Abstract
BACKGROUND
Dysregulated hedgehog signaling is the pivotal molecular abnormality underlying basal-cell carcinomas. Vismodegib is a new orally administered hedgehog-pathway inhibitor that produces objective responses in locally advanced and metastatic basal-cell carcinomas.
METHODS
We tested the anti–basal-cell carcinoma efficacy of vismodegib in a randomized, double-blind, placebo-controlled trial in patients with the basal-cell nevus syndrome at three clinical centers from September 2009 through January 2011. The primary end point was reduction in the incidence of new basal-cell carcinomas that were eligible for surgical resection (surgically eligible) with vismodegib versus placebo after 3 months; secondary end points included reduction in the size of existing basal-cell carcinomas.
RESULTS
In 41 patients followed for a mean of 8 months (range, 1 to 15) after enrollment, the per-patient rate of new surgically eligible basal-cell carcinomas was lower with vismodegib than with placebo (2 vs. 29 cases per group per year, P<0.001), as was the size (percent change from baseline in the sum of the longest diameter) of existing clinically significant basal-cell carcinomas (−65% vs. −11%, P = 0.003). In some patients, all basal-cell carcinomas clinically regressed. No tumors progressed during treatment with vismodegib. Patients receiving vismodegib routinely had grade 1 or 2 adverse events of loss of taste, muscle cramps, hair loss, and weight loss. Overall, 54% of patients (14 of 26) receiving vismodegib discontinued drug treatment owing to adverse events. At 1 month, vismodegib use had reduced the hedgehog target-gene expression by basal-cell carcinoma by 90% (P<0.001) and diminished tumor-cell proliferation, but apoptosis was not affected. No residual basal-cell carcinoma was detectable in 83% of biopsy samples taken from sites of clinically regressed basal-cell carcinomas.
CONCLUSIONS
Vismodegib reduces the basal-cell carcinoma tumor burden and blocks growth of new basal-cell carcinomas in patients with the basal-cell nevus syndrome. The adverse events associated with treatment led to discontinuation in over half of treated patients. (Funded by Genentech and others; ClinicalTrials.gov number, NCT00957229.)
Basal-cell carcinomas are the most common cancer in the United States, with an estimated annual incidence of 0.1 to 0.5%.1 The rare, heritable basal-cell nevus (Gorlin) syndrome (Online Mendelian Inheritance in Man number, 109400) may cause hundreds to thousands of basal-cell carcinomas in a single patient, and affected persons are at increased risk for medulloblastomas and rhabdomyosarcomas.2 Patients with the basal-cell nevus syndrome inherit one defective copy of the tumor-suppressor gene encoding patched 1 (PTCH1), which acts as a primary inhibitor of the hedgehog signaling pathway.3–5 PTCH1 mutations and loss of the remaining wild-type allele also occur in sporadic basal-cell carcinomas6–8; essentially all basal-cell carcinomas, whether or not they are associated with identifiable mutations of PTCH1 or the smoothened gene (SMO), the target of PTCH1 inhibition, have enhanced hedgehog signaling. Currently, no pharmacologic therapy is consistently efficacious for basal-cell carcinomas, and the quality of life for patients with the basal-cell nevus syndrome is severely diminished by the need for frequent, repetitive, scarring surgical procedures.
Vismodegib (Erivedge, Genentech–Curis) is a low-molecular-weight systemic inhibitor of the hedgehog signaling pathway that was approved by the Food and Drug Administration in January 2012 for the treatment of locally advanced or metastatic basal-cell carcinomas.9 The efficacy of the drug against basal-cell carcinoma, along with its low incidence of serious adverse effects, suggested that vismodegib might be suitable for patients with the basal-cell nevus syndrome, whose large numbers of basal-cell carcinomas represent a substantial physical and psychological burden. Hence, we tested the anti–basal-cell carcinoma efficacy and safety of vismodegib in patients with the basal-cell nevus syndrome. Here, we report the results from the planned interim analysis of our phase 2 trial.
METHODS
STUDY CONDUCT
This study was designed by the authors and was overseen by an independent data and safety monitoring board that met semiannually to conduct three planned interim analyses. The study investigators designed the protocol, enrolled and examined the patients, managed and analyzed all the clinical data, and wrote the manuscript. Genentech supplied the medication, performed pharmacodynamic and pharmacokinetic measurements, and read drafts of the manuscript for clarity. No one who is not an author contributed to the writing of the manuscript.
