Abstract
Women are more sensitive to the harmful effects of alcohol (EtOH) abuse than men, yet the underlying mechanisms remain poorly understood. Previous gene expression analysis of the medial prefrontal cortex (mPFC) following a chronic intoxication paradigm using continuous 72 h vapor inhalation found that females, but not males, exhibit an inflammatory response at peak withdrawal that is associated with cell damage. Given that glucocorticoids can function as anti-inflammatories, are known to increase with EtOH exposure, and influence neurotoxicity, we hypothesized that males and females may exhibit an altered corticosterone (CORT) response following chronic intoxication. Analysis of serum CORT levels revealed the expected increase during withdrawal with no difference between males and females, while control males but not females exhibited higher CORT concentrations than naive animals. Glucocorticoid signaling characterized using focused qPCR arrays identified a sexually dimorphic response in the mPFC during withdrawal, particularly among astrocyte-enriched genes. These genes include aquaporin-1 (Aqp1), sphingosine kinase 1 (Sphk1) and connective tissue growth factor (Ctgf); genes associated with inflammatory signaling, and tissue damage and repair. Bioinformatic analysis also revealed activation of inflammatory signaling and cell death pathways in females. Confirmation studies showed that female mice exhibited significant neuronal degeneration within the anterior cingulate cortex (ACC). By contrast, EtOH exposure lead to a significant reduction in cell death in males. Thus, distinct glucocorticoid signaling pathways are associated with sexually dimorphic neurotoxicity, suggesting one mechanism by which EtOH-exposed females are particularly vulnerable to the damaging effects of alcohol in the CNS.
Keywords: alcohol, neurodegeneration, glucocorticoid signaling, neurotoxicity, astrocyte
1. Introduction
Alcohol (ethanol; EtOH) abuse has well recognized detrimental health consequences and was responsible for approximately 3.3 million deaths in 2012, nearly 6% of the total global death rate (World Health Organization, 2014). Chronic EtOH abuse increases rates of injury, cardiovascular-, digestive-, respiratory- and endocrine disease, cancer, and mental disorders (Centers for Disease Control and Prevention, 2014). The brain is also a target of EtOH effects (Zahr et al., 2011), as neurotoxicity is observed within the frontal cortex of alcoholics (Kril et al., 1997) along with cognitive impairment (Fein et al., 1990). The frontal cortex is involved in executive function and inhibitory control, and dysfunction here contributes to the EtOH addiction cycle (Koob and Volkow, 2010) making this region particularly important for study. However, the mechanism(s) of neuronal damage and neurodegeneration associated with EtOH abuse in the central nervous system (CNS) remain unclear.
Neurodegeneration may either be a consequence or the cause of neuroinflammation, but the impact of EtOH exposure on inflammatory signaling and cell death in the CNS are poorly described. The hippocampus is one region of EtOH-induced inflammation (He and Crews, 2008), but little is known about the relationship between EtOH-induced inflammation and neurodamage. Overall, the immunological consequences of EtOH abuse are complex and likely target-organ dependent. For example, EtOH consumption can induce immunosuppression associated with increased infection rates in some tissues, and in contrast inflammation and cell death in other tissues (Cook, 1998). Consistent with an immune compromised state, alcoholics exhibit increased rates and severity of infectious diseases, particularly those with lung involvement such as pneumonia and tuberculosis (MacGregor and Louria, 1997). Alcoholic liver disease however, is characterized by infiltration of immune cells, an inflammatory response associated with toll-like receptor 4 (TLR-4) signaling, tumor necrosis factor-α (TNF-α) production, and cell death (Albano, 2008). With the exception of the hippocampus, associations between EtOH abuse and neuroinflammation are only recently being characterized (Liu et al., 2007, McBride et al., 2012, Lippai et al., 2013, Vetreno et al., 2013).
Inflammation is strongly influenced by glucocorticoids and the potential exists that glucocorticoid responses may play an important role of EtOH-induced neurodegeneration. Glucocorticoids tightly control the body's immune response (Sorrells and Sapolsky, 2007), and therapeutically, glucocorticoids are used to suppress inflammation in conditions such as asthma, autoimmune disease, and sepsis (Lam et al., 2013, Barnes and Ulrik, 2014, Lutalo et al., 2014). Glucocorticoid medications are also employed in the treatment of centrally-mediated diseases, including for multiple sclerosis particularly during active phase relapse (Myhr and Mellgren, 2009) and for edema associated with brain tumors (Dietrich et al., 2011). Notably, EtOH abuse is associated with hypothalamic-pituitary adrenal (HPA) dysregulation and abnormal HPA activity is a risk factor for developing an EtOH abuse disorder (Stephens and Wand, 2012). Cortisol (induced by HPA axis activation) is associated with relapse in alcoholics (Badrick et al., 2008). In the CNS, long -term elevations in glucocorticoid concentrations within the brain are associated with neurotoxicity (Sapolsky, 2000, Erickson et al., 2003) and with enhanced vulnerability to excitotoxic insult (Mulholland et al., 2005). Some immune effects of acute EtOH exposure can be blocked by antagonism of the glucocorticoid receptor (Weiss et al., 1996). Although plasma glucocorticoid levels in males are increased with acute or chronic EtOH exposure (Adinoff et al., 2003) and during EtOH withdrawal (Adinoff et al., 1998), glucocorticoid response profiles associated with EtOH administration in females are not as well characterized. Combined, these studies highlight the nexus between EtOH actions and glucocorticoid signaling, and the involvement of glucocorticoids in both neurotoxicity and immune regulation in the CNS.
The identification and characterization of sex differences has recently become an area of intense research focus (Couzin-Frankel, 2014), and sex differences are observed with respect to glucocorticoid signaling. Sexually dimorphic glucocorticoid signaling has been reported within the liver, where gene expression analysis of liver tissue from glucocorticoid treated rats indicated that males were more responsive to glucocorticoid treatment than females (Duma et al., 2010). Interestingly in this same study, treatment with dexamethasone (a synthetic glucocorticoid) two hours after injection of lipopolysaccharide (LPS; a TLR4 agonist) reduced plasma concentrations of TNF-α and interleukin 6 (IL-6) as well as overall mortality in male but not in female rats. Consistent with these results, a recent review suggests that females are resistant to the glucocorticoid-mediated effects of stress on immune suppression (Bourke et al., 2012). Sex differences in the response to glucocorticoids in the CNS have also been observed, with male mice demonstrating an enhanced inflammatory response during treatment of acute brain injury (Sorrells et al., 2013). In addition, knock out of glucocorticoid receptors in forebrain cortico-limbic sites including the PFC, hippocampus and basolateral amygdala results in significant sex differences in behavior, with male knockout mice demonstrating increased depression-like behavior and reduced sucrose preference, whereas female mice were unaffected (Solomon et al., 2012). While numerous sex differences are also associated with EtOH abuse (Nolen-Hoeksema, 2004, Wiren, 2013), the characterization of neuroinflammation and neurotoxicity associated with EtOH abuse in male vs female alcoholics remains poorly characterized.
Given a lifetime of chronic abuse, nutritional deficits, family discord and poor health choices, it can be difficult to develop a more mechanistic understanding of neurotoxicity in the EtOH dependent individual. Thus, models of vulnerability to the damaging effects of EtOH are needed. Employing a paradigm which focuses on initial vulnerability to the effects of EtOH, we have observed sex differences in the immune response to EtOH in mice exposed chronically to high intoxication and subjected to a single synchronized withdrawal from chronic EtOH exposure (Hashimoto and Wiren, 2008, Wilhelm et al., 2014). Several lines of evidence ranging from the single gene level to complex pathway analyses of both males and females consistently indicated immunological involvement in mPFC tissue from female mice early during withdrawal. Notably, lines of mice selected for extremes in alcohol withdrawal severity (the Withdrawal Seizure-Resistant and Withdrawal Seizure-Prone lines) showed no significant difference in this response, i.e., pathway analysis revealed inflammatory activation in females but not males of both selected lines irrespective of genotype/phenotype (Hashimoto and Wiren, 2008, Wilhelm et al., 2014). Additional bioinformatics analysis identified nuclear factor kappa-light-chain-enhancer of activated B cells (NF-κB) signaling hubs at peak EtOH withdrawal in mPFC in both males and females, but with completely distinct interacting gene networks (Wilhelm et al., 2014), again indicative of a dimorphic immune response. Consistent with this result, gene expression analysis of hippocampal tissue harvested post-mortem from alcoholics indicated induction of immune related genes with a signaling network centered on NF-κB (McClintick et al., 2013). NF-κB is a transcription factor which plays a key role in both immune signaling and cell survival (Li and Verma, 2002), and is involved in numerous cellular processes including neurodevelopment, synapse function and neuroplasticity (O'Neill and Kaltschmidt, 1997). Nevertheless, the relationship between EtOH-induced immune dysregulation, altered glucocorticoid response and neurodamage remains unclear.
Thus, the present study sought to characterize the potential for sexually dimorphic glucocorticoid signaling in the CNS following chronic intoxication. Our previous gene expression profiling analysis had examined sex and selected line genotype/phenotype effects on transcriptional differences in mPFC during peak withdrawal following chronic EtOH intoxication (Hashimoto and Wiren, 2008, Wilhelm et al., 2014). At peak withdrawal, sex was the most influential factor underlying the effects of chronic EtOH on gene expression, with cell death pathways prominent in females. Confirmation analysis demonstrated enhanced toxicity within the lateral parietal cortex in females but not males (Hashimoto and Wiren, 2008). Notably, expression profiles in females were consistent with a pro-inflammatory response, while males exhibited an immunosuppressed response (Wilhelm et al., 2014). Based on these sexually dimorphic immune and neurotoxicity responses following EtOH, the elevation in glucocorticoids associated with EtOH exposure, and the involvement of glucocorticoids in neurotoxicity and immune regulation, we hypothesized that differences in glucocorticoids or glucocorticoid signaling may be associated with the observed immunosuppressed response in males but inflammatory response in females associated with cell death in the CNS. In the studies presented here, we begin to characterize sexually dimorphic glucocorticoid signaling patterns during withdrawal from chronic EtOH.
2. Methods
2.1 Animals
Withdrawal Seizure-Resistant (WSR) mice were provided by the laboratory of Dr. John Crabbe in Portland, OR. WSR mice were used because previous data indicated that sex, not genotype/phenotype was the strongest influence on gene expression at peak withdrawal (Hashimoto and Wiren, 2008), and because WSR mice are resistant to the potential confound of EtOH-withdrawal induced seizures. Replicate-1 mice were employed for confirmation analysis as a similar transcriptional response was observed in Replicate-2 mice (Hashimoto and Wiren, 2008). Body weights prior to EtOH exposure ranged from 19.7 – 31.4 g, with a mean body weight of 24.5 ± 0.37 g for females, and males weighing more at 28.3 ± 0.39 g (p < 0.001, t-test). Following EtOH exposure, female mice lost 7.2 ± 0.8%, while males lost slightly more at 10.2 ± 1.2% of their body weights (p < 0.05, t-test). This compares with similar body weight losses of 2.2 ± 1.4% by control females and 3.9 ± 0.4% for control males (p > 0.05, t-test). Mice were maintained in groups of 2 – 4 (occasionally females were housed in groups of 5) under a standard light/dark cycle with lights on between 0600 – 1800 h. Females were not evaluated for estrous cycle in these studies, as we and others have demonstrated that chronic intoxication and withdrawal disrupts normal estrous cycling and can result in a persistence of diestrus (Veatch et al., 2007, Forquer et al., 2011). Water and standard lab chow were available ad libitum. Room temperatures were maintained at 22 ± 1°C. Animal procedures were approved by the Portland Oregon VA Medical Center Institutional Animal Care and Use Committee and followed US National Institutes of Health and animal welfare guidelines.
