Abstract
Purpose
The objective of this work is to characterize and quantify the impact of respiratory-induced prostate motion.
Methods and Materials
Real-time intrafraction motion is observed with the Calypso 4-dimensional nonradioactive electromagnetic tracking system (Calypso Medical Technologies, Inc. Seattle, Washington). We report the results from a total of 1024 fractions from 31 prostate cancer patients. Wavelet transform was used to decompose the signal to extract and isolate the respiratory-induced prostate motion from the total prostate displacement.
Results
Our results show that the average respiratory motion larger than 0.5 mm can be observed in 68% of the fractions. Fewer than 1% of the patients showed average respiratory motion of less than 0.2 mm, whereas 99% of the patients showed average respiratory-induced motion ranging between 0.2 and 2 mm. The maximum respiratory range of motion of 3 mm or greater was seen in only 25% of the fractions. In addition, about 2% patients showed anxiety, indicated by a breathing frequency above 24 times per minute.
Conclusions
Prostate motion is influenced by respiration in most fractions. Real-time intrafraction data are sensitive enough to measure the impact of respiration by use of wavelet decomposition methods. Although the average respiratory amplitude observed in this study is small, this technique provides a tool that can be useful if one moves to smaller treatment margins (≤5 mm). This also opens ups the possibility of being able to develop patient specific margins, knowing that prostate motion is not unpredictable.
Introduction
Conformal external beam therapy can effectively enhance the local control of prostate cancer (1, 2). The knowledge of organ motion is essential to ensure conformed dose delivery to the target and minimize toxicity to the surrounding organs at risk during prostate radiation therapy (3, 4). Prostate intrafraction motion causes the dose distribution to be smeared. Even with perfect external immobilization techniques and minimized whole pelvic motion controlled by patient consciousness, the intrafraction internal organ motion during treatment will contribute to smearing of the dose distribution and increase toxicity of surrounding tissue significantly (5, 6). In general, prostate motion is a combination of the contribution from bladder and rectum filling and respiratory motion. Currently, there is very limited understanding of how prostate movement is affected by the respiratory cycle because most intrafraction tracking techniques cannot separate the contribution from respiratory-induced prostate motion from the bladder and rectum filling. In this article we attempt to isolate the respiratory motion from the total prostate displacement and characterize the contribution of respiratory-induced prostate motion using a wavelet transform technique. To our knowledge, this is the first study of this type.
High-precision, real-time intrafraction motion is obtained with the Calypso 4-dimensional nonradioactive electromagnetic position tracking system (Calypso Medical Technologies, Inc. Seattle, Washington). Three electromagnetic transponders (“beacon”) are implanted into the prostate. During daily radiation treatment, the beacon transponders communicate with the Calypso system through nonionizing radiofrequency signal, and the prostate isocenter displacement in the superoinferior, anteroposterior, and lateral directions is recorded at a frequency of 10 Hz. The objective of this work is to characterize and quantify the impact of respiratory-induced prostate motion from these tracking data. We report the results from a total of 1024 fractions from 31 prostate cancer patients who underwent daily intensity modulated radiation therapy (IMRT) treatment.
Methods and Materials
Patient and Calypso implantation procedure
A total of 31 subjects clinically diagnosed with prostate cancer and treated with IMRT at Winship Cancer Institute, Atlanta, Georgia, were included in the study. The real-time tracking technique, which was instrumental in initial Food and Drug Administration approval for the Calypso system, has been previously described (7–9). In brief, all patients underwent implantation of 3 beacon electromagnetic transponders into the prostate via transrectal ultrasound guidance before the radiation treatment. All patients were treated in the supine position as follows: a band was placed around the feet, a wedge under the knees, a ring for the hands on the chest, and a Vac-Loc bag for daily immobilization. All patients were instructed to drink 500 mL of fluid 4 hours before simulation and during daily treatments. No specific bowel preparation instructions were provided. A low-residue diet was encouraged during treatments, and patients were encouraged to have a bowel movement before daily treatments.
