | 1.92 | 2.0-8.0 | Sugita et al 2010 | |
| DCPH-P-Na(I) | 2.0 | 5.9 | Yumita et al 2010 | |
| NPe6 | 1.92 | 4.5 | Yumita et al 2011 | |
| polyhydroxy fullerenes | 1.92 | 4.5 | Yumita et al 2013 | |
| (ii) hepatic | hematoporphyrin | 1.92 | 1.27-3.18 | Yumita et al 1989 |
| II | 1.43 | 1.0-4.0 | Liu et al 2008[b] | |
| titanium nanoparticles | 1.0 | 0.1 | Ninomiya et al 2012 | |
| II | 0.5,1.0 | 0.8, 0.4 | Ninomiya et al 2014 | |
| hypocrellin-B | 1.7 | 0.46 | Wang et al 2012[a] | |
| (iii) nasopharyngeal | curcumin | 1.7 | 0.46 | Wang et al 2011[b] |
| II | 1.7 | 0.46 | Wang et al 2011[c] | |
| II | 1.7 | 0.46 | Wang et al 2012[b] | |
| (iv) glioma | ZnPcS2P2 | 1.0 | 0.5 | Chen et al 2012[b] |
| HMME | 1.0 | 0.5 | Li et al 2013 | |
| photofrin | 1.0 | 0.5 | Xu et al 2012 | |
| II | 1.0 | 0.5 | Xu et al 2013 | |
| titanium nanoparticles | 1.0 | 1.0 | Yamaguchi et al 2011[b] | |
| (v) human breast | protoporphyrin IX | 1.1 | 1.0 | Li et al 2012[a] |
| chlorin e6 | 1.0 | 0.36, 0.72 | Wang et al 2013[a] | |
| chlorin e6 + adriamycin | 1.0 | 0.5-2.0 | Gao et al 2010 | |
| (vi) ovarian | cisplantin | 1.0 | 2.0 | Bernard et al 2011 |
| methylene blue | 1.7 | 0.46 | Xiang et al 2011 | |
| (vii) other | ||||
| acute myeloid leukemia | chlorine e6 | 1.1 | 1.0 | Su et al 2013[b] |
| cholangiocarcinoma | hematoporphyrin | |||
| + doxorubicin | 1.2 | 0.5-2.0 | Liang et al 2013 | |
| Ehrlich ascites | protoporphyrin IX | 1.34 | 1.0-5.0 | Zhao et al 2009 |
| gastric | antibody/porphyrin | 1.0 | 1.0 | Abe et al 2002 |
| human leukemia | protoporphyrin IX | 1.1 | 1.0 | Su et al 2014 |
| human melanoma | cisplatin | 1.0 | 1.0 | Bernard et al 2012 |
| histiocytic lymphoma | hematoporphyrin | 1.1 | 1.0 | Su et al 2013[a] |
| murine leukemia | protoporphyrin IX | 1.1 | 0.64-2.1 | Wang et al 2013[b] |
| murine mammary | chlorine e6 | 1.0 | 0.36, 0.72 | Li et al 2013 |
| tongue | 5-aminolevulinic acid | 1.0 | 0.6, 0.8 | Lv et al 2012 |
| multiple cell lines | porphyrin derivative | 1.0 | 1.0 | Tsuru et al 2012 |
| porphyrin derivative | 1.0 | 0.5-2.0 | Hachimine et al 2007 | |
| (viii) non-neoplastic (endothelial cells) |
5-aminolevulinic acid | 1.0 | 1.0 | Gao et al 2013 |
Key: ATX-S10 = 4-formyloximethylidene-3-hydroxy-2-vinyl-deuterio-porphynyl(IX)-6,7-diaspartic acid; DCPH-P-Na(I) = 13,17-bis(1-carboxyethyl)-8-[2-(2,4-dichlorophenyl-hydrazono)ethylidene]-3-ethenyl-7-hydroxy-2,7,12,18-tetramethylchlorin; Pe6 = mono-l-aspartyl chlorin e6; HMME = hematoporphyrin monomethyl ether
Table 2.
Sonodynamic therapy of tumors
| Tumor | Sonosensitizer | Insonation parameters | Reference | |
|---|---|---|---|---|
| MHz | W.cm−2 | |||
| (i) murine sarcoma 180 | hematoporphyrin | 1.92 | 1.7 | Yumita et al 1990 |
| pheobromide a | 1.92 | 3.0 | Umemurra et al 1996[b] | |
| protoporphyrin IX | 2.2 | 5.0 | Liu et al 2007[a,b] | |
| sinoporphyrin sodium | 1.9 | 2.0-6.0 | Li et al 2013 | |
| (II) colon | ATX-S10 | 2.0 | 3.0 | Yumita et al 2000[a] |
| photofrin II | 1.92 | 1.0-5.0 | Yumita et al 2000[b] | |
| protoporphyrin IX/nanoparticles | 1.1 | 2.0 | Sazgarnia et al 2011 | |
| protoporphyrin IX/nanoparticles | 1.1 | 2.0 | Shanei et al 2012 | |
| NPe6 | 2.0 | 3.0 | Yumita et al 2011 | |
| polyhydroxy fullerenes | 2.0 | 3.0 | Yumita et al 2013 | |
| (iii) hepatic | hematoporphyrin | 1.43 | 2.0 | Liu et al 2008[b] |
| protoporphyrin IX | 1.43 | 3.0 | Wang et al 2011[a] | |
| titanium oxide nanoparticles | 1.0 | 1.0 | Ninomiya et al 2012 | |
| hematoporphyrin microbubbles | 1.0 | 2.0 | Zheng et al 2012 | |
| Chlorin e6 | 1.56 | 4.0 | Shi et al 2011 | |
| (iv) glioma (rats) | 5-aminolevulinic acid | 1.04 | 10.0 | Ohmura et al 2011 |
| 5-aminolevulinic acid | 1.0 | 2.65 | Jeong et al 2012 | |
| chlorin e6/polyvinyl pyrrolidone | 1.0 | 0.4-1.0 | Tserkovsky et al 2012 | |
| (v) breast | photofrin | .015/1.0 | 0.2,2.0 | Barati et al 2010 |
| no sensitizer | .015/1.0 | 0.2,2.0 | Barati et al 2009 | |
| (vi) gastric | antibody / gallium-porphyrin | 1.0 | 2.0 | Abe et al 2002 |
| porphyrin derivative | 1.0 | 2.0 | Tsuru et al 2012 | |
| (vii) other | ||||
| squamous cell | gallium-porphyrin/pheophorbide-a | 1.0 | 0.51 | Jin et al 2000 |
| osteosarcoma (rats) | hematoporphyrin | 10.5 | 0.8 | Tian et al 2009 |
| small cell lung | chlorin e6 | 1.0 | 0.4-1.6 | Chen et al 2013[b] |
| tongue | 5-aminolevulinic acid | 1.1 | 2.0 | Gao et al 2013 |
| human MKN-45 | DCPH-PNa(I) | 1.0 | 2.0 | Hachimine et al 2013 |
Unless otherwise stated all tumors were in mice. Key: ATX-S10 = 4-formyloximethylidene-3-hydroxy-2-vinyl-deuterio-porphynyl(IX)-6,7-dia spartic acid; NPe6 = mono-l-aspartyl chlorin e6
Following the initial descriptions of sonodynamic therapy by Yumita et al (1989) and Umemura et al (1990), there have been numerous confirming reports which further demonstrated the bioeffects of the therapy. In contrast to the earlier reviews, we have grouped the research studies according to the type of cancer cell and the accompanying sonsensitizer that were insonated; the aim was to provide a guide to previous sonodynamic studies in which the type of cancer receiving therapy is emphasized (Tables 1 and 2). Considerable data have been published over the past 25 years using many different sonosensitizers and involving many types of cancer (Tables 1 and 2), and each report has consistently shown the significant bioeffects of sonodynamic therapy. The relative merits of each of these numerous sonodynamic agents are, however, difficult to determine as each of the agents was investigated in isolation without comparing the efficacy of one against another. Thus key questions remain to be answered - for example, are the recently developed nanoparticle sonosensitizers any more effective than the original porphyrins in killing cancer cells?
As a routine, sonodynamic therapy caused apoptosis in cancer cell suspensions and cultures, and inhibited tumor growth in the animal models of cancer. The therapy's effectiveness has also been demonstrated in more deeply located tumors including those of the central nervous system (Ohmura et al 2011; Gao et al 2013; Jeong et al 2012). Post-therapy histologic studies have consistently shown damage to the ultrastructure of the cancer cells including destruction of cell membranes, mitochondrial swelling and chromatin condensation (Liu et al 2006[b], 2007[a, b], 2008; Wang et al 2008[a], 2011[c], 2012[b]); it was considered that these changes induced by the therapy may have mediated cancer cell death. Combining photodynamic therapy with sonodynamic therapy had a synergistic effect in solid tumors with additional post-therapy tumor necrosis, inhibition of tumor growth and increased survival times (Jin et al 2000; Tserkovsky et al 2012).
As many of the intravenously injected sonosensitizers (Tables 1 and 2), were initially developed for use in photodynamic therapy, it is important that those used in the future for sonodynamic therapy should have low or no sensitivity to light and cause minimal cutaneous side effects (Shibaguchi et al 2011; Ninomiya et al 2012; Tsuru et al 2012; Gao et al 2013). Preliminary data from solid tumors supports the administration of a regimen of multiple therapies to further enhance the bioeffects and so reduce tumor growth and size; fractionation will also reduce the thermal affects of therapy (Jeong et al 2012).
The combining of the sonosensitizer with a microbubble contrast agent may lead to important future developments in sonodynamic therapy (Zheng et al 2012). The combined agent can then be classified as a theranostic agent as the entry of the loaded microbubbles into the tumor vasculature can be monitored by ultrasound imaging and, once detected within the tumor by diagnostic ultrasound, sonodynamic therapy can be initiated. A therapeutic-diagnostic platform is established which can monitor the efficacy of therapy (Lionetti and Paddeu 2010). Further, insonation of the microbubble may lead to an additional significant local thermal bioeffects with destruction of the endothelial cells lining the tumor vasculature and a decrease in tumor vascularity (Levenback et al 2012).
To date the in vivo observations have been performed in implanted subcutaneous tumors of laboratory animals (mice and rats) so that there remains a need for future studies in a larger mammal, perhaps using sonodynamic therapy for treating naturally occurring cancers. If successful, these additional studies could lead to initial human clinical trials.
ULTRASOUND MEDIATED CHEMOTHERAPY
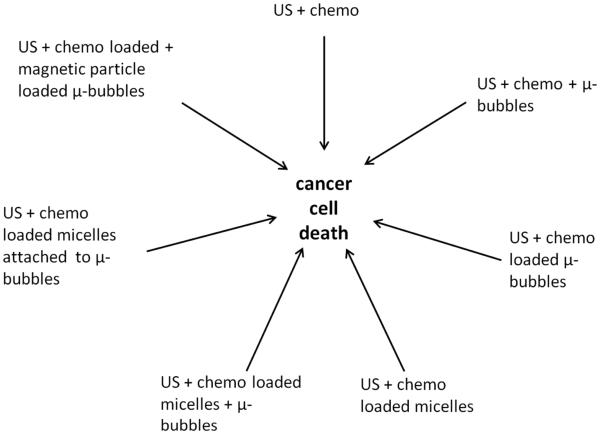
In cancer therapy there is interest in utilizing low intensity ultrasound to enhance the delivery of chemotherapeutic agents to a solid tumor (Table 3). The agents may be non-specific in that they do not selectively target neoplastic cells and thus high levels of the cytotoxic drug will also be present in normal tissues with possible adverse side effects (Nomikou et al 2010 [a]). Further, factors including poor vascularity and defective lymphatic drainage can result in a high interstitial fluid pressure within the tumor and prevent the uptake of therapeutic levels of the drug in the tumor (Nomikou et al 2010 [a]). Insonation of a tumor in the presence of the chemotherapeutic agent provides the potential for enhancing the delivery of the agent to the cancer cells whilst minimizing the cytotoxic effects in the contiguous normal tissues. The delivery of chemotherapeutic agents has been studied in combination with ultrasound alone, with ultrasound and microbubbles, and with drug-loaded microbubbles. Additional chemotherapeutic investigations have been made following insonation of drug-loaded liposomes in the presence of microbubbles and ultrasound, and insonation of drug-loaded liposomes attached to microbubbles (Table 3). In addition to the role of microbubbles in the delivery of chemotherapy via these direct effects, other effects related to antivascular activity have also been described and they are discussed later (see Antivascular Ultrasound). Also magnetic microbubbles have been developed where the drug and iron oxide were co-encapsulated into a microbubble which could be imaged by both ultrasound and magnetic resonance (MR) imaging. This review has been centered on the results of recently published research studies; for a general overview of the topic the reader is referred to papers by Nomikou and McHale 2010[b] and Trendowski (2013).
Table 3.
Ultrasound mediated chemotherapy
| Method | in vitro/in vivo | Insonation parameters | Reference | |||
|---|---|---|---|---|---|---|
| MHz | W.cm−2 | PW/CW | MPa | |||
| Chemotherapy + US | ||||||
| in vitro | 0.24 | 5.76 | CW | Yu et al 2004[a] | ||
| II | 1.0 | 0.2-0.5 | PW | - | Yoshida et al 2008 | |
| II | 1.0 | 0.2-0.5 | PW | - | Hassan et al 2012 | |
| II | 1.0 | 0.05 | PW | - | Li et al 2013 | |
| In vivo | 1.0 | 2.0 | CW | - | Tomizawa et al 2001 | |
| II | 0.24 | 7.84 | CW | - | Yu et al 2004b | |
| II | 1.0 | 1.0 | PW | - | Nomikou et al 2010a | |
| II | 1.0 | 0.01-0.12 | PW | - | Li et al 2013 | |
| Chemotherapy in presence of microbubbles + US | ||||||
| in vitro | 1.0 | 0.5-1.0 | PW | - | Watanabe et al 2008 | |
| II | 1.0 | - | - | 0.4-0.8 | Escoffre et al 2011 | |
| II | 1.0 | - | PW | 0.5 | Heath et al 2012 | |
| II | 0.5-2.25 | - | PW | - | Sorace et al 2012 | |
| II | 1.1 | 2.0-4.0 | CW | Yang et al 2014 | ||
| In vivo | 1.0 | 3.0 | PW | - | Watanabe et al 2008 | |
| II | 1.0 | 3.0 | cont | - | Lu et al 2011 | |
| II | 1.011 | 0.64 | PW | Matsuo et al 2011 | ||
| II | 1.0 | - | PW | 0.5 | Heath et al 2012 | |
| II | 1.0 | - | PW | 0.1-2.0 | Sorace et al 2012 | |
| Chemotherapy-loaded microbubbles +US | ||||||
| in vitro | 3.0 | 3.0 | PW | - | Chumakova et al 2006 | |
| II | 1.0 | 1.0 | PW | - | Tinkov et al 2010b | |
| II | 1.0 | 1.0 | PW | - | Yan et al 2011 | |
| II | 5.0 | - | PW | 0.45 | Cochran et al 2011 | |
| II | 0.8 | 2.56 | PW | - | Ren et al 2013 | |
| in vivo | 1.3 | - | PW | 1.2 | Tinkov et al 2010a | |
| II | 0.3 | 2.0 | PW | - | Kang et al 2010 | |
| II | 5.0 | - | PW | - | Cochran et al 2011 | |
| II | 1.0 | 2.0 | PW | - | Li et al 2012[b] | |
| II | 1.0 | - | PW | 0.7 | Ting et al 2012 | |
| Chemotherapy-loaded micelles/liposomes +US | ||||||
| in vivo | 1.0 | 3.4 | PW | - | Gao et al 2005 | |
| II | 1.0 | 3.4 | CW | - | Rappoport et al 2009 | |
| II | 0.029 | 5.9 | CW | - | Schroeder et al 2009[a] | |
| II | 0.02 | 1.0 | CW | 0.173 | Staples et al 2010 | |
| II | 2.25 | - | PW | 1.9 | Yan et al 2013 | |
| Chemotherapy-loaded liposomes in presence of microbubbles + US | ||||||
| in vivo | 1.0 | - | - | 1.2 | Lin et al 2012[a] | |
| II | 1.0 | 0.3 | PW | - | Zhao et al 2012 | |
| Chemotherapy-loaded liposomes attached to microbubbles + US | ||||||
| in vitro | 1.0 | 2.0 | PW | - | Lentacker et al 2010 | |
| II | 1.0 | 2.0 | PW | - | Geers et al 2011 | |
| II | 1.0 | - | - | 0.2-0.6 | Escoffre et al 2013 | |
| in vivo | 3.0 | 2.0 | PW | - | Rapoport et al 2007 | |
| Chemotherapy and magnetic nanoparticles co-encapsulated onto microbubbles + US | ||||||
| in vivo | 0.3 | 2.0 | PW | - | Niu et al 2013 | |
Key: PW= pulsed wave; CW = continuous wave; - = parameter not available; US = ultrasound
Chemotherapy in the presence of ultrasound
The potential for low intensity ultrasound to increase the sensitivity of cancer cells to a chemotherapeutic agent has been investigated both in vitro and in vivo.
in vitro studies
Insonation of tumor cell suspensions and cultures in the presence of a chemotherapeutic agent can facilitate the cellular uptake of the agent; the inertial cavitation induced by ultrasound beam leads to the formation of microjets that carry the agent directly into the cell or disrupt the cell membranes permitting the inflow of extracellulary located agents (Feril and Tachibana 2012).
Yoshida et al (2008) reported in human myelomonocytic cells a synergistic enhancement of cell killing and increased apoptosis when they were insonated in the presence of doxorubicin. Only a few studies have evaluated the differences in the response between chemosensitive and chemoresistant cancer cells. Yu et al (2004[a]) insonated Adriamycin and cisplatin resistant sub-strains of human ovarian cancer cell lines. They found differing bioeffects between chemosensitive and chemoresistive cells. Cell proliferation and clone forming in the chemoresistive cell populations were suppressed by ultrasound whereas the chemosensitive cells were unaffected. In another study of doxorubicin, Hassan et al (2012) studied human uterine carcinoma cells and a multidrug resistant phenotype. The order of application of insonation and doxorubicin demonstrated differences in the sensitivity of the carcinoma cells. The authors observed that the parent carcinoma cells could either be desensitized or the resistant cell sensitized to doxorubicin depending on the time of sonication. Insonation of human tongue carcinoma cells in the presence of scutellarin significantly enhanced cell injury with irregular shaped and fractured microvilli, and formation of apoptotic bodies on scanning electron microscopy; there was inhibition of cancer cell growth and induction of cell apoptosis (Li et al 2013).