STUDY DESIGN
We enrolled 42 patients with the basal-cell nevus syndrome at three clinical centers from September 2009 through January 2011. After providing written informed consent, patients were randomly assigned, in a 2:1 ratio, to receive oral vismodegib (2-chloro-N-[4-chloro-3-(2-pyridinyl)phenyl]-4-[methylsulfonyl]benzamide) at a dose of 150 mg daily or placebo for a planned 18 months. The primary statistical end point was the comparative rate of appearance of new basal-cell carcinomas that were eligible for surgical resection (surgically eligible) — those with a diameter of 3 mm or greater on the nose or periorbital skin, 5 mm or greater elsewhere on the face, or 9 mm or greater on the trunk or limbs (excluding the leg below the knees, which was not monitored). Secondary end points included a reduction in the rate of appearance of smaller basal-cell carcinomas (5 mm or less) on the upper back with vismodegib versus placebo, reduction in size of existing surgically eligible basal-cell carcinomas, duration of the effect against basal-cell carcinoma after drug discontinuation, change in hedgehog target-gene expression in basal-cell carcinomas, and between-group differences in adverse events. The complete protocol is available with the full text of this article at NEJM.org.
STUDY PATIENTS AND TREATMENT
All patients had received a clinical diagnosis of the basal-cell nevus syndrome on the basis of at least two major criteria,10 and all had a total of at least 10 surgically eligible basal-cell carcinomas present at study entry or removed during the previous 2 years. Patients were randomly assigned according to a computer-generated randomization list and were not stratified on the basis on the prior rate of development of basal-cell carcinomas.
Each patient received the study drug for a maximum of 18 months until the occurrence of intolerable toxic effects or clinical worsening of disease (defined as >60 new surgically eligible basal-cell carcinomas or doubling of the cumulative longest diameter of existing or new surgically eligible basal-cell carcinomas).
ASSESSMENTS
Per protocol, we assessed tumor response by examining the skin at study visits, monthly for the first 3 months, every other month for the next 6 months, and every 3 months for the final 9 months of the 18-month study period. We identified basal-cell carcinoma lesions clinically, used calipers to measure their longest diameter, and used clinical photos from previous visits to ensure consistency of clinical examination. The principal investigator trained all study dermatologists and participated in early study visits to ensure consistent assessments of surgically eligible basal-cell carcinomas. We discontinued the participation of one patient because of clinical worsening of disease (Fig. 1 in the Supplementary Appendix, available at NEJM.org). We characterized adverse events according to the National Cancer Institute Common Terminology Criteria for Adverse Events (version 3.0).
IN VITRO STUDIES
We assessed the drug-induced inhibition of hedgehog signaling by means of quantitative polymerase-chain-reaction analysis of the levels of messenger RNA encoding the hedgehog target gene glioma-associated oncogene homolog 1 (zinc finger protein) (GLI1) in skin-shave–biopsy samples of basal-cell carcinomas taken at baseline and 1 month after the start of vismodegib treatment, calculated by the 2−ΔCt method, in which the cycling threshold (Ct) of GLI1 was normalized to the Ct of glyceraldehyde-3-phosphate dehydrogenase (GAPDH) and expressed as a power of 2 (2Ct[GLI1]-Ct[GAPDH]) (see the Supplementary Appendix).
STATISTICAL ANALYSIS
All the analyses presented were prespecified before the data were unblinded and included data from all patients who were randomly assigned to a study group (Fig. 1 in the Supplementary Appendix). We estimated that with 20 patients receiving vismodegib and 10 receiving placebo, the study would have 80% power to detect a difference of 50 percentage points between the two groups in the primary end point at an overall alpha level of 0.05 (two-tailed). We anticipated a 20% dropout rate and planned to enroll a total of 41 patients. We used the generalized linear model11 to analyze the rate of new surgically eligible basal-cell carcinomas. Because the number of new surgically eligible basal-cell carcinomas is a count (i.e., non-continuous) variable, we used the Poisson distribution and applied the natural log link. The natural log of the amount of follow-up time for any patient was included as an offset to account for differential follow-up among study patients. The clinical site and the number of surgically eligible basal-cell carcinomas at baseline were included as covariates to account for variability among study patients. Three interim analyses assessing efficacy and adverse events were planned for unblinded data managed by an in-house biostatistician supervised by the data safety and monitoring board, which consisted of three dermatologic clinician–scientists and a biostatistician not otherwise involved in the trial and unrelated to the study centers. We used the Lan–DeMets alpha-spending approach with the Pocock boundaries to determine the alpha level to be applied at each of the three interim analyses and the final analysis to ensure that the overall type I error rate was maintained at an alpha level of 0.05. We report here the results of the second interim analysis, in December 2010, when the data safety and monitoring board concluded that the predetermined threshold for a significant difference (P<0.0113) between the two groups had been reached.