2.2 Ethanol Exposure and Tissue Harvest
Mice were made dependent upon EtOH using a 72 h vapor inhalation method to achieve chronic exposure with continuous high intoxication as described previously (Beadles-Bohling and Wiren, 2006), employing the EtOH dehydrogenase inhibitor pyrazole (Pyz). Briefly, on day 1 EtOH animals were weighed, injected i.p. with EtOH (20% v/v) at 1.5 g/kg with 1 mmol/kg Pyz to reduce variations in blood EtOH concentration (BEC) and placed into vapor inhalation chambers. On days 2 and 3, mice were briefly removed from the chambers to be weighed, given i.p. injections of Pyz dissolved in 0.9% saline, have tail blood samples drawn (EtOH animals only) and placed immediately back into vapor inhalation chambers. The vapor inhalation paradigm thus provides the advantage of temporal separation of chronic intoxication from initiation of withdrawal. A saline-only control was not included because previous analysis using an unbiased PCR differential display screening method that included WSR mice failed to identify significant differences in gene expression across a broad spectrum of genes between saline and Pyz-treated animals in this paradigm (Schafer et al., 1998). BEC was determined as previously described (Beadles-Bohling and Wiren, 2006). The EtOH vapor inhalation method employed for these studies rapidly induces dependence as assessed by increased hyperexcitability, reduced exploratory behavior and increased tremor (Kosobud and Crabbe, 1986), increased motor impairment (Philibin et al., 2008), EtOH tolerance (Goldstein and Zaechelein, 1983), increased anxiety (Kliethermes et al., 2004), and increased voluntary withdrawal-induced relapse drinking (Hashimoto et al., 2011) after a single continuous 72 h period of chronic intoxication. Average BECs over the 72 h EtOH exposure period were 2.16 ± 0.09 mg/ml for EtOH exposed females and 1.98 ± 0.08 mg/ml for EtOH exposed males (unpaired t-test p > 0.05 comparing male vs. female). Previous studies have not found differences in EtOH metabolism between females and males (Kosobud and Crabbe, 1986).
For RNA analyses, tissue was harvested via cervical dislocation 8 h after removal from the chambers corresponding to peak withdrawal severity following high levels of intoxication (Finn and Crabbe, 1999). Brains were briefly placed in ice-cold isotonic buffer after which the medial prefrontal cortex (mPFC) was dissected with a coronal slice ≈2 mm deep to include tissue +2.0 mm from Bregma to the front of the brain (after removal of olfactory bulb and tubercle) and 2.0 mm lateral from midline. Tissue in the mPFC dissection includes the following brain regions: frontal association cortex, prelimbic cortex, infralimbic cortex, rostral portions of secondary motor cortex, anterior cingulate cortex, primary motor cortex, medial, ventral and lateral orbital cortex, agranular insular cortex and dorsolateral orbital cortex. Blood samples for CORT measurements were collected via cardiac puncture from deeply anesthetized animals [mouse cocktail: ketamine (7.5 mg/ml), xylazine (0.75 mg/ml) and acepromazine (0.15 mg/ml)]. After centrifugation, the serum was transferred to a clean Eppendorf tube and stored at -20°C.
2.3 Chemicals
Most chemicals were purchased from Sigma-Aldrich Chemical Co. (St. Louis, MO). EtOH (ethyl EtOH, 200 proof) for use in vapor chambers and injections was purchased from Pharmco Products Inc. (Brookfield, CT).
2.4 RNA Isolation and Glucocorticoid Signaling Focused qPCR Array Analysis
Following dissection, RNA isolation from the mPFC was carried out following manufacturer's recommendations for the RNA Stat-60 reagent (Tel-Test, Inc. Friendswood, TX) with modifications as previously described (Hashimoto and Wiren, 2008). Contaminating genomic DNA was eliminated by digestion with RNase-free DNase followed by Zymo-spin column purification as described in the manufacturer's protocol (Zymo Research, Orange, CA) and verified by qPCR. RNA integrity was assessed using a 1% agarose gel stained with SYBR Gold (Life Technologies, Grand Island, NY) and quantified using Quant-iT RiboGreen (Life Technologies, Grand Island, NY). Synthesis of cDNA was carried out on 1 μg of RNA as described in the iScript Advanced cDNA synthesis Kit for RT-qPCR (Bio-Rad Laboratories, Hercules, CA). Amplification of transcripts for focused qPCR array analysis (Bio-Rad Laboratories, Hercules, CA) was carried out as described in the product manuals with approximately 10 ng of cDNA per well in a CFX 96 PCR machine (Bio-Rad Laboratories, Hercules, CA). The focused qPCR platform was designed to assess overall glucocorticoid signaling (genes are listed in Supplemental Table 2) and was modified to include the transcription factors irf4 and irf5. This array probes a large variety of functional gene groupings influenced by glucocorticoids including genes involved in: glucose and fatty acid metabolism, cytokines and chemokines, growth factors, extracellular matrix, cytoskeleton regulation, endocytosis and exocytosis, cell surface receptors, channels and transporters, signal transduction, stress responses, transcription factors, circadian rhythm, RNA processing, protein folding and trafficking, metallothioneins, nucleotide metabolism and cotranscription factors. Bioinformatic analyses were conducted by uploading genes regulated by 1.5 fold or more to the Ingenuity Pathway Analyses (IPA) website (www.ingenuity.com). Analysis of the uploaded genes was carried out using the proprietary IPA software, with significance determined based on the relative enrichment of the uploaded genes to biological function, pathway, or network using Fishers exact test.
2.5 Assessment of EtOH-Induced Brain Damage
For histological analysis, animals were deeply anesthetized with mouse cocktail and perfused with cold 0.9% saline followed by 4% paraformaldehyde. Brains were removed, placed in 4% paraformaldehyde overnight at 4°C, and then paraffin-embedded. Sagittal sections (6 μm) including the mPFC were placed on positively charged microscope slides, and stained with hematoxylin and eosin (H&E) as previously described (Hashimoto and Wiren, 2008). Images were captured that encompassed the mPFC of both the right and the left hemispheres using a Leica DM5000B microscope at 10X magnification. After visual examination for regions of consistent cell damage, counts were determined in the ACC using the threshold function in MetaMorph software (MetaMorph Premier Image Analysis software v7.7.7, Universal Imaging Corporation, Downingtown, PA, USA) with the experimenter blind to treatment and sex.
2.6 Confocal Microscopy
Slides were deparaffinized and re-hydrated through a graded series of EtOH. Antigen retrieval was carried out in 0.01M Citrate Buffer (pH 6.0) heated in a microwave on high for 15 min and then allowed to cool for 1.5 h at room temperature. Slides were subsequently washed, blocked with normal goat serum, and then probed with primary antibody (Mouse anti-NeuN; Millipore, Billerica, MA; MAB377at 1:1000) overnight at 4°C. Sections were washed, probed with secondary antibody (goat anti-Mouse Alexafluor 647; Life Technologies, Grand Island, NY; A-21236 at 1:200) for 2 h at room temperature, and washed again. Finally, samples were exposed to 0.1% acetic acid and 0.0004% Fluor-Jade B (Millipore, formerly Chemicon, Billerica, MA; AG310) for 40 min on a shaker, and then rinsed and dried at 45°C for 40 min protected from light. Immunofluorescently labeled tissue samples were scanned using a Leica TCS SP5 confocal microscope (Leica Microsystems, Buffalo Grove, IL) with 405 Diode, Argon, and HeNe 633 lasers using a 40X objective. Images of serial optical sections were acquired at 0.5 μm intervals and each channel was scanned sequentially. Master gain and digital offset system settings were optimized for producing the images without saturation. Images are shown using the 2-D maximum intensity projection image to highlight the brightest pixel value of the entire image z-stack at each pixel location. Leica LAS AF software with the co-localization module was used to analyze the images.
2.7 Corticosterone ELISA
Levels of CORT were examined in mouse serum by competitive enzyme-linked immunosorbent assay (ELISA) as described in the manufacturer's protocol (Enzo Life Sciences, Farmingdale, NY). Samples and unknowns were assayed in duplicate with the average value used for subsequent calculations.
2.8 Statistical Analysis
Prism v6.04 (Graphpad Software, Inc., La Jolla, CA) was used for Analysis of Variance (ANOVA), and correlation analyses and Microsoft Excel (Microsoft Corp., Redmond, WA) was used for t-tests. Data is shown as mean ± standard error of the mean (SEM).
3. Results
3.1 Sexually dimorphic glucocorticoid signaling following chronic EtOH intoxication and withdrawal
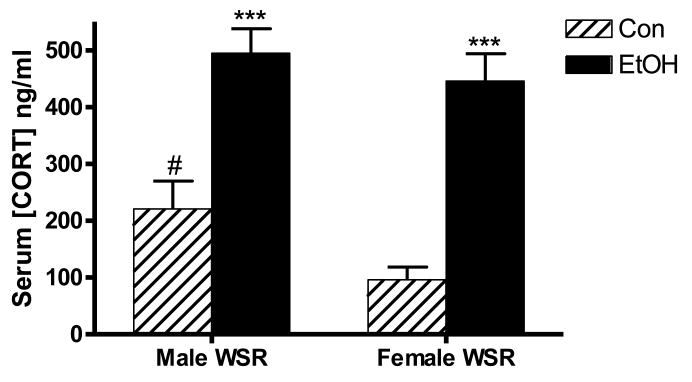
We first examined the glucocorticoid response in males and females by determining serum CORT concentrations at peak EtOH withdrawal following chronic intoxication in this paradigm, as the response in males vs. females has not been previously characterized. As shown in Figure 1, CORT levels in both males and females were increased during withdrawal. Two-way ANOVA of serum CORT concentrations indicated significant main effects of treatment (p < 0.001) and sex (p < 0.05), with EtOH exposure resulting in increased serum CORT concentration in both males and females during withdrawal. The effect of sex was driven by a significant difference in serum CORT concentration in control mice between males and females, where males exhibited elevated serum CORT relative to females (p < 0.05). Further, control male mice exhibited an increase in CORT levels compared to naïve male mice that did not reach statistical significance (naïve: 35.1 ± 13.5 ng/ml, Con: 220.6 ± 49.1 ng/ml; p = 0.07). Consistent with other literature (Galea et al., 1997) naïve female mice had increased serum CORT compared with naïve males that did not reach statistical significance (naïve female: 101.9 ± 22.5 ng/ml, p = 0.06), but no difference compared with Con females (Con female: 95.8 ± 22.5 ng/ml, p > 0.90). Increased CORT in male mice under control conditions may be due to stress induced by housing in inhalation chambers near other male cohorts, as males may be more reactive to certain stressful stimuli than females (Mashoodh et al., 2008, Hudson et al., 2014, Westenbroek et al., 2013). The vapor inhalation chambers are constructed from a wire mesh and are capable of housing up to 8 cages of animals per chamber. Though physically separated by the wire mesh, animals are able to smell and potentially communicate with other animals. Of note, female cages were placed in chambers housing other females, while males were housed exclusively with males. Importantly, elevations in serum CORT concentrations were similar between EtOH-exposed male and female animals (p > 0.05), such that serum CORT levels were not significantly different between males and females during withdrawal.