In vivo studies
The efficacy of chemotherapy and ultrasound has also been studied in mouse tumor models. In a murine lymphoma, Tomizawa et al (2001) found that the combination of intraperitoneal bleomycin and ultrasound lead to a suppression of tumor growth. When camptothecin was injected directly into a fibrosarcoma which was then insonated it was hypothesized that the resultant decrease in tumor growth may have followed the ultrasound-dispersion of the chemotherapeutic drug throughout the tumor including the more poorly vascularized regions (Nomikou et al 2010[a]). Chemosensitive and chemoresistant ovarian cancers, implanted in the murine kidney, were insonated 15 min after the intraperitoneal injection of adriamycin (Yu et al 2004[b]). Ultrasound potentiated the efficacy of adriamycin in both types of cancer and thus it was suggested that ultrasound reversed the adriamycin resistance in ovarian cancer cells. In studies of a human tongue squamous cell carcinoma, scutellarin was orally administered prior to tumor insonation and the combined therapy resulted in an inhibition of tumor growth, angiogenesis and lymphangiogenesis (Li et al 2013).
Chemotherapy in the presence of microbubbles and ultrasound
Chemotherapy in the presence of microbubbles and ultrasound has the potential to enhance the in vivo delivery of the agent to a tumor and also to minimize harmful systemic side effects in normal tissues (Heath et al 2012). Watanabe et al (2008) observed that the chemoeffect of cisplatin was enhanced in the presence of ultrasound. They believed that at low intensities the enhanced anti-tumor effect of the chemotherapeutic agent was associated with collapsing and cavitating microbubbles. At very low intensities, however, it remains to be demonstrated whether the microbubbles collapse or simply undergo volume oscillations. The pressures thus generated cause transient increases in the permeability of cell membranes allowing exogenous molecules such as chemotherapeutic agents to enter the cell. Further, the intracellular delivery of therapeutic compounds may be facilitated by endocytosis and pore formation involving the endothelial cells lining blood vessels. Meijering et al (2009) reported that, following insonation (1.0 MHz, 0.22 MPa) of normal rat femoral arteries in the presence of circulating microbubbles, dextran molecules became localized in intracellular vesicles indicating uptake of macromolecules by endocytosis. Also, endothelial cell pore formation was demonstrated by influx of calcium ions and cellular release of dextrans. In an in vitro study using ultrasound alone (1.5 MHz, 0.03 W.cm−2, 0.1 MPa), it was also found that endocytotic vesicles and clathrin (a protein that plays a major role in the formation of coated vesicles) coated pits formed in fibroblasts (Hauser et al 2009) and again provided a means for the uptake of drugs in addition to sonoporation.
It should also be noted that the release of the chemotherapeutic agent from the intravascular microbubbles will also impact on the blood vessels, killing the vessels and permitting the therapeutic agent to leave the vessel. The direct effects of microbubbles alone affecting the vasculature and its role in cancer therapy have been proposed as an antivascular therapy (Wood et al 2007; Goertz et al 2008; see Section IV). Thus the relative contributions of chemotherapeutics to the total observed change needs to be determined,
In vitro studies
Ultrasound has been applied directly to cell cultures in the presence of microbubbles and a chemotherapeutic agent. When murine colon carcinoma and murine mammary carcinoma cells were insonated in the presence of cisplatin and microbubbles, apoptosis was induced (Watanabe et al 2008). Also, when head and neck cancer cell lines were insonated in the presence of a microbubble and cisplatin or cetuximab, Heath et al (2012) reported increased cancer cell permeability, and enhanced drug uptake and apoptosis. Sorace et al (2012) insonated a human breast cancer cell line in the presence of microbubbles, a fluorescent dye (calcein) and taxol, and found that maximum uptake of the extracellular tracer occurred at 1.0 MHz and that cell death was increased by 50% (when compared to the controls). They hypothesized that the microbubble mediated ultrasound therapy increased cell membrane permeability through the generation of small pores which increased passive intracellular delivery of taxol. Similarly, increased membrane permeability was found by Yang et al (2014) following insonation of a human myelogenous leukemia cell line in the presence of doxorubicin and microbubbles. The enhanced delivery of the chemotherapeutic agent resulted in cytotoxicity, cellular necrosis and DNA damage. Their findings supported those of an earlier study by Escoffre et al (2011) who had reported an increased incidence of apoptosis in human glioblastoma and breast cancer cells following insonation in the presence of doxorubicin and microbubbles.
In vivo studies
In vivo experiments have reported a reduction in tumor size following insonation after the chemotherapeutic agent and microbubbles were either injected directly into the tumor or given intravenously. Cisplatin (cis-diamminedichloroplatinum II) and microbubbles were directly injected into human colon carcinoma cell implanted subcutaneously in mice (Watanabe et al 2008). The tumor was then insonated and a consequent reduction in tumor volume was observed. Similar studies of a subcutaneous murine melanoma were performed by Matsuo et al (2011) using melphalon as the chemotherapeutic agent and again resulted in tumor regression. Lu et al (2011) also made intratumoral (murine colon cancer) injections of microbubbles prior to the intravenous injection of epirubicin and tumor insonation. The authors described retarded tumor growth and increased survival times and reported that cavitation contributed to the tumor growth inhibition. Intratumoral injections of microbubbles may not, however, be suitable for clinical practice as access to tumors may not be easy and a uniform distribution of the microbubbles throughout the tumor parenchyma is often difficult to achieve. Observations of the insonation of a squamous cell carcinoma in mice (Heath et al 2012) following the intravenous injection of cisplatin or cetuximab and a microbubble contrast agent (Definity) showed a reduction in tumor size, apoptosis and increased cell membrane destruction. Human breast cancer cells were implanted subcutaneously in the flank of mice and the resultant tumors were insonated in the presence of intravenously injected microbubbles and taxol (Sorace et al 2012). A pressure amplitude of 0.5 MPa resulted in the highest impediment to tumor growth over the three weeks period and also produced the highest degree of tumor necrosis.
Role of microbubbles in opening blood-brain barrier force rebral chemotherapy
There is increasing interest in the use of focused ultrasound in the presence of a circulating microbubble to temporarily open the blood-brain barrier (BBB). The barrier is characterized by tight junctions between the endothelial cells lining the blood vessels in the central nervous system so that the passage of nutrients into the brain via cellular pathways can be closely regulated and brain homeostasis can be maintained. Further, the barrier also prevents toxic substances as well as other pathogens from entering the brain. Temporarily increasing the permeability of the barrier will permit the passage of chemotherapeutic agents into the neural tissue and it is this feature which is of particular relevance in cancer therapy. The underlying bioeffect of insonation is stable cavitation as energy is transferred to the circulating microbubble causing it to expand and contract (oscillate) with resultant damage to the contiguous endothelial cell and their tight junctions with resultant increases in the permeability of the BBB. The topic has recently been extensively reviewed by Burgess and Hynynen (2014) and Liu et al (2014). In Table 4 other recent publications have been listed together with the insonation parameters that were used to increase the permeability of the barrier in normal rodent brains and in those with implanted cerebral neoplasms.
Table 4.
Opening of blood-brain barrier with pulsed focused ultrasound and microbubbles in rodents
| Brain | Insonation parameters | Reference | ||
|---|---|---|---|---|
| MHz | W.cm−2 | MPa | ||
| Normal brain | ||||
| 0.4 | 5.0-10.0 | 0.98-1.35 | Liao et al 2012 | |
| - | - | 0.45-0.60 | Chen et al 2013 | |
| 1.5 | - | 0.45-0.60 | Samiotaki &Konofagou 2013 | |
| Implanted brain tumor | ||||
| glioma | 0.4 | 1.0-10.0 | 0.45-1.35 | Liu et al 2010 |
| breast cancer | 0.69 | 0.32 | 0.69 | Park et al 2012 |
| glioma | 1.0 | - | 0.7 | Ting et al 2012 |
| gliosarcoma | 1.7 | - | 1.2 | Treat et al 2012 |
| glioma | 0.69 | - | 0.55-0.81 | Aryl et al 2013 |
| glioma | 0.40 | 4.0 | 0.325 | Fan et al 2013[a] |
| glioma | 1.0 | 3.0 | 0.6 | Wei et al 2013 |
| glioma | 0.61 | - | 0.4 | Kovacs et al 2014 |
| astrocytoma (unfocused US) | 0.5 | - | 0.4 | II |
Key: US = ultrasound
In normal rat brains, Liao et al (2012) used MR imaging to demonstrate regions of breakdown of the BBB following cerebral insonation in the presence of albumin-shelled Gd-DTPA circulating microbubbles and showed that a threshold acoustic pressure of 0.98MPa was required to open the BBB. In further MR observations, Samiotaki and Konofagou (2013) reported an opening threshold in mice of 0.45MPa in the presence of circulating Definity microbubbles. A similar threshold was also found in mice (Chen et al 2013) where at 0.45 MPa there was an homogeneous breakdown of the BBB in the insonated hippocampus in the presence of circulating microbubbles. Additional observations with circulating phase-shift nanodroplets (formed by pressurizing microbubbles so that the gas core was converted into a liquid phase) showed higher acoustic pressures were required to open the BBB but the incidence of inertial cavitation was reduced (Chen et al 2013).
Further in vivo observations have been made in rodents following chemotherapy of implanted brain neoplasms. Liu et al (2010) used MR imaging to follow tumor progression after opening of the BBB by focused sonication in the presence of circulating microbubbles and treatment with carmustine (bis-chloroethylnitrosourea). Tumor growth was controlled and animal survival times were increased. In an additional study, carmustine was loaded onto microbubbles and brain insonation released the chemotherapeutic agent at the target site (Ting et al 2012). MR imaging demonstrated control of tumor progression and improved animal survival times were again shown. In multiple investigations utilizing doxorubicin (either given alone [Kovacs et al 2014], encapsulated in liposomes [Treat et al 2012; Aryal et al 2013], or conjugated with supermagnetic iron oxide nanoparticles [Fan et al 2013a]), focused ultrasound therapy of a brain neoplasm in the presence of microbubbles also resulted in reduced tumor growth and an increase in median animal survival times. These observations have also been confirmed using other chemotherapeutic agents including trastuzumab (Park et al 2012; treatments given weekly for six weeks) and temozolomide (Wei et al 2013). Burgess and Hynynen (2014) have, however, raised the concern that the intravenous drug concentrations selected for a successful therapeutic outcome in the brain may have to be adjusted to avoid potential peripheral toxicity.
It should be noted, however, that the normal BBB is damaged by a brain neoplasm and this is the reason that iodine-containing contrast agents in the case of computed tomography and paramagnetic contrast agents in the case of magnetic resonance imaging can cross the leaky barrier and demonstrate the cerebral pathology in each of these imaging systems. Thus the future clinical role of ultrasound therapy may be to further increase the permeability of an already leaky barrier and perhaps permit the egress of larger chemotherapeutic molecules than is normally possible from the vascular lumen into the cancer tissue.
Chemotherapy-loaded microbubbles and ultrasound
The chemotherapeutic drug may be loaded or bound to a microbubble whose diameter is less than that of an erythrocyte so that it easily enters the neovasculature of a tumor (Liu et al 2006[a]). On insonation of a tumor, ultrasound targeted microbubble destruction occurs which has the advantages of being externally controlled and localized to the tumor site. The formation of microbubbles and their potential as carriers for drugs, small molecules, nucleic acids and proteins has been reviewed by Tinkov et al (2009). The structure of the shell of microbubble is important; lipophilic chemotherapeutic drugs including doxorubicin, paclitaxel and docetaxel can be incorporated into the lipid layer of a microbubble (Kang and Yeh 2012). It is believed that such soft-shelled microbubbles may be not be capable of stably incorporating large volumes of drug molecules because of their relatively thin shells; the use of polymer-based hard-shelled microbubbles permits the entrapment of hydrophilic (rhodamine-B) and hydrophobic (coumarine-6) chemotherapeutic agents in the shell and may enhance the efficacy of cancer therapy (Fokong et al 2012).
The concept of a theranostic microbubble which can combine a chemotherapeutic role with ultrasound imaging is receiving increased attention (Peyman et al 2013; Zhao et al 2013). The bubble is loaded with a chemotherapeutic agent that is released in a tumor under the action of low-intensity ultrasound; the bubbles may also act as a contrast agent and used for contrast enhanced power Doppler ultrasound imaging of the tumor vasculature. Stride and Coussios (2010) have provided an extensive review of the basic physics and cavitation of microbubbles used in therapy and imaging and how, when acoustically driven, they can be destroyed at a tumor site and release a chemotherapeutic agent. The mechanism underlying the delivery of a drug from the microbubble to a tumor was investigated using a light emitting compound loaded onto a microbubble (Liao et al 2012). On insonation (1 MHz, pulsed, 3.0 W.cm−2) the compound was released into tumor capillaries and entered the neoplastic cells.
The incorporation of drugs onto the surface of microbubble has been described (Liu et al 2006[a]; Mayer et al 2008; Ferrara et al 2009). During tumor insonation, cavitation ruptures the bubble so releasing the drug and delivering it into the tumor - it may impart a "ballistic effect" to drive the drug through the wall of the tumor capillary (Liu et al 2006[a]). Also sonication can oscillate the microbubbles resulting in an increase in the permeability of the contiguous cell membranes including those of the endothelial cells lining tumor capillaries and further enhance the entry of a locally released chemotherapeutic agent into a cancer cell (Feril and Tachibana 2012; Stride and Coussios 2010; Zhao et al 2013). Following insonation (1.5 MHz, pulsed, 1.0 W.cm−2), of a rat glioma cell culture, fibered confocal fluourescence microscopy demonstrated in real time the intracellular delivery of an impermeable green dye from the microbubble into the cancer cell (Derieppe et al 2013).
In vitro studies
Insonation in the presence of microbubbles of a breast cancer cell line treated with 5-fluorouracil resulted in damage to the cells and it was presumed that the chemotherapeutic agent bound to the albumin shell of the microbubble (Chumakova et al 2006). Doxorubicin was embedded in the shell of the microbubble and its insonation in a culture of a human renal carcinoma cells resulted in an enhanced cytotoxic activity when compared to free doxorubicin and doxorubicin-loaded liposomes (Tinkov et al 2010[b]). Yan et al (2011) studied the bioeffects of paclitaxel-loaded lipid microbubble coated with a breast tumor homing peptide; the microbubble attached to human breast cancer cells and insonation of the culture resulted in reduced cell viability. Using a polymer microbubble Cochran et al (2011) found that, paclitaxel could be loaded to a much greater extent than doxorubicin and on insonation of cultures containing the loaded microbubble and human breast cancer cell line there was significant release of paclitaxel presumably as the microbubble ruptured and leading to the observed reduction in cell viability. Ren et al (2013) encapsulated docetaxil, a hydrophilic molecule, in a lipid microbubble and demonstrated that insonation inhibited the proliferation of human colon carcinoma cells.
In vivo studies
In power Doppler ultrasound images of liver cancer in rabbits, Cochran et al (2011) showed that doxorubicin loaded microbubbles could permeate the vasculature of the tumor. Their observation confirmed the findings of Tinkov et al (2010[a]) who used doxorubicin loaded microbubbles to treat a murine pancreatic carcinoma that had been implanted subcutaneously in rats. Following insonation, there was a lowering of the tumor growth rate related to an increase in local drug concentration. In comparison to the untreated controls, a reduction in tumor growth rate, accompanied by an increased survival time and reduction in the number of metastases, was also reported in a liver tumor in rabbits insonated following the intravenous injection of docetaxel loaded lipid microbubbles (Kang et al 2010); ultrasound therapy ruptured the microbubbles and released the drug locally in the tumor tissues.
When 10-hydroxycamptothecin-loaded microbubbles were injected intravenously, they were also detectable in power Doppler images of a subcutaneously located murine hepatic tumor (Li et al 2012[b]). During insonation of the tumor the microbubbles fragmented to nanoparticles, the fragments accumulated in the tumor and the chemotherapeutic agent was slowly released. The low blood flow in tumors and their large blood volume were favorable for the infusion and destruction of microbubbles within the tumor. Significant drug levels were delivered locally and the growth rate of the tumor was reduced; there was, however, no reduction in size of tumors receiving unloaded microbubbles.
Chemotherapy-loaded polymeric micelles or liposomes and ultrasound
An alternative approach to loading a chemotherapeutic agent onto a microbubble is to instead use a polymeric micelle or liposome. The micelles have been used to improve the delivery of chemotherapeutic drugs to a tumor and the aim is to release the agent from the micelle under the action of ultrasound. The topic of ultrasound activation of micelles has recently been reviewed (Husseini and Pitt 2009; Schroeder et al 2009[b]; Rapoport 2012). It has been postulated that inertial cavitation improves micelle delivery by creating holes in the membranes of neoplastic cells so that the micelles can diffuse into the neoplastic cell. Inertial cavitation may also open the micelles to release the now intracellular chemotherapeutic drug (Husseini and Pitt 2009). It has also been suggested that the insonation induces a mild hyperthermia which may enhance the movement of the micelles from the tumor capillaries into the interstitium of the tumor (Rapoport 2012).
There have been a series of in vivo observations of the effectiveness of the therapy method. Schroeder et al (2009[a]) reported that insonation of a colon adenocarcinoma implanted in the feet of mice following the intravenous injection of cisplatin loaded liposomes inhibited tumor growth and the tumors regressed over time. In studies of intraperitoneal or subcutaneous ovarian carcinomas in mice, Gao et al (2005) reported that ultrasound triggered an intracellular uptake of polymeric micelles encapsulated with doxorubicin; the drug had previously been injected intravenously and had accumulated in the interstitium of the tumor. There was also a decreased growth rate of the subcutaneous tumor. Further, Yan et al (2013) intravenously injected liposome-microbubble complexes containing paclitaxil prior to insonation of mice with a subcutaneous breast tumor. They reported an increase in drug concentration in the tumor and an inhibition of tumor growth.
In a subcutaneous colorectal epithelial cancer cell line in rats, Staples et al (2010) reported that the infusion of doxorubicin labeled micelles and insonation (20 kHz, continuous, 1.0 W.cm−2 and 476 kHz, pulsed, 23.61 W.cm−2) lead to higher concentrations of the chemotherapeutic agent within the tumor for 30 min post-insonation; at later times similar levels of the agent were present in insonated and non-insonated tissues. The authors suggested that cavitation events may have released the doxorubicin from circulating and extravasated micelles into the tumor and insonation could have transiently increased the permeability of the tumor neovasculature. With the progression of time, however, cavitation could occur at slower rates as the micelles are cleared from the circulatory system and also the tumor capillaries will regain their normal permeability thus explaining the observed similar levels of doxorubicin in cancerous and normal tissues.