After daily localization with minimal initial residual, intrafractional motion of the prostate during the treatment is monitored at a 10-Hz frequency as the lateral (RL), anteroposterior (AP), and superoinferior (SI) displacement of the isocenter with the Calypso 4-dimensional tracking system (7, 8). An action threshold of 3 mm of prostate motion or greater lasting for more than 30 seconds was instituted. If this was exceeded, the beam and prostate tracking was stopped and the patient was repositioned with minimal residual before treatment was resumed. A typical IMRT course consisted of 38 to 39 fractions, and the mean fraction length was 7 minutes 6 seconds (426 seconds). Only the fractions consisting of a minimum of 3 minutes of continuous tracking data were included in the analysis, resulting in a total of 1024 fractions. The tracking data obtained from the Calypso system and analyzed within this work are limited to intrafraction prostate motion obtained after initial prostate localization with near 0 initial residual.
Prostate motion characterization and quantification using wavelet analysis
Normally, Fourier transform is extremely useful for extracting frequency content of the respiratory sinusoidal signal. However, the major drawback is the requirement for the signal to be stationary. Unfortunately, prostate motion tracking data are non-stationary, characterized by drifting, abrupt changes, and multiple excursions (7). To overcome the limitation of Fourier transformation, wavelet transform is used as a more suitable tool. Wavelet analysis allows the use of stretched wavelets to extract low-frequency information and compressed wavelets to extract high-frequency information (10, 11). As a result, the particular power spectral band relevant to respiration motion can be effectively separated from high-frequency noise through this multi-resolution time-frequency analysis.
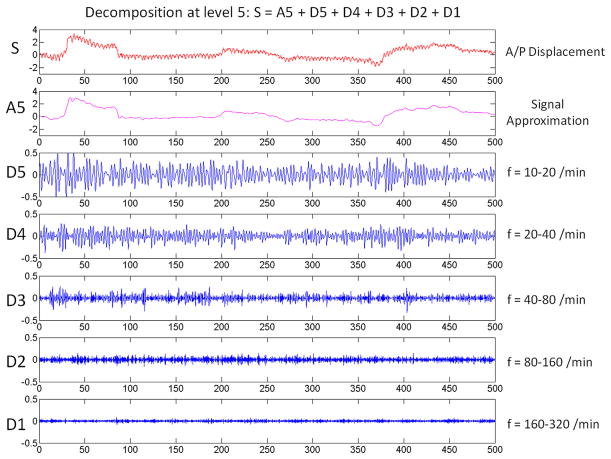
Uniform samples at 10 Hz were obtained from the real-time Calypso tracking data. Then, the data were wavelet transformed with the discontinuous wavelet transform algorithm to 5 levels by use of db4 wavelets (Daubechies wavelets) (13). The use of 10 Hz results in the following frequency bands: D5, 0.1563 to 0.3125 Hz; D4, 0.3125 to 0.625 Hz; D3, 0.625 to 1.25 Hz; D2, 1.25 to 2.5 Hz; and D1, 2.5 to 5 Hz. The frequency band corresponds to respiratory frequency as follows: D5, 10 to 20 times per minute; D4, 20 to 40 times per minute; D3, 40 to 80 times per minute; D2, 80 to 160 times per minute; and D1, 160 to 320 times per minute. The typical breathing pattern is around 10 to 24 breaths per minute, and thus extracted signals at D5 and D4 bands are mainly used for further analysis. It is essential to include the D4 band because some patients may have anxiety or respiratory distress during radiation, resulting in accelerated breathing frequency above 24 times per minute. On the other hand, the frequency ranges of D1 through D3 are apparently too high for respiratory motion. Power spectral density analysis was performed to extract the peak breathing frequency and the average total power. The average total power is defined as the area under the power spectral density curve.
Results
Prostate motion characterization and quantification using wavelet analysis
The general pattern of the prostate motion may not be predicable because the frequency and magnitude of the effect from bladder and rectum filling are unknown. Various excursion patterns have shown that it may be futile to predict the general prostate motion behavior (7). However, a closer look at the tracking data, as shown in Figure 1, clearly shows that there exists an oscillation pattern, especially in the AP and SI directions. This pattern exists in all fractions for all patients, and the difference is just a matter of magnitude rather than existence of the pattern. Graphically, the oscillation is about 16 to 18 times per minute and consistently present throughout the entire tracking period. To effectively analyze this respiratory-induced prostate motion, this periodical signal must be extracted and separated from the general prostate motion. For that purpose, wavelet decomposition is used in our study because it is ideal for situations where a repetitive pattern caused by respiration exists amidst background noise caused by prostate drifting and bladder or rectal filling. We have shown in our previous publication (12) that the prostate motion in the AP/SI direction is highly correlated, which is consistent with the longitudinal oblique motion of the prostate, and likely due to the effect of respiration on an organ confined between the bladder and rectum. For this reason, the magnitude and peak frequency of the respiratory motion are extracted from the displacement in the oblique direction ( ) (y-axis for AP and z-axis for SI).
