Abstract
Pancreatic cancer is one of the deadliest human malignancies due to its early metastatic spread and resistance to therapy. The mechanisms regulating pancreatic cancer metastasis are so far poorly understood. Here, using both in vitro and in vivo approaches, it is demonstrated that CD44, a transmembrane glycoprotein expressed on a subset of pancreatic cancer cells, is required for the induction of epithelial-mesenchymal transition (EMT) and the activation of an invasive program in pancreatic cancer. Mechanistically, the transcription factor Snail1 (SNAI1), a regulator of the EMT program, is a downstream target of CD44 in primary pancreatic cancer cells and regulates membrane bound metalloproteinase (MMP14/MT1-MMP) expression. In turn, MT1-MMP expression is required for pancreatic cancer invasion. Thus, these data establish the CD44-Snail-MMP axis as a key regulator of the EMT program and of invasion in pancreatic cancer. (135)
IMPLICATIONS
This study sets the stage for CD44 and MT1-MMP as therapeutic targets in pancreatic cancer, for which small molecule or biologic inhibitors are available.
Keywords: CD44, MT1-MMP, pancreatic cancer, EMT, invasion
INTRODUCTION
Pancreatic cancer is one of the deadliest human malignancies, with a 5-year survival of less than 6%, and a prognosis largely unchanged over the course of the past 40 years. Most pancreatic cancer patients present with metastases at diagnosis. Thus, understanding the drivers of metastasis in pancreatic cancer is of key therapeutic value.
Pancreatic cancer is almost invariably associated to oncogenic mutations in the Kras gene(1, 2). Expression of mutant Kras in combination with mutations in the tumor suppressor p53 in the pancreas leads to mouse models that closely recapitulate the step-wise progression of human tumors (3). Recently, elegant mouse model studies revealed that a fraction of the pancreatic epithelial cells undergo epithelial-mesenchymal transition (EMT) and invade the surrounding fibrotic matrix even during early neoplastic stages (4). These cells enter the bloodstream and seed in the liver. Intriguingly, the cells able to escape the epithelial layer expressed CD44, a membrane protein previously associated with human pancreatic cancer stem cells (5).
CD44 is a transmembrane glycoprotein adhesion receptor that binds hyaluronic acid and is involved in many cellular processes including growth, survival, adhesion, and cell migration (6–8). Different isoforms of CD44 exist based on alternative RNA splicing. The standard isoform of CD44 (CD44s) is the smallest isoform with all the variable regions in the extracellular domain removed through alternative splicing. The variant isoforms of CD44 (CD44v) contain multiple combinations of exons 6 – 15 (v1 – v10) which are located within the stalk-like region of the extracellular domain of the molecule (7–9). Either isoform can be metastasis-promoting depending on the cellular context. The functional significance and requirement of CD44 in pancreatic cancer has not been explored, and addressing it was the purpose of this study.
MATERIALS AND METHODS
Cell Culture
The primary cell lines used in this study were isolated from pancreatic tumors in iKras*p53* mice and cultured as previously described (10). The cells were always maintained in doxycycline (1 ug/ml of doxycycline, Sigma), to activate expression of oncogenic Kras, unless otherwise stated. The human pancreatic cancer cell line Hs766T is available through ATCC. UM2, UM18 and 1319 are primary human pancreatic cancer cells derived from patients with confirmed diagnosis of pancreatic ductal adenocarcinoma (University of Michigan).
Western-blot analysis
Cells were lysed in RIPA buffer (Sigma-Aldrich, R0278) and protease inhibitor (Sigma-Aldrich, P8340). Western blot analysis was performed as previously described (11). The rat anti-mouse CD44 antibody KM114 from BD bioscience was used at a 1:1000 dilution.
Quantitative RT-PCR
RNA extraction and RT-PCR were performed as previously described (11). Gapdh was used as the housekeeping gene expression control. A list of the primers used is provided in Supplemental Table 1.
Short Interfering RNA Transfection
Control or target-specific siRNAs were purchased from Sigma (St. Louis, MO) and transfected at a concentration of 20-nM using Lipofectamine RNAi MAX kit (Invitrogen) according to the manufacturer’s instructions. Knock-down level of target genes was determined using qRT-PCR.