RESULTS
STUDY PATIENTS
From September 2009 through January 2011, we enrolled 42 patients with the basal-cell nevus syndrome. One patient withdrew from the study before receiving any study medication. Patients randomly assigned to receive placebo or vismodegib were similar with regard to age, weight, and baseline number of surgically eligible basal-cell carcinomas (Table 1). At the planned second interim analysis in December 2010, the data safety and monitoring board recommended ending the placebo treatment owing to statistically significant differences in efficacy favoring the vismodegib group. The data cutoff date was February 17, 2011, when the institutional review board of the Children’s Hospital Oakland Research Institute approved the recommendation. On this date, all 41 patients who received a dose of the study drug had undergone at least one follow-up visit for tumor and safety assessment, 38 patients had completed at least 3 months of follow-up visits, and patients had been followed for a mean of 8 months (range, 1 to 15).
Table 1.
Characteristics of the 41 Patients with the Basal-Cell Nevus Syndrome.*
| Characteristic | Vismodegib (N = 26) | Placebo (N = 15) | P Value |
|---|---|---|---|
| Age — yr | 54±8 | 53±8 | 0.94 |
| Sex — no. (%) | 0.74 | ||
| Female | 8 (31) | 6 (40) | |
| Male | 18 (69) | 9 (60) | |
| Weight at baseline — kg | 100±24 | 100±29 | 1.00 |
| Last study visit — mo | 9±4 | 7±4 | 1.00 |
| Number of surgically eligible basal-cell carcinomas at baseline | 44±41 | 37±50 | 0.79 |
Plus–minus values are means ±SD.
CLINICAL RESPONSE
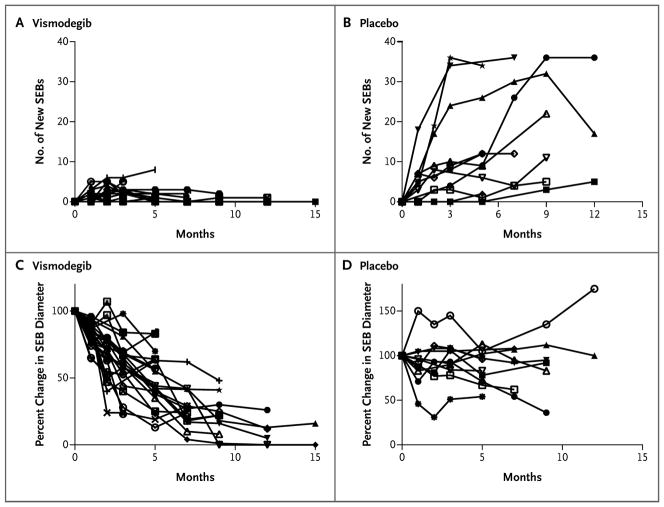
During the study period, we tracked more than 2000 existing, and 694 new, surgically eligible basal-cell carcinomas. A total of 38 of the 41 patients (93%) completed at least 3 months of follow-up visits and had data for the primary end point (reduction in the rate of new surgically eligible basal-cell carcinomas) at 3 months after receiving study medication. We chose this end point expecting that at least 2 months of therapy would be required to discern an antitumor effect; however, we found a reduction in the numbers of new surgically eligible basal-cell carcinomas with vismodegib versus placebo at 1 month (Fig. 1A and 1B). Vismodegib significantly reduced the per-patient rate of new surgically eligible basal-cell carcinomas below that of the placebo group (mean, 2 vs. 29 new surgically eligible basal-cell carcinomas per year; median, 2 vs. 25 new surgically eligible basal-cell carcinomas per year; P<0.001). Vismodegib also reduced the size of existing surgically eligible basal-cell carcinomas, expressed as the percent change from baseline in the sum of the longest diameters (mean, −65%, vs. −11% with placebo; median, −71%, vs. −21% with placebo; P = 0.003) (Fig. 2).
Figure 1. Surgically Eligible Basal-Cell Carcinomas (SEBs) in 36 Patients Followed for More Than 2 Months, According to Study Group.