Figure 1. Increased serum corticosterone (CORT) levels at peak withdrawal.
CORT levels were evaluated by ELISA in male and female WSR mice at peak withdrawal. Both males and females exhibit a strong induction of CORT 8 h after EtOH exposure, with peak values not different between the sexes. Control (Con) males exhibit a relatively increased serum concentration of CORT compared to Con females. N = 10 – 17. ***, p < 0.001 compared to appropriate Con; #, p < 0.05 compared to Con females.
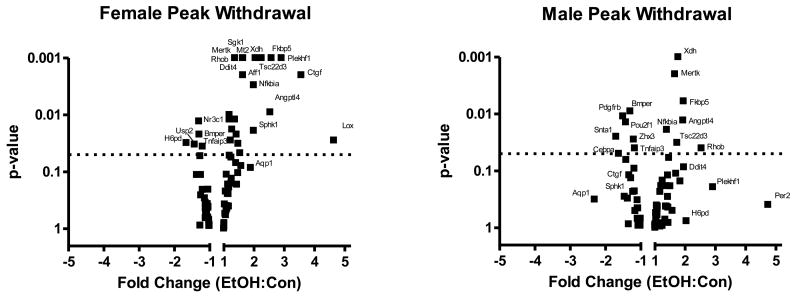
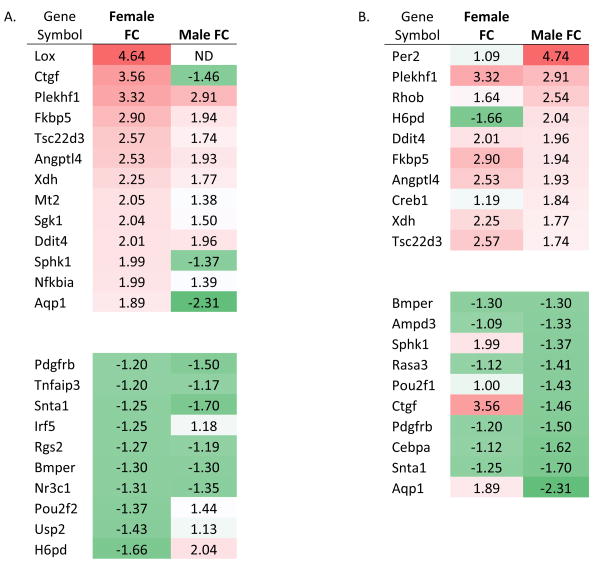
Although CORT levels were not different between males and females, recent studies suggest that glucocorticoid signaling may differ between males and females, at least in some tissues (Duma et al., 2010). Therefore, we explored pathways known to be modulated by CORT to identify patterns of sex-specific signaling in the CNS. For this work, we used focused qPCR arrays designed to characterize overall CORT signaling patterns (see Supplemental Table 1 for complete results), using mRNA harvested from mPFC at peak withdrawal to compare male and female gene expression differences. We note that the elevated CORT levels in male mice may lead to a relative reduction in the activation of CORT responsive genes during EtOH withdrawal, therefore, we have focused on genes that were regulated in a divergent fashion between male and female mice (i.e., up-regulation in females; down-regulation in males), which could not be explained by elevated CORT in Con vs. naïve males, or by differences between Con males and Con females (Supplemental Table 2, Supplemental Figure 1). Several CORT-regulated genes were similarly influenced by EtOH between males and females (Table 1; Figure 2). However, many CORT-responsive genes exhibited a dimorphic response, with ≈33% of the regulated transcripts responding distinctly between males and females. The most dimorphic responses, i.e., those transcripts that were regulated in opposite directions between males and females, included: H6pd (hexose-6-phosphate dehydrogenase), Ctgf (connective tissue growth factor), Aqp1 (aquaporin-1), and Sphk1 (sphingosine kinase-1). In addition, there were several genes that were regulated in one sex but unaffected in the other. Overall, females exhibit a skew toward up-regulation of several of the glucocorticoid target genes examined, while males exhibit a more balanced response, with induction of expression of some genes but inhibition of others (Figure 2). To further characterize and better understand expression pattern differences in the CORT response, we employed bioinformatics analysis using IPA of genes regulated by 1.5 fold or more (Thibault et al., 2000, Mayfield et al., 2002). While males exhibited disease and disorder pathways associated with endocrine (p < 0.01) and neurological diseases (p < 0.01), females instead exhibited pathways associated with immunological (p < 0.01) and inflammatory diseases (p < 0.01) (Supplemental Table 3). Analysis of upstream regulators using IPA analysis in females revealed activation of both immune and cell death-related regulators including transforming growth factor β1 (TGFB1), interleukin-6 (IL6), p38 mitogen-activated protein kinase (MAPK), and TP53 (Supplemental Table 4). Thus, expression differences in the CORT response observed in females indicated activation of both immune and cell death pathways, consistent with cell death at peak withdrawal in the mPFC in females but not males.
Table 1.
Heat-map of EtOH-regulated genes as a function of sex, sorted by fold regulation in females (A), or males (B). ND denotes a gene that was detected below a minimum cycle threshold of 35. FC denotes fold change for gene of interest. The intensity of red (up-regulated) or green (down-regulated) indicates the relative change in expression in EtOH-exposed animals relative to control animals.
|
Figure 2. Volcano plots visualizing distinct glucocorticoid signaling patterns in males and females at peak withdrawal.
Focused qPCR analysis with mRNA harvested from male and female WSR mice at peak withdrawal was used to characterize overall glucocorticoid signaling. Females exhibit a skewed response at peak withdrawal with more up-regulation while males exhibit a more balanced response at peak withdrawal with both up- and down-regulation observed. Several genes exhibit similar regulation following EtOH exposure in both males and females (e.g. C-mer proto-oncogene tyrosine kinase (Mertk), FK506 binding protein 5 (Fkbp5), and Angiopoietin-like 4 (Angptl4). A number of astrocytic genes were regulated in a dimorphic fashion including Connective tissue growth factor (Ctgf), Sphingosine kinase 1 (Sphk1), and Aquaporin-1 (Aqp1). N = 3 – 4. The dashed line denotes the p-value = 0.05, thus genes above this line were regulated in a statistically significant manner.
3.2 EtOH-induced neurotoxicity in female, but not male mice
Given activation of cell death pathways in females, we sought biological confirmation of tissue damage in females using H&E staining. Analysis was performed 5 d after withdrawal, as peak cell death has previously been observed 5-10 days after excitotoxic insult (Panegyres, 1998). Immunohistochemical analyses focused on the mPFC, as this is a known region of damage in the alcoholic brain (Kril et al., 1997). Based on a visual survey, the anterior cingulate cortex (ACC) was characterized as it was the brain region with the most widespread and consistent damage. As predicted from pathway analyses, females exhibited increased neurodegeneration within the ACC (Figure 3A,B). Quantification of dead and dying cells (Figure 3C) revealed a significant increase in dead cells in the ACC in females. In contrast, males had reduced neurodegeneration, consistent with relative protection from EtOH-induced damage in this paradigm. Similar results were observed in the Withdrawal Seizure-Prone (WSP) selected line (data not shown), and this phenomena has also been observed outside the mPFC in the lateral parietal cortex (Hashimoto and Wiren, 2008). Interestingly, average BEC was not correlated with neurotoxicity in either male or female mice (p's > 0.1). These results underscore the sexually dimorphic response to EtOH and are consistent with gene expression analysis identifying sex as the critical determinant of EtOH-induced changes in biological response in early withdrawal stages irrespective of genetic background (Hashimoto and Wiren, 2008).
Figure 3. Increased cell death in female mice following EtOH within the ACC.
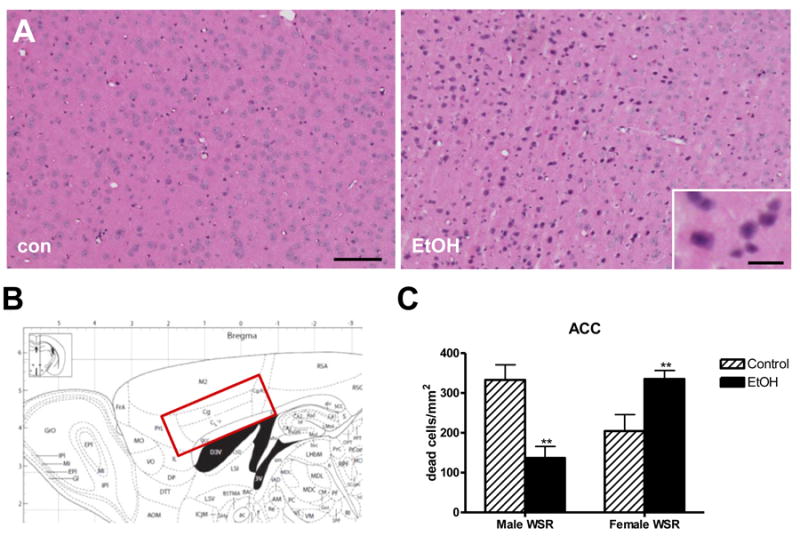
Dead and dying cells were identified by pyknotic nuclei and cytoplasmic hypereosinophilia using H&E staining. A. Female mice exhibit increased toxicity following EtOH exposure. B. Atlas showing region of interest. C. Quantitation of dead cells. Females show significant elevation in dead and dying cells. By contrast EtOH-exposed male mice exhibit a reduction in the total number of dead cells within the ACC. Data shown are the mean ± SEM. N = 8 – 16. **, t-test p < 0.01 compared to same sex Con. Scale bars, 300 μm, inset 30 μm. Magnification, 10X.
To identify the cell type damaged by EtOH, confocal microscopy using double immunofluorescence was employed. Damage was characterized in the ACC in WSR female mice 5 d after withdrawal. For this analysis, neurons were first labeled with NeuN. Fluoro-Jade B, an anionic tribasic derivative of fluorescein and highly acidic analog of eosin, was then used to identify degenerating cells (Schmued and Hopkins, 2000). Consistent with other studies (Savaskan et al., 2000), Fluoro-Jade B staining was relatively absent from the nucleus and instead was localized in the cytoplasm. As shown in Figure 4, NeuN and Fluoro-Jade B staining were exclusively co-localized, indicating that the degenerating cells in female mice were neurons. Other models of EtOH-induced damage have also identified EtOH-induced neuronal degeneration using this technique (Qin and Crews, 2012). Thus we observed a sexually dimorphic response in the mPFC, with increased neurodegeneration in female mice following chronic intoxication. This data provides biological confirmation of the enhanced tissue damage outcome predicted by the divergent CORT signaling analysis.
Figure 4. Ethanol withdrawal results in neuronal cell death in females.