Fig. 1.
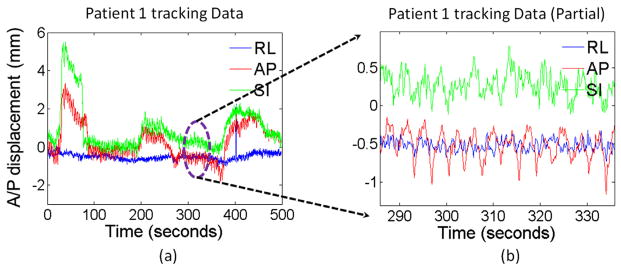
(a) Example of tracking data. (b) A zoom-in view of a portion of the tracking data shows the respiratory-induced oscillation pattern especially in the anteroposterior (AP) and superoinferior (SI) directions. (RL, lateral.)
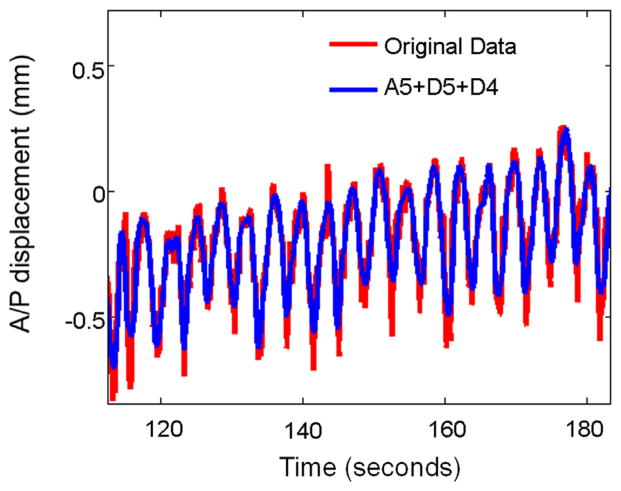
An example of the wavelet decomposition is shown in Figure 2. The original signal is decomposed to a low-frequency approximation at level 5 (A5) and high-frequency details at 5 levels (D5-D1). It is obvious that details D1 through D3 are beyond the range of respiratory frequency, and the D4 and D5 frequency range (10–40 breaths per minute) represents the range for most of the patients. An example of the D4-plus-D5 oscillation superimposed on the A5 approximation in comparison to its original signal is shown in Figure 3. Detail at levels 4 and 5 (D4 plus D5) well represents the major oscillation pattern while disregarding the higher-frequency noises.
Fig. 2.
Example of wavelet decomposition. The signal (S) is decomposed to 5 levels. (f, frequency.)
Fig. 3.
D4-plus-D5 oscillation superimposed on A5 approximation in comparison to its original signal.
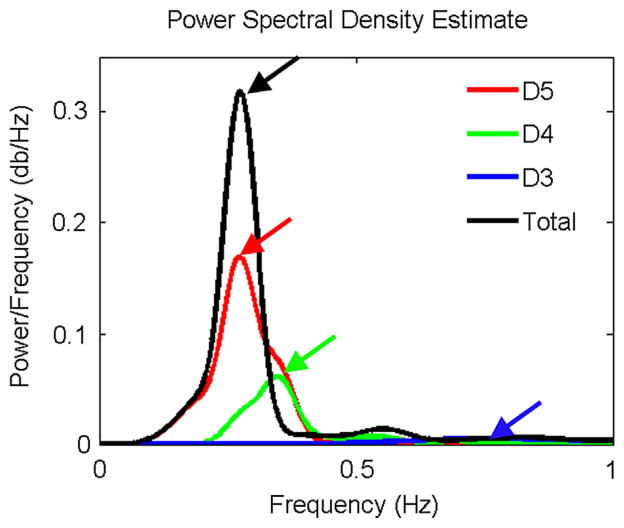
The power spectral density analysis for D3, D4, and D5 is shown in Figure 4. The total represents the sum of all 5 levels of details. D5 showed the highest and most dominant signal strength compared with signal at the D4 and D3 frequency range, indicating that the oscillation signal is mainly at the D5 frequency range. For a total of 1024 fractions, there are only 157 fractions (15%) during which the patient’s respiratory frequency is above 20 times per minute. Moreover, only 24 fractions (2%) are higher than 24 times per minute, which may indicate the increased anxiety level of the patient due to the treatment.