Data are shown for each of the 25 patients receiving vismodegib and 11 patients receiving placebo; individual curves cannot always be distinguished. Panels A and B show the cumulative number of new SEBs during the study period; Panels C and D show the percent change from baseline in the sums of the longest diameters of SEBs.
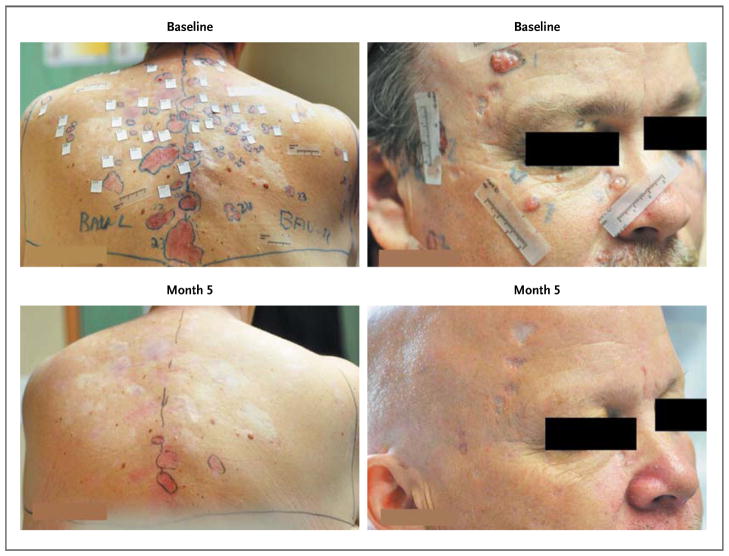
Figure 2. Surgically Eligible Basal-Cell Carcinomas in Two Patients Receiving Vismodegib.
Reduction in the baseline sizes of existing superficial (back) and nodular (face) carcinomas (top row) is evident in the two patients at 5 months (bottom row).
During the trial, patients were allowed to have tumors surgically removed at the discretion of their primary dermatologist. Patients receiving vismodegib had fewer surgeries as part of standard care as compared with patients receiving placebo (mean number of surgeries per patient, 0.31, vs. 4.4 with placebo; median, 0, vs. 1.0 for placebo; P<0.001). All tumors responded to vismodegib treatment, with nearly complete clinical remission in some patients. Palmar and plantar pits, which are pathognomonic signs of the basal-cell nevus syndrome, also disappeared during vismodegib therapy, often within the first month (Fig. 2 in the Supplementary Appendix).
Pharmacokinetic assessment of vismodegib was performed on plasma samples collected at 1 month. The median (±SE) total plasma drug level was 25±7 μmol per liter (range, 13 to 42). We found no correlation between these levels and tumor response at 1 or 3 months.
ADVERSE EVENTS
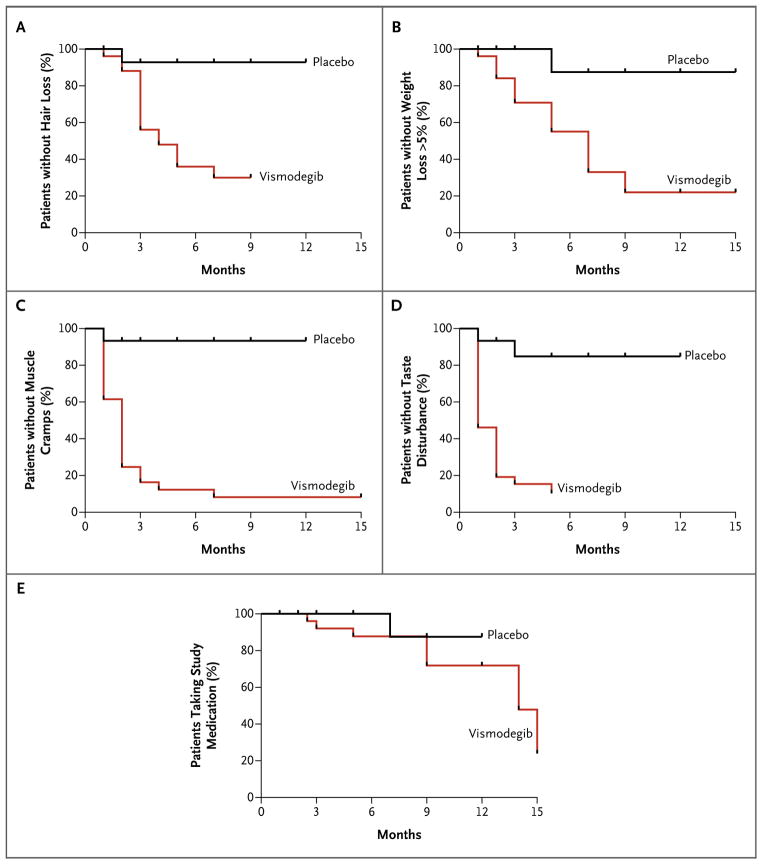
As of the data cutoff date of February 17, 2011, 27% of patients receiving vismodegib (7 of 26) had stopped the drug, owing to adverse events, during a mean observation time of 8 months (Fig. 1 in the Supplementary Appendix). We ended the participation of 1 patient in the placebo group because of disease progression. Most adverse events were mild or moderate in severity, and no grade 5 events were observed. Patients receiving vismodegib were significantly more likely to have grade 1 or 2 dysgeusia, muscle cramps, hair loss, and weight loss, as compared with those receiving placebo (Fig. 3, and Table 1 in the Supplementary