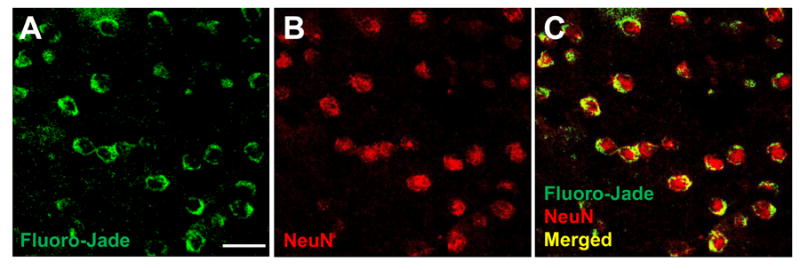
ACC sections from female WSR mice at 5 days after withdrawal, double stained with A. Fluoro-Jade B (green) and B. NeuN (red) C. NeuN/Fluoro-Jade B double-stained (yellow) cells in merged image represent neurons undergoing degeneration. Representative images show overwhelming co-localization of the neuronal marker NeuN with the neurodegeneration marker Fluoro-Jade B, with calculated co-localization = 98% using the Leica co-localization module. Scale bar, 25 μm. Magnification, 40X.
4. Discussion
At peak withdrawal following chronic intoxication, differences in CORT signaling and neurodegeneration in mPFC between males and females were observed. Alterations in the CORT response were examined here to better characterize a potential role in the previously observed cell death and inflammatory differences identified between males and females (Hashimoto and Wiren, 2008, Wilhelm et al., 2014). Serum CORT concentrations increased at peak withdrawal after chronic intoxication but were similar for both sexes. In contrast, down-stream signaling events within the mPFC during withdrawal were differentially influenced by sex, where females exhibited changes in gene expression consistent with activation of inflammation and cell death associated pathways including changes in Il-6, p38 MAPK, TGF-β and TP53 levels. Following EtOH exposure, increases in the number of dead or dying cells within the ACC were observed in females while decreases were seen in males. Similar results were previously observed within the lateral parietal cortex (Hashimoto and Wiren, 2008). Using confocal microscopy, neurons were identified as the cells exhibiting damage, confirming a neurotoxic response in the immediate withdrawal period following chronic intoxication in females. The observed reduction in neurotoxicity in males following EtOH exposure in this vulnerability paradigm was somewhat unexpected, however previous analysis in hippocampal slice preparations found protection from CORT-induced damage associated with combined EtOH and CORT exposure in males, but instead enhanced CORT and EtOH-induced toxicity in females (Walls et al., 2013). Furthermore, sexually dimorphic EtOH-induced brain damage with EtOH use disorder has been reported, as late adolescent females exhibit reduced PFC volumes while adolescent males have larger PFC volumes, compared to same-sex controls (Medina et al., 2008), a finding consistent with the present results. Thus females are particularly vulnerable to the damaging effects of EtOH during early withdrawal while males are relatively insensitive, a phenomenon which may be influenced by sex-differences in the combined effects of EtOH and CORT in the CNS.
4.1 Role of glucocorticoids in EtOH-induced neurodegeneration
Glucocorticoids likely play a role in brain damage induced by EtOH (Rose et al., 2010), but there is little characterization of sexually dimorphic responses and the key mediators have not been identified. An altered dose-response relationship between males and females could explain differential signaling, as “acute” stress levels of glucocorticoids protect against toxicity (Nadeau and Rivest, 2003), but “high” stress levels of glucocorticoids enhance both inflammation and damage (Sapolsky and Pulsinelli, 1985). In this context and as noted in the introduction, work indicates that sexually dimorphic responses to glucocorticoids are observed. For example, dexamethasone-treated peripheral blood cells from men exhibit reduced LPS-induced TNF-α and IL-6 production, while peripheral blood cells from women exhibit increased cytokine production following the same treatment (Rohleder et al., 2001). These results suggest that glucocorticoids may enhance inflammatory signaling in females, but induce immune suppression in males. Consistent with this hypothesis, our data indicate that females exhibit a pro-inflammatory and neurotoxic response to EtOH that is not suppressed by elevations in CORT, and may even be exacerbated by glucocorticoid-mediated events. Previous work indicates that the cortex is relatively resistant to LPS-induced inflammation in male rats (Kim et al., 2000); however, females may respond differently. Although changes in both glucocorticoid concentrations (Burgess and Handa, 1992) and inflammatory signaling (Straub, 2007) are associated with alteration in estrogen levels, we have previously shown that chronic intoxication and withdrawal modestly suppressed 17β-estradiol levels in both males and females (Forquer et al., 2011) and are thus unlikely to influence the sexually dimorphic pattern in immune response following EtOH exposure. In addition, expression levels of glucocorticoid and mineralocorticoid receptors within the cortex are similar between males and females and at a level comparable to receptor expression within most of the hippocampus (Owen and Matthews, 2003), suggesting that differences in receptor levels are unlikely to underlie sexually dimorphic responses.
The chronic EtOH exposure and withdrawal paradigm employed for these studies highlights the susceptibility of females to neurodamage after chronic intoxication in the immediate withdrawal period, consistent with other studies which have also observed increased vulnerability to EtOH-induced brain damage in females (Alfonso-Loeches et al., 2013). In contrast, studies in chronic alcoholics (Zou and Crews, 2012) and those that have employed large volume gavage, with repeated dosing and multiple withdrawals, observe damage in males in the hippocampus (Crews et al., 2013), but have largely left females and other brain regions unexamined. It may be that the longer-term binge EtOH administration methods reflect a more advanced condition of EtOH exposure, perhaps due to the waxing and waning of blood EtOH levels in such procedures, or elevated damage to the gut. Thus, though long-term alcoholics of both genders exhibit neurotoxicity (Zahr et al., 2011), distinct molecular signaling and divergent CORT responses suggest underlying mechanisms are sexually dimorphic. In addition, few studies have focused on the mPFC or females, with most analysis describing inflammatory responses in males, in the hippocampus. Future studies will be needed to explore whether CORT levels are correlated with EtOH-induced neurotoxicity in this setting. Given the strongly dimorphic response in gene expression differences and divergent signaling pathways activated after chronic intoxication, these results suggest that therapeutic targets may be distinct between males and females and advocate for a sex-specific approach to treatment.
4.2 Gene expression differences associated with EtOH-induced neurotoxicity
Despite several studies demonstrating increased vulnerability to the detrimental effects of EtOH in women, this observation remains understudied (Schuckit et al., 2012, Sharrett-Field et al., 2013, Wiren, 2013), with the mechanisms underlying this phenomenon unclear. Women have smaller bodily EtOH distribution volumes but faster elimination rates (Jones, 2010), thus pharmacokinetic differences are unlikely to explain sex differences in tissue damage. Females also demonstrate fewer withdrawal symptoms following ethanol administration (Deshmukh et al., 2003). It should be noted that basal gene expression differences of some genes (See Supplemental Table 2, Supplemental Figure 1), such as H6pd, may contribute to the observed sexually dimorphic responses with for example the relatively higher expression of H6pd in females compared to males, as H6pd also exhibited the largest fold-reduction in expression in females vs induction in males. Nevertheless, such caveats do not apply to other genes, such as Sphk1, which was nominally higher in control females relative to control males, then induced following EtOH in females and suppressed following EtOH in males. Thus, genes that were differentially regulated as a consequence of EtOH exposure between males and females can provide insight into underlying molecular mechanisms. For example, the observed differences in CTGF expression between males and females following chronic EtOH exposure may be an important target in this model, as CTGF, which is transcriptionally regulated by the synthetic glucocorticoid dexamethasone (Wang et al., 2008), is involved in fibrosis and scaring (Mori et al., 1999), an important component of alcoholic liver disease (Gao and Bataller, 2011). CTGF expression is also enhanced in models of EtOH-induced chronic pancreatitis (Lawrencia et al., 2009), and is highly expressed in astrocytes, particularly when associated with gliosis and scar formation in response to injury or excitotoxic insult (Schwab et al., 2001). Both CTGF (Sanchez-Lopez et al., 2009) and Sphk1 (Vann et al., 2002) induce expression of inflammatory markers such as TNF-α. Sphk1 is an enzyme that phosphorylates the lipid sphingosine to sphingosine-1-phosphate (S1P), and S1P induces CTGF expression (Muehlich et al., 2004). Inflammation and tissue damage are intricately linked, and increases in S1P have also been observed with neuroapoptosis in models of fetal EtOH syndrome (Chakraborty et al., 2012). Increased expression of CTGF and Sphk1 in females is consistent with EtOH-induced astrocyte dysfunction and activation of inflammatory and cell death pathways in females during withdrawal, and CTGF and Sphk1 may be important mediators of damage in the CNS following chronic intoxication.
4.3 Astrocytes are a target of EtOH action in females
Mounting data now indicate that astrocytes play an important role in several neurodegenerative diseases including Alzheimer's disease, amyotrophic lateral sclerosis, Parkinson's disease and Huntington's disease (Maragakis and Rothstein, 2006). Because astrocytes are critical components of neuronal homeostasis, dysfunction of these cells can lead to neuronal stress and toxicity via several mechanisms including: excitotoxicity due to impaired glutamate regulation (Maragakis and Rothstein, 2004), protein aggregation and axonal pathology (Forman et al., 2005), failure of the blood brain barrier (Abbott et al., 2006) and disruption of lipid homeostasis and programmed cell death (Gu et al., 2013). To our knowledge, there are no consistent reports of significant gliosis in uncomplicated human alcoholics, nor has a potential for sexually dimorphic responses been evaluated. However, data indicates that astrocyte function can be disrupted in chronic alcoholics (Cullen and Halliday, 1994, Miguel-Hidalgo et al., 2002, Ikegami et al., 2003, Lewohl et al., 2005) and recent studies have found a link between craving during abstinence and glutamatergic dysfunction within the ACC (Bauer et al., 2013). In the present study, several astrocyte-encirched genes were influenced by EtOH exposure in females. EtOH induces CTGF in primary astrocyte cultures (Pignataro et al., 2013), and Sphingosine-1-receptors are primarily localized to brain astrocytes (Choi et al., 2011). Consistent with the involvement of astrocytes in EtOH-induced brain damage, increased levels of pro-inflammatory molecules such as the chemokine MCP-1, as well as brain-region specific increases in microglial and/or activated astrocyte markers (the resident immune cells within the brain) within the cingulate cortex, ventral tegmental area and midbrain are observed in a predominantly male population alcoholics (He and Crews, 2008). In our paradigm, there is no indication that microglia are reactive (data not shown), consistent with a lack of microglial activation observed in other models (Marshall et al., 2013). Some have suggested that microglia may actually be protective in the gavage model of EtOH-induced neurodegeneration (Marshall et al., 2013), thus leaving astrocytes as a likely candidate for mediating EtOH-induced damage and inflammation. Finally, we and others have identified astrocytes as a target of EtOH-induced changes in brain gene expression in bioinformatics analyses, including in chronically-exposed female mice in our vapor inhalation paradigm (Wilhelm et al., 2014) and studies within the frontal cortex of cirrhotic alcoholics (Liu et al., 2007). Astrocytes play a critical role in CNS homeostasis, but their importance in EtOH abuse and other neurodegenerative diseases has only recently been appreciated (Brambilla et al., 2013).