Transfection of CD44s Plasmid
Plasmids expressing cDNA of CD44s (ORF) or MT1-MMP (ORF) in pCMV6 and control vector pCMV6 were purchased from OriGene.
Collagen Invasion assay
Type I collagen was prepared from rat tail (BD Bioscience) in 0.2% acetic acid to a final concentration of 2.7 mg/ml; gelling was induced in upper well of a 6-well transwell dish (3-mm pore size; Corning, Inc.). After gelling was complete (45 min at 37 °C), 1.5 X105 cells in complete medium were added to the upper well and 2.5 ml of medium was added to the lower chamber. Invasion assays were routinely terminated after 3 days. Invasion depths were measured from digitally captured images of hematoxylin and eosin-stained cross-sections.
Orthotopic pancreatic cancer xenograft
Two groups of cells, control shRNA infected cells and CD44 shRNA infected cells (5×105), were injected in the pancreatic tail of NOD/SCID mice (6 per group). Mice were monitored daily and sacrificed when the control group became moribund.
Detailed Experimental procedures are provided in the Supplemental Methods.
RESULTS AND DISCUSSION
CD44 expression in mouse pancreatic cancer correlates with EMT
CD44 expression was previously detected in a subset of mouse pancreatic cancer cells in the KPCY mouse model (4). In order to determine its expression in iKras*p53* mice we stained primary tumors and metastases samples by immunohistochemistry. Both primary pancreatic cancer and liver metastasis contained CD44-positive cells; in contrast, CD44 expression was not detected in normal pancreatic and liver tissue (Fig. S1A).
In order to determine the specific isoform of CD44 expressed in pancreatic cancer cells, we used primary mouse pancreatic cancer cells derived from iKras*p53* mice (10), which can be subdivided in two sub-groups based on morphology. The epithelial group forms stroma-rich tumors when transplanted in host mice. In contrast, mesenchymal lines have a fibroblast-like morphology and form tumors largely devoid of stroma (10). Interestingly, these subsets are reminiscent of the “epithelial” or “classical” and “quasi-mesenchymal” subtypes of human pancreatic cancer recently described (12). For the current study, used a panel of four primary cell lines, two classified as epithelial (iKras*p53*#1a, iKras*p53*#3) and two as mesenchymal (iKras*p53*#2, iKras*p53*#1b) based on morphology (Fig. 1A). Of note, the iKras*p53*#1a and iKras*p53*#1b cell lines were derived from the same primary tumor; the mesenchymal iKras*p53*#1b subclone was isolated from a mostly epithelial initial culture. The use of cell lines derived from the iKras*p53* model gave us the opportunity to modulate the expression of oncogenic Kras by administering, or withdrawing, doxycycline from the growth medium (10).
Figure 1. EMT and invasion correlate with CD44 expression in primary mouse pancreatic cancer cells.
(A) Morphology of primary pancreatic cell lines derived from iKras*p53* mice in culture. (B) qRT-PCR analysis of epithelial and mesenchymal genes. Mesenchymal cells versus epithelial cells, p < 0.001. (C) Expressions of CD44 isoforms (CD44s and CD44v) by qRT-PCR (left, mesenchymal cells versus epithelial cells p < 0.01), RT-PCR (middle) and Western Blot (right). (D) Collagen invasion assay (left) and quantification (right, Mesenchymal cells versus epithelial cells p < 0.005).
In order to determine whether the different morphological characteristics of the cells correlated with a specific gene signature, we measured the expression of epithelial (E-cadherin) and EMT markers (Snail1, Zeb1 and Twist1) by RT-PCR (Fig. 1B). Consistent with the noted morphological features, E-cadherin mRNA level was significantly lower in the mesenchymal cell lines (iKras*p53*#1b and iKras*p53*#2) and higher in the epithelial cell lines. Conversely, Snail1, Zeb1, and Twist1 expression were higher in the mesenchymal cell lines (iKras*p53*#1b, iKras*p53*#2), compared to the epithelial cell lines (iKras*p53*#1a, iKras*p53*#3). Thus, the gene signature of the cell lines confirmed the morphology-based classification. These findings are consistent with the notion that human pancreatic tumors can be classified in subtypes with epithelial or mesenchymal characteristics (12).