Fig. 4.
Power spectral density analysis for D3, D4, and D5 and sum of details.
The quantification of the respiratory motion amplitude is then determined by the average and maximum respiratory range of motion in the oblique direction. To isolate the total displacement and the impact of respiratory motion, we used the following steps (the notations can be found in Fig. 2): (1) The oscillation details (D4 plus D5) were extracted for both AP (y-axis) and SI (z-axis) directions. (2) Regardless of the displacement for the approximation component (A5), the respiratory motion in oblique direction is defined as . This way, we only calculate the contribution of the amplitude from the respiratory motion rather than total displacement. (3) The average absolute amplitude (RMmean) and the difference between maximum and minimum amplitude (RMmax) of the oblique motion obtained in step 2 is calculated for each fraction. (4) The average respiratory range of motion is then determined as 4 × RMmean, taking into account the positive and negative signs of the data. For instance, an oscillation wave with an amplitude of 2 mm (± 1 mm) has an average absolute amplitude of 0.5 mm. Meanwhile, the maximum respiratory range of motion is RMmax. The results are shown in Table 1.
Table 1.
Results for amplitude of respiratory prostate motion
| No. of fractions | |
|---|---|
| Average respiratory range of motion | |
| 0–0.2 mm | 4 (<1%) |
| 0.2–0.5 mm | 322 (31%) |
| 0.5–1.0 mm | 688 (67%) |
| 1.0–2.0 mm | 10 (1%) |
| >2.0 mm | 0 (0%) |
| Maximum respiratory range of motion | |
| 0–0.5 mm | 16 (2%) |
| 0.5–1.0 mm | 252 (25%) |
| 1.0–2.0 mm | 348 (34%) |
| 2.0–3.0 mm | 145 (14%) |
| >3.0 mm | 263 (25%) |
It is worth noting that the amplitude presented in this article is only the oscillation component of the tracking data. The actual average displacement of the signal can be much higher than 1 or 2 mm. For this reason, the amplitude reported in this article is much smaller than what is normally reported in the literature on deep breathing (14).
Discussion
The small amplitude of respiratory-induced prostate motion extracted from wavelet analysis should not be confused with the overall prostate motion, which has been investigated by several groups. The Calypso real-time tracking data showed that the continuous prostate motion can be unpredictable, ranging greatly from constant drifting, abrupt changes, or a combination thereof, given the unpredictability of bladder filling, rectum filling, and flatulence (7). Shah et al (15) reported prostate motion greater than 3 mm and greater than 5 mm for 12.6% and 2.9% of fractions in the supine position, respectively. Similarly, Langen et al (16) reported prostate motion greater than 3 mm and greater than 5 mm for 14% and 3% of fractions, respectively. Willoughby et al (8) reported prostate displacements of 0.9 ± 0.35 mm, 3.61 ± 3.13 mm, and 3.92 ± 4.32 mm in the lateral, AP, and SI directions, respectively.