4.4 The importance of the anterior cingulate cortex in EtOH abuse
The mPFC was a region of interest in these studies due to its involvement in addiction disorders (Koob and Volkow, 2010), its association with behavioral (inhibitory) control, and because it is a site of damage (Zahr et al., 2011) and neurodegeneration in EtOH abusers (e.g., Perry et al., 2011). The ACC, within the mPFC, is also involved in error detection, performance monitoring, and anticipation and detection of pain (Bush et al., 2000, MacDonald et al., 2000, Kosaki and Watanabe, 2012). EtOH abusers demonstrate reductions in gamma amino-butyric acid (GABA) and N-acetyl-aspartate levels (Silveri et al., 2014), and short-term increases in glutamate levels within the ACC (Hermann et al., 2012). Alcoholics also exhibit reductions in decision making scores and gray matter volume within several brain regions including the cingulate cortex (Le Berre et al., 2014). Furthermore, abstinent (one to two months) alcoholics exhibit hyperactivation of the ACC following exposure to neutral or relaxing cues, but hypoactivation of the ACC during cues designed to induce stress, suggesting altered stress reactivity and functional alterations that persist during abstinence (Seo et al., 2013). The ACC also plays an important role in maintaining cognitive flexibility, as well as reducing the influence of irrelevant stimuli (Dias and Aggleton, 2000, Ragozzino and Rozman, 2007). One aspect of addiction, a rigid adherence to pursuit of drug, is partially born out of a lack of cognitive flexibility (Zinn et al., 2004), a behavioral feature frequently observed in female alcoholics (Flannery et al., 2007). Consistent with this, female rodents exhibit increased escalation of drug seeking and drug-primed reinstatement (models of relapse) relative to males (Carroll and Anker, 2010). Thus, damage to the mPFC and ACC in particular is likely to further entrench EtOH abusers in the addiction cycle due to neurochemical, structural and behavioral impairments associated with dysregulation of this brain region.
5. Conclusion
In summary, this is the first study to compare CORT response in males and females during withdrawal from chronic EtOH intoxication and to identify distinct EtOH-induced glucocorticoid signaling within the mPFC of males and females. Although elevations of CORT were similar between males and females, glucocorticoid signaling and EtOH-induced neurodamage were sexually dimorphic following chronic EtOH intoxication. Neurotoxicity may lie at the interface of inflammatory signaling and CORT signaling, with alcoholic females exhibiting a glucocorticoid-induced potentiation of inflammatory signaling and alcoholic males exhibiting a more classical immune-suppressing glucocorticoid response during the early stages of withdrawal. Sex differences in the response to EtOH are a frequently overlooked phenomenon, to the great detriment of women's health, and awareness of the need to include females in research is growing (Couzin-Frankel, 2014). It is increasingly clear that males and females show robust differences in biology, signaling pathways and response to treatment. In addition to the observation that sex/gender strongly influences immune function and the inflammatory response (Fish, 2008), previous reports have demonstrated that sex influences the biological response to EtOH (Ceylan-Isik et al., 2010). Compared to males, females exhibit: decreased risk for the development of ethanol dependence and addiction; increased ethanol sensitivity and ethanol consumption; reduced withdrawal severity; and increased risk of recidivism and relapse (Brady and Randall, 1999, Devaud and Chadda, 2001, Sershen et al., 2002, Wang et al., 2003, Carroll et al., 2004, Prescott et al., 2005, Becker and Hu, 2008, Potts et al., 2013). Evidence also indicates that ethanol is more rewarding in females (Blanchard et al., 1993, Torres et al., 2014). Notably, females are also more sensitive to the harmful effects of EtOH, a phenomenon known as “telescoping”. Females exhibit similar or increased damage after a shorter period of ethanol abuse compared to males (Brady and Randall, 1999, Mann et al., 2005), where the degree of cardiomyopathy (Fernandez-Sola and Nicolas-Arfelis, 2002), peripheral neuropathy (Ammendola et al., 2000) and cirrhosis (Loft et al., 1987) is worse in female alcoholics than in males. Females also appear more vulnerable to brain damage in animal models of dependence (Hashimoto and Wiren, 2008, Alfonso-Loeches et al., 2013) and human alcoholics (Hommer et al., 2001, Mann et al., 2005). Most concerning is that annualized death rates are also substantially elevated for female alcoholics relative to male alcoholics (Batty et al., 2009, John et al., 2013). Unfortunately, rigorous characterization of the potential for sexually dimorphic responses to EtOH in the clinical literature has been lacking (see Schuckit et al., 2012), with many studies underpowered to examine the issue. Our results in preclinical animal models indicate that in the early stages of addiction, females exhibit activation of pathways and underlying molecular mediators that can exacerbate ethanol-induced neurodegeneration in the mPFC, which are fundamentally distinct from male responses (Hashimoto and Wiren, 2008, Wilhelm et al., 2014). Combined, these results indicate that males and females exhibit distinct biological responses to intoxication (see Wiren, 2013), some of which may be due to baseline differences (Yang et al., 2006) and some of which may be the result of more complicated signaling differences observed here (and see Hashimoto and Wiren, 2008, Hashimoto et al., 2011, Wilhelm et al., 2014). Given the burden of significantly increased mortality in female alcoholics, a greater understanding of these fundamental sex differences is needed as they represent distinct, targetable pathways that may prove more effective candidates for the development of therapeutics for treatment during withdrawal and for the maintenance of sobriety.
Supplementary Material
Acknowledgments
This research was supported by grants from the Department of Veterans Affairs (BX001172 (KMW) and BX001294 (CJW)) and from the NIH/NIAAA (R01AA021468 (KMW)). Additionally, this material is the result of work supported with resources and the use of facilities at the Portland VA Medical Center (KMW) and the Research Career Scientist Program (KMW). We thank Melissa Andrew for assistance with the vapor exposure procedure and acknowledge support from NIAAA for the Portland Alcohol Research Center (P60AA010760) and for the maintenance of colonies of WSR and WSP mice (R24AA020245) used in the present studies.
Abbreviations
- ACC
anterior cingulate cortex
- ANOVA
Analysis of Variance
- BEC
blood alcohol concentration
- CNS
central nervous system
- Con
control
- CORT
corticosterone
- ELISA
enzyme-linked immunosorbent assay
- EtOH
alcohol/ethanol
- GABA
gamma amino-butyric acid
- H&E
hematoxylin and eosin
- HPA
hypothalamic-pituitary-adrenal axis
- IL-6
interleukin 6
- IPA
Ingenuity Pathway Analysis
- LPS
lipopolysaccharide
- PRRs
pattern recognition receptors
- mPFC
medial prefrontal cortex
- MAPK
mitogen-activated protein kinase
- NF-κB
nuclear factor kappa-light-chain-enhancer of activated B cells
- Pyz
pyrazole
- S1P
sphingosine-1-phosphate
- TGFB1
transforming growth factor β1
- TLR-4
toll-like receptor 4 (TLR-4)
- TNF-α
tumor necrosis factor-α
- WSP
Withdrawal Seizure-Prone
- WSR
Withdrawal Seizure-Resistant
References
- Abbott NJ, Ronnback L, Hansson E. Astrocyte-endothelial interactions at the blood-brain barrier. Nature reviews Neuroscience. 2006;7:41, 53. doi: 10.1038/nrn1824. [DOI] [PubMed] [Google Scholar]
- Adinoff B, Iranmanesh A, Veldhuis J, Fisher L. Disturbances of the stress response: the role of the HPA axis during alcohol withdrawal and abstinence. Alcohol health and research world. 1998;22:67–72. [PMC free article] [PubMed] [Google Scholar]
- Adinoff B, Ruether K, Krebaum S, Iranmanesh A, Williams MJ. Increased salivary cortisol concentrations during chronic alcohol intoxication in a naturalistic clinical sample of men. Alcoholism, clinical and experimental research. 2003;27:1420–1427. doi: 10.1097/01.ALC.0000087581.13912.64. [DOI] [PubMed] [Google Scholar]
- Albano E. Oxidative mechanisms in the pathogenesis of alcoholic liver disease. Molecular aspects of medicine. 2008;29:9–16. doi: 10.1016/j.mam.2007.09.004. [DOI] [PubMed] [Google Scholar]
- Alfonso-Loeches S, Pascual M, Guerri C. Gender differences in alcohol-induced neurotoxicity and brain damage. Toxicology. 2013;311:27–34. doi: 10.1016/j.tox.2013.03.001. [DOI] [PubMed] [Google Scholar]
- Ammendola A, Gemini D, Iannaccone S, Argenzio F, Ciccone G, Ammendola E, Serio L, Ugolini G, Bravaccio F. Gender and peripheral neuropathy in chronic alcoholism: a clinical-electroneurographic study. Alcohol and alcoholism. 2000;35:368–371. doi: 10.1093/alcalc/35.4.368. [DOI] [PubMed] [Google Scholar]
- Badrick E, Bobak M, Britton A, Kirschbaum C, Marmot M, Kumari M. The relationship between alcohol consumption and cortisol secretion in an aging cohort. The Journal of clinical endocrinology and metabolism. 2008;93:750–757. doi: 10.1210/jc.2007-0737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barnes CB, Ulrik CS. Asthma and Adherence to Inhaled Corticosteroids: Current Status and Future Perspectives. Respiratory care. 2014 doi: 10.4187/respcare.03200. [DOI] [PubMed] [Google Scholar]
- Batty GD, Hunt K, Emslie C, Lewars H, Gale CR. Alcohol problems and all-cause mortality in men and women: predictive capacity of a clinical screening tool in a 21-year follow-up of a large, UK-wide, general population-based survey. Journal of psychosomatic research. 2009;66:317–321. doi: 10.1016/j.jpsychores.2008.09.021. [DOI] [PubMed] [Google Scholar]
- Bauer J, Pedersen A, Scherbaum N, Bening J, Patschke J, Kugel H, Heindel W, Arolt V, Ohrmann P. Craving in alcohol-dependent patients after detoxification is related to glutamatergic dysfunction in the nucleus accumbens and the anterior cingulate cortex. Neuropsychopharmacology : official publication of the American College of Neuropsychopharmacology. 2013;38:1401–1408. doi: 10.1038/npp.2013.45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beadles-Bohling AS, Wiren KM. Anticonvulsive effects of kappa-opioid receptor modulation in an animal model of ethanol withdrawal. Genes, brain, and behavior. 2006;5:483–496. doi: 10.1111/j.1601-183X.2005.00200.x. [DOI] [PubMed] [Google Scholar]