We then determined the expression of different CD44 isoforms in the pancreatic cancer cell lines. We used RT-PCR and Western blot to measure the expression of CD44s and CD44v, respectively, in the iKras*p53*#2, iKras*p53*#1a, iKras*p53*#3 and iKras*p53*#1b cells (Fig. 1C). Interestingly, the mesenchymal cells (iKras*p53*#2, iKras*p53*#1b) expressed high levels of the standard CD44 mRNA and protein, in addition to the variant isoforms of CD44. In contrast, the epithelial cells (iKras*p53*#1a) did not express CD44s at the mRNA or protein level.
To complete the characterization of these cell lines, we investigated their invasive potential using a Boyden Chamber Matrigel Invasion Assay. In this assay, the epithelial iKras*p53*#1a cells showed no invasive capabilities (Fig. S1B). In contrast, the mesenchymal iKras*p53*#1b cells invaded through Matrigel, in a Kras-dependent manner (Fig. S1B–C). The results were confirmed and further expanded with additional cell lines using a Collagen Invasion Assay, which is more relevant to the in vivo situation given the collagen-rich matrix of pancreatic tumors. The epithelial iKras*p53*#1a and iKras*p53*#3 cells did not invade through the collagen; however, the mesenchymal iKras*p53*#1b and iKras*p53*#2 cells were able to invade (Fig. 1D). Thus, we identified a correlation between the expression of the CD44s isoform, mesenchymal phenotype and invasive capabilities of the pancreatic cancer cells in vitro. This result parallels previously described findings in mouse models of pancreatic cancer (4).
CD44s induces EMT in pancreatic cancer cells
In order to determine whether there was a causative link between CD44s expression and the acquisition of a mesenchymal phenotype, we experimentally manipulated the expression of CD44s. Transfection of iKras*p53*#1a cells with a CD44s expression vector produced a shift to a mesenchymal morphology compared to control transfected cells (Fig. 2A). We next evaluated whether CD44s expression led to changes in the expression of epithelial and mesenchymal genes. We found that expression of CD44s in iKras*p53*#1a cells resulted in near complete inhibition of E-cadherin mRNA levels (Fig. 2B), while expression of the mesenchymal marker Vimentin was increased ~2-fold. Then, we measured the expression of three transcription factors linked to the EMT process: Twist1, Zeb1 and Snail1. We found no change in expression in the first two genes but a significant increase in Snail1 expression upon overexpression of CD44s. Thus, CD44 expression activated the expression of at least one EMT regulator and caused a change in phenotype consistent with EMT.
Figure 2. CD44 induces EMT and is required for invasion in pancreatic cancer cells.
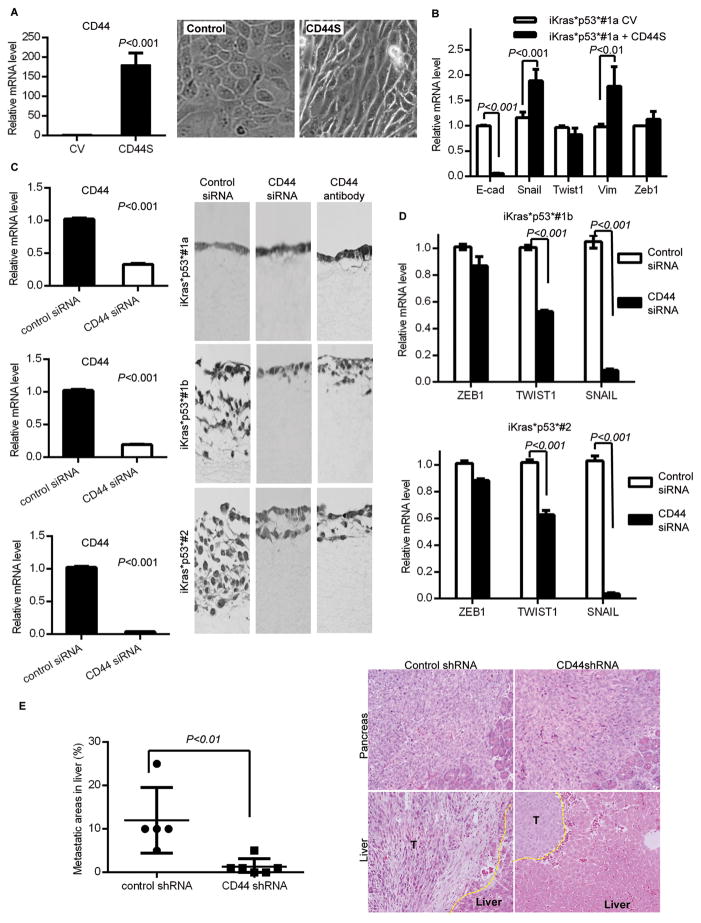
(A) qRT-PCR analysis for CD44 in iKras*p53*#1a cells transfected with control or CD44s expression vector (left) and morphology change of iKras*p53*#1a cells expressing control or CD44s vector (right). (B) qRT-PCR analysis for EMT genes. (C) qRT-PCR analysis for cells transfected with control siRNA or anti-CD44 siRNA. (D) Collagen invasion assay. (E) H&E staining of pancreas (top) and liver (bottom) following orthotopic transplantation of control shRNA or anti-CD44 shRNA infected iKras*p53*#1b cells. Quantification of the metastatic area.