Rapoport et al (2009) followed a different approach where nano-micelles loaded with paclitaxel or gemcitabine were infused into the tumor vasculature and transformed by ultrasound into microbubbles after the nano-emulsions had extravasated into the interstitium. Ovarian, breast and pancreatic cancers were studied in mice and their results showed enhanced delivery of the chemotherapeutic agents to the tumors. There was also a reduction in tumor growth, although the initial reduction was significant subsequent treatments were less effective possibly due to the development of drug resistance by the cancer cells.
In order to minimize the potential side effects a chemotherapeutic agent on normal tissues, Ibsen et al (2012) developed "shockwave ruptured nanopayload carriers” in which a microbubble was encapsulated with a protective outer liposome. Tissue phantom studies were performed using 2.25 MHz ultrasound at the relatively higher pressure amplitude of 1.5 MPa. Insonation destroyed the microbubble of perfluorocarbon gas and ruptured the outer liposome membrane and so had the potential to release a highly concentrated chemotherapeutic payload from within the particle.
Chemotherapy-loaded liposomes in presence of microbubbles and ultrasound
Inertial cavitation induced by the insonation of microbubbles can augment the intracellular absorption of a chemotherapeutic agent. Zhoa et al 2012 insonated breast cancer cells in the presence of microbubbles and doxorubicin-loaded liposomes; they reported that ultrasound mediated cavitation lead to the observed reduction in cell viability in the treated cell cultures. In an in vivo study, Lin et al (2012[a]) used polyethylene glycol coated liposomes loaded with doxorubicin to treat a murine colorectal adenocarcinoma implanted subcutaneously in mice. Microbubbles were injected intravenously and the tumor was insonated at a higher pressure amplitude of 1.2 MPa, prior to the injection of the chemotherapeutic agent. Following therapy there were significantly enhanced drug levels in the tumor and delayed tumor growth. Also, it was found in other experiments that the temperature of the tumor increased by of 5.0°C when insonation occurred in the presence of circulating microbubbles but by only 2.5 °C in their absence. It was concluded that the absorption of ultrasound energy was enhanced by the oscillation and cavitation of microbubbles within the insonated tumor. Zhao et al (2012) utilized an intratumoral injection of microbubbles and the intravenous injection of doxorubicin loaded liposomes, and found that the growth of breast cancer tumor in mice was reduced when it was insonated. It was also concluded that cavitation was an important bioeffect of insonation and enhanced the absorption of doxorubicin.
Chemotherapy-loaded liposomes attached to microbubbles and ultrasound
The feasibility of the targeted delivery of a theranostic agent under image guidance is addressed by the attachment of drug-loaded liposomes to a microbubble of ultrasound contrast medium. Diagnostic ultrasound can be used to confirm the presence of the loaded microbubbles within the tumor vasculature; the application of low-intensity ultrasound then ruptures the bubbles accurately restricting the delivery of the chemotherapeutic agent to the neoplastic cells.
The cytotoxicity of doxorubicin containing liposomes coupled to the surface of a microbubble has been demonstrated in vitro. Lentacker et al (2010) using melanoma cells described an almost instantaneous cellular entry of the chemotherapeutic agent following insonation which was thought to be related to sonoporation of cell membranes. In a subsequent study also of melanoma cells, Geers et al (2011) showed that 600-1300 doxorubicin loaded liposomes were attached to the surface of each microbubble and insonation increased the killing of the cancer cells. In another study of glioblastoma cells, insonated in the presence of similar liposomes linked to microbubble, Escoffre et al (2013) reported a decrease in cancer cell viability.
The therapeutic potential of combining drug-loaded liposomes, microbubbles and ultrasound was demonstrated by Rapoport et al (2007) when polyethylene glycol coated liposomes loaded with doxorubicin and perfluoropentane microbubbles were used to treat breast cancer tumors in mice. Following the intravenous injection of the liposome/microbubble mixture and subsequent insonation, increased uptake of doxorubicin in the tumor was observed. The uptake of the drug was greater when microbubbles and drug-loaded liposomes were used than when the liposomes were administered alone. The observed increase was attributed to inertial cavitation. Tissue heating to 41° C was also demonstrated in normal mice by Cool et al (2013) when indocyanine green containing liposomes where coupled to the surface of intravenously injected microbubbles – insonation (1 MHz, 2.0-5.0 W.cm−2) caused release of the dye and it was concluded that the technique may have applications in improving ultrasound mediated drug delivery.
An alternative approach has been reviewed by Ibsen et al (2013) where the chemotherapeutic agent is carried on the surface of the microbubble and the loaded microbubble is in turn encapsulated within a liposome. The potential advantages include the rapid release of the encapsulated chemotherapeutic drug within the tumor and a low leak rate of the agent into normal tissues. The microbubble can also be used as an imaging contrast agent i.e. a theranostic agent, as it is involved in both therapy and diagnosis.
Chemotherapy in the presence of ultrasound and magnetic nanoparticles loaded onto microbubbles (magnetic microbubbles)
The encapsulation of iron oxide nanoparticles into the microbubble enabled the development of multi-modality imaging i.e. magnetic resonance (MR) imaging in addition to ultrasound imaging; the topic has recently been briefly reviewed (Owen et al 2012; Zhao et al 2013). Niu et al (2013) developed such a multifunctional theranostic agent in which pelvic limb lymph node metastases from an implanted squamous cell carcinoma in rabbits were imaged by both MR and ultrasound. Doxorubicin and iron oxide nanoparticles were co-encapsulated into the microbubble and insonation of the nodes triggered the release of the chemotherapeutic agent. The therapy increased apoptosis, and decreased tumor proliferation and micro blood vessel and lymphatic density.
Additional comments
In summary, investigations of ultrasound mediated chemotherapy have evaluated the efficiency of the delivery of a wide range of chemotherapeutic agents to cancer cell cultures and suspensions, and solid tumors. Across all delivery methods consistent findings have been a potentiation of the efficacy of the delivery of chemotherapeutic agents with resultant necrosis of neoplastic cells, inhibition of tumor growth and increased animal survival times. The precise mechanisms underlying the ultrasound mediated chemotherapy are, however, frequently based on speculation rather than direct evidence. The insonation of a solid tumor with low intensity ultrasound in the presence of an intravascular chemotherapeutic agent can potentiate and localize the cytotoxic affects to the cancer cells, whilst minimizing side effects in the adjacent normal tissue. Traditional chemotherapy is given in low doses over time and future studies may show whether ultrasound mediated chemotherapy can substantially reduce the overall dose of the agent and decrease its toxicity. Although some in vivo experimental studies have used intratumoral or intraperitoneal injections of the chemotherapeutic agent or microbubbles, intravenous injections should result in a more even and generalized distribution of these substances within the tumor and an improved response to therapy.
Although large numbers of studies have demonstrated the efficacy of ultrasound mediated chemotherapy, relatively few have dealt with the issue of the biodistribution of the agents and their elimination from the body. Those chemotherapeutic agent-loaded microbubbles not destroyed by an ultrasound beam which has been localized to a tumor will continue to circulate in the vascular system and may be retained in a major organ. Toft et al (2006) studied the biodistribution of the perfluorobutane gas contained in a microbubble at different time points following its intravenous injection in rats. They observed that the highest concentration of perfluorobutane was in the spleen followed by decreasing levels respectively in the liver, lung, kidney and other tissues. They also demonstrated that 50% of the perfluorobutane was recorded in the exhaled air by 20 min after injection and 96% was recovered in 24 hours. Retention of microbubbles in the sinusoidal spaces within the liver has been reported and they are phagocytosed by Kupffer cells (liver specific macrophages; Cosgrove 2006; Yanagisawa et al 2007; Liu et al 2008[a]). In in vitro studies, acoustic pressures of 0.63 MPa were required to destroy microbubbles adherent to Kupffer cells and 0.73 MPa destroyed those microbubbles that had been phagocytosed (Liu et al 2008[a]). There is also an uptake of microbubbles by the spleen where they may be phagocytosed by macrophages or simply trapped within the splenic parenchyma (Lim et al 2004). Further, it has been demonstrated that lipid-shelled microbubbles are retained in the renal cortex of mice and humans (Liu et al 2013[a]). The microbubbles were located in the murine glomerular microvessels following of a complement-mediated interaction with the vascular endothelium.
Given that the majority of the microbubbles will be destroyed during the insonation of the tumor neovasculature, it is likely that non-clinically significant levels of the chemotherapeutic agent will be retained in the liver, spleen or kidney. In considering future developments in ultrasound mediated chemotherapy it will be important, however, to fully understand the behavior and eventual outcome of these phagocytosed or adherent microbubbles, especially as their associated chemotherapeutic agent has the potential to cause toxic side effects in these major organs.
The ability to image, whether by diagnostic ultrasound or MR imaging, the distribution of the chemotherapeutic agent within the tumor has significant clinical implications. By loading the chemotherapeutic agent onto the surface of a microbubble of ultrasound contrast medium, localized insonation of such a theranostic agent could lead to an accurate delivery of the drug to a tumor and to minimization of unwanted cytotoxic effects in the adjacent normal tissues. Further, it may be feasible to reduce the systemic dose of the chemotherapeutic agent and also lead to a reduction in clinical side effects. In considering the underlying bioeffects of such theranostic methods the emphasis has been placed on the role of inertial cavitation, little attention has been given, however, to other possible mechanisms including accompanying thermal effects on the tumor vasculature. As the in vivo studies have usually been performed in rat and mouse tumor models, there is a need to progress to investigations in naturally occurring neoplasms which could then lead to initial clinical trials.
ULTRASOUND MEDIATED GENE TRANSFECTION
Recently there has been considerable interest in the emerging area of using ultrasound and microbubbles to facilitate gene delivery to neoplastic cells (Liang et al 2010; Geis et al 2012; Sirsi and Borden 2012). It has been hypothesized that the use of focused ultrasound to target DNA-loaded microbubbles located within the lumen of a tumor’s neovasculature can result in destruction of the microbubble and release or transfection of genetic material locally into the tumor parenchyma. It is further proposed that insonation also causes the process of sonoporation in which transient pores form in the cancer cell membranes through which molecules are able to enter the cell (Haag et al 2006; Li et al 2014). In each case it is considered that insonation is the driving force responsible for the transfection of the nucleic acids and the subsequently observed therapeutic effects in the cancer cells. It remains uncertain, however, as to exactly how genes pass through the endothelial barrier to reach the tumor. Sporadic capillary rupture and increased vascular permeability as well as enhanced permeability of cell membranes may play a role in the observed efficacy of therapy. Some of the more recent studies have been listed in Table 5; the acoustic pressures required to promote gene transfection are usually greater than 0.3 MPa and so fall into a general classification of moderate ultrasound intensities. We have provided an introductory, preliminary review of the topic, and the reader is encouraged to evaluate the results of new investigations as further data accumulates.
Table 5.
Insonation parameters used for ultrasound and microbubble mediated gene delivery
| Cells / tumor | Insonation parameters | Treatment | Reference | ||||
|---|---|---|---|---|---|---|---|
| MHz | W.cm−2 | MI | MPa | PW/CW | |||
| in vitro | |||||||
| hepatocellular carcinoma | 1.0 | 0.5 | - | - | PW | IFNβ | Sakakima et al 2005 |
| prostate | 1.75 | - | 1.9 | 1.44* | PW | antisense oligodeoxynucleotide | Haag et al 2006 |
| hepatoma | 1.0 | 1.0 | - | - | PW | pEGFP | Zhang et al 2011 |
| prostate | 0.021 | 4.6 | - | - | PW | pEGFP DNA | Bai et al 2012[b] |
| endothelial cell | 1.0 | 2.0 | - | - | PW | Click beetle luciferase | Wang et al 2012[c] |
| breast cancer | 2.0 | 0.75 | - | - | CW | KDRP-CD/TK | Li et al 2012[c] |
| ovarian | 1.0 | 0.5 | - | - | PW | PEGFP-N1-wtp53 | Chang et al 2013 |
| in vivo | |||||||
| hepatocellular carcinoma | 1.0 | 2.0 | - | - | PW | IFNβ | Sakakima et al 2005 |
| prostate | - | - | 1.9 | - | PW | antisense oligodeoxynucleotide | Haag et al 2006 |
| hepatoma | 1.0 | 2.0 | - | - | PW | HSV-TK | Zhou et al 2010 |
| squamous cell carcinoma | 1.3 | - | 1.6 | 1.40* | PW | TK | Carson et al 2011 |
| melanoma | 1.011 | 0.22 | - | - | PW | IFNβ | Yamaguchi et al 2011[a] |
| prostate | 1.0 | 1.0 | - | 0.12 | PW | IL-27 | Zolochevska et al 2011 |
| squamous cell carcinoma | 1.3 | - | 1.6 | 1.40* | PW | sIRNA | Carson et al 2012 |
| nephroblastoma | 1.0 | 1.0 | - | - | CW | DNA | Sirsi et al 2012 |
| hepatic | 1.0 | 3.0 | - | - | PW | KDR-TK, AFP-TK | Tang et al 2012 |
| endothelial cell | 1.0 | 2.0 | - | - | PW | Click beetle luciferase | Wang et al 2012[c] |
| mammary adenocarcinoma | 1.3 | - | - | 2.1 | PW | shRNA | Fujii et al 2013 |
| hepatoma | 1.3 | - | 1.3 | 1.14* | PW | HSV-TK/GCV | Yu et al 2013 |
| renal carcinoma | 1.0 | 2.0 | - | - | PW | recombinant adeno-associate virus | Li et al 2014 |
Key: MI = mechanical index; PW = pulsed wave; CW = continuous wave; - = parameter not available;
represents calculated negative peak pressure = .
As a routine, gene therapy was easily targeted to a tumor and the investigations showed successful transfection of genetic material resulting in apoptosis of cancer cells, both in vitro and in vivo, and reduced tumor growth. Following insonation, the wide distribution of transfection within the tumor lead to a more efficient therapeutic response and Fujii et al (2013) were unable to detect transfection in organs outside ultrasound beam. Microbubbles loaded with genes or inhibiting RNA (directed to epidermal growth factor) have been shown to effectively reduce tumor growth (Carson et al 2011, 2012). The underlying hypothesis is to deliver genes that inhibit signaling processes to specific sites in the tumor while sparing the non-targeted areas. By this approach selective payloads of these materials can be delivered non-invasively and repeatedly.
On some occasions the genetic material and microbubbles were injected separately into a tumor (Tang et al 2012) or alternatively they were mixed and then added to the tissue culture or injected into the tumor (Li et al 2012[c]; Li et al 2014; Sakakima et al 2005; Yamaguchi et al 2011[a]; Zhang et al 2011; Zolochevska et al 2011). An improved distribution throughout the parenchyma of the tumor would, however, be expected when the nucleotide and microbubble are linked and injected intravenously. As the phosphate backbone of DNA and RNA is highly anionic, microbubbles with cationic shells have been utilized so that there is spontaneous formation by electrostatic binding of a complex based on the charge interactions (Wang et al 2012[c]; Yu et al 2013). In using such complexes, Wang et al (2012) showed significantly enhanced transfection in both cell cultures and tumors. They observed that the enhancement in transfection efficiency with cationic microbubbles was more pronounced in cell culture studies than in tumors. Possibly more DNA was delivered in tissue cultures than in vivo but a further reason for the difference could be the extent of cavitation activity – there may have been more cavitation in vitro than in vivo.
In these ultrasound-mediated methods, the transfection/transduction of genetic material was usually accomplished using non-viral techniques, but using a virus as a carrier with microbubbles was also successful (Li et al 2014; Tang et al 2012). Compared with viral vectors, non viral techniques have increased safety but are disadvantaged by low transfection efficiencies (Wang et al 2012[c]), however, microbubbles are good carriers of genes with a greater capacity for antisense oligonucleotides, and fragment of DNA and even the entire chromosome (Li et al 2012[c]).
As far as the underlying bioeffects are concerned, during insonation the in vivo cavitation activity is confined to microbubbles within the lumens of the tumor neovasculature and it remains to be elucidated how these cavitation events induce sonoporation and vesicle formation in the extravascularly located cancer cells. In summary, ultrasound-mediated gene therapy is non-invasive and repeated deliveries can be performed to achieve a more sustained transfection, and also multiple genes can be delivered to achieve a synergistic therapeutic response (Fujii et al 2013). The method is site specific so providing a distinct advantage over systemic cancer therapies for tumors with their associated effects on normal tissues (Carson et al 2012; Sirsi et al 2012). It should be remembered, however, that following insonation the transfection of genes could result in unexpected morphologies and abnormalities in normal cells contiguous with tumor. In the future, animal investigations could be aimed at following tumors over longer time periods post-therapy and making repeated deliveries of genetic material. Also, a multigene approach using a combination of antiangiogenic and pro-apoptotic gene therapies could be performed (Fujii et al 2013).
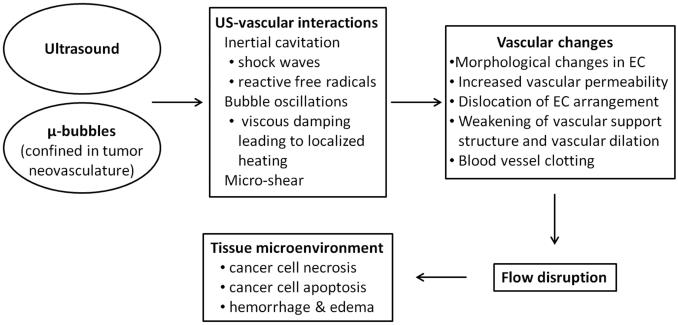
ANTIVASCULAR ULTRASOUND
It has been proposed that a solid tumor should be considered to have two compartments of cells, one containing the neoplastic cells and the other the endothelial cells of the tumor neovasculature (Folkman 2001; Siemann 2006). As a solid tumor exceeds a cubic millimeter in volume it must establish its own vascular supply in order to ensure the continuing viability of its cancer cells. It is well established that the tumor neovascular differs in structure from that of normal blood vessels - tumor vessels are fragile, leaky and have abnormal branching patterns (Vaupel 2006).
Ultrasound imaging utilizing a circulating microbbuble has become a method of choice to demonstrate the tumor neovasculature and to enable differentiations to be made between vascular and avascular regions within a tumor (Wood et al 2005, 2006; Perini et al 2008; Anderson et al 2011; Hyvelin et al 2013). The technique can also be used to assess the efficacy of an antivascular therapy. Following the intravenous injection of microbubbles, the vascular regions within a tumor can be identified in routine B-mode (delta-projection imaging - Sehgal et al 2009) and power Doppler images (visual inspection, percentage area of flow and color weighted flow area, cumulative histogram area - Wood et al 2007; Mason et al 2011). Alternative approaches using high frequency Doppler imaging without the use of microbubbles have also been developed. Goertz et al (2002) and Chen et al (2011, 2012[a]) demonstrated the vascularity of murine tumors in power Doppler images made at 25-38 MHz, and in a study of a prostatic adenocarcinoma implanted in mice, vascular perfusion was assessed at 25 MHz in power Doppler images by measuring the ratio of the color weighted pixels to the total tumor area or percentage area of flow (Chen et al 2013[a]).