Appendix). As of January 31, 2012, 54% of patients receiving vismodegib (14 of 26) had discontinued the medication owing to side effects, and only 1 of 5 eligible patients was able to continue vismodegib for 18 months. Patients receiving vismodegib had more grade 3 or 4 adverse events as compared with patients receiving placebo (Table 1 in the Supplementary Appendix). When vismodegib was withdrawn, dysgeusia and muscle cramps ceased within 1 month, and scalp and body hair started to regrow within 3 months.
Figure 3. Kaplan–Meier Estimates of Freedom from the Most Common Grade 1 or 2 Adverse Events and Study-Drug Use, According to Study Group.
Panels A, B, C, and D show freedom from each of the four most common adverse events in the study: hair loss, weight loss of more than 5%, muscle cramps, and taste disturbance (dysgeusia), respectively. Panel E shows the continued receipt of the assigned study drug (with stopping due to adverse events).
HISTOLOGIC AND MOLECULAR STUDIES
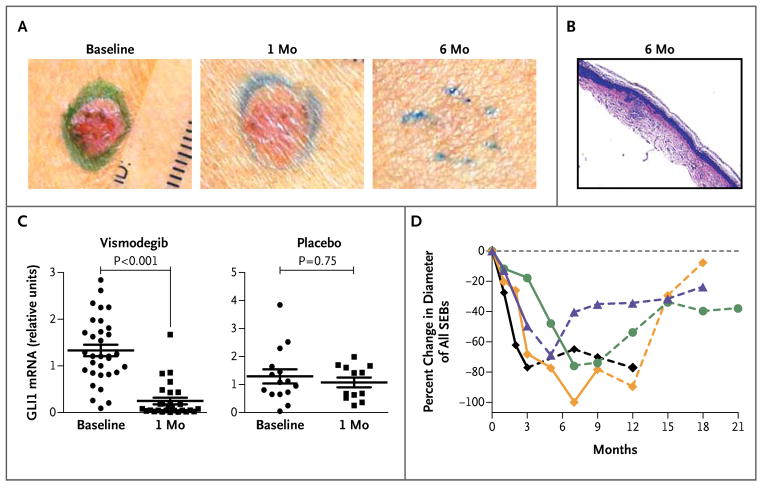
We obtained biopsy specimens from basal-cell carcinoma tumors to determine the histologic correlates of visible changes in the tumors. Residual microscopic basal-cell carcinomas were present in 88% of random specimens (22 of 25) from basal-cell carcinomas that were clinically raised (plaques or papules) in patients treated with vismodegib for 1 month. Among patients receiving vismodegib for more than 3 months, 46% of biopsy specimens (6 of 13) revealed residual tumors detected in random histologic sections. Among lesions that appeared to be clinically resolved (i.e., flat and scarlike without erythema), residual tumors were present in only 17% of biopsy samples (1 of 6) of basal-cell carcinomas (Fig. 4A and 4B). The effect of vismodegib against basal-cell carcinoma was associated with a decrease in hedgehog signaling, as shown by a 90% decrease (P<0.001) in GLI1 messenger RNA in biopsy specimens from basal-cell carcinomas in patients treated for 1 month (Fig. 4C). One month of vismodegib treatment also significantly reduced tumor proliferation, as assessed by Ki67 expression, but caused no change in apoptosis, as assessed by cleaved caspase 3 (Fig. 3 in the Supplementary Appendix).