- Becker JB, Hu M. Sex differences in drug abuse. Frontiers in neuroendocrinology. 2008;29:36–47. doi: 10.1016/j.yfrne.2007.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blanchard BA, Steindorf S, Wang S, Glick SD. Sex differences in ethanol-induced dopamine release in nucleus accumbens and in ethanol consumption in rats. Alcoholism, clinical and experimental research. 1993;17:968–973. doi: 10.1111/j.1530-0277.1993.tb05650.x. [DOI] [PubMed] [Google Scholar]
- Bourke CH, Harrell CS, Neigh GN. Stress-induced sex differences: adaptations mediated by the glucocorticoid receptor. Hormones and behavior. 2012;62:210–218. doi: 10.1016/j.yhbeh.2012.02.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brady KT, Randall CL. Gender differences in substance use disorders. The Psychiatric clinics of North America. 1999;22:241–252. doi: 10.1016/s0193-953x(05)70074-5. [DOI] [PubMed] [Google Scholar]
- Brambilla L, Martorana F, Rossi D. Astrocyte signaling and neurodegeneration: new insights into CNS disorders. Prion. 2013;7:28–36. doi: 10.4161/pri.22512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burgess LH, Handa RJ. Chronic estrogen-induced alterations in adrenocorticotropin and corticosterone secretion, and glucocorticoid receptor-mediated functions in female rats. Endocrinology. 1992;131:1261–1269. doi: 10.1210/endo.131.3.1324155. [DOI] [PubMed] [Google Scholar]
- Bush G, Luu P, Posner MI. Cognitive and emotional influences in anterior cingulate cortex. Trends in cognitive sciences. 2000;4:215–222. doi: 10.1016/s1364-6613(00)01483-2. [DOI] [PubMed] [Google Scholar]
- Carroll ME, Anker JJ. Sex differences and ovarian hormones in animal models of drug dependence. Hormones and behavior. 2010;58:44–56. doi: 10.1016/j.yhbeh.2009.10.001. [DOI] [PubMed] [Google Scholar]
- Carroll ME, Lynch WJ, Roth ME, Morgan AD, Cosgrove KP. Sex and estrogen influence drug abuse. Trends in pharmacological sciences. 2004;25:273–279. doi: 10.1016/j.tips.2004.03.011. [DOI] [PubMed] [Google Scholar]
- Centers for Disease Control and Prevention. Fact Sheets- Alcohol Use and Healt. 2014;2014 [Google Scholar]
- Ceylan-Isik AF, McBride SM, Ren J. Sex difference in alcoholism: who is at a greater risk for development of alcoholic complication? Life sciences. 2010;87:133–138. doi: 10.1016/j.lfs.2010.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chakraborty G, Saito M, Shah R, Mao RF, Vadasz C, Saito M. Ethanol triggers sphingosine 1-phosphate elevation along with neuroapoptosis in the developing mouse brain. Journal of neurochemistry. 2012;121:806–817. doi: 10.1111/j.1471-4159.2012.07723.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choi JW, Gardell SE, Herr DR, Rivera R, Lee CW, Noguchi K, Teo ST, Yung YC, Lu M, Kennedy G, Chun J. FTY720 (fingolimod) efficacy in an animal model of multiple sclerosis requires astrocyte sphingosine 1-phosphate receptor 1 (S1P1) modulation. Proceedings of the National Academy of Sciences of the United States of America. 2011;108:751–756. doi: 10.1073/pnas.1014154108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cook RT. Alcohol abuse, alcoholism, and damage to the immune system--a review. Alcoholism, clinical and experimental research. 1998;22:1927–1942. [PubMed] [Google Scholar]
- Couzin-Frankel J. National Institutes of Health. Needed: more females in animal and cell studies. Science. 2014;344:679. doi: 10.1126/science.344.6185.679. [DOI] [PubMed] [Google Scholar]
- Crews FT, Qin L, Sheedy D, Vetreno RP, Zou J. High mobility group box 1/Toll-like receptor danger signaling increases brain neuroimmune activation in alcohol dependence. Biological psychiatry. 2013;73:602–612. doi: 10.1016/j.biopsych.2012.09.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cullen KM, Halliday GM. Chronic alcoholics have substantial glial pathology in the forebrain and diencephalon. Alcohol Alcohol Suppl. 1994;2:253–257. [PubMed] [Google Scholar]
- Deshmukh A, Rosenbloom MJ, Sassoon S, O'Reilly A, Pfefferbaum A, Sullivan EV. Alcoholic men endorse more DSM-IV withdrawal symptoms than alcoholic women matched in drinking history. Journal of studies on alcohol. 2003;64:375–379. doi: 10.15288/jsa.2003.64.375. [DOI] [PubMed] [Google Scholar]
- Devaud LL, Chadda R. Sex differences in rats in the development of and recovery from ethanol dependence assessed by changes in seizure susceptibility. Alcoholism, clinical and experimental research. 2001;25:1689–1696. [PubMed] [Google Scholar]
- Dias R, Aggleton JP. Effects of selective excitotoxic prefrontal lesions on acquisition of nonmatching- and matching-to-place in the T-maze in the rat: differential involvement of the prelimbic-infralimbic and anterior cingulate cortices in providing behavioural flexibility. The European journal of neuroscience. 2000;12:4457–4466. doi: 10.1046/j.0953-816x.2000.01323.x. [DOI] [PubMed] [Google Scholar]
- Dietrich J, Rao K, Pastorino S, Kesari S. Corticosteroids in brain cancer patients: benefits and pitfalls. Expert review of clinical pharmacology. 2011;4:233–242. doi: 10.1586/ecp.11.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duma D, Collins JB, Chou JW, Cidlowski JA. Sexually dimorphic actions of glucocorticoids provide a link to inflammatory diseases with gender differences in prevalence. Science signaling. 2010;3:ra74. doi: 10.1126/scisignal.2001077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Erickson K, Drevets W, Schulkin J. Glucocorticoid regulation of diverse cognitive functions in normal and pathological emotional states. Neuroscience and biobehavioral reviews. 2003;27:233–246. doi: 10.1016/s0149-7634(03)00033-2. [DOI] [PubMed] [Google Scholar]
- Fein G, Bachman L, Fisher S, Davenport L. Cognitive impairments in abstinent alcoholics. The Western journal of medicine. 1990;152:531–537. [PMC free article] [PubMed] [Google Scholar]
- Fernandez-Sola J, Nicolas-Arfelis JM. Gender differences in alcoholic cardiomyopathy. The journal of gender-specific medicine : JGSM : the official journal of the Partnership for Women's Health at Columbia. 2002;5:41–47. [PubMed] [Google Scholar]
- Finn DA, Crabbe JC. Chronic ethanol differentially alters susceptibility to chemically induced convulsions in withdrawal seizure-prone and -resistant mice. The Journal of pharmacology and experimental therapeutics. 1999;288:782–790. [PubMed] [Google Scholar]
- Fish EN. The X-files in immunity: sex-based differences predispose immune responses. Nature reviews Immunology. 2008;8:737–744. doi: 10.1038/nri2394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flannery B, Fishbein D, Krupitsky E, Langevin D, Verbitskaya E, Bland C, Bolla K, Egorova V, Bushara N, Tsoy M, Zvartau E. Gender differences in neurocognitive functioning among alcohol-dependent Russian patients. Alcoholism, clinical and experimental research. 2007;31:745–754. doi: 10.1111/j.1530-0277.2007.00372.x. [DOI] [PubMed] [Google Scholar]
- Forman MS, Lal D, Zhang B, Dabir DV, Swanson E, Lee VM, Trojanowski JQ. Transgenic mouse model of tau pathology in astrocytes leading to nervous system degeneration. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2005;25:3539–3550. doi: 10.1523/JNEUROSCI.0081-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forquer MR, Hashimoto JG, Roberts ML, Wiren KM. Elevated testosterone in females reveals a robust sex difference in altered androgen levels during chronic alcohol withdrawal. Alcohol. 2011;45:161–171. doi: 10.1016/j.alcohol.2010.08.013. [DOI] [PubMed] [Google Scholar]
- Galea LA, McEwen BS, Tanapat P, Deak T, Spencer RL, Dhabhar FS. Sex differences in dendritic atrophy of CA3 pyramidal neurons in response to chronic restraint stress. Neuroscience. 1997;81:689–697. doi: 10.1016/s0306-4522(97)00233-9. [DOI] [PubMed] [Google Scholar]
- Gao B, Bataller R. Alcoholic liver disease: pathogenesis and new therapeutic targets. Gastroenterology. 2011;141:1572–1585. doi: 10.1053/j.gastro.2011.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldstein DB, Zaechelein R. Time course of functional tolerance produced in mice by inhalation of ethanol. The Journal of pharmacology and experimental therapeutics. 1983;227:150–153. [PubMed] [Google Scholar]
- Gu L, Huang B, Shen W, Gao L, Ding Z, Wu H, Guo J. Early activation of nSMase2/ceramide pathway in astrocytes is involved in ischemia-associated neuronal damage via inflammation in rat hippocampi. Journal of neuroinflammation. 2013;10:109. doi: 10.1186/1742-2094-10-109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hashimoto JG, Forquer MR, Tanchuck MA, Finn DA, Wiren KM. Importance of genetic background for risk of relapse shown in altered prefrontal cortex gene expression during abstinence following chronic alcohol intoxication. Neuroscience. 2011;173:57–75. doi: 10.1016/j.neuroscience.2010.11.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hashimoto JG, Wiren KM. Neurotoxic consequences of chronic alcohol withdrawal: expression profiling reveals importance of gender over withdrawal severity. Neuropsychopharmacology : official publication of the American College of Neuropsychopharmacology. 2008;33:1084–1096. doi: 10.1038/sj.npp.1301494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- He J, Crews FT. Increased MCP-1 and microglia in various regions of the human alcoholic brain. Experimental neurology. 2008;210:349–358. doi: 10.1016/j.expneurol.2007.11.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hermann D, Frischknecht U, Heinrich M, Hoerst M, Vollmert C, Vollstadt-Klein S, Tunc-Skarka N, Kiefer F, Mann K, Ende G. MR spectroscopy in opiate maintenance therapy: association of glutamate with the number of previous withdrawals in the anterior cingulate cortex. Addiction biology. 2012;17:659–667. doi: 10.1111/j.1369-1600.2010.00290.x. [DOI] [PubMed] [Google Scholar]
- Hommer D, Momenan R, Kaiser E, Rawlings R. Evidence for a gender-related effect of alcoholism on brain volumes. The American journal of psychiatry. 2001;158:198–204. doi: 10.1176/appi.ajp.158.2.198. [DOI] [PubMed] [Google Scholar]
- Ikegami Y, Goodenough S, Inoue Y, Dodd PR, Wilce PA, Matsumoto I. Increased TUNEL positive cells in human alcoholic brains. Neuroscience letters. 2003;349:201–205. doi: 10.1016/s0304-3940(03)00826-7. [DOI] [PubMed] [Google Scholar]
- John U, Rumpf HJ, Bischof G, Hapke U, Hanke M, Meyer C. Excess mortality of alcohol-dependent individuals after 14 years and mortality predictors based on treatment participation and severity of alcohol dependence. Alcoholism, clinical and experimental research. 2013;37:156–163. doi: 10.1111/j.1530-0277.2012.01863.x. [DOI] [PubMed] [Google Scholar]