This review centers on techniques utilizing the combination of ultrasound and microbubbles as a vascular disrupting agent; the tumor vasculature is damaged, leading to necrosis of the neoplastic cells with a consequent reduction in tumor growth and lengthened survival time. Although the focus of this review is on the role of low-intensity ultrasound as part of such an antivascular tumor therapy, we have also included references to the use of moderately higher intensity insonations (Table 6). The antivascular responses to these differing approaches are probably related to major differences in their bioeffects – lower intensities are reported to create more thermal effects and higher intensities more cavitation effects. Such divisions, however, may be considered as artificial as the two effects are intertwined and seldom is it true that one class of effects can be disregarded (Baker et al 2001).
Table 6.
Antivascular ultrasound therapy of tumors in presence of circulating microbubbles
| Type of microbubble | tumor | Insonation parameters | Reference | |||
|---|---|---|---|---|---|---|
| MHz | W.cm−2 | PW/CW | MPa | |||
| Unloaded | ||||||
| melanoma | 1.0-3.0 | 2.0 | CW | 0.28 | Wood et al 2005,2006,2007,2008,2010 | |
| 3 tumor types | 1.0 | - | PW | 0.74 | Goertz et al, 2008,2009 | |
| adenocarcinoma | 1.2 | - | PW | 5.0 | Chin et al 2009 | |
| colon carcinoma | 1.5 | 133* | - | - | Zhong et al 2010 | |
| glioma | 1.0 | - | PW | 1.0-1.2 | Burke et al 2011 | |
| adenocarcinoma | 1.0 | 2.0 | PW | - | Lin et al 2012[a] | |
| Walker-256 | 0.83 | 0.4, 1.36 PW | 2.6, 4.8 | Liu et al 2012 | ||
| colon carcinoma | 0.24 | - | PW | 0.5 | Huang et al 2013 | |
| hepatoma | 1.75 | 539 | - | - | Wang et al 2013 | |
| breast cancer | 5.0 | - | PW | 4.0 | Hu et al 2012 | |
| epidermoid | 1.22 | 0.89 | PW | 4.6 | Peijing et al 2014 | |
|
| ||||||
| Unloaded +antianglogenic genes | prostate | 1.0 | 2.0 | PW | - | Duvshani-Eshet et al 2007 |
| hepatoma | 1.0 | 2.0 | PW | - | Nie et al 2008 | |
|
| ||||||
| Loaded | ||||||
| docetaxel | squamous carc. | 0.3 | 2.0 | - | - | Kang et al 2010 |
| II | prostate | 1.0 | - | PW | 1.65 | Goertz et al 2012 |
| doxorubicin | breast cancer | 3.0 | 2.0 | PW | - | Rapoport et al 2007 |
| II | colorectal | 1.0 | 2.0 | PW | 0.6 | Lin et al 2012b |
| carmestine +antiangiogenic complex | glioma | 1.0 | - | PW | 0.5 | Fan et al 2013 |
| antiangiogenic agent | colon cancer | 0.24 | - | PW | 0.5 | Zhang et al 2014 |
Key: PW= pulsed wave; CW = continuous wave; - = details not provided; carc. = carcinoma
= estimated;
Predominantly thermal bioeffects
In multiple studies of a murine melanoma model, it was consistently demonstrated that tumor insonation (1-3 min treatment time) in the presence of a circulating microbubble had a significant antivascular effect (Wood et al 2005, 2006, 2007, 2008, 2010). Each minute of insonation decreased tumor perfusion by ≈25% (Wood et al 2005). Of particular interest in these theranostic studies was the observation that the normal vasculature in the adjacent tissues and organs appeared unaffected by the therapy. The tumor increased in echogenicity following therapy; it appeared to be related at least in part to tissue inhomogeneities formed following the disruption of the tumor neovasculature (Wood et al 2009). In detailed histological observations, the predominant effect of insonation was an irreparable dilation of the tumor capillaries with associated intercellular edema (Bunte et al 2006). There was also hemorrhage and increased intercellular fluid. A day after insonation liquefactive necrosis of the neoplastic cells had occurred and was related to a generalized tumor ischemia following the effects of therapy on the neoplasm's vascular channels. It should be noted that this histologic finding differs from that reported following tumor insonation with high intensity focused ultrasound where acute coagulative necrosis occurs, not only of the neoplastic cells, but also of the tumor vasculature (Van Leenders et al 2005; Wu et al 2001, 2002). These observed bioeffects resulted in a reduced tumor growth rate and a 28% increase in survival time following a single 3 minutes tumor therapy (Wood et al 2010). The antivascular action of low-intensity ultrasound was increased when tumor insonation was at 3 MHz rather than 1 MHz and the temperature of the tumor increased by 50C.min−1 and 20C.min−1 respectively (Wood et al 2008). Thus it was considered that a thermal affect on the endothelial cells lining the tumor capillaries may be important, although other bioeffects, sonochemical reactions and cellular responses could also play a role (Wood et al 2005, 2008). In recent studies, Hunt et al (2014) found that the effects of antivascular ultrasound go beyond direct cytotoxicity to include intratumoral immune activation.
In modeling blood flow in a tumor, Levenback et al (2012) considered the tissue as a continuum of microbubble-filled vasculature, cells and interstitial fluids with compressibility equal to the sum of the compressibility of each component. The mathematical simulations established that the absorption of ultrasound by viscous dampening of the microbubble oscillations induced local heating of the neovasculature. Microbubble enhanced heating was also modeled and observed in a phantom by Razansky et al (2006). Blood flow in tumor neovasculature is slower than in the normal vasculature of healthy tissues and thus in tumors there is additional time for an interaction between ultrasound and microbubbles with resultant thermally-induced damage to their endothelial cell linings, leading to the observed antivascular affect of therapy (Levenback et al 2012). Such damage will be significantly diminished in normal capillaries where the circulation is faster and any temperature rise will be much smaller and have no biological effect.
In a study of normal rabbits, McDannold et al (2006) insonated the brain using 1.5 MHz focused, continuous and pulsed ultrasound in the presence of circulating microbubbles. Small focal regions (1mm in diameter) were sonicated at intensities significantly > 5.0 W.cm−2. Magnetic resonance observations demonstrated temperature increases in the brain following insonation which had caused necrosis of the entire sonicated area following interruption of the vascular supply.
In a further simulation of tissue heating by microbubbles at 1mW.cm−2, Umemura et al (2005) found that the increased absorption of ultrasound was primarily at the resonance frequency; with ultrasonic intensities of 1 W.cm−2, increased absorption occurred at both resonance and sub-harmonic frequencies. At intensities greater than 1 W.cm−2 ultrasound absorption was observed at two or three broad-band resonance peaks. They concluded that microbubble enhanced absorption doubled at microbubble concentrations as low as 8 bubbles.mm−3. In vivo experiments in rat kidneys at high spatial peak intensities of up to 290 W.cm−2 in the focal range increased temperature by three to five times and caused coagulative necrosis of the tissue. In another study, Klotz et al (2012) modeled microbubble oscillations in a simulated microvascular network of the rat brain using focused ultrasound. The Pennes bioheat transfer equation was used to calculate the heat absorbed during the microbubble oscillations and they reported that the temperature increased most around the bubbles and to lesser extent throughout the tissues. In the presence of 107 microbubbles.mL−1, the ideal insonation parameters were found to be 1.5 MHz and pressure amplitudes of 0.8 - 1.4 MPa.
Predominantly cavitation bioeffects
In a review of in vitro observations of the affects of insonation of cancer cells in the presence of microbubbles, Bai et al (2012[a]) concluded that microbubble heating at < 0.2 MPa may be related to stable cavitation; at higher acoustic pressures of > 0.6 MPa inertial cavitation becomes important. Utilizing a low intensity insonation in the presence of microbubbles similar to that used by Wood et al (2007; 2008), Lin et al (2012[a]) found in rabbits that the growth of colorectal adenocarcinoma was inhibited. They hypothesized, however, that during insonation oscillation of the microbubble and its collapse with violent cavitation may disrupt the walls of the tumor neovasculature and so delay tumor growth.
Intravascular inertial cavitation was observed in rabbit ears by Tu et al (2006) using focused ultrasound at 1.17MHz and peak rarefaction pressures of 1-9 MPa (estimated intensity = 40-1,000 W.cm−2). Other in vivo studies of tumor models have centered on the antivascular actions of pulsed ultrasound beams at higher intensities and pressure amplitudes where the predominant effect on circulating microbubbles has been inertial cavitation. Using pulsed ultrasound, Goertz et al (2008, 2009) insonated various murine tumors following the intravenous injection of microbubles and found a reduction in tumor blood flow - when weekly treatments were given the growth of the tumor was inhibited (Goertz et al 2009). They reported that the signals emitted from the microbubbles during tumor insonation were consistent with the occurrence of inertial cavitation (Goertz et al 2009). Cavitation was also considered to be the bioeffect leading to reduced tumor growth, tumor necrosis and decreased expression of an angiogenesis marker (CD31; Huang et al 2013).
Zhong et al (2010) observed that ultrasound at a high mechanical index of 1.7 (an estimated intensity of 133 W.cm−2) and microbubbles inhibited hepatic metastases from a colon carcinoma in the spleen of rats. The metastases were smaller in number and size. Histology of the spleen showed a decrease in the number of tumor cells, hemorrhage and necrosis. It was concluded that cavitation had damaged the capillaries, activated coagulation systems and induced thromboses leading to avascular regions and blockage of metastatic channels.
Using higher peak intensities (2.6 and 4.8 MPa), Liu et al (2012) insonated a rat tumor following the intravenous injection of microbubbles and described disruption of the tumor microvasculature. Therapy was accompanied by decreases in tumor temperature and tumor mean gray scale. It was believed that the vascular effects were due to cavitation; these findings were supported by additional observations by Peijing et al 2014 in a rabbit tumor. Ultrasound at 4.3 MPa in combination with microbubbles enhanced liver ethanol ablation in rabbits and was accompanied by temporary interruption of perfusion (Liu et al 2013[b]). Wang et al (2013[c]) investigated the effect of focused ultrasound (mechanical index (MI) of 0.1-0.3, ISPTA = 539 mW.cm−2) and microbubbles on the permeability of the capillaries of a rat hepatoma; Evans blue was the marker of vascular permeablility. They suggested that the treatment regimen could induce intravascular cavitation so increasing the permeability of the neovasculature; higher mechanical index sonications caused more cavitation and induced greater vascular permeability.
Role of other bioeffects, chemotherapeutic and antiangiogenic agents, and antiangiogenic genes
Other bioeffects of therapy have also been considered. Following repeated daily treatments (over about 12 days) of a mouse colon adenocarcinoma in the presence of circulating microbubbles, Chin et al (2009) found that the tumors were smaller and insonation caused no significant temperature increases . They hypothesized that it was unlikely that tumor cells or vascular endothelium were destroyed acutely during treatment as tumor blood flow was restored after each therapy. They attributed the reduced tumor growth to an inflammatory response. After the intravenous injection of microbubbles, Burke et al (2011) insonated, (treatment time = 1 hour) a C6 rat glioma tumor implanted subcutaneously in mice using very short duty cycles (0.00002-0.01s). A week following therapy tumor necrosis and tumor growth inhibition was greatest when the longest duty cycles were used and the temperature of the tumor also increased post-therapy (an increase of 5.40C at a duty cycle of 0.01s). It was hypothesized that mechanical bioeffects were more important than thermal effects in the antivascular actions.
In another study of breast cancer cell model in mice, microbubbles conjugated with peptides were injected and bound to tumor endothelial cell receptors (Hu et al 2012). Insonation resulted in reduced tumor blood flow which returned to normal within 30 minutes. It was hypothesized that platelet activation was the likely mechanism for flow reduction in the neovasculature.
It was shown by Goertz et al (2012) that the antivascular action is enhanced by using the chemotherapeutic agent docetaxel at an higher pressure amplitude (1.65 MPa) and it was believed that inertial cavitation was the major source of the antivascular effects. By labeling a drug-loaded (carmestine) microbubble with an antigen to vascular endothelial growth factor, Fan et al (2013[b]) enhanced the delivery of the chemotherapeutic agent to the tumor neovasculature. The use of the antiangiogenic complex was studied in a rat glioma model where the blood-brain-barrier was broken down in presence of insonation and the circulating microbubble. The local delivery of the chemotherapeutic agent was enhanced with resultant suppression of the growth of the glioma and the animals' survival time was increased. The improved antitumor activity provided by anticancer drugs in the presence of ultrasound and microbubbles was also demonstrated by Rapoport et al (2007; 2.0W.cm−2), Burke and Price (2010; undefined chemotherapeutic agent and ultrasound intensities) and Lin et al (2012[b]; 0.6MPa). Antitumor activity was also superior when metronomic chemotherapy was combined with ultrasound and microbubbles at an ultrasound pressure amplitude of 1.65 MPa (Todorova et al 2013).
Zhang et al (2014) conjugated an antiangiogenic drug (Endostar) with a microbubble and retro-orbitally injected it in mice with a subcutaneous human colon cancer prior to insonation (238 kHz, pulsed, 0.5MPa). They reported an inhibition of tumor blood flow and a delay in tumor growth related to decreased expression of VEGFR-2 and alpha-v beta-3 integrin.
In observations of a murine colorectal adenocarcinoma, neoplastic cells were implanted in the ear (Lin et al 2012[b]). Polyethylene glycol coated liposomes loaded with doxorubicin were intravenously injected, followed by intravenous microbubbles and insonation (1.0 MHz, pulsed, 2.0 W. cm−2). Tumor growth was significantly inhibited and it was considered that the therapy disrupted tumor blood vessels and enhanced the delivery of drug into tumor tissue i.e. the antivascular effect trapped the doxorubicin in the tumor. Because of the vascular damage within the tumor, Lin et al (2012[b]) have raised the possibility that their treatment may facilitate the release of neoplastic cells into the general circulation leading to metastatic disease. Kang et al (2010) found, however, that although metastases from a liver tumor in rabbits still occurred their extent was reduced following therapy with docetaxel-loaded microbubbles and ultrasound.
There is also interest in a primarily antivascular role of gene therapy techniques. In a murine hepatocellular carcinoma model, Nie et al (2008) intravenously injected plasmid DNA with microbubbles and then insonated the subcutaneous tumor. Transference of an anti-angiogenic gene resulted in a significant antivascular affect with reduced microvessel density and tumor growth. Unlike the majority of studies where microbubbles were injected intravenously, Duvshani-Eshet et al (2007) used intratumoral injections of Optison to facilitate delivery of an angiogenesis inhibiting gene; the murine prostate cancer was insonated at 2.0 W.cm−2 and tumor growth was inhibited especially when weekly treatments were given over four weeks. Many antivascular effects are mediated through affects on the endothelial cells of the tumor neovasculature. In vitro culture studies by Al-Mahrouki et al (2012) showed that microbubbles stimulated at 0.57 MPa upregulated the genes involved in apoptosis pathways.
Additional comments
The combination of the use of microbubbles for both antivascular therapy and vascular imaging is an important innovation as such a theranostic agent exploits the use of ultrasound imaging for monitoring the success of anti-cancer therapy. Targeting the neovascular-cell compartment of a tumor with low intensity ultrasound in the presence of a circulating microbubble of ultrasound contrast agent consistently resulted in loss a of tumor vascular perfusion. As a result, the neoplastic cell compartment within the avascular regions underwent liquefactive necrosis with a resultant decrease in tumor volume and an increase in survival times of the laboratory animals. The velocity of blood flow in the tumor neovasculature is slower than in the vessels of the normal contiguous tissues; thus explaining why the bioeffects resulting from the interaction between ultrasound and a microbubble are so much greater within a neoplasm and why the circulation of the adjacent normal tissues appears unaffected. Thus the therapy technique accurately targets the neovasculature of the tumor whilst sparing the normal blood vessels in the adjacent healthy tissues.
In the antivascular treatment of a neoplasm it is essential to deliver sufficient energy to the circulating microbubbles within the lumens of the tumor neovasculature. The microbubbles provide a mechanism for delivering the acoustic energy at sites contiguous with the endothelial cells lining the neovasculature, resulting in their disruption. Both thermal and cavitation effects have been implicated as biophysical mechanisms of interaction. Those studies utilizing continuous wave ultrasound have emphasized thermal bioeffects whereas the pulsed ultrasound observations have emphasized cavitation. It is difficult to separate the two effects. In all likelihood both effects are likely to play a role, although by varying the nature of sonication conditions one or the other mechanism may dominate. For example, low intensities and long tone bursts or continuous wave ultrasound may emphasize microbubble oscillations for long intervals and thus lead to significant heating effects. Higher intensities with shorter pulses may on the other hand emphasize bubble collapse and thus inertial cavitation. In addition, other non-thermal effects including micro-shear associated with microbubble oscillations, radiation pressure and sonochemical reactions may also play a role, although to date the evidence for these mechanisms is limited.
Cancer cell death is a secondary effect of antivascular ultrasound – it has both benefits and limitations. The benefit is that the effect is generic and not specific to any tumor type. Further, it does not require treatment of individual cancer cells, since the survival of several thousand cells depends on an individual blood vessel; disrupting a few blood vessels could trigger cell death in many cancer cells. Antivascular ultrasound only requires access to the surface of the endothelial cells, unlike antivascular drugs that need to penetrate the cells to affect their cytoskeleton. Also, when compared to HIFU, the antivascular ultrasound instruments are likely to be less costly, simpler to design, and safer to use in clinical settings. In antivascular ultrasound the insonating beam in unfocused so that the entire neoplasm can be treated directly without the need to “paint” the lesion with successive small regions of treatment. A limitation of antivascular ultrasound is that susceptible tumor neovasculature has to be present for the therapy to be effective.
Future studies could be aimed at further investigating the potential for inciting an intra-tumoral immune response (Hunt et al 2014) and enhancing chemotherapeutic retention. Following antivascular ultrasound therapy of murine melanomas, Hunt et al (2014) described a lymphocyte-mediated inflammation characterized by the presence of T-cell lymphocytes within the tumors, which raises the possibility of the induction of a systemic post-therapy immune response against the cancer cells. In post-therapy MR observations of the melanomas it was found by Hunt et al (2014) that the washout of the MR contrast agent, gadolinium, was decreased and it was retained with the tumor, thus supporting the work of Goertz et al (2012) and demonstrating the potential role of antivascular ultrasound in trapping a simultaneously administered therapeutic agent within the tumor (Goertz et al 2012).