Figure 4. Results from Histologic and Molecular Studies.
Panel A shows clinical resolution of a typical basal-cell carcinoma lesion between baseline and 6 months. Panel B (hematoxylin and eosin) shows a biopsy specimen from a basal-cell carcinoma lesion at 6 months, with no obvious groups of tumor cells visible microscopically in random histologic sections after review by a dermatopathologist. (Scar and inflammatory cells are visible near the epidermis.) Panel C shows the messenger-RNA (mRNA) expression of the hedgehog target gene glioma-associated oncogene homolog 1 (zinc finger protein) (GLI1) in basal-cell carcinomas at baseline and at 1 month, according to study group. Data for individual patients are shown; the horizontal line indicates the mean, and the I bar indicates the standard error. Panel D shows the percent change from baseline in the sum of the longest diameters of existing surgically eligible basal-cell carcinomas (SEBs) in each of the first four patients (of seven in total) who stopped vismodegib before the 18-month end point and had over 3 months of follow-up. Solid segments of each curve indicate months during vismodegib treatment; and dashed segments, months after vismodegib was stopped.
OBSERVATIONS AFTER UNBLINDING
Figure 4D illustrates the percent change in cumulative diameter of existing surgically eligible basal-cell carcinomas during and after vismodegib therapy in the first four patients who stopped vismodegib and were monitored for more than 3 months after stopping. The sums of the longest diameters returned to baseline levels several months after vismodegib was stopped (Fig. 4 in the Supplementary Appendix), as did the palmar and plantar pits. Basal-cell carcinomas and pits appear to recur at the original sites, and very few new surgically eligible basal-cell carcinomas developed after the medication was stopped. In a post hoc analysis of seven patients who stopped vismodegib, 0.69 new surgically eligible basal-cell carcinomas developed per month, a rate that is considerably less than that among patients receiving placebo (2.4 per month).
DISCUSSION
Vismodegib significantly reduced the rate of appearance of new surgically eligible basal-cell carcinomas among patients with the basal-cell nevus syndrome. Vismodegib also reduced the initially substantial burden of surgically eligible basal-cell carcinomas, even to the point of clinical resolution; however, most surgically eligible basal-cell carcinomas regrew once the drug was stopped. In addition, the drug was associated with adverse events; as of January 31, 2012, 54% of patients had discontinued vismodegib owing to side effects. The adverse events were similar to those reported in phase 1 trials9 and phase 2 trials12 of vismodegib and other hedgehog inhibitors (LDE225),13 suggesting that the events are due to class-specific inhibition of the hedgehog signaling pathway. In contrast to vismodegib, the use of topical LDE225 did not cause taste loss and muscle cramps after 4 weeks of treatment.14
Our results illustrate the potential for using molecularly targeted drugs for cancer chemoprevention. The high rate of discontinuing the drug because of adverse effects is unlikely to be ameliorated by altering the chemical structure, as the toxic effects appear to be related to inhibition of the drug target, not off-target effects. It is uncertain whether vismodegib is as effective in the 10% of basal-cell carcinomas driven by activating SMO mutations.15–18 Thus, our results for vismodegib use are consistent with the concept of cancer “interception” — the blocking of further growth of tumors at a stage before they become clinically apparent.19
The lack of any demonstrable tumor progression or resistance to vismodegib during treatment contrasts with the development of resistance in other hedgehog-driven tumors like medulloblastomas17 and with the frequent development of resistance to therapies targeted to other dysregulated oncologic pathways (e.g., epidermal growth factor receptor and the tyrosine kinase BCR-ABL).20,21 Perhaps the lack of development of resistance in basal-cell carcinomas during treatment in this study and the rarity of metastatic behavior correlate with the relative genomic stability of these cancers.22 In contrast, essentially all surgically eligible basal-cell carcinomas recur after vismodegib treatment is stopped, suggesting the possibility of drug-tolerant tumor cells with “cancer stem cell” characteristics in basal-cell carcinomas. More broadly, any finding that tumor stem cells are resistant to hedgehog inhibition contradicts the concept that cancer stem cells might depend at least partially on hedgehog activation.23,24 However, we cannot rule out the possibility that all clinically regressed tumors harbor residual groups of tumor cells and that these account for the regrowth of tumors after stopping vismodegib or the possibility that a small number of morphologically normal (histologically unrecognizable) dormant tumor cells remain after examination of random histologic sections. This phenomenon of regression of basal-cell carcinoma after the shutdown of the hedgehog pathway, followed by regrowth after hedgehog-pathway reactivation, recapitulates findings in a conditional mouse model of basal-cell carcinoma.25
Overall, our findings confirm the essential role of the hedgehog pathway in basal-cell carcinomas and indicate that vismodegib is efficacious in preventing and treating basal-cell carcinomas in patients with the basal-cell nevus syndrome.