Given the complexity of prostate motion, we present the first attempt to isolate the high-frequency respiratory-induced motion from the general prostate motion. The amplitude of respiratory motion alone will be the minimum amount of planning target volume margin required and is likely a function of how frequent prostate motion is imaged during treatment delivery (9). Malone et al (17) quantified and characterized the respiratory motion using implanted gold fiducial markers. A total length of a 20-second interval of fluoroscopy was analyzed, and the maximum displacement in the AP and SI directions was measured. For a total of 20 patients, mean displacement was 1.6 mm and 2.9 mm in the AP and SI directions, respectively. Dinkel et al (14) evaluated respiratory-induced prostate motion using cine–magnetic resonance imaging. The temporal resolution was 3 frames per second, and the total acquisition time was 15 seconds. From the magnetic resonance imaging measurement, the mean displacement of the prostate during deep breathing in the SI and AP directions was 2.7 mm and 1.8 mm, respectively. The prostate displacement for abdominal contraction (via a coughing maneuver) was significantly higher: mean SI displacement was 8.4 mm, and mean AP movement was 8.3 mm. Our results show that most patients have a mean prostate motion below 1 mm, which is a much lower number than the numbers in previous studies. The 2 main reasons are (1) our data were acquired during the actual treatment with normal breathing, and thus deep breathing or conscious abdominal contraction is not included; and (2) the respiratory component is effectively extracted by use of wavelet decomposition, thus excluding the possible large displacement caused by other factors such as bladder/rectum filling or flatulence.
Udrescu et al (18) investigated respiratory prostate motion using 4-dimensional computed tomography and concluded that there is no respiratory motion observed. In this study the SI direction displacement is, in general, unattainable because of the 2.5-mm slice thickness. In both the AP and lateral directions, the prostate motion stayed below 1 mm, with an average of 0.27 mm for a period of 2 minutes. The amplitude of the respiratory motion is consistent with our results, where 99% patients had a mean prostate motion below 1 mm.
The use of high-frequency, real-time tracking data allowed us to evaluate the respiratory motion quantitatively. Our approach has several distinct improvements over respiratory-induced prostate motion analysis in the literature (13, 17, 18). First, the oscillation component of the prostate motion is identified through wavelet analysis, which effectively isolates the respiratory-induced prostate motion from the total prostate displacement. Second, previous publications reported the results from visual observation because of the limited length and sampling rate of data. The 10-Hz real-time tracking data during treatment give sufficient information to extract the frequency and amplitude of the respiratory-induced prostate motion through wavelet frequency-time analysis. Indeed, this is the first time wavelet analysis has been used to characterize respiratory-induced prostate motion during radiation therapy.
Conclusions
Prostate motion is influenced by respiration in most fractions. Real-time intrafraction data are sensitive enough to measure the impact of respiration by use of wavelet decomposition methods. Although the average respiratory amplitude observed in this study is small, this technique provides a tool that can be useful if one moves to smaller treatment margins (≤5 mm). This also opens up the possibility of being able to develop patient-specific margins, knowing that prostate motion is not unpredictable. Moving toward 0-mm margins will only be possible if deformation change of the prostate is also taken into account.
Summary.
The objective of this work is to characterize and quantify respiratory-induced prostate motion using wavelet transform of the Calypso tracking system. Our results show that prostate motion is influenced by respiration in mostfractions, and this technique provides a tool that can be useful if one moves toward smaller margins (≤5 mm). This also opens ups the possibility of being able to develop patient-specific margins, knowing that prostate motion is not unpredictable.
Footnotes
Conflict of Interest: none.