The antivascular effects on the tumor neovasculature are potent, but there remains, however, a need for additional experiments to optimize the bioeffects; the antivascular effects should be maximized whilst continuing to minimize the collateral damage in the adjacent normal tissues. The differences in mechanisms of interaction for different sonicaton intensities also need to be investigated.
CONCLUSIONS
Sonodynamic therapy, ultrasound mediated chemotherapy and gene delivery, and antivascular ultrasound therapy, all of which utilized low-intensity ultrasound, consistently produced bioeffects which resulted in the death of cancer cells. Low-intensity applications of ultrasound are believed to be safe, non-toxic, can easily be applied to a tumor, and the apparatus is relatively inexpensive. It is probable that more than a single bioeffect resulted in the efficacy of a therapy although, in animal tumor models, the importance of thermal and inertial cavitation bioeffects have been established. In some situations the accessibility of the tumor to insonation is a limitation as the cancer cells may be contiguous with a gas-containing structure or bone and not easily reached by the ultrasound beam. In such clinical situations, this limitation can be overcome by the use of intracavity transducers for imaging and therapy. Furthermore, the currently designed microbubbles have a limited loading capacity to deliver therapeutic agents at the required dosages. These limitations could be overcome by an improved design of microbubbles so that they can carry greater payloads. Co-administration of therapeutic agents along with the microbubbles could also be exploited as a means to overcome the issue of sufficient payload.
There is a generally held assumption that the agents used with ultrasound and microbubbles are not themselves directly affected by the insonation. In the presence of microbubble collapse and cavitation, sonochemical effects of ultrasound may also occur (Verrall and Sehgal 1988). Thus it remains to be shown whether injected agents including chemotherapeutics, genes or any other sonosensitizer are not directly altered by ultrasound in the presence of microbubbles; the lack of any significant direct insonation effect on these agents could potentially accelerate their clinical acceptance and introduction. Further, the underlying mechanisms of action and transport of the agent from the tumor neovasculature to their site of action in the extravascular space of the tumor could be further explored. Finally, because the neovasculature plays such a crucial role in the delivery of the agents, the in vitro cellular studies that are commonly performed by direct insonation should also, in future, take into consideration the role of tumor neovasculature.
The combination of the use of microbubbles for both therapy and vascular imaging is an important innovation as such a theranostic agent establishes a therapeutic-diagnostic platform which can monitor the success of anti-cancer therapy. The literature contains a large amount of data on the effects of low-intensity ultrasound therapy on cancer cells and mostly subcutaneously located tumor models in mice and rats. Future animal cancer trials should include longitudinal studies in which repeated treatments are given over time as these would mimic the likely clinical treatment regimens. There is a paucity of data, however, on the value of each of these therapies in naturally occurring cancers and in larger mammals; if the results of such future investigations were encouraging they could promptly be applied in a clinical trial.
Fig. 1.
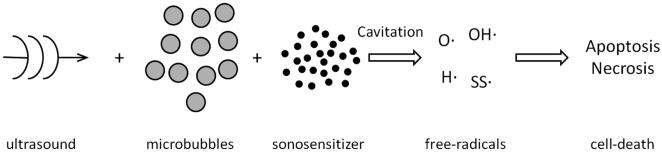
Schema of sonodynamic therapy. Low intensity insonation of cancer cells in the presence of a sonosensitizer causes cavitation leading to the production of free radicals with resultant cell death by apoptosis and necrosis.
Fig. 2.
Outline of different approaches for ultrasound mediated chemotherapy. Utilizing a variety of techniques, low intensity ultrasound is used to enhance the delivery of a chemotherapeutic agent to cancer cells. Key: US = ultrasound; chemo = chemotherapeutic agent.
Fig. 3.
Schema of antivascular ultrasound. Microbubbles contained in the neovasculature of a tumor are insonated with low intensity ultrasound causing a series of bioeffects which lead to vascular damage. Blood flow to the tumor is disrupted and there is consequential necrosis and apoptosis of cancer cells.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- Abe H, Kuroki M, Tachibana K, Li T, Awasthi A, Ueno A, Matsumoto H, Imakiire T, Yamauchi Y, Yamada H, Ariyoshi A, Kuroki M. Targeted sonodynamic therapy of cancer using a photosensitizer conjugated with antibody against carcinoembryonic antigen. Anticancer Res. 2002;22:1575–1580. [PubMed] [Google Scholar]
- Al-Mahrouki AA, Karshafian R, Giles A, Czarnota GJ. Bioeffects of ultrasound-stimulated microbubbles on endothelial cells: gene expression changes associated with radiation enhancement in vitro. Ultrasound Med Biol. 2012;38:1958–1969. doi: 10.1016/j.ultrasmedbio.2012.07.009. [DOI] [PubMed] [Google Scholar]
- Anderson CR, Hu X, Zhang H, Tlaxca J, Decléves AE, Houghtaling R, Sharma K, Lawrence M, Ferrara KW, Rychak JJ. Ultrasound molecular imaging of tumor angiogenesis with an integrin targeted microbubble contrast agent. Invest Radiol. 2011;46:215–224. doi: 10.1097/RLI.0b013e3182034fed. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aryal M, Vykhodtseva N, Zhang YZ, Park J, McDannold N. Multiple treatments with liposomal doxorubicin and ultrasound-induced disruption of blood-tumor and blood-brain barriers improve outcomes in a rat glioma model. J Control Release. 2013;169:103–111. doi: 10.1016/j.jconrel.2013.04.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baker KG, Robertson VJ, Duck FA. A review of therapeutic ultrasound: biophysical effects. Phys Ther. 2001;81:1351–1358. [PubMed] [Google Scholar]
- Bai WK, Shen E, Hu B. The induction of the apoptosis of cancer cell by sonodynamic therapy: a review. Chin J Cancer Res. 2012[a];24:368–373. doi: 10.3978/j.issn.1000-9604.2012.08.03. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bai WK, Wu ZH, Shen E, Zhang JZ, Hu B. The improvement of liposome-mediated transfection of pEGFP DNA into human prostate cancer cells by combining low-frequency and low-energy ultrasound with microbubbles. Oncol Rep. 2012[b];27:475–480. doi: 10.3892/or.2011.1510. [DOI] [PubMed] [Google Scholar]
- Barati AH, Mokhtari-Dizaji M, Mozdarani H, Bathaie SZ, Hassan ZM. Treatment of murine tumors using dual-frequency ultrasound in an experimental in vivo model. Ultrasound Med Biol. 2009;35:756–763. doi: 10.1016/j.ultrasmedbio.2008.11.004. [DOI] [PubMed] [Google Scholar]
- Barati AH, Mokhtari-Dizaji M. Ultrasound dose fractionation in sonodynamic therapy. Ultrasound Med Biol. 2010;36:880–887. doi: 10.1016/j.ultrasmedbio.2010.03.010. [DOI] [PubMed] [Google Scholar]
- Bernard V, Fojt L, Skorpíková J, Mornstein V. Determination of free cisplatin in medium by differential pulse polarography after ultrasound andcisplatin treatment of a cancer cell culture. Indian J Biochem Biophys. 2011;48:59–62. [PubMed] [Google Scholar]
- Bernard V, Mornstein V, Škorpíková J, Jaroš J. Ultrasound and cisplatin combined treatment of human melanoma cells A375--the study of sonodynamic therapy. Ultrasound Med Biol. 2012;38:1205–1211. doi: 10.1016/j.ultrasmedbio.2012.02.006. [DOI] [PubMed] [Google Scholar]
- Bunte RM, Ansaloni S, Sehgal CM, Lee WM, Wood AK. Histopathological observations of the antivascular effects of physiotherapy ultrasound on a murine neoplasm. Ultrasound Med Biol. 2006;32:453–461. doi: 10.1016/j.ultrasmedbio.2005.12.005. [DOI] [PubMed] [Google Scholar]
- Burgess A, Hynynen K. Drug delivery across the blood-brain barrier using focused ultrasound. Expert Opin Drug Deliv. 2014;11:711–721. doi: 10.1517/17425247.2014.897693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burke CW, Price RJ. Contrast ultrasound targeted treatment of gliomas in mice via drug-bearing nanoparticle delivery and microvascular ablation. J Vis Exp. 2010:46. doi: 10.3791/2145. doi:pii: 2145. 10.3791/2145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burke CW, Klibanov AL, Sheehan JP, Price RJ. Inhibition of glioma growth by microbubble activation in a subcutaneous model using low duty cycle ultrasound without significant heating. J Neurosurg. 2011;114:1654–61. doi: 10.3171/2010.11.JNS101201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carson AR, McTiernan CF, Lavery L, Hodnick A, Grata M, Leng X, Wang J, Chen X, Modzelewski RA, Villanueva FS. Gene therapy of carcinoma using ultrasound-targeted microbubble destruction. Ultrasound Med Biol. 2011;37:393–402. doi: 10.1016/j.ultrasmedbio.2010.11.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carson AR, McTiernan CF, Lavery L, Grata M, Leng X, Wang J, Chen X, Villanueva FS. Ultrasound-targeted microbubble destruction to deliver siRNA cancer therapy. Cancer Res. 2012;72:6191–6199. doi: 10.1158/0008-5472.CAN-11-4079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang S, Guo J, Sun J, Zhu S, Yan Y, Zhu Y, Li M, Wang Z, Xu RX. Targeted microbubbles for ultrasound mediated gene transfection and apoptosis induction in ovarian cancer cells. Ultrason Sonochem. 2013;20:171–179. doi: 10.1016/j.ultsonch.2012.06.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen CC, Sheeran PS, Wu SY, Olumolade OO, Dayton PA, Konofagou EE. Targeted drug delivery with focused ultrasound-induced blood-brain barrier opening using acoustically-activated nanodroplets. J Control Release. 2013;172:795–804. doi: 10.1016/j.jconrel.2013.09.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen JJ, Chen JJ, Chiang CS, Hong JH, Yeh CK. Assessment of tumor vasculature for diagnostic and therapeutic applications in a mouse model in vivo using 25-MHz power Doppler imaging. Ultrasonics. 2011;51:925–931. doi: 10.1016/j.ultras.2011.05.007. [DOI] [PubMed] [Google Scholar]
- Chen JJ, Fu SY, Chiang CS, Hong JH, Yeh CK. Characterization of tumor vasculature distributions in central and peripheral regions based on Doppler ultrasound. Med Phys. 2012[a];39:7490–7498. doi: 10.1118/1.4762683. [DOI] [PubMed] [Google Scholar]
- Chen JJ, Fu SY, Chiang CS, Hong JH, Yeh CK. A preclinical study to explore vasculature differences between primary and recurrent tumors using ultrasound Doppler imaging. Ultrasound Med Biol. 2013[a];39:860–869. doi: 10.1016/j.ultrasmedbio.2012.11.005. [DOI] [PubMed] [Google Scholar]
- Chen Z, Li J, Song X, Wang Z, Yue W. Use of a novel sonosensitizer in sonodynamic therapy of U251 glioma cells in vitro. Exp Ther Med. 2012[b];3:273–278. doi: 10.3892/etm.2011.390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen H, Zhou X, Gao Y, Zheng B, Tang F, Huang J. Recent progress in development of new sonosensitizers for sonodynamic cancer therapy. Drug Discov Today. 2014 Jan 30;pii:S1359–6446. doi: 10.1016/j.drudis.2014.01.010. [DOI] [PubMed] [Google Scholar]
- Chen B, Zheng R, Liu D, Li B, Lin J, Zhang W. The tumor affinity of chlorin e6 and its sonodynamic effects on non-small cell lung cancer. Ultrason Sonochem. 2013[b];20:667–673. doi: 10.1016/j.ultsonch.2012.09.008. [DOI] [PubMed] [Google Scholar]
- Chin CT, Raju BI, Shevchenko T, Klibanov AL. Control and reversal of tumor growth by ultrasound activated microbubbles. IEEE International Ultrasonics Symposium Proceedings. 2009:77–80. [Google Scholar]
- Chumakova OV, Liopo AV, Evers BM, Esenaliev RO. Effect of 5-fluorouracil, Optison and ultrasound on MCF-7 cell viability. Ultrasound Med Biol. 2006;32:751–758. doi: 10.1016/j.ultrasmedbio.2006.01.011. [DOI] [PubMed] [Google Scholar]
- Cochran MC, Eisenbrey J, Ouma RO, Soulen M, Wheatley MA. Doxorubicin and paclitaxel loaded microbubbles for ultrasound triggered drug delivery. Int J Pharm. 2011;414:161–170. doi: 10.1016/j.ijpharm.2011.05.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cool SK, Geers B, Roels S, Stremersch S, Vanderperren K, Saunders JH, De Smedt SC, Demeester J, Sanders NN. Coupling of drug containing liposomes to microbubbles improves ultrasound triggered drug delivery in mice. J Control Release. 2013 Dec;172:885–893. doi: 10.1016/j.jconrel.2013.09.014. [DOI] [PubMed] [Google Scholar]
- Cosgrove D. Ultrasound contrast agents: an overview. Eur J Radiol. 2006;60:324–330. doi: 10.1016/j.ejrad.2006.06.022. [DOI] [PubMed] [Google Scholar]
- Derieppe M, Yudina A, Lepetit-Coiffé M, de Senneville BD, Bos C, Moonen C. Real-time assessment of ultrasound-mediated drug delivery using fibered confocal fluorescence microscopy. Mol Imaging Biol. 2013;15:3–11. doi: 10.1007/s11307-012-0568-9. [DOI] [PubMed] [Google Scholar]
- Duvshani-Eshet M, Benny O, Morgenstern A, Machluf M. Therapeutic ultrasound facilitates antiangiogenic gene delivery and inhibits prostate tumor growth. Mol Cancer Ther. 2007;6:2371–2382. doi: 10.1158/1535-7163.MCT-07-0019. [DOI] [PubMed] [Google Scholar]
- Escoffre JM, Mannaris C, Geers B, Novell A, Lentacker I, Averkiou M, Bouakaz A. Doxorubicin liposome-loaded microbubbles for contrast imaging and ultrasound-triggered drug delivery. IEEE Trans Ultrason Ferroelectr Freq Control. 2013;60:78–87. doi: 10.1109/TUFFC.2013.2539. [DOI] [PubMed] [Google Scholar]
- Escoffre JM, Piron J, Novell A, Bouakaz A. Doxorubicin delivery into tumor cells with ultrasound and microbubbles. Mol Pharm. 2011;8:799–806. doi: 10.1021/mp100397p. [DOI] [PubMed] [Google Scholar]
- Fan CH, Ting CY, Lin HJ, Wang CH, Liu HL, Yen TC, Yeh CK. SPIO-conjugated, doxorubicin-loaded microbubbles for concurrent MRI and focused-ultrasound enhanced brain-tumor drug delivery. Biomaterials. 2013[a];34:3706–3715. doi: 10.1016/j.biomaterials.2013.01.099. [DOI] [PubMed] [Google Scholar]
- Fan CH, Ting CY, Liu HL, Huang CY, Hsieh HY, Yen TC, Wei KC, Yeh CK. Antiangiogenic-targeting drug-loaded microbubbles combined with focused ultrasound for glioma treatment. Biomaterials. 2013[b];34:2142–2155. doi: 10.1016/j.biomaterials.2012.11.048. [DOI] [PubMed] [Google Scholar]
- Feril LB, Ogawa R, Tachibana K, Kondo T. Optimized ultrasound-mediated gene transfection in cancer cells. Cancer Sci. 2006;97:1111–1114. doi: 10.1111/j.1349-7006.2006.00286.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feril LB, Tachibana K, Kondo T, Campbell PA. Sonodynamic therapy. In: Frenkel V, editor. Therapeutic ultrasound mechanisms to applications. Nova Sciences Publishers, Inc; New York,NY: 2011. pp. 279–295. [Google Scholar]
- Feril LB, Tachibana K. Use of ultrasound in drug delivery systems: emphasis on experimental methodology and mechanisms. Int J Hyperthermia. 2012;28:282–289. doi: 10.3109/02656736.2012.668640. [DOI] [PubMed] [Google Scholar]
- Ferrara KW, Borden MA, Zhang H. Lipid-shelled vehicles: engineering for ultrasound molecular imaging and drug delivery. Acc Chem Res. 2009;42:881–892. doi: 10.1021/ar8002442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fokong S, Theek B, Wu Z, Koczera P, Appold L, Jorge S, Resch-Genger U, van Zandvoort M, Storm G, Kiessling F, Lammers T. Image-guided, targeted and triggered drug delivery to tumors using polymer-based microbubbles. J Control Release. 2012;163:75–81. doi: 10.1016/j.jconrel.2012.05.007. [DOI] [PubMed] [Google Scholar]
- Folkman J. Angiogenesis. In: Braunwald E, Fauci AS, Kasper DL, Hauser SL, Longo DL, Jameson JL, editors. Harrison’s textbook of internal medicine. Mc Graw-Hill; New York: 2001. pp. 517–530. [Google Scholar]