Supplementary Material
Acknowledgments
Supported by Genentech, a Clinical and Translational Science Award from the National Institutes of Health (UL1RR02413), grants from the National Institute of Arthritis and Musculoskeletal and Skin Diseases (5P30AR044535-11, to Dr. Bickers; 1K23AR056736, to Dr. Tang) and the National Cancer Institute (R01CA109584, to Dr. Epstein), a Damon Runyon Cancer Research Foundation Clinical Investigator Award (CI-54-11, to Dr. Tang), and funding from the Swim across America Foundation and the Michael J. Rainen Family Foundation.
Under the ongoing collaboration agreement between Genen-tech, a member of the Roche Group, and Curis, vismodegib was discovered by Genentech and was jointly validated through a series of preclinical studies.
We thank the enrollees; Kristi Burr and associates of the Basal Cell Carcinoma Nevus Syndrome Life Support Network; the members of the data and safety monitoring board: Barbara Gilchrest, Lowell Goldsmith, Russell Hall, and James Rochon; the staff of the Oakland Children’s Hospital Pediatric General Clinical Research Center (particularly biostatistician Ginny Gildengoren); the University of California, San Francisco, Clinical and Translational Science Institute (particularly Steve Hulley and Charles McCulloch); Lee Rubin and Robert Gwynn for helpful discussions; and Chris Callahan, Rick Graham, Tom Januario, Ron Firestein, and Natasha Miley of Genentech for support of the molecular analyses.
Footnotes
Disclosure forms provided by the authors are available with the full text of this article at NEJM.org.
References
- 1.Miller DL, Weinstock MA. Nonmelanoma skin cancer in the United States: incidence. J Am Acad Dermatol. 1994;30:774–8. doi: 10.1016/s0190-9622(08)81509-5. [DOI] [PubMed] [Google Scholar]
- 2.Gorlin RJ. Nevoid basal cell carcinoma syndrome. Medicine (Baltimore) 1987;66:98–113. doi: 10.1097/00005792-198703000-00002. [DOI] [PubMed] [Google Scholar]
- 3.Johnson RL, Rothman AL, Xie J, et al. Human homolog of patched, a candidate gene for the basal cell nevus syndrome. Science. 1996;272:1668–71. doi: 10.1126/science.272.5268.1668. [DOI] [PubMed] [Google Scholar]
- 4.Hahn H, Wicking C, Zaphiropoulous PG, et al. Mutations of the human homolog of Drosophila patched in the nevoid basal cell carcinoma syndrome. Cell. 1996;85:841–51. doi: 10.1016/s0092-8674(00)81268-4. [DOI] [PubMed] [Google Scholar]
- 5.Epstein EH. Basal cell carcinomas: attack of the hedgehog. Nat Rev Cancer. 2008;8:743–54. doi: 10.1038/nrc2503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Gailani MR, Ståhle-Bäckdahl M, Leffell DJ, et al. The role of the human homologue of Drosophila patched in sporadic basal cell carcinomas. Nat Genet. 1996;14:78–81. doi: 10.1038/ng0996-78. [DOI] [PubMed] [Google Scholar]
- 7.Aszterbaum M, Rothman A, Johnson RL, et al. Identification of mutations in the human PATCHED gene in sporadic basal cell carcinomas and in patients with the basal cell nevus syndrome. J Invest Dermatol. 1998;110:885–8. doi: 10.1046/j.1523-1747.1998.00222.x. [DOI] [PubMed] [Google Scholar]
- 8.Ling G, Ahmadian A, Persson A, et al. PATCHED and p53 gene alterations in sporadic and hereditary basal cell cancer. Oncogene. 2001;20:7770–8. doi: 10.1038/sj.onc.1204946. [DOI] [PubMed] [Google Scholar]
- 9.Von Hoff DD, LoRusso PM, Rudin CM, et al. Inhibition of the hedgehog pathway in advanced basal-cell carcinoma. N Engl J Med. 2009;361:1164–72. doi: 10.1056/NEJMoa0905360. [DOI] [PubMed] [Google Scholar]