References
- 1.Pollack A, Zagars GK. External beam radiotherapy dose response of prostate cancer. Int J Radiat Oncol Biol Phys. 1997;39:1011–1018. doi: 10.1016/s0360-3016(97)00508-7. [DOI] [PubMed] [Google Scholar]
- 2.Nilsson S, Norlén BJ, Widmark A. A systematic overview of radiation therapy effects in prostate cancer. Acta Oncol. 2004;43:316–381. doi: 10.1080/02841860410030661. [DOI] [PubMed] [Google Scholar]
- 3.Nederveen AJ, van der Heide UA, Dehnad H, et al. Measurements and clinical consequences of prostate motion during a radiotherapy fraction. Int J Radiat Oncol Biol Phys. 2002;53:206–214. doi: 10.1016/s0360-3016(01)02823-1. [DOI] [PubMed] [Google Scholar]
- 4.Huang E, Dong L, Chandra A, et al. Intrafraction prostate motion during IMRT for prostate cancer. Int J Radiat Oncol Biol Phys. 2002;53:261–268. doi: 10.1016/s0360-3016(02)02738-4. [DOI] [PubMed] [Google Scholar]
- 5.Zelefsky MJ, Levin EJ, Hunt M, et al. Incidence of late rectal and urinary toxicities after three-dimensional conformal radiotherapy and intensity-modulated radiotherapy for localized prostate cancer. Int J Radiat Oncol Biol Phys. 2008;70:1124–1129. doi: 10.1016/j.ijrobp.2007.11.044. [DOI] [PubMed] [Google Scholar]
- 6.Guckenberger M, Ok S, Polat B, et al. Toxicity after intensity-modulated, image-guided radiotherapy for prostate cancer. Strahlenther Onkol. 2010;186:535–543. doi: 10.1007/s00066-010-2144-z. [DOI] [PubMed] [Google Scholar]
- 7.Kupelian P, Willoughby T, Mahadevan A, et al. Multi-institutional clinical experience with the Calypso System in localization and continuous, real-time monitoring of the prostate gland during external radiotherapy. Int J Radiat Oncol Biol Phys. 2007;67:1088–1098. doi: 10.1016/j.ijrobp.2006.10.026. [DOI] [PubMed] [Google Scholar]
- 8.Willoughby TR, Kupelian PA, Pouliot J, et al. Target localization and real-time tracking using the Calypso 4D localization system in patients with localized prostate cancer. Int J Radiat Oncol Biol Phys. 2006;65:528–534. doi: 10.1016/j.ijrobp.2006.01.050. [DOI] [PubMed] [Google Scholar]
- 9.Curtis W, Khan M, Magnelli A, et al. Relationship of imaging frequency and planning margin to account for intrafraction prostate motion: analysis based on real-time monitoring data. Int J Radiat Oncol Biol Phys. 2013;85:700–706. doi: 10.1016/j.ijrobp.2012.05.044. [DOI] [PubMed] [Google Scholar]
- 10.Houtveen JH, Molenaar PCM. Comparison between the Fourier and Wavelet methods of spectral analysis applied to stationary and nonstationary heart period data. Psychophysiology. 2001;38:729–735. [PubMed] [Google Scholar]
- 11.Pichot V, Gaspoz J-M, Molliex S, et al. Wavelet transform to quantify heart rate variability and to assess its instantaneous changes. J Appl Physiol. 1999;86:1081–1091. doi: 10.1152/jappl.1999.86.3.1081. [DOI] [PubMed] [Google Scholar]
- 12.Lin, Liu, Yang, et al. The Non-Gaussian Nature of Prostate Motion Based on Real-Time Intrafraction Tracking. Int J Radiat Oncol Biol Phys. 2013 doi: 10.1016/j.ijrobp.2013.05.019. Epub July 9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Daubechies I. Ten Lectures on Wavelets. Philadelphia, PA: Society for Industrial and Applied Mathematics; 1992. [Google Scholar]
- 14.Dinkel J, Thieke C, Plathow C, et al. Respiratory-induced prostate motion. Strahlenther Onkol. 2011;187:426–432. doi: 10.1007/s00066-011-2201-2. [DOI] [PubMed] [Google Scholar]
- 15.Shah AP, Kupelian PA, Willoughby TR, et al. An evaluation of intrafraction motion of the prostate in the prone and supine positions using electromagnetic tracking. Radiother Oncol. 2011;99:37–43. doi: 10.1016/j.radonc.2011.02.012. [DOI] [PubMed] [Google Scholar]
- 16.Langen KM, Willoughby TR, Meeks SL, et al. Observations on real-time prostate gland motion using electromagnetic tracking. Int J Radiat Oncol Biol Phys. 2008;71:1084–1090. doi: 10.1016/j.ijrobp.2007.11.054. [DOI] [PubMed] [Google Scholar]
- 17.Malone S, Crook JM, Kendal WS, et al. Respiratory-induced prostate motion: quantification and characterization. Int J Radiat Oncol Biol Phys. 2000;48:105–109. doi: 10.1016/s0360-3016(00)00603-9. [DOI] [PubMed] [Google Scholar]
- 18.Udrescu C, Jalade P, de Bari B, et al. Evaluation of the respiratory prostate motion with four-dimensional computed tomography scan acquisitions using three implanted markers. Radiother Oncol. 2012;103:266–269. doi: 10.1016/j.radonc.2012.03.016. [DOI] [PubMed] [Google Scholar]