- Fujii H, Matkar P, Liao C, Rudenko D, Lee PJ, Kuliszewski MA, Prud'homme GJ, Leong-Poi H. Optimization of Ultrasound-mediated Anti-angiogenic Cancer Gene Therapy. Mol Ther Nucleic Acids. 2013 May 21;2:e94. doi: 10.1038/mtna.2013.20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao ZG, Fain HD, Rapoport N. Controlled and targeted tumor chemotherapy by micellar-encapsulated drug and ultrasound. J Control Release. 2005;102:203–222. doi: 10.1016/j.jconrel.2004.09.021. [DOI] [PubMed] [Google Scholar]
- Gao HJ, Zhang WM, Wang XH, Zheng RN. Adriamycin enhances the sonodynamic effect of chlorin e6 against the proliferation of human breast cancer MDA-MB-231 cells in vitro. J South Med Univ. 2010;30:2291–2294. [PubMed] [Google Scholar]
- Gao Z, Zheng J, Yang B, Wang Z, Fan H, Lv Y, Li H, Jia L, Cao W. Sonodynamic therapy inhibits angiogenesis and tumor growth in a xenograft mouse model. Cancer Lett. 2013;335:93–99. doi: 10.1016/j.canlet.2013.02.006. [DOI] [PubMed] [Google Scholar]
- Geers B, Lentacker I, Sanders NN, Demeester J, Meairs S, De Smedt SC. Self-assembled liposome-loaded microbubbles: The missing link for safe and efficient ultrasound triggered drug-delivery. J Control Release. 2011;152:249–256. doi: 10.1016/j.jconrel.2011.02.024. [DOI] [PubMed] [Google Scholar]
- Geis NA, Katus HA, Bekeredjian R. Microbubbles as a vehicle for gene and drug delivery: current clinical implications and future perspectives. Curr Pharm Des. 2012;18:2166–2183. doi: 10.2174/138161212800099946. [DOI] [PubMed] [Google Scholar]
- Goertz DE, Yu JL, Kerbel RS, Burns PN, Foster FS. High-frequency Doppler ultrasound monitors the effects of antivascular therapy on tumor blood flow. Cancer Res. 2002;62:6371–6375. [PubMed] [Google Scholar]
- Goertz DE, Karshafian R, Hynynen K. Antivascular effects of pulsed low intensity ultrasound and microbubbles in mouse tumors. IEEE International Ultrasonics Symposium Proceedings. 2008:670–673. [Google Scholar]
- Goertz DE, Karshafian R, Hynynen K. Investigating the effects of pulsed low intensity ultrasound and microbubbles in mouse tumors. IEEE International Ultrasonics Symposium Proceedings. 2009:89–92. [Google Scholar]
- Goertz DE, Todorova M, Mortazavi O, Agache V, Chen B, Karshafian R, Hynynen K. Antitumor effects of combining docetaxel (taxotere) with the antivascular action of ultrasound stimulated microbubbles. PLoS One. 2012;7:e52307. doi: 10.1371/journal.pone.0052307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haag P, Frauscher F, Gradl J, Seitz A, Schäfer G, Lindner JR, Klibanov AL, Bartsch G, Klocker H, Eder IE. Microbubble-enhanced ultrasound to deliver an antisense oligodeoxynucleotide targeting the human androgen receptor into prostate tumours. J Steroid Biochem Mol Biol. 2006;102:103–113. doi: 10.1016/j.jsbmb.2006.09.027. [DOI] [PubMed] [Google Scholar]
- Hachimine K, Shibaguchi H, Kuroki M, Yamada H, Kinugasa T, Nakae Y, Asano R, Sakata I, Yamashita Y, Shirakusa T, Kuroki M. Sonodynamic therapy of cancer using a novel porphyrin derivative, DCPH-P-Na(I), which is devoid of photosensitivity. Cancer Sci. 2007;98:916–920. doi: 10.1111/j.1349-7006.2007.00468.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hassan MA, Furusawa Y, Minemura M, Rapoport N, Sugiyama T, Kondo T. Ultrasound-induced new cellular mechanism involved in drug resistance. PLoS One. 2012;7:e48291. doi: 10.1371/journal.pone.0048291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hauser J, Ellisman M, Steinau HU, Stefan E, Dudda M, Hauser M. Ultrasound enhanced endocytotic activity of human fibroblasts. Ultrasound Med Biol. 2009;35:2084–2092. doi: 10.1016/j.ultrasmedbio.2009.06.1090. [DOI] [PubMed] [Google Scholar]
- Heath CH, Sorace A, Knowles J, Rosenthal E, Hoyt K. Microbubble therapy enhances anti-tumor properties of cisplatin and cetuximab in vitro and in vivo. Otolaryngol Head Neck Surg. 2012;146:938–945. doi: 10.1177/0194599812436648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu X, Kheirolomoom A, Mahakian LM, Beegle JR, Kruse DE, Lam KS, Ferrara KW. Insonation of targeted microbubbles produces regions of reduced blood flow within tumor vasculature. Invest Radiol. 2012;47:398–405. doi: 10.1097/RLI.0b013e31824bd237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang P, You X, Pan M, Li S, Zhang Y, Zhao Y, Wang M, Hong Y, Pu Z, Chen L, Yang G, Guo Y. A novel therapeutic strategy using ultrasound mediated microbubbles destruction to treat colon cancer in a mouse model. Cancer Lett. 2013;335:183–190. doi: 10.1016/j.canlet.2013.02.011. [DOI] [PubMed] [Google Scholar]
- Hunt SJ, Gade T, Soulen MC, Pickup S, Sehgal CM. Antivascular ultrasound therapy: MR validation and activation of immune response in murine melanoma. J Ultrasound Med. 2014 doi: 10.7863/ultra.34.2.275. (in press) [DOI] [PubMed] [Google Scholar]
- Husseini GA, Pitt WG. Ultrasonic-activated micellar drug delivery for cancer treatment. J Pharm Sci. 2009;98:795–811. doi: 10.1002/jps.21444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hyvelin JM, Tardy I, Arbogast C, Costa M, Emmel P, Helbert A, Theraulaz M, Nunn AD, Tranquart F. Use of ultrasound contrast agent microbubbles in preclinical research: recommendations for small animal imaging. Invest Radiol. 2013;48:570–583. doi: 10.1097/RLI.0b013e318289f854. [DOI] [PubMed] [Google Scholar]
- Ibsen S, Benchimol M, Simberg D, Esener S. Ultrasound mediated localized drug delivery. Adv Exp Med Biol. 2012;733:145–153. doi: 10.1007/978-94-007-2555-3_14. [DOI] [PubMed] [Google Scholar]
- Ibsen S, Schutt CE, Esener S. Microbubble-mediated ultrasound therapy: a review of its potential in cancer treatment. Drug Des Devel Ther. 2013;7:375–388. doi: 10.2147/DDDT.S31564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jeong EJ, Seo SJ, Ahn YJ, Choi KH, Kim KH, Kim JK. Sonodynamically induced antitumor effects of 5-aminolevulinic acid and fractionated ultrasound irradiation in an orthotopic rat glioma model. Ultrasound Med Biol. 2012;38:2143–2150. doi: 10.1016/j.ultrasmedbio.2012.07.026. [DOI] [PubMed] [Google Scholar]
- Jin ZH, Miyoshi N, Ishiguro K, Umemura S, Kawabata K, Yumita N, Sakata I, Takaoka K, Udagawa T, Nakajima S, Tajiri H, Ueda K, Fukuda M, Kumakiri M. Combination effect of photodynamic and sonodynamic therapy on experimental skin squamous cell carcinoma in C3H/HeN mice. J Dermatol. 2000;27:294–306. doi: 10.1111/j.1346-8138.2000.tb02171.x. [DOI] [PubMed] [Google Scholar]
- Kang J, Wu X, Wang Z, Ran H, Xu C, Wu J, Wang Z, Zhang Y. Antitumor effect of docetaxel-loaded lipid microbubbles combined with ultrasound-targeted microbubble activation on VX2 rabbit liver tumors. J Ultrasound Med. 2010;29:61–70. doi: 10.7863/jum.2010.29.1.61. [DOI] [PubMed] [Google Scholar]
- Kang ST, Yeh CK. Ultrasound microbubble contrast agents for diagnostic and therapeutic applications: current status and future design. Chang Gung Med J. 2012;35:125–139. doi: 10.4103/2319-4170.106159. [DOI] [PubMed] [Google Scholar]
- Kovacs Z, Werner B, Rassi A, Sass JO, Martin-Fiori E, Bernasconi M. Prolonged survival upon ultrasound-enhanced doxorubicin delivery in two syngenic glioblastoma mouse models. J Control Release. 2014;187:74–82. doi: 10.1016/j.jconrel.2014.05.033. [DOI] [PubMed] [Google Scholar]
- Klotz AR, Lindvere L, Stefanovic B, Hynynen K. Temperature change near microbubbles within a capillary network during focused ultrasound. Phys Med Biol. 2010 Mar 21;55(6):1549–61. doi: 10.1088/0031-9155/55/6/001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuroki M, Hachimine K, Abe H, Shibaguchi H, Kuroki M, Maekawa S, Yanagisawa J, Kinugasa T, Tanaka T, Yamashita Y. Sonodynamic therapy of cancer using novel sonosensitizers. Anticancer Res. 2007;27:3673–3677. [PubMed] [Google Scholar]
- Lentacker I, Geers B, Demeester J, De Smedt SC, Sanders NN. Design and evaluation of doxorubicin-containing microbubbles for ultrasound-triggered doxorubicin delivery: cytotoxicity and mechanisms involved. Mol Ther. 2010;18:101–108. doi: 10.1038/mt.2009.160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levenback BJ, Sehgal CM, Wood AK. Modeling of thermal effects in antivascular ultrasound therapy. J Acoust Soc Am. 2012;131:540–549. doi: 10.1121/1.3662048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li H, Fan H, Wang Z, Zheng J, Cao W. Potentiation of scutellarin on human tongue carcinoma xenograft by low-intensity ultrasound. PLoS One. 2013;8:e59473. doi: 10.1371/journal.pone.0059473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Y, Wang P, Zhao P, Zhu S, Wang X, Liu Q. Apoptosis induced by sonodynamic treatment by protoporphyrin IX on MDA-MB-231 cells. Ultrasonics. 2012[a];52:490–496. doi: 10.1016/j.ultras.2011.10.013. [DOI] [PubMed] [Google Scholar]
- Li P, Zheng Y, Ran H, Tan J, Lin Y, Zhang Q, Ren J, Wang Z. Ultrasound triggered drug release from 10-hydroxycamptothecin-loaded phospholipid microbubbles for targeted tumor therapy in mice. J Control Release. 2012[b];162:349–354. doi: 10.1016/j.jconrel.2012.07.009. [DOI] [PubMed] [Google Scholar]
- Li XH, Zhou P, Wang LH, Tian SM, Qian Y, Chen LR, Zhang P. The targeted gene (KDRP-CD/TK) therapy of breast cancer mediated by SonoVue and ultrasound irradiation in vitro. Ultrasonics. 2012[c];52:186–191. doi: 10.1016/j.ultras.2011.08.002. [DOI] [PubMed] [Google Scholar]
- Li F, Jin L, Wang H, Wei F, Bai M, Shi Q, Du L. The dual effect of ultrasound-targeted microbubble destruction in mediating recombinant adeno-associated virus delivery in renal cell carcinoma: transfection enhancement and tumor inhibition. J Gene Med. 2014;16:28–39. doi: 10.1002/jgm.2755. [DOI] [PubMed] [Google Scholar]
- Liang HD, Tang J, Halliwell M. Sonoporation, drug delivery, and gene therapy. Proc Inst Mech Eng H. 2010;224:343–361. doi: 10.1243/09544119JEIM565. [DOI] [PubMed] [Google Scholar]
- Liang L, Xie S, Jiang L, Jin H, Li S, Liu J. The Combined effects of hematoporphyrin monomethyl ether-SDT and doxorubicin on the proliferation of QBC939 cell lines. Ultrasound Med Biol. 2013;39:146–160. doi: 10.1016/j.ultrasmedbio.2012.08.017. [DOI] [PubMed] [Google Scholar]
- Liao AH, Li YK, Lee WJ, Wu MF, Liu HL, Kuo ML. Estimating the delivery efficiency of drug-loaded microbubbles in cancer cells with ultrasound and bioluminescence imaging. Ultrasound Med Biol. 2012;38:1938–1948. doi: 10.1016/j.ultrasmedbio.2012.07.013. [DOI] [PubMed] [Google Scholar]
- Liao AH, Liu HL, Su CH, Hua MY, Yang HW, Weng YT, Hsu PH, Huang SM, Wu SY, Wang HE, Yen TC, Li PC. Paramagnetic perfluorocarbon-filled albumin-(Gd-DTPA) microbubbles for the induction of focused-ultrasound-induced blood-brain barrier opening and concurrent MR and ultrasound imaging. Phys Med Biol. 2012;57:2787–2802. doi: 10.1088/0031-9155/57/9/2787. [DOI] [PubMed] [Google Scholar]
- Lim AK, Patel N, Eckersley RJ, Taylor-Robinson SD, Cosgrove DO, Blomley MJ. Evidence for spleen-specific uptake of a microbubble contrast agent: a quantitative study in healthy volunteers. Radiology. 2004;231:785–788. doi: 10.1148/radiol.2313030544. [DOI] [PubMed] [Google Scholar]
- Lin CY, Li JR, Tseng HC, Wu MF, Lin WL. Enhancement of focused ultrasound with microbubbles on the treatments of anticancer nanodrug in mouse tumors. Nanomedicine. 2012[a];8:900–907. doi: 10.1016/j.nano.2011.10.005. [DOI] [PubMed] [Google Scholar]
- Lin CY, Tseng HC, Shiu HR, Wu MF, Chou CY, Lin WL. Ultrasound sonication with microbubbles disrupts blood vessels and enhances tumor treatments of anticancer nanodrug. Int J Nanomedicine. 2012[b];7:2143–2152. doi: 10.2147/IJN.S29514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lionetti V, Paddeu S. Paradossi G, Pellegretti P, Trucco A, editors. Towards ultrasound molecular imaging. Ultrasound contrast agents. Targeting and processing methods for theranostics. 2010:1. [Google Scholar]
- Liu Y, Miyoshi H, Nakamura M. Encapsulated ultrasound microbubbles: therapeutic application in drug/gene delivery. J Control Release. 2006[a];114:89–99. doi: 10.1016/j.jconrel.2006.05.018. [DOI] [PubMed] [Google Scholar]
- Liu Q, Wang X, Wang P, Qi H, Zhang K, Xiao L. Sonodynamic effects of protoporphyrin IX disodium salt on isolated sarcoma 180 cells. Ultrasonics. 2006[b];45:56–60. doi: 10.1016/j.ultras.2006.06.063. [DOI] [PubMed] [Google Scholar]
- Liu Q, Wang X, Wang P, Xiao L. Sonodynamic antitumor effect of protoporphyrin IX disodium salt on S180 solid tumor. Chemotherapy. 2007 (a);53:429–436. doi: 10.1159/000110008. [DOI] [PubMed] [Google Scholar]
- Liu Q, Wang X, Wang P, Xiao L, Hao Q. Comparison between sonodynamic effect with protoporphyrin IX and hematoporphyrin on sarcoma 180. Cancer Chemother Pharmacol. 2007 (b);60:671–680. doi: 10.1007/s00280-006-0413-4. [DOI] [PubMed] [Google Scholar]
- Liu GJ, Moriyasu F, Hirokawa T, Rexiati M, Yamada M, Imai Y. Optical microscopic findings of the behavior of perflubutane microbubbles outside and insideKupffer cells during diagnostic ultrasound examination. Invest Radiol. 2008[a];43:829–836. doi: 10.1097/RLI.0b013e3181852719. [DOI] [PubMed] [Google Scholar]
- Liu Q, Li X, Xiao L, Wang P, Wang X, Tang W. Sonodynamically induced antitumor effect of hematoporphyrin on Hepatoma 22. Ultrason Sonochem. 2008[b];15:943–948. doi: 10.1016/j.ultsonch.2008.04.001. [DOI] [PubMed] [Google Scholar]
- Liu HL, Hua MY, Chen PY, Chu PC, Pan CH, Yang HW, Huang CY, Wang JJ, Yen TC, Wei KC. Blood-brain barrier disruption with focused ultrasound enhances delivery of chemotherapeutic drugs for glioblastoma treatment. Radiology. 2010;255:415–425. doi: 10.1148/radiol.10090699. [DOI] [PubMed] [Google Scholar]
- Liu Z, Gao S, Zhao Y, Li P, Liu J, Li P, Tan K, Xie F. Disruption of tumor neovasculature by microbubble enhanced ultrasound: a potential new physical therapy of anti-angiogenesis. Ultrasound Med Biol. 2012;38:253–261. doi: 10.1016/j.ultrasmedbio.2011.11.007. [DOI] [PubMed] [Google Scholar]
- Liu YN, Khangura J, Xie A, Belcik JT, Qi Y, Davidson BP, Zhao Y, Kim S, Inaba Y, Lindner JR. Renal retention of lipid microbubbles: a potential mechanism for flank discomfort during ultrasound contrast administration. J Am Soc Echocardiogr. 2013[a];26:1474–1481. doi: 10.1016/j.echo.2013.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Q, Zhao H, Wu S, Zhao X, Zhong Y, Li L, Liu Z. Impact of microbubble-enhanced ultrasound on liver ethanol ablation. Ultrasound Med Biol. 2013[b];39:1039–1046. doi: 10.1016/j.ultrasmedbio.2013.01.007. [DOI] [PubMed] [Google Scholar]
- Liu HL, Fan CH, Ting CY, Yeh CK. Combining microbubbles and ultrasound for drug delivery to brain tumors: current progress and overview. Theranostics. 2014;4:432–444. doi: 10.7150/thno.8074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu CT, Zhao YZ, Wu Y, Tian XQ, Li WF, Huang PT, Li XK, Sun CZ, Zhang L. Experiment on enhancing antitumor effect of intravenous epirubicin hydrochloride by acoustic cavitation in situ combined with phospholipid-based microbubbles. Cancer Chemother Pharmacol. 2011;68:343–348. doi: 10.1007/s00280-010-1489-4. [DOI] [PubMed] [Google Scholar]
- Lv Y, Fang M, Zheng J, Yang B, Li H, Xiuzigao Z, Song W, Chen Y, Cao W. Low-intensity ultrasound combined with 5-aminolevulinic acid administration in the treatment of human tongue squamous carcinoma. Cell Physiol Biochem. 2012;30:321–333. doi: 10.1159/000339067. [DOI] [PubMed] [Google Scholar]
- Mason RP, Zhao D, Liu L, Trawick ML, Pinney KG. A perspective on vascular disrupting agents that interact with tubulin: preclinical tumor imaging and biological assessment. Integr Biol (Camb) 2011;3:375–387. doi: 10.1039/c0ib00135j. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsuo M, Yamaguchi K, Feril LB, Jr, Endo H, Ogawa K, Tachibana K, Nakayama J. Synergistic inhibition of malignant melanoma proliferation by melphalan combined with ultrasound and microbubbles. Ultrason Sonochem. 2011;18:1218–1224. doi: 10.1016/j.ultsonch.2011.03.005. [DOI] [PubMed] [Google Scholar]