- Jones AW. Evidence-based survey of the elimination rates of ethanol from blood with applications in forensic casework. Forensic science international. 2010;200:1–20. doi: 10.1016/j.forsciint.2010.02.021. [DOI] [PubMed] [Google Scholar]
- Kim WG, Mohney RP, Wilson B, Jeohn GH, Liu B, Hong JS. Regional difference in susceptibility to lipopolysaccharide-induced neurotoxicity in the rat brain: role of microglia. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2000;20:6309–6316. doi: 10.1523/JNEUROSCI.20-16-06309.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kliethermes CL, Cronise K, Crabbe JC. Anxiety-like behavior in mice in two apparatuses during withdrawal from chronic ethanol vapor inhalation. Alcoholism, clinical and experimental research. 2004;28:1012–1019. doi: 10.1097/01.alc.0000131976.40428.8f. [DOI] [PubMed] [Google Scholar]
- Koob GF, Volkow ND. Neurocircuitry of addiction. Neuropsychopharmacology : official publication of the American College of Neuropsychopharmacology. 2010;35:217–238. doi: 10.1038/npp.2009.110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kosaki Y, Watanabe S. Dissociable roles of the medial prefrontal cortex, the anterior cingulate cortex, and the hippocampus in behavioural flexibility revealed by serial reversal of three-choice discrimination in rats. Behavioural brain research. 2012;227:81–90. doi: 10.1016/j.bbr.2011.10.039. [DOI] [PubMed] [Google Scholar]
- Kosobud A, Crabbe JC. Ethanol withdrawal in mice bred to be genetically prone or resistant to ethanol withdrawal seizures. The Journal of pharmacology and experimental therapeutics. 1986;238:170–177. [PubMed] [Google Scholar]
- Kril JJ, Halliday GM, Svoboda MD, Cartwright H. The cerebral cortex is damaged in chronic alcoholics. Neuroscience. 1997;79:983–998. doi: 10.1016/s0306-4522(97)00083-3. [DOI] [PubMed] [Google Scholar]
- Lam SW, Bauer SR, Guzman JA. Septic shock: the initial moments and beyond. Cleveland Clinic journal of medicine. 2013;80:175–184. doi: 10.3949/ccjm.80a.12002. [DOI] [PubMed] [Google Scholar]
- Lawrencia C, Charrier A, Huang G, Brigstock DR. Ethanol-mediated expression of connective tissue growth factor (CCN2) in mouse pancreatic stellate cells. Growth factors. 2009;27:91–99. doi: 10.1080/08977190902786319. [DOI] [PubMed] [Google Scholar]
- Le Berre AP, Rauchs G, La Joie R, Mezenge F, Boudehent C, Vabret F, Segobin S, Viader F, Allain P, Eustache F, Pitel AL, Beaunieux H. Impaired decision-making and brain shrinkage in alcoholism. European psychiatry : the journal of the Association of European Psychiatrists. 2014;29:125–133. doi: 10.1016/j.eurpsy.2012.10.002. [DOI] [PubMed] [Google Scholar]
- Lewohl JM, Wixey J, Harper CG, Dodd PR. Expression of MBP, PLP, MAG, CNP, and GFAP in the Human Alcoholic Brain. Alcoholism, clinical and experimental research. 2005;29:1698–1705. doi: 10.1097/01.alc.0000179406.98868.59. [DOI] [PubMed] [Google Scholar]
- Li Q, Verma IM. NF-kappaB regulation in the immune system. Nature reviews Immunology. 2002;2:725–734. doi: 10.1038/nri910. [DOI] [PubMed] [Google Scholar]
- Lippai D, Bala S, Csak T, Kurt-Jones EA, Szabo G. Chronic alcohol-induced microRNA-155 contributes to neuroinflammation in a TLR4-dependent manner in mice. PloS one. 2013;8:e70945. doi: 10.1371/journal.pone.0070945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu J, Lewohl JM, Harris RA, Dodd PR, Mayfield RD. Altered gene expression profiles in the frontal cortex of cirrhotic alcoholics. Alcoholism, clinical and experimental research. 2007;31:1460–1466. doi: 10.1111/j.1530-0277.2007.00444.x. [DOI] [PubMed] [Google Scholar]
- Loft S, Olesen KL, Dossing M. Increased susceptibility to liver disease in relation to alcohol consumption in women. Scandinavian journal of gastroenterology. 1987;22:1251–1256. doi: 10.3109/00365528708996472. [DOI] [PubMed] [Google Scholar]
- Lutalo PM, Jordan N, D'Cruz DP. Which dose of steroids and which cytotoxics for severe lupus? Presse medicale. 2014;43:e157–165. doi: 10.1016/j.lpm.2014.03.001. [DOI] [PubMed] [Google Scholar]
- MacDonald AW, 3rd, Cohen JD, Stenger VA, Carter CS. Dissociating the role of the dorsolateral prefrontal and anterior cingulate cortex in cognitive control. Science. 2000;288:1835–1838. doi: 10.1126/science.288.5472.1835. [DOI] [PubMed] [Google Scholar]
- MacGregor RR, Louria DB. Alcohol and infection. Current clinical topics in infectious diseases. 1997;17:291–315. [PubMed] [Google Scholar]
- Mann K, Ackermann K, Croissant B, Mundle G, Nakovics H, Diehl A. Neuroimaging of gender differences in alcohol dependence: are women more vulnerable? Alcoholism, clinical and experimental research. 2005;29:896–901. doi: 10.1097/01.alc.0000164376.69978.6b. [DOI] [PubMed] [Google Scholar]
- Maragakis NJ, Rothstein JD. Glutamate transporters: animal models to neurologic disease. Neurobiology of disease. 2004;15:461–473. doi: 10.1016/j.nbd.2003.12.007. [DOI] [PubMed] [Google Scholar]
- Maragakis NJ, Rothstein JD. Mechanisms of Disease: astrocytes in neurodegenerative disease. Nature clinical practice Neurology. 2006;2:679–689. doi: 10.1038/ncpneuro0355. [DOI] [PubMed] [Google Scholar]
- Marshall SA, McClain JA, Kelso ML, Hopkins DM, Pauly JR, Nixon K. Microglial activation is not equivalent to neuroinflammation in alcohol-induced neurodegeneration: The importance of microglia phenotype. Neurobiology of disease. 2013;54:239–251. doi: 10.1016/j.nbd.2012.12.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mayfield RD, Lewohl JM, Dodd PR, Herlihy A, Liu J, Harris RA. Patterns of gene expression are altered in the frontal and motor cortices of human alcoholics. Journal of neurochemistry. 2002;81:802–813. doi: 10.1046/j.1471-4159.2002.00860.x. [DOI] [PubMed] [Google Scholar]
- McBride WJ, Kimpel MW, McClintick JN, Ding ZM, Hyytia P, Colombo G, Edenberg HJ, Lumeng L, Bell RL. Gene expression in the ventral tegmental area of 5 pairs of rat lines selectively bred for high or low ethanol consumption. Pharmacology, biochemistry, and behavior. 2012;102:275–285. doi: 10.1016/j.pbb.2012.04.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McClintick JN, Xuei X, Tischfield JA, Goate A, Foroud T, Wetherill L, Ehringer MA, Edenberg HJ. Stress-response pathways are altered in the hippocampus of chronic alcoholics. Alcohol. 2013;47:505–515. doi: 10.1016/j.alcohol.2013.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Medina KL, McQueeny T, Nagel BJ, Hanson KL, Schweinsburg AD, Tapert SF. Prefrontal cortex volumes in adolescents with alcohol use disorders: unique gender effects. Alcoholism, clinical and experimental research. 2008;32:386–394. doi: 10.1111/j.1530-0277.2007.00602.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miguel-Hidalgo JJ, Wei J, Andrew M, Overholser JC, Jurjus G, Stockmeier CA, Rajkowska G. Glia pathology in the prefrontal cortex in alcohol dependence with and without depressive symptoms. Biological psychiatry. 2002;52:1121–1133. doi: 10.1016/s0006-3223(02)01439-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mori T, Kawara S, Shinozaki M, Hayashi N, Kakinuma T, Igarashi A, Takigawa M, Nakanishi T, Takehara K. Role and interaction of connective tissue growth factor with transforming growth factor-beta in persistent fibrosis: A mouse fibrosis model. Journal of cellular physiology. 1999;181:153–159. doi: 10.1002/(SICI)1097-4652(199910)181:1<153::AID-JCP16>3.0.CO;2-K. [DOI] [PubMed] [Google Scholar]
- Muehlich S, Schneider N, Hinkmann F, Garlichs CD, Goppelt-Struebe M. Induction of connective tissue growth factor (CTGF) in human endothelial cells by lysophosphatidic acid, sphingosine-1-phosphate, and platelets. Atherosclerosis. 2004;175:261–268. doi: 10.1016/j.atherosclerosis.2004.04.011. [DOI] [PubMed] [Google Scholar]
- Mulholland PJ, Self RL, Harris BR, Little HJ, Littleton JM, Prendergast MA. Corticosterone increases damage and cytosolic calcium accumulation associated with ethanol withdrawal in rat hippocampal slice cultures. Alcoholism, clinical and experimental research. 2005;29:871–881. doi: 10.1097/01.alc.0000163509.27577.da. [DOI] [PubMed] [Google Scholar]
- Myhr KM, Mellgren SI. Corticosteroids in the treatment of multiple sclerosis. Acta neurologica Scandinavica Supplementum. 2009:73–80. doi: 10.1111/j.1600-0404.2009.01213.x. [DOI] [PubMed] [Google Scholar]
- Nadeau S, Rivest S. Glucocorticoids play a fundamental role in protecting the brain during innate immune response. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2003;23:5536–5544. doi: 10.1523/JNEUROSCI.23-13-05536.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nolen-Hoeksema S. Gender differences in risk factors and consequences for alcohol use and problems. Clinical psychology review. 2004;24:981–1010. doi: 10.1016/j.cpr.2004.08.003. [DOI] [PubMed] [Google Scholar]
- O'Neill LA, Kaltschmidt C. NF-kappa B: a crucial transcription factor for glial and neuronal cell function. Trends in neurosciences. 1997;20:252–258. doi: 10.1016/s0166-2236(96)01035-1. [DOI] [PubMed] [Google Scholar]
- Owen D, Matthews SG. Glucocorticoids and sex-dependent development of brain glucocorticoid and mineralocorticoid receptors. Endocrinology. 2003;144:2775–2784. doi: 10.1210/en.2002-0145. [DOI] [PubMed] [Google Scholar]
- Panegyres PK. The effects of excitotoxicity on the expression of the amyloid precursor protein gene in the brain and its modulation by neuroprotective agents. Journal of neural transmission. 1998;105:463–478. doi: 10.1007/s007020050070. [DOI] [PubMed] [Google Scholar]
- Perry JL, Joseph JE, Jiang Y, Zimmerman RS, Kelly TH, Darna M, Huettl P, Dwoskin LP, Bardo MT. Prefrontal cortex and drug abuse vulnerability: translation to prevention and treatment interventions. Brain research reviews. 2011;65:124–149. doi: 10.1016/j.brainresrev.2010.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Philibin SD, Cameron AJ, Metten P, Crabbe JC. Motor impairment: a new ethanol withdrawal phenotype in mice. Behavioural pharmacology. 2008;19:604–614. doi: 10.1097/FBP.0b013e32830ded27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pignataro L, Varodayan FP, Tannenholz LE, Protiva P, Harrison NL. Brief alcohol exposure alters transcription in astrocytes via the heat shock pathway. Brain and behavior. 2013;3:114–133. doi: 10.1002/brb3.125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Potts JR, Howard MR, Verma S. Recurrent severe alcoholic hepatitis: clinical characteristics and outcomes. European journal of gastroenterology & hepatology. 2013;25:659–664. doi: 10.1097/MEG.0b013e32835d83d9. [DOI] [PubMed] [Google Scholar]