Then, taking the opposite approach, namely inhibition of CD44, we sought to determine whether there was a functional relationship between CD44 expression, expression of EMT markers, and invasive ability. Anti-CD44 siRNA transfection effectively reduced CD44 (all isoforms) expression (Fig. 2C, left).
Moreover, cells transfected with the anti-CD44 siRNA had only a moderate decrease in Twist1 expression and no change in Zeb1 expression, but a significant decrease in the expression of Snail1, MMP2 and MMP-9, and conversely an increase in E-cadherin expression (Fig. 2D and Fig. S2A-B). Finally, to further investigate whether CD44s levels regulate Snail, and thus MT1-MMP expression, we targeted the splicing factor ESRP1 (epithelial splicing regulatory protein 1). ESRP1 promotes CD44 alternative splicing, thus reducing the levels of CD44s (13). Indeed, our data show that siRNA-mediated inactivation in iKras*p53*#1a cells results in an increase of CD44s, and a corresponding increase in Snail and MT1MMP mRNA. (Fig. S3D). Taken together, there data support the notion that CD44s regulates Snail in pancreatic cancer.
Our results indicate thatCD44 is a regulator of Snail expression. We therefore sought to investigate the mechanism of this regulation. Several effector pathways are activated downstream of CD44 in different contexts, including p-AKT, MAPK and Yap1 (8), but Western blot and qRT-PCR analysis did not reveal significant changes in any of those (Fig. S3A and data not shown). In contrast, we observed changes in Wnt/β-catenin signaling following modulation of CD44. The expression of the Wnt target gene Lef1 was reduced upon CD44 knock-down; consistently, β-catenin levels were reduced upon CD44 knock-down and increased upon CD44 overexpression. Phospho-GSK-3β (Ser9) levels increased upon CD44 overexpression (Fig. S3B), consistent with decreased GSK-3β activity. GSK-3β is a known negative regulator of β-catenin. Moreover, GSK-3β is a negative regulator of Snail1 (14, 15). Thus, our data is consistent with previous work identifying Wnt signaling as a key modulator of the EMT gene program (15). Moreover, it identifies and potential positive feedback loop between CD44 and Wnt signaling in pancreatic cancer.
CD44s regulates invasion in pancreatic cancer cells
Finally, we performed collagen assays to determine whether inhibition of CD44 led to changes in the invasive properties of the cells. As expected, no change was observed in the non-invasive iKras*p53*#1a cell line. However, in both iKras*p53*#1b and iKras*p53*#2 cells anti-CD44 siRNA transfection led to almost complete abrogation of invasion compared to control siRNA-treated cells (Fig. 2C, right). We then confirmed this result using the CD44 blocking antibody KM114. Thus, CD44 expression is required to confer invasive properties in pancreatic cancer cells in vitro.