- Mayer CR, Geis NA, Katus HA, Bekeredjian R. Ultrasound targeted microbubble destruction for drug and gene delivery. Expert Opin Drug Deliv. 2008;5:1121–1138. doi: 10.1517/17425247.5.10.1121. [DOI] [PubMed] [Google Scholar]
- McDannold NJ, Vykhodtseva NI, Hynynen K. Microbubble contrast agent with focused ultrasound to create brain lesions at low power levels: MR imaging and histologic study in rabbits. Radiology. 2006;241:95–106. doi: 10.1148/radiol.2411051170. [DOI] [PubMed] [Google Scholar]
- Meijering BD, Juffermans LJ, van Wamel A, Henning RH, Zuhorn IS, Emmer M, Versteilen AM, Paulus WJ, van Gilst WH, Kooiman K, de Jong N, Musters RJ, Deelman LE, Kamp O. Ultrasound and microbubble-targeted delivery of macromolecules is regulated by induction of endocytosis and pore formation. Circ Res. 2009;104:679–687. doi: 10.1161/CIRCRESAHA.108.183806. [DOI] [PubMed] [Google Scholar]
- Misík V, Riesz P. Free radical intermediates in sonodynamic therapy. N Y Acad Sci. 2000;899:335–348. doi: 10.1111/j.1749-6632.2000.tb06198.x. [DOI] [PubMed] [Google Scholar]
- Nie F, Xu HX, Lu MD, Wang Y, Tang Q. Anti-angiogenic gene therapy for hepatocellular carcinoma mediated by microbubble-enhanced ultrasound exposure: an in vivo experimental study. J Drug Target. 2008;16:389–395. doi: 10.1080/10611860802088846. [DOI] [PubMed] [Google Scholar]
- Ninomiya K, Ogino C, Oshima S, Sonoke S, Kuroda S, Shimizu N. Targeted sonodynamic therapy using protein-modified TiO2 nanoparticles. Ultrason Sonochem. 2012;19:607–614. doi: 10.1016/j.ultsonch.2011.09.009. [DOI] [PubMed] [Google Scholar]
- Ninomiya K, Noda K, Ogino C, Kuroda S, Shimizu N. Enhanced OH radical generation by dual-frequency ultrasound with TiO2 nanoparticles: its application to targeted sonodynamic therapy. Ultrason Sonochem. 2014;21:289–294. doi: 10.1016/j.ultsonch.2013.05.005. [DOI] [PubMed] [Google Scholar]
- Niu C, Wang Z, Lu G, Krupka TM, Sun Y, You Y, Song W, Ran H, Li P, Zheng Y. Doxorubicin loaded superparamagnetic PLGA-iron oxide multifunctional microbubbles for dual-mode US/MR imaging and therapy of metastasis in lymph nodes. Biomaterials. 2013;34:2307–2317. doi: 10.1016/j.biomaterials.2012.12.003. [DOI] [PubMed] [Google Scholar]
- Nomikou N, Li YS, McHale AP. Ultrasound-enhanced drug dispersion through solid tumours and its possible role in aiding ultrasound-targeted cancer chemotherapy. Cancer Lett. 2010[a];288:94–98. doi: 10.1016/j.canlet.2009.06.028. [DOI] [PubMed] [Google Scholar]
- Nomikou N, McHale AP. Exploiting ultrasound-mediated effects in delivering targeted, site-specific cancer therapy. Cancer Lett. 2010[b];296:133–143. doi: 10.1016/j.canlet.2010.06.002. [DOI] [PubMed] [Google Scholar]
- Ohmura T, Fukushima T, Shibaguchi H, Yoshizawa S, Inoue T, Kuroki M, Sasaki K, Umemura S. Sonodynamic therapy with 5-aminolevulinic acid and focused ultrasound for deep-seated intracranial glioma in rat. Anticancer Res. 2011;31:2527–2533. [PubMed] [Google Scholar]
- Owen J, Pankhurst Q, Stride E. Magnetic targeting and ultrasound mediated drug delivery: benefits, limitations and combination. Int J Hyperthermia. 2012;28:362–373. doi: 10.3109/02656736.2012.668639. [DOI] [PubMed] [Google Scholar]
- Park EJ, Zhang YZ, Vykhodtseva N, McDannold N. Ultrasound-mediated blood-brain/blood-tumor barrier disruption improves outcomes with trastuzumab in a breast cancer brain metastasis model. J Control Release. 2012;163:277–284. doi: 10.1016/j.jconrel.2012.09.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peijing L, Mei Z, Yali X, Yang Z, Shunji G, Gao Yun-hua G. Impact of microbubble enhanced, pulsed, focused ultrasound on tumor circulation of subcutaneous VX2 cancer. Chinese Medical Journal. 2014;127:2605–2611. [PubMed] [Google Scholar]
- Perini R, Choe R, Yodh AG, Sehgal C, Divgi CR, Rosen MA. Non-invasive assessment of tumor neovasculature: techniques and clinical applications. Cancer Metastasis Rev. 2008;27:615–630. doi: 10.1007/s10555-008-9147-6. [DOI] [PubMed] [Google Scholar]
- Peyman SA, Abou-Saleh RH, Evans SD. Research Spotlight: Microbubbles for therapeutic delivery. Ther Deliv. 2013;4:539–542. doi: 10.4155/tde.13.23. [DOI] [PubMed] [Google Scholar]
- Preston RC. Hydrophone-based measurements on a specific acoustic pulse. Part 1: Field characterization. In: Preston RC, editor. Output measurements for medical ultrasound. Springer-Verlag; London, UK: 1991. p. 95. [Google Scholar]
- Rapoport N. Ultrasound-mediated micellar drug delivery. Int J Hyperthermia. 2012;28:374–385. doi: 10.3109/02656736.2012.665567. [DOI] [PubMed] [Google Scholar]
- Rapoport N, Gao Z, Kennedy A. Multifunctional nanoparticles for combining ultrasonic tumor imaging and targeted chemotherapy. J Natl Cancer Inst. 2007;99:1095–2152. doi: 10.1093/jnci/djm043. [DOI] [PubMed] [Google Scholar]
- Rapoport NY, Kennedy AM, Shea JE, Scaife CL, Nam KH. Controlled and targeted tumor chemotherapy by ultrasound-activated nanoemulsions/microbubbles. J Control Release. 2009;138:268–276. doi: 10.1016/j.jconrel.2009.05.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Razansky D, Einziger PD, Adam DR. Enhanced heat deposition using ultrasound contrast agent--modeling and experimental observations. IEEE Trans Ultrason Ferroelectr Freq Control. 2006;53:137–147. doi: 10.1109/tuffc.2006.1588399. [DOI] [PubMed] [Google Scholar]
- Ren ST, Liao YR, Kang XN, Li YP, Zhang H, Ai H, Sun Q, Jing J, Zhao XH, Tan LF, Shen XL, Wang B. The antitumor effect of a new docetaxel-loaded microbubble combined with low-frequency ultrasound in vitro: preparation and parameter analysis. Pharm Res. 2013 Feb;:16. doi: 10.1007/s11095-013-0996-5. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- Rosenthal I, Sostaric JZ, Riesz P. Sonodynamic therapy--a review of the synergistic effects of drugs and ultrasound. Ultrason Sonochem. 2004;11:349–363. doi: 10.1016/j.ultsonch.2004.03.004. [DOI] [PubMed] [Google Scholar]
- Sakakima Y, Hayashi S, Yagi Y, Hayakawa A, Tachibana K, Nakao A. Gene therapy for hepatocellular carcinoma using sonoporation enhanced by contrast agents. Cancer Gene Ther. 2005;12:884–889. doi: 10.1038/sj.cgt.7700850. [DOI] [PubMed] [Google Scholar]
- Samiotaki G, Konofagou EE. Dependence of the reversibility of focused-ultrasound-induced blood-brain barrier opening on pressure and pulse length in vivo. IEEE Trans Ultrason Ferroelectr Freq Control. 2013;60:2257–2265. doi: 10.1109/TUFFC.2013.6644731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sazgarnia A, Shanei A, Meibodi NT, Eshghi H, Nassirli H. A novel nanosonosensitizer for sonodynamic therapy: in vivo study on a colon tumor model. J Ultrasound Med. 2011;30:1321–1329. doi: 10.7863/jum.2011.30.10.1321. [DOI] [PubMed] [Google Scholar]
- Schroeder A, Honen R, Turjeman K, Gabizon A, Kost J, Barenholz Y. Ultrasound triggered release of cisplatin from liposomes in murine tumors. J Control Release. 2009[a];137:63–8. doi: 10.1016/j.jconrel.2009.03.007. [DOI] [PubMed] [Google Scholar]
- Schroeder A, Kost J, Barenholz Y. Ultrasound, liposomes, and drug delivery: principles for using ultrasound to control the release of drugs from liposomes. Chem Phys Lipids. 2009[b];162:1–16. doi: 10.1016/j.chemphyslip.2009.08.003. [DOI] [PubMed] [Google Scholar]
- Sehgal CM, Cary TW, Arger PH, Wood AKW. Delta-projection imaging on contrast-enhanced ultrasound to quantify tumor microvasculature and perfusion. Acad Radiol. 2009;16:71–78. doi: 10.1016/j.acra.2008.07.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shanei A, Sazgarnia A, Tayyebi Meibodi N, Eshghi H, Hassanzadeh-Khayyat M, Esmaily H, Attaran Kakhki N. Sonodynamic Therapy Using Protoporphyrin IX Conjugated to Gold Nanoparticles: An In Vivo Study on a Colon Tumor Model. Iran J Basic Med Sci. 2012;15:759–767. [PMC free article] [PubMed] [Google Scholar]
- Shi H, Liu Q, Qin X, Wang P, Wang X. Pharmacokinetic study of a novel sonosensitizer chlorin-e6 and its sonodynamic anti-cancer activity in hepatoma-22 tumor-bearing mice. Biopharm Drug Dispos. 2011;32:319–332. doi: 10.1002/bdd.761. [DOI] [PubMed] [Google Scholar]
- Shibaguchi H, Tsuru H, Kuroki M, Kuroki M. Sonodynamic cancer therapy: a non-invasive and repeatable approach using low-intensity ultrasound with a sonosensitizer. Anticancer Res. 2011;31:2425–2429. [PubMed] [Google Scholar]
- Siemannn DW. Tumor vasculature: a target for anticancer therapies. Vascular-targeted therapies in oncology. In: Siemann DW, editor. John Wiley and Sons Ltd; Chichester: 2006. pp. 1–8. [Google Scholar]
- Sirsi SR, Borden MA. Advances in ultrasound mediated gene therapy using microbubble contrast agents. Theranostics. 2012;2:1208–1222. doi: 10.7150/thno.4306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sirsi SR, Hernandez SL, Zielinski L, Blomback H, Koubaa A, Synder M, Homma S, Kandel JJ, Yamashiro DJ, Borden MA. Polyplex-microbubble hybrids for ultrasound-guided plasmid DNA delivery to solid tumors. J Control Release. 2012;157:224–234. doi: 10.1016/j.jconrel.2011.09.071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sorace AG, Warram JM, Umphrey H, Hoyt K. Microbubble-mediated ultrasonic techniques for improved chemotherapeutic delivery in cancer. J Drug Target. 2012;20:43–54. doi: 10.3109/1061186X.2011.622397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Staples BJ, Pitt WG, Roeder BL, Husseini GA, Rajeev D, Schaalje GB. Distribution of doxorubicin in rats undergoing ultrasonic drug delivery. J Pharm Sci. 2010;99:3122–3131. doi: 10.1002/jps.22088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stride EP, Coussios CC. Cavitation and contrast: the use of bubbles in ultrasound imaging and therapy. Proc Inst Mech Eng H. 2010;224:171–191. doi: 10.1243/09544119JEIM622. [DOI] [PubMed] [Google Scholar]
- Su X, Wang P, Wang X, Cao B, Li L, Liu Q. Apoptosis of U937 cells induced by hematoporphyrin monomethyl ether-mediated sonodynamic action. Cancer Biother Radiopharm. 2013[a];28:207–217. doi: 10.1089/cbr.2012.1190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Su X, Wang P, Wang X, Guo L, Li S, Liu Q. Involvement of MAPK activation and ROS generation in human leukemia U937 cells undergoing apoptosis in response to sonodynamic therapy. Int J Radiat Biol. 2013[b];89:915–927. doi: 10.3109/09553002.2013.817700. [DOI] [PubMed] [Google Scholar]
- Su X, Li Y, Wang P, Wang X, Liu Q. Protoporphyrin IX-mediated sonodynamic action induces apoptosis of K562 cells. Ultrasonics. 2014;54:275–284. doi: 10.1016/j.ultras.2013.07.015. [DOI] [PubMed] [Google Scholar]
- Sugita N, Iwase Y, Yumita N, Ikeda T, Umemura S. Sonodynamically induced cell damage using rose bengal derivative. Anticancer Res. 2010;30:3361–3366. [PubMed] [Google Scholar]
- Tang W, Liu Q, Wang X, Mi N, Wang P, Zhang J. Membrane fluidity altering and enzyme inactivating in sarcoma 180 cells post the exposure to sonoactivated hematoporphyrin in vitro. Ultrasonics. 2008 [a];48:66–73. doi: 10.1016/j.ultras.2007.10.002. [DOI] [PubMed] [Google Scholar]
- Tang W, Liu Q, Wang X, Wang P, Cao B, Mi N, Zhang J. Involvement of caspase 8 in apoptosis induced by ultrasound-activated hematoporphyrin in sarcoma 180 cells in vitro. J Ultrasound Med. 2008 [b];27:645–656. doi: 10.7863/jum.2008.27.4.645. [DOI] [PubMed] [Google Scholar]
- Tang W, Liu Q, Wang X, Zhang J, Wang P, Mi N. Ultrasound exposure in the presence of hematoporphyrin induced loss of membrane integral proteins and inactivity of cell proliferation associated enzymes in sarcoma 180 cells in vitro. Ultrason Sonochem. 2008 [c];15:747–754. doi: 10.1016/j.ultsonch.2007.12.002. [DOI] [PubMed] [Google Scholar]
- Tang Q, He X, Liao H, He L, Wang Y, Zhou D, Ye S, Chen Q. Ultrasound microbubble contrast agent-mediated suicide gene transfection in the treatment of hepatic cancer. Oncol Lett. 2012;4:970–972. doi: 10.3892/ol.2012.845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tian Z, Quan X, Xu C, Dan L, Guo H, Leung W. Hematoporphyrin monomethyl ether enhances the killing action of ultrasound on osteosarcoma in vivo. J Ultrasound Med. 2009;28:1695–1702. doi: 10.7863/jum.2009.28.12.1695. [DOI] [PubMed] [Google Scholar]
- Ting CY, Fan CH, Liu HL, Huang CY, Hsieh HY, Yen TC, Wei KC, Yeh CK. Concurrent blood-brain barrier opening and local drug delivery using drug-carrying microbubbles and focused ultrasound for brain glioma treatment. Biomaterials. 2012;33:704–712. doi: 10.1016/j.biomaterials.2011.09.096. [DOI] [PubMed] [Google Scholar]
- Tinkov S, Bekeredjian R, Winter G, Coester C. Microbubbles as ultrasound triggered drug carriers. J Pharm Sci. 2009;98:1935–1961. doi: 10.1002/jps.21571. [DOI] [PubMed] [Google Scholar]
- Tinkov S, Coester C, Serba S, Geis NA, Katus HA, Winter G, Bekeredjian R. New doxorubicin-loaded phospholipid microbubbles for targeted tumor therapy: in-vivo characterization. J Control Release. 2010[a];148:368–372. doi: 10.1016/j.jconrel.2010.09.004. [DOI] [PubMed] [Google Scholar]
- Tinkov S, Winter G, Coester C, Bekeredjian R. New doxorubicin-loaded phospholipid microbubbles for targeted tumor therapy: Part I--Formulation development and in-vitro characterization. J Control Release. 2010[b];143:143–150. doi: 10.1016/j.jconrel.2009.12.026. [DOI] [PubMed] [Google Scholar]
- Todorova M, Agache V, Mortazavi O, Chen B, Karshafian R, Hynynen K, Man S, Kerbel RS, Goertz DE. Antitumor effects of combining metronomic chemotherapy with the antivascular action of ultrasound stimulated microbubbles. Int J Cancer. 2013;132:2956–2966. doi: 10.1002/ijc.27977. [DOI] [PubMed] [Google Scholar]
- Toft KG, Hustvedt SO, Hals PA, Oulie I, Uran S, Landmark K, Normann PT, Skotland T. Disposition of perfluorobutane in rats after intravenous injection of Sonazoid. Ultrasound Med Biol. 2006;32:107–114. doi: 10.1016/j.ultrasmedbio.2005.09.008. [DOI] [PubMed] [Google Scholar]
- Tomizawa M, Ebara M, Saisho H, Sakiyama S, Tagawa M. Irradiation with ultrasound of low output intensity increased chemosensitivity of subcutaneous solid tumors to an anti-cancer agent. Cancer Lett. 2001;173:31–35. doi: 10.1016/s0304-3835(01)00687-5. [DOI] [PubMed] [Google Scholar]
- Treat LH, McDannold N, Zhang Y, Vykhodtseva N, Hynynen K. Improved anti-tumor effect of liposomal doxorubicin after targeted blood-brain barrier disruption by MRI-guided focused ultrasound in rat glioma. Ultrasound Med Biol. 2012;38:1716–1725. doi: 10.1016/j.ultrasmedbio.2012.04.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trendowski M. The promise of sonodynamic therapy. Cancer Metastasis Rev. 2013 Dec;:18. doi: 10.1007/s10555-013-9461-5. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- Tserkovsky DA, Alexandrova EN, Chalau VN, Istomin YP. Effects of combined sonodynamic and photodynamic therapies with photolon on a glioma C6 tumor model. Exp Oncol. 2012;34:332–335. [PubMed] [Google Scholar]
- Tsuru H, Shibaguchi H, Kuroki M, Yamashita Y, Kuroki M. Tumor growth inhibition by sonodynamic therapy using a novel sonosensitizer. Free Radic Biol Med. 2012;53:464–472. doi: 10.1016/j.freeradbiomed.2012.04.025. [DOI] [PubMed] [Google Scholar]
- Tu J, Hwang JH, Matula TJ, Brayman AA, Crum LA. Intravascular inertial cavitation activity detection and quantification in vivo with Optison. Ultrasound Med Biol. 2006;32:1601–1609. doi: 10.1016/j.ultrasmedbio.2006.07.015. [DOI] [PubMed] [Google Scholar]
- Umemura S, Yumita N, Nishigaki R, Umemura K. Mechanism of cell damage by ultrasound in combination with hematoporphyrin. Jpn J Cancer Res. 1990;81:962–966. doi: 10.1111/j.1349-7006.1990.tb02674.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Umemura S, Kawabata K, Sasaki K, Yumita N, Umemura K, Nishigaki R. Recent advances in sonodynamic approach to cancer therapy. Ultrason Sonochem. 1996 [a];3:S187–S191. [Google Scholar]