- Prescott CA, Caldwell CB, Carey G, Vogler GP, Trumbetta SL, Gottesman II. The Washington University Twin Study of alcoholism. American journal of medical genetics Part B, Neuropsychiatric genetics : the official publication of the International Society of Psychiatric Genetics. 2005;134B:48–55. doi: 10.1002/ajmg.b.30124. [DOI] [PubMed] [Google Scholar]
- Qin L, Crews FT. NADPH oxidase and reactive oxygen species contribute to alcohol-induced microglial activation and neurodegeneration. Journal of neuroinflammation. 2012;9:5. doi: 10.1186/1742-2094-9-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ragozzino ME, Rozman S. The effect of rat anterior cingulate inactivation on cognitive flexibility. Behavioral neuroscience. 2007;121:698–706. doi: 10.1037/0735-7044.121.4.698. [DOI] [PubMed] [Google Scholar]
- Rohleder N, Schommer NC, Hellhammer DH, Engel R, Kirschbaum C. Sex differences in glucocorticoid sensitivity of proinflammatory cytokine production after psychosocial stress. Psychosomatic medicine. 2001;63:966–972. doi: 10.1097/00006842-200111000-00016. [DOI] [PubMed] [Google Scholar]
- Rose AK, Shaw SG, Prendergast MA, Little HJ. The importance of glucocorticoids in alcohol dependence and neurotoxicity. Alcoholism, clinical and experimental research. 2010;34:2011–2018. doi: 10.1111/j.1530-0277.2010.01298.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanchez-Lopez E, Rayego S, Rodrigues-Diez R, Rodriguez JS, Rodrigues-Diez R, Rodriguez-Vita J, Carvajal G, Aroeira LS, Selgas R, Mezzano SA, Ortiz A, Egido J, Ruiz-Ortega M. CTGF promotes inflammatory cell infiltration of the renal interstitium by activating NF-kappaB. Journal of the American Society of Nephrology : JASN. 2009;20:1513–1526. doi: 10.1681/ASN.2008090999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sapolsky RM. Glucocorticoids and hippocampal atrophy in neuropsychiatric disorders. Archives of general psychiatry. 2000;57:925–935. doi: 10.1001/archpsyc.57.10.925. [DOI] [PubMed] [Google Scholar]
- Sapolsky RM, Pulsinelli WA. Glucocorticoids potentiate ischemic injury to neurons: therapeutic implications. Science. 1985;229:1397–1400. doi: 10.1126/science.4035356. [DOI] [PubMed] [Google Scholar]
- Savaskan NE, Eyupoglu IY, Brauer AU, Plaschke M, Ninnemann O, Nitsch R, Skutella T. Entorhinal cortex lesion studied with the novel dye fluoro-jade. Brain research. 2000;864:44–51. doi: 10.1016/s0006-8993(00)02148-x. [DOI] [PubMed] [Google Scholar]
- Schafer GL, Crabbe JC, Wiren KM. Identification of neuroendocrine-specific protein as an ethanol-regulated gene with mRNA differential display. Mammalian genome : official journal of the International Mammalian Genome Society. 1998;9:979–982. doi: 10.1007/s003359900910. [DOI] [PubMed] [Google Scholar]
- Schmued LC, Hopkins KJ. Fluoro-Jade B: a high affinity fluorescent marker for the localization of neuronal degeneration. Brain research. 2000;874:123–130. doi: 10.1016/s0006-8993(00)02513-0. [DOI] [PubMed] [Google Scholar]
- Schuckit MA, Smith TL, Trim RS, Kuperman S, Kramer J, Hesselbrock V, Bucholz KK, Nurnberger JI, Jr, Hesselbrock M, Saunders G. Sex differences in how a low sensitivity to alcohol relates to later heavy drinking. Drug and alcohol review. 2012;31:871–880. doi: 10.1111/j.1465-3362.2012.00469.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwab JM, Beschorner R, Nguyen TD, Meyermann R, Schluesener HJ. Differential cellular accumulation of connective tissue growth factor defines a subset of reactive astrocytes, invading fibroblasts, and endothelial cells following central nervous system injury in rats and humans. Journal of neurotrauma. 2001;18:377–388. doi: 10.1089/089771501750170930. [DOI] [PubMed] [Google Scholar]
- Seo D, Lacadie CM, Tuit K, Hong KI, Constable RT, Sinha R. Disrupted ventromedial prefrontal function, alcohol craving, and subsequent relapse risk. JAMA psychiatry. 2013;70:727–739. doi: 10.1001/jamapsychiatry.2013.762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sershen H, Hashim A, Vadasz C. Strain and sex differences in repeated ethanol treatment-induced motor activity in quasi-congenic mice. Genes, brain, and behavior. 2002;1:156–165. doi: 10.1034/j.1601-183x.2002.10303.x. [DOI] [PubMed] [Google Scholar]
- Sharrett-Field L, Butler TR, Reynolds AR, Berry JN, Prendergast MA. Sex differences in neuroadaptation to alcohol and withdrawal neurotoxicity. Pflugers Archiv : European journal of physiology. 2013;465:643–654. doi: 10.1007/s00424-013-1266-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silveri MM, Cohen-Gilbert J, Crowley DJ, Rosso IM, Jensen JE, Sneider JT. Altered anterior cingulate neurochemistry in emerging adult binge drinkers with a history of alcohol-induced blackouts. Alcoholism, clinical and experimental research. 2014;38:969–979. doi: 10.1111/acer.12346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Solomon MB, Furay AR, Jones K, Packard AE, Packard BA, Wulsin AC, Herman JP. Deletion of forebrain glucocorticoid receptors impairs neuroendocrine stress responses and induces depression-like behavior in males but not females. Neuroscience. 2012;203:135–143. doi: 10.1016/j.neuroscience.2011.12.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sorrells SF, Caso JR, Munhoz CD, Hu CK, Tran KV, Miguel ZD, Chien BY, Sapolsky RM. Glucocorticoid signaling in myeloid cells worsens acute CNS injury and inflammation. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2013;33:7877–7889. doi: 10.1523/JNEUROSCI.4705-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sorrells SF, Sapolsky RM. An inflammatory review of glucocorticoid actions in the CNS. Brain, behavior, and immunity. 2007;21:259–272. doi: 10.1016/j.bbi.2006.11.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stephens MA, Wand G. Stress and the HPA axis: role of glucocorticoids in alcohol dependence. Alcohol research : current reviews. 2012;34:468–483. [PMC free article] [PubMed] [Google Scholar]
- Straub RH. The complex role of estrogens in inflammation. Endocrine reviews. 2007;28:521–574. doi: 10.1210/er.2007-0001. [DOI] [PubMed] [Google Scholar]
- Thibault C, Lai C, Wilke N, Duong B, Olive MF, Rahman S, Dong H, Hodge CW, Lockhart DJ, Miles MF. Expression profiling of neural cells reveals specific patterns of ethanol-responsive gene expression. Molecular pharmacology. 2000;58:1593–1600. doi: 10.1124/mol.58.6.1593. [DOI] [PubMed] [Google Scholar]
- Torres OV, Walker EM, Beas BS, O'Dell LE. Female rats display enhanced rewarding effects of ethanol that are hormone dependent. Alcoholism, clinical and experimental research. 2014;38:108–115. doi: 10.1111/acer.12213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vann LR, Payne SG, Edsall LC, Twitty S, Spiegel S, Milstien S. Involvement of sphingosine kinase in TNF-alpha-stimulated tetrahydrobiopterin biosynthesis in C6 glioma cells. The Journal of biological chemistry. 2002;277:12649–12656. doi: 10.1074/jbc.M109111200. [DOI] [PubMed] [Google Scholar]
- Veatch LM, Wright TM, Randall CL. Only male mice show sensitization of handling-induced convulsions across repeated ethanol withdrawal cycles. Alcoholism, clinical and experimental research. 2007;31:477–485. doi: 10.1111/j.1530-0277.2006.00328.x. [DOI] [PubMed] [Google Scholar]
- Vetreno RP, Qin L, Crews FT. Increased receptor for advanced glycation end product expression in the human alcoholic prefrontal cortex is linked to adolescent drinking. Neurobiology of disease. 2013;59:52–62. doi: 10.1016/j.nbd.2013.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walls SA, Rosenwasser AM, Devaud LL. Sex and regional differences in effects of chronic intermittent ethanol exposure on subsequent excitotoxic challenges in hippocampal slice cultures. Neuroscience letters. 2013;550:6–11. doi: 10.1016/j.neulet.2013.05.011. [DOI] [PubMed] [Google Scholar]
- Wang GJ, Volkow ND, Fowler JS, Franceschi D, Wong CT, Pappas NR, Netusil N, Zhu W, Felder C, Ma Y. Alcohol intoxication induces greater reductions in brain metabolism in male than in female subjects. Alcoholism, clinical and experimental research. 2003;27:909–917. doi: 10.1097/01.ALC.0000071740.56375.BA. [DOI] [PubMed] [Google Scholar]
- Wang W, Morrison B, Galbaugh T, Jose CC, Kenney N, Cutler ML. Glucocorticoid induced expression of connective tissue growth factor contributes to lactogenic differentiation of mouse mammary epithelial cells. Journal of cellular physiology. 2008;214:38–46. doi: 10.1002/jcp.21159. [DOI] [PubMed] [Google Scholar]
- Weiss PA, Collier SD, Pruett SB. Role of glucocorticoids in ethanol-induced decreases in expression of MHC class II molecules on B cells and selective decreases in spleen cell number. Toxicology and applied pharmacology. 1996;139:153–162. doi: 10.1006/taap.1996.0154. [DOI] [PubMed] [Google Scholar]
- Westenbroek C, Perry AN, Becker JB. Pair housing differentially affects motivation to self-administer cocaine in male and female rats. Behavioural brain research. 2013;252:68–71. doi: 10.1016/j.bbr.2013.05.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilhelm CJ, Hashimoto JG, Roberts ML, Sonmez MK, Wiren KM. Understanding the addiction cycle: A complex biology with distinct contributions of genotype vs. sex at each stage. Neuroscience. 2014;279C:168–186. doi: 10.1016/j.neuroscience.2014.08.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wiren KM. Males and females are just different: sexually dimorphic responses to chronic ethanol exposure in hippocampal slice cultures. Neuroscience letters. 2013;550:1–5. doi: 10.1016/j.neulet.2013.06.030. [DOI] [PubMed] [Google Scholar]
- Organization, W. H, editor. World Health Organization. WHO Alcohol. 2014. [Google Scholar]
- Yang X, Schadt EE, Wang S, Wang H, Arnold AP, Ingram-Drake L, Drake TA, Lusis AJ. Tissue-specific expression and regulation of sexually dimorphic genes in mice. Genome research. 2006;16:995–1004. doi: 10.1101/gr.5217506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zahr NM, Kaufman KL, Harper CG. Clinical and pathological features of alcohol-related brain damage. Nature reviews Neurology. 2011;7:284–294. doi: 10.1038/nrneurol.2011.42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zinn S, Stein R, Swartzwelder HS. Executive functioning early in abstinence from alcohol. Alcoholism, clinical and experimental research. 2004;28:1338–1346. doi: 10.1097/01.alc.0000139814.81811.62. [DOI] [PubMed] [Google Scholar]
- Zou J, Crews FT. Inflammasome-IL-1beta Signaling Mediates Ethanol Inhibition of Hippocampal Neurogenesis. Frontiers in neuroscience. 2012;6:77. doi: 10.3389/fnins.2012.00077. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.