CD44 regulates pancreatic cancer metastasis in vivo
Next we evaluated the requirement of CD44 in pancreatic cancer liver metastasis. Mouse pancreatic cancer cells iKras*p53*#1b were infected with either control vector or CD44 shRNA and then orthotopically injected to the pancreatic tail of NOD/SCID mice (6 mice per group). Pancreatic, liver, and lung tissue were harvested and analyzed at the time of death or at 2 weeks. All the animals had large primary tumors at the site of injection. Moreover, the mice injected with pancreatic cancer cells infected with control vectors had extensive liver metastasis (in average 10% of the total liver area in each section analyzed). These animals died or reached humane euthanasia endpoints due to elevated disease burden. Mice injected with pancreatic cancer cells infected with CD44 shRNA appeared healthy at the same time point, but were sacrificed to compare tumor growth. Although we did not observed changes in the primary tumor, we observed a significant reduction in the metastatic burden, with only rare, small metastases (Fig. 2E). Thus, this set of experiments provides in vivo evidence that CD44 is required for pancreatic cancer invasion.
CD44 regulates pancreatic cancer cell invasion through MT1-MMP
Our data so far indicate that Snail1 expression is activated downstream of CD44. Snail1 has been shown to regulate the expression of MT1-MMP, a membrane matrix metalloproteinase with strong links to tumor invasion (16). Interestingly, we observed that MT1-MMP was expressed 300–750 fold higher in the mesenchymal cell lines compared to the epithelial lines (Fig. S2C). Inactivation of CD44 with siRNA inhibited the expression of MT1-MMP by >95% in the mesenchymal iKras*p53*#1b and iKras*p53*#2 cells (Fig. 3B and Fig. S2D), while overexpression of CD44s in the epithelial iKras*p53*#1a cells significantly increased the expression of MT1-MMP (Fig. S2E). Thus, MT1-MMP expression is regulated downstream of CD44 in pancreatic cancer cells. Re-expression of the transcription factor Snail1, in the mesenchymal cells with CD44 knockdown, rescued the expression of MT1-MMP (Fig. 3A). In contrast, re-expression of Zeb1 or Zeb1 knock-down had no effect on MT1-MMP expression (Fig. S3C). These data indicate that CD44 regulates MT1-MMP expression through Snail1.
Figure 3. A CD44-Snail1-MT1-MMP axis regulates pancreatic cancer invasion.
(A) MT1-MMP mRNA level upon CD44 knockdown alone or combined with re-expression of Snail1. p < 0.01. (B) Collagen invasion assays in control cells, or cells treated with a MT1-MMP siRNA or inhibitor. (C) Collagen invasion assays upon CD44 knock-down alone or combined with re-expression of MT1-MMP. (D) CD44S expression in the resected human pancreatic cancer and matched adjacent uninvolved pancreas. (E) CD44S and MT1-MMP expression in human pancreatic cancer cell lines. (F) Matrigel invasion assay (left) of human pancreatic cancer cell lines treated with MT1-MMP inhibitor and quantification (right).
Inhibition of MT1-MMP with either siRNA or a small molecule inhibitor resulted in significant inhibition of collagen invasion (Fig. 3B), indicating that MT1-MMP is necessary for pancreatic cancer invasion. To determine if MT1-MMP expression could rescue the invasive potential of cancer cells when CD44 was inactivated, we overexpressed MT1-MMP in mesenchymal cell lines treated with either CD44 or control shRNA. As shown previously, inactivation of CD44 in the mesenchymal cell lines inhibited collagen invasion. However, re-expression of MT1-MMP in mesenchymal cell lines with CD44 knockdown rescued the invasive phenotype (Fig. 3C). MT1-MMP drives invasion in breast cancer (16–18). Moreover, MT1-MMP has been associated with pancreatic cancer fibrosis and desmoplastic reaction (18, 19). Our data identifies a CD44-Snail1-MT1-MMP axis that regulates EMT and invasion in pancreatic cancer.
MT1-MMP is expressed in human pancreatic cancer cells and regulates invasion
In order to determine the relevance of our findings to human pancreatic cancer, we first analyzed the expression of CD44s in RNA extracted from resected pancreatic cancer and matched adjacent uninvolved pancreas. We found that in two out of three sample sets CD44s was significantly overexpressed in the tumor compared to the non-tumor tissue (Fig. 3D). Similar variability was observed when CD44s expression was analyzed in a panel of four human pancreatic cancer cell lines (Fig. 3E). MT1-MMP expression was detected only in the two human cell lines that had high CD44s expression (Fig. 3E). Finally, we performed Matrigel invasion assays with the two human cell lines that had MT1-MMP expression, with or without MT1-MMP inhibitor. In both cell lines, MT1-MMP inhibition significantly decreased the invasion ability of the cells (Fig. 3F). Thus, MT1-MMP is important in migration at least in a subset of human pancreatic cancer cells. However, human samples have higher variability than their mouse counterpart, possibly due to their higher genome complexity.