- Umemura K, Yumita N, Nishigaki R, Umemura S. Sonodynamically induced antitumor effect of pheophorbide a. Cancer Lett. 1996[b];102:151–157. doi: 10.1016/0304-3835(96)04174-2. [DOI] [PubMed] [Google Scholar]
- Umemura S, Kawabata K, Sasaki K. In vivo acceleration of ultrasonic tissue heating by microbubble agent. IEEE Trans Ultrason Ferroelectr Freq Control. 2005;52:1690–1698. doi: 10.1109/tuffc.2005.1561623. [DOI] [PubMed] [Google Scholar]
- Van Leenders GJLH, Beerlage HP, Th Ruijter E, de la Rosette JJMCH, van de Kaa CA. Histopathological changes associated with high intensity focused ultrasound (HIFU) treatment for localised adenocarcinoma of the prostate. J Clin Pathol. 2000;53:391–394. doi: 10.1136/jcp.53.5.391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vaupel P. Abnormal microvasculature and defective microcirculatory function in solid tumors. In: Siemann DW, editor. Vascular-targeted therapies in oncology. John Wiley and Sons Ltd; Chichester: 2006. pp. 9–29. [Google Scholar]
- Verrall RE, Sehgal CM. Sonoluminescence. In: Suslick KS, editor. Utrasound. Its chemical, physical and biological effects. VCH Publishers Inc; New York: 1988. pp. 227–286. [Google Scholar]
- Wang P, Wang XB, Liu QH, Tang W, Li T. Enhancement of ultrasonically induced cytotoxic effect by hematoporphyrin in vitro. Chemotherapy. 2008 [a];54:364–371. doi: 10.1159/000151704. [DOI] [PubMed] [Google Scholar]
- Wang XB, Liu QH, Wang P, Tang W, Hao Q. Study of cell killing effect on S180 by ultrasound activating protoporphyrin IX. Ultrasonics. 2008 [b];48:135–140. doi: 10.1016/j.ultras.2007.11.001. [DOI] [PubMed] [Google Scholar]
- Wang XB, Liu QH, Wang P, Zhang K, Tang W, Wang BL. Enhancement of apoptosis by sonodynamic therapy with protoporphyrin IX in isolate sarcoma 180 cells. Cancer Biother Radiopharm. 2008 [c];23:238–246. doi: 10.1089/cbr.2007.0436. [DOI] [PubMed] [Google Scholar]
- Wang X, Liu Q, Wang Z, Wang P, Zhao P, Zhao X, Yang L, Li Y. Role of autophagy in sonodynamic therapy-induced cytotoxicity in S180 cells. Ultrasound Med Biol. 2010;36:1933–46. doi: 10.1016/j.ultrasmedbio.2010.06.022. [DOI] [PubMed] [Google Scholar]
- Wang X, Wang Y, Wang P, Cheng X, Liu Q. Sonodynamically induced anti-tumor effect with protoporphyrin IX on hepatoma-22 solid tumor. Ultrasonics. 2011 [a];51:539–546. doi: 10.1016/j.ultras.2010.12.001. [DOI] [PubMed] [Google Scholar]
- Wang X, Xia X, Leung AW, Xiang J, Jiang Y, Wang P, Xu J, Yu H, Bai D, Xu C. Ultrasound induces cellular destruction of nasopharyngeal carcinoma cells in the presence of curcumin. Ultrasonics. 2011 [b];51:165–170. doi: 10.1016/j.ultras.2010.07.006. [DOI] [PubMed] [Google Scholar]
- Wang X, Xia X, Xu C, Xu J, Wang P, Xiang J, Bai D, Leung AW. Ultrasound-induced cell death of nasopharyngeal carcinoma cells in the presence of curcumin. Integr Cancer Ther. 2011 [c];10:70–76. doi: 10.1177/1534735410377197. [DOI] [PubMed] [Google Scholar]
- Wang X, Leung AW, Jiang Y, Yu H, Li X, Xu C. Hypocrellin B-mediated sonodynamic action induces apoptosis of hepatocellular carcinoma cells. Ultrasonics. 2012 [a];52:543–546. doi: 10.1016/j.ultras.2011.11.007. [DOI] [PubMed] [Google Scholar]
- Wang X, Leung AW, Luo J, Xu C. TEM observation of ultrasound-induced mitophagy in nasopharyngeal carcinoma cells in the presence of curcumin. Exp Ther Med. 2012 [b];3:146–148. doi: 10.3892/etm.2011.365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang DS, Panje C, Pysz MA, Paulmurugan R, Rosenberg J, Gambhir SS, Schneider M, Willmann JK. Cationic versus neutral microbubbles for ultrasound-mediated gene delivery in cancer. Radiology. 2012[c];264:721–732. doi: 10.1148/radiol.12112368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang H, Liu Q, Zhang K, Wang P, Xue Q, Li L, Wang X. Comparison between sonodynamic and photodynamic effect on MDA-MB-231 cells. J Photochem Photobiol B. 2013[a];127:182–191. doi: 10.1016/j.jphotobiol.2013.08.015. [DOI] [PubMed] [Google Scholar]
- Wang X, Wang P, Zhang K, Su X, Hou J, Liu Q. Initiation of autophagy and apoptosis by sonodynamic therapy in murine leukemia L1210 cells. Toxicol In Vitro. 2013[b];27:1247–1259. doi: 10.1016/j.tiv.2012.12.023. [DOI] [PubMed] [Google Scholar]
- Wang G, Zhuo Z, Xia H, Zhang Y, He Y, Tan W, Gao Y. Investigation into the impact of diagnostic ultrasound with microbubbles on the capillary permeability of rat hepatomas. Ultrasound Med Biol. 2013[c];39:628–637. doi: 10.1016/j.ultrasmedbio.2012.11.004. [DOI] [PubMed] [Google Scholar]
- Watanabe Y, Aoi A, Horie S, Tomita N, Mori S, Morikawa H, Matsumura Y, Vassaux G, Kodama T. Low-intensity ultrasound and microbubbles enhance the antitumor effect of cisplatin. Cancer Sci. 2008;99:2525–2531. doi: 10.1111/j.1349-7006.2008.00989.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wei KC, Chu PC, Wang HY, Huang CY, Chen PY, Tsai HC, Lu YJ, Lee PY, Tseng IC, Feng LY, Hsu PW, Yen TC, Liu HL. Focused ultrasound-induced blood-brain barrier opening to enhance temozolomide delivery for glioblastoma treatment: a preclinical study. PLoS One. 2013;8:e58995. doi: 10.1371/journal.pone.0058995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wood AK, Ansaloni S, Ziemer LS, Lee WM, Feldman MD, Sehgal CM. The antivascular action of physiotherapy ultrasound on murine tumors. Ultrasound Med Biol. 2005;31:1403–1410. doi: 10.1016/j.ultrasmedbio.2005.06.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wood AK, Bunte RM, Ansaloni S, Lee WM, Sehgal CM. The antivascular actions of mild intensity ultrasound on a murine neoplasm. In: Clement GT, McDannold NJ, Hynynen K, editors. Therapeutic ultrasound: 5th International Symposium on Therapeutic Ultrasound.2006. pp. 467–470. [Google Scholar]
- Wood AK, Bunte RM, Cohen JD, Tsai JH, Lee WM, Sehgal CM. The antivascular action of physiotherapy ultrasound on a murine tumor: role of a microbubble contrast agent. Ultrasound Med Biol. 2007;33:1901–1910. doi: 10.1016/j.ultrasmedbio.2007.06.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wood AK, Bunte RM, Price HE, Deitz MS, Tsai JH, Lee WM, Sehgal CM. The disruption of murine tumor neovasculature by low-intensity ultrasound-comparison between 1- and 3-MHz sonication frequencies. Acad Radiol. 2008;15:1133–1141. doi: 10.1016/j.acra.2008.04.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wood AK, Bunte RM, Schultz SM, Sehgal CM. Acute increases in murine tumor echogenicity after antivascular ultrasound therapy: a pilot preclinical study. J Ultrasound Med. 2009;28:795–800. doi: 10.7863/jum.2009.28.6.795. [DOI] [PubMed] [Google Scholar]
- Wood AK, Schultz SM, Lee WM, Bunte RM, Sehgal CM. Antivascular ultrasound therapy extends survival of mice with implanted melanomas. Ultrasound Med Biol. 2010;36:853–857. doi: 10.1016/j.ultrasmedbio.2010.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu F, Chen WZ, Bai J, Zou JZ, Wang ZL, Zhu H, Wang ZB. Pathological changes in human malignant carcinoma treated with high intensity focused ultrasound. Ultrasound Med Biol. 2001;27:1099–1106. doi: 10.1016/s0301-5629(01)00389-1. [DOI] [PubMed] [Google Scholar]
- Wu F, Chen W-Z, Bai J, Zou JZ, Wang ZL, Zhu H, Wang ZB. Tumor vessel disruption resulting from high-intensity focused ultrasound in patients with solid malignancies. Ultrasound Med Biol. 2002;28:535–542. doi: 10.1016/s0301-5629(01)00515-4. [DOI] [PubMed] [Google Scholar]
- Xiang J, Xia X, Jiang Y, Leung AW, Wang X, Xu J, Wang P, Yu H, Bai D, Xu C. Apoptosis of ovarian cancer cells induced by methylene blue-mediated sonodynamic action. Ultrasonics. 2011;51:390–395. doi: 10.1016/j.ultras.2010.11.005. [DOI] [PubMed] [Google Scholar]
- Xu ZY, Li XQ, Chen S, Cheng Y, Deng JM, Wang ZG. Glioma stem-like cells are less susceptible than glioma cells to sonodynamic therapy with photofrin. Technol Cancer Res Treat. 2012;11:615–623. doi: 10.7785/tcrt.2012.500277. [DOI] [PubMed] [Google Scholar]
- Xu ZY, Wang K, Li XQ, Chen S, Deng JM, Cheng Y, Wang ZG. The ABCG2 transporter is a key molecular determinant of the efficacy of sonodynamic therapy with Photofrin in glioma stem-like cells. Ultrasonics. 2013;53:232–238. doi: 10.1016/j.ultras.2012.06.005. [DOI] [PubMed] [Google Scholar]
- Yamaguchi K, Feril LB, Jr, Tachibana K, Takahashi A, Matsuo M, Endo H, Harada Y, Nakayama J. Ultrasound-mediated interferon β gene transfection inhibits growth of malignant melanoma. Biochem Biophys Res Commun. 2011[a];411:137–142. doi: 10.1016/j.bbrc.2011.06.115. [DOI] [PubMed] [Google Scholar]
- Yamaguchi S, Kobayashi H, Narita T, Kanehira K, Sonezaki S, Kudo N, Kubota Y, Terasaka S, Houkin K. Sonodynamic therapy using water-dispersed TiO2-polyethylene glycol compound on glioma cells: comparison of cytotoxic mechanism with photodynamic therapy. Ultrason Sonochem. 2011[b];18:1197–1204. doi: 10.1016/j.ultsonch.2010.12.017. [DOI] [PubMed] [Google Scholar]
- Yan F, Li L, Deng Z, Jin Q, Chen J, Yang W, Yeh CK, Wu J, Shandas R, Liu X, Zheng H. Paclitaxel-liposome-microbubble complexes as ultrasound-triggered therapeutic drug delivery carriers. J Control Release. 2013;166:246–255. doi: 10.1016/j.jconrel.2012.12.025. [DOI] [PubMed] [Google Scholar]
- Yan F, Li X, Jin Q, Jiang C, Zhang Z, Ling T, Qiu B, Zheng H. Therapeutic ultrasonic microbubbles carrying paclitaxel and LyP-1 peptide: preparation, characterization and application to ultrasound-assisted chemotherapy in breast cancer cells. Ultrasound Med Biol. 2011;37:768–779. doi: 10.1016/j.ultrasmedbio.2011.02.006. [DOI] [PubMed] [Google Scholar]
- Yanagisawa K, Moriyasu F, Miyahara T, Yuki M, Iijima H. Phagocytosis of ultrasound contrast agent microbubbles by Kupffer cells. Ultrasound Med Biol. 2007;33:318–325. doi: 10.1016/j.ultrasmedbio.2006.08.008. [DOI] [PubMed] [Google Scholar]
- Yang S, Wang P, Wang X, Su X, Liu Q. Activation of microbubbles by low-level therapeutic ultrasound enhances the antitumor effects of doxorubicin. Eur Radiol. 2014 Aug;:6. doi: 10.1007/s00330-014-3334-3. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- Yoshida T, Kondo T, Ogawa R, Feril LB, Jr, Zhao QL, Watanabe A, Tsukada K. Combination of doxorubicin and low-intensity ultrasound causes a synergistic enhancement in cell killing and an additive enhancement in apoptosis induction in human lymphoma U937 cells. Cancer Chemother Pharmacol. 2008;61:559–567. doi: 10.1007/s00280-007-0503-y. [DOI] [PubMed] [Google Scholar]
- Yu T, Bai J, Hu K, Wang Z. Biological effects of ultrasound exposure on adriamycin-resistant and cisplatin-resistant human ovarian carcinoma cell lines in vitro. Ultrason Sonochem. 2004[a];11:89–94. doi: 10.1016/S1350-4177(03)00160-3. [DOI] [PubMed] [Google Scholar]
- Yu T, Huang X, Hu K, Bai J, Wang Z. Treatment of transplanted adriamycin-resistant ovarian cancers in mice by combination of adriamycin and ultrasound exposure. Ultrason Sonochem. 2004[b];11:287–291. doi: 10.1016/j.ultsonch.2003.07.002. [DOI] [PubMed] [Google Scholar]
- Yu T, Wang Z, Mason TJ. A review of research into the uses of low level ultrasound in cancer therapy. Ultrason Sonochem. 2004[c];11:95–103. doi: 10.1016/S1350-4177(03)00157-3. [DOI] [PubMed] [Google Scholar]
- Yu BF, Wu J, Zhang Y, Sung HW, Xie J, Li RK. Ultrasound-targeted HSVtk and Timp3 gene delivery for synergistically enhanced antitumor effects in hepatoma. Cancer Gene Ther. 2013;20:290–297. doi: 10.1038/cgt.2013.19. [DOI] [PubMed] [Google Scholar]
- Yumita N, Nishigaki R, Umemura K, Umemura S. Hematoporphyrin as a sensitizer of cell-damaging effect of ultrasound. Jpn J Cancer Res. 1989;80:219–222. doi: 10.1111/j.1349-7006.1989.tb02295.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yumita N, Nishigaki R, Umemura K, Umemura S. Synergistic effect of ultrasound and hematoporphyrin on sarcoma 180. Jpn J Cancer Res. 1990;81:304–308. doi: 10.1111/j.1349-7006.1990.tb02565.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yumita N, Nishigaki R, Sakata I, Nakajima S, Umemura S. Sonodynamically induced antitumor effect of 4-formyloximethylidene-3-hydroxy-2-vinyl-deuterio-porphynyl(IX)-6,7-dia spartic acid (ATX-S10) Jpn J Cancer Res. 2000 [a];91:255–60. doi: 10.1111/j.1349-7006.2000.tb00939.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yumita N, Nishigaki R, Umemura S. Sonodynamically induced antitumor effect of Photofrin II on colon 26 carcinoma. J Cancer Res Clin Oncol. 2000 [b];126:601–606. doi: 10.1007/PL00008471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yumita N, Umemura S, Nishigaki R. Ultrasonically induced cell damage enhanced by photofrin II: mechanism of sonodynamic activation. In Vivo. 2000 [c];14:425–429. [PubMed] [Google Scholar]
- Yumita N, Iwase Y, Nishi K, Ikeda T, Umemura S, Sakata I, Momose Y. Sonodynamically induced cell damage and membrane lipid peroxidation by novel porphyrin derivative, DCPH-P-Na(I) Anticancer Res. 2010;30:2241–2246. [PubMed] [Google Scholar]
- Yumita N, Iwase Y, Nishi K, Ikeda T, Komatsu H, Fukai T, Onodera K, Nishi H, Takeda K, Umemura S, Okudaira K, Momose Y. Sonodynamically-induced antitumor effect of mono-l-aspartyl chlorin e6 (NPe6) Anticancer Res. 2011;31:501–506. [PubMed] [Google Scholar]
- Yumita N, Iwase Y, Imaizumi T, Sakurazawa A, Kaya Y, Nishi K, Ikeda T, Umemura S, Chen FS, Momose Y. Sonodynamically-induced anticancer effects by functionalized fullerenes. Anticancer Res. 2013;33:3145–3151. [PubMed] [Google Scholar]
- Zhang X, Li K, Cui X, Hu L, Chen Y. Combined pluronic P85- and ultrasound contrast agents-mediated gene transfection to HepG2 cells. J Huazhong Univ Sci Technolog Med Sci. 2011;31:842–845. doi: 10.1007/s11596-011-0688-5. [DOI] [PubMed] [Google Scholar]
- Zhang C, Huang P, Zhang Y, Chen J, Shentu W, Sun Y, Yang Z, Chen S. Anti-tumor efficacy of ultrasonic cavitation is potentiated by concurrent delivery of anti-angiogenic drug in colon cancer. Cancer Lett. 2014;347:105–1013. doi: 10.1016/j.canlet.2014.01.022. [DOI] [PubMed] [Google Scholar]
- Zhao YZ, Dai DD, Lu CT, Lv HF, Zhang Y, Li X, Li WF, Wu Y, Jiang L, Li XK, Huang PT, Chen LJ, Lin M. Using acoustic cavitation to enhance chemotherapy of DOX liposomes: experiment in vitro and in vivo. Drug Dev Ind Pharm. 2012;38:1090–1098. doi: 10.3109/03639045.2011.640332. [DOI] [PubMed] [Google Scholar]
- Zhao YZ, Du LN, Lu CT, Jin YG, Ge SP. Potential and problems in ultrasound-responsive drug delivery systems. Int J Nanomedicine. 2013;8:1621–1633. doi: 10.2147/IJN.S43589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao X, Liu Q, Tang W, Wang X, Wang P, Gong L, Wang Y. Damage effects of protoporphyrin IX - sonodynamic therapy on the cytoskeletal F-actin of Ehrlich ascites carcinoma cells. Ultrason Sonochem. 2009;16:50–56. doi: 10.1016/j.ultsonch.2008.05.005. [DOI] [PubMed] [Google Scholar]
- Zheng Y, Zhang Y, Ao M, Zhang P, Zhang H, Li P, Qing L, Wang Z, Ran H. Hematoporphyrin encapsulated PLGA microbubble for contrast enhanced ultrasound imaging and sonodynamic therapy. J Microencapsul. 2012;29:437–444. doi: 10.3109/02652048.2012.655333. [DOI] [PubMed] [Google Scholar]
- Zhong H, Li R, Hao YX, Guo YL, Hua X, Zhang XH, Chen ZH. Inhibition effects of high mechanical index ultrasound contrast on hepatic metastasis of cancer in a rat model. Acad Radiol. 2010;17:1345–1349. doi: 10.1016/j.acra.2010.06.013. [DOI] [PubMed] [Google Scholar]
- Zhou S, Li S, Liu Z, Tang Y, Wang Z, Gong J, Liu C. Ultrasound-targeted microbubble destruction mediated herpes simplex virus-thymidine kinase gene treats hepatoma in mice. J Exp Clin Cancer Res. 2010 Dec 23;29:170. doi: 10.1186/1756-9966-29-170. (pages 1-6) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zolochevska O, Xia X, Williams BJ, Ramsay A, Li S, Figueiredo ML. Sonoporation delivery of interleukin-27 gene therapy efficiently reduces prostate tumor cell growth in vivo. Hum Gene Ther. 2011;22:1537–1550. doi: 10.1089/hum.2011.076. [DOI] [PMC free article] [PubMed] [Google Scholar]