- 10.Kimonis VE, Goldstein AM, Pastakia B, et al. Clinical manifestations in 105 persons with nevoid basal cell carcinoma syndrome. Am J Med Genet. 1997;69:299–308. [PubMed] [Google Scholar]
- 11.McCullagh P, Nelder JA. Generalized linear models. 2. London: Chapman and Hall; 1989. [Google Scholar]
- 12.Sekulic A, Migden MR, Oro AE, et al. Efficacy and safety of vismodegib in advanced basal-cell carcinoma. N Engl J Med. 2012;366:2171–9. doi: 10.1056/NEJMoa1113713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ahnert JR, Baselga J, Tawbi HA, et al. A phase I dose-escalation study of LDE225, a smoothened (Smo) antagonist, in patients with advanced solid tumors. Presented at the 2010 annual meeting of the American Society of Clinical Oncology; Chicago. June 4–8, 2010; abstract. [Google Scholar]
- 14.Skvara H, Kalthoff F, Meingassner JG, et al. Topical treatment of basal cell carcinomas in nevoid basal cell carcinoma syndrome with a Smoothened inhibitor. J Invest Dermatol. 2011;131:1735–44. doi: 10.1038/jid.2011.48. [DOI] [PubMed] [Google Scholar]
- 15.Xie J, Murone M, Luoh SM, et al. Activating Smoothened mutations in sporadic basal-cell carcinoma. Nature. 1998;391:90–2. doi: 10.1038/34201. [DOI] [PubMed] [Google Scholar]
- 16.Reifenberger J, Wolter M, Weber RG, et al. Missense mutations in SMOH in sporadic basal cell carcinomas of the skin and primitive neuroectodermal tumors of the central nervous system. Cancer Res. 1998;58:1798–803. [PubMed] [Google Scholar]
- 17.Yauch RL, Dijkgraaf GJP, Alicke B, et al. Smoothened mutation confers resistance to a hedgehog pathway inhibitor in medulloblastoma. Science. 2009;326:572–4. doi: 10.1126/science.1179386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Chen JK, Taipale J, Young KE, Maiti T, Beachy PA. Small molecule modulation of Smoothened activity. Proc Natl Acad Sci U S A. 2002;99:14071–6. doi: 10.1073/pnas.182542899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Blackburn EH. Cancer interception. Cancer Prev Res (Phila) 2011;4:787–92. doi: 10.1158/1940-6207.CAPR-11-0195. [DOI] [PubMed] [Google Scholar]
- 20.Engelman JA, Zejnullahu K, Mitsudomi T, et al. MET amplification leads to gefitinib resistance in lung cancer by activating ERBB3 signaling. Science. 2007;316:1039–43. doi: 10.1126/science.1141478. [DOI] [PubMed] [Google Scholar]
- 21.Gorre ME, Mohammed M, Ellwood K, et al. Clinical resistance to STI-571 cancer therapy caused by BCR-ABL gene mutation or amplification. Science. 2001;293:876–80. doi: 10.1126/science.1062538. [DOI] [PubMed] [Google Scholar]
- 22.Davari P, Hebert JL, Albertson DG, et al. Loss of Blm enhances basal cell carcinoma and rhabdomyosarcoma tumorigenesis in Ptch1+/− mice. Carcinogenesis. 2010;31:968–73. doi: 10.1093/carcin/bgp309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Takezaki T, Hide T, Takanaga H, Nakamura H, Kuratsu J, Kondo T. Essential role of the Hedgehog signaling pathway in human glioma-initiating cells. Cancer Sci. 2011;102:1306–12. doi: 10.1111/j.1349-7006.2011.01943.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Zhao C, Chen A, Jamieson CH, et al. Hedgehog signalling is essential for maintenance of cancer stem cells in myeloid leukaemia. Nature. 2009;458:776–9. doi: 10.1038/nature07737. [Erratum, Nature 2009;460:652.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hutchin ME, Kariapper MST, Grachtchouk M, et al. Sustained Hedgehog signaling is required for basal cell carcinoma proliferation and survival: conditional skin tumorigenesis recapitulates the hair growth cycle. Genes Dev. 2005;19:214–23. doi: 10.1101/gad.1258705. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.