SIGNIFICANCE
CD44 and MT1-MMP are surface molecules, and as such valid therapeutic targets. In fact, blocking antibodies against CD44 (20–24) and MT1-MMP inhibitors (22) have been described, and new ones are likely to be developed. Our work provides rationale for further exploration of these molecules for pancreatic cancer treatment.
Supplementary Material
Acknowledgments
Financial Support: This project was supported by 2012 Career Development Award of the GI Spore (F033121) and 2013–2015 ACS Faculty Research Fellowship. Research in the MPdM is supported by NCI-1R01CA151588–01. MAC was supported by a University of Michigan Program in Cellular and Molecular Biology training grant (NIH T32 GM07315) and by a University of Michigan Center for Organogenesis Training Grant (5-T32-HD007515).
We thank Kim Bologna for her assistance with editing the manuscript and Marsha Thomas for laboratory support.
Footnotes
Conflict of interest statement: The authors have no conflicts of interest.
References
- 1.Jones S, Zhang X, Parsons DW, Lin JC, Leary RJ, Angenendt P, et al. Core signaling pathways in human pancreatic cancers revealed by global genomic analyses. Science. 2008;321:1801–6. doi: 10.1126/science.1164368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hruban RH, Adsay NV, Albores-Saavedra J, Compton C, Garrett ES, Goodman SN, et al. Pancreatic intraepithelial neoplasia: a new nomenclature and classification system for pancreatic duct lesions. Am J Surg Pathol. 2001;25:579–86. doi: 10.1097/00000478-200105000-00003. [DOI] [PubMed] [Google Scholar]
- 3.Hingorani SR, Wang L, Multani AS, Combs C, Deramaudt TB, Hruban RH, et al. Trp53R172H and KrasG12D cooperate to promote chromosomal instability and widely metastatic pancreatic ductal adenocarcinoma in mice. Cancer Cell. 2005;7:469–83. doi: 10.1016/j.ccr.2005.04.023. [DOI] [PubMed] [Google Scholar]
- 4.Rhim AD, Mirek ET, Aiello NM, Maitra A, Bailey JM, McAllister F, et al. EMT and dissemination precede pancreatic tumor formation. Cell. 2012;148:349–61. doi: 10.1016/j.cell.2011.11.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Li C, Heidt DG, Dalerba P, Burant CF, Zhang L, Adsay V, et al. Identification of pancreatic cancer stem cells. Cancer Res. 2007;67:1030–7. doi: 10.1158/0008-5472.CAN-06-2030. [DOI] [PubMed] [Google Scholar]
- 6.Aruffo A, Stamenkovic I, Melnick M, Underhill CB, Seed B. CD44 is the principal cell surface receptor for hyaluronate. Cell. 1990;61:1303–13. doi: 10.1016/0092-8674(90)90694-a. [DOI] [PubMed] [Google Scholar]
- 7.Marhaba R, Zoller M. CD44 in cancer progression: adhesion, migration and growth regulation. J Mol Histol. 2004;35:211–31. doi: 10.1023/b:hijo.0000032354.94213.69. [DOI] [PubMed] [Google Scholar]
- 8.Zoller M. CD44: can a cancer-initiating cell profit from an abundantly expressed molecule? Nat Rev Cancer. 2011;11:254–67. doi: 10.1038/nrc3023. [DOI] [PubMed] [Google Scholar]
- 9.Orian-Rousseau V. CD44, a therapeutic target for metastasising tumours. Eur J Cancer. 2010;46:1271–7. doi: 10.1016/j.ejca.2010.02.024. [DOI] [PubMed] [Google Scholar]
- 10.Collins MA, Brisset JC, Zhang Y, Bednar F, Pierre J, Heist KA, et al. Metastatic pancreatic cancer is dependent on oncogenic Kras in mice. PLoS One. 2012;7:e49707. doi: 10.1371/journal.pone.0049707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Collins MA, Bednar F, Zhang Y, Brisset JC, Galban S, Galban CJ, et al. Oncogenic Kras is required for both the initiation and maintenance of pancreatic cancer in mice. J Clin Invest. 2012;122:639–53. doi: 10.1172/JCI59227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Collisson EA, Sadanandam A, Olson P, Gibb WJ, Truitt M, Gu S, et al. Subtypes of pancreatic ductal adenocarcinoma and their differing responses to therapy. Nat Med. 2011;17:500–3. doi: 10.1038/nm.2344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Brown RL, Reinke LM, Damerow MS, Perez D, Chodosh LA, Yang J, et al. CD44 splice isoform switching in human and mouse epithelium is essential for epithelial-mesenchymal transition and breast cancer progression. J Clin Invest. 2011;121:1064–74. doi: 10.1172/JCI44540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Yook JI, Li XY, Ota I, Hu C, Kim HS, Kim NH, et al. A Wnt-Axin2-GSK3beta cascade regulates Snail1 activity in breast cancer cells. Nat Cell Biol. 2006;8:1398–406. doi: 10.1038/ncb1508. [DOI] [PubMed] [Google Scholar]
- 15.Yook JI, Li XY, Ota I, Fearon ER, Weiss SJ. Wnt-dependent regulation of the E-cadherin repressor snail. J Biol Chem. 2005;280:11740–8. doi: 10.1074/jbc.M413878200. [DOI] [PubMed] [Google Scholar]
- 16.Ota I, Li XY, Hu Y, Weiss SJ. Induction of a MT1-MMP and MT2-MMP-dependent basement membrane transmigration program in cancer cells by Snail1. Proc Natl Acad Sci U S A. 2009;106:20318–23. doi: 10.1073/pnas.0910962106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Sabeh F, Ota I, Holmbeck K, Birkedal-Hansen H, Soloway P, Balbin M, et al. Tumor cell traffic through the extracellular matrix is controlled by the membrane-anchored collagenase MT1-MMP. J Cell Biol. 2004;167:769–81. doi: 10.1083/jcb.200408028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Shields MA, Dangi-Garimella S, Krantz SB, Bentrem DJ, Munshi HG. Pancreatic cancer cells respond to type I collagen by inducing snail expression to promote membrane type 1 matrix metalloproteinase-dependent collagen invasion. J Biol Chem. 2011;286:10495–504. doi: 10.1074/jbc.M110.195628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Krantz SB, Shields MA, Dangi-Garimella S, Cheon EC, Barron MR, Hwang RF, et al. MT1-MMP cooperates with Kras(G12D) to promote pancreatic fibrosis through increased TGF-beta signaling. Mol Cancer Res. 2011;9:1294–304. doi: 10.1158/1541-7786.MCR-11-0023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Jin L, Hope KJ, Zhai Q, Smadja-Joffe F, Dick JE. Targeting of CD44 eradicates human acute myeloid leukemic stem cells. Nat Med. 2006;12:1167–74. doi: 10.1038/nm1483. [DOI] [PubMed] [Google Scholar]
- 21.Charrad RS, Gadhoum Z, Qi J, Glachant A, Allouche M, Jasmin C, et al. Effects of anti-CD44 monoclonal antibodies on differentiation and apoptosis of human myeloid leukemia cell lines. Blood. 2002;99:290–9. doi: 10.1182/blood.v99.1.290. [DOI] [PubMed] [Google Scholar]
- 22.Marangoni E, Lecomte N, Durand L, de Pinieux G, Decaudin D, Chomienne C, et al. CD44 targeting reduces tumour growth and prevents post-chemotherapy relapse of human breast cancers xenografts. Br J Cancer. 2009;100:918–22. doi: 10.1038/sj.bjc.6604953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Du L, Wang H, He L, Zhang J, Ni B, Wang X, et al. CD44 is of functional importance for colorectal cancer stem cells. Clin Cancer Res. 2008;14:6751–60. doi: 10.1158/1078-0432.CCR-08-1034. [DOI] [PubMed] [Google Scholar]
- 24.Guo Y, Ma J, Wang J, Che X, Narula J, Bigby M, et al. Inhibition of human melanoma growth and metastasis in vivo by anti-CD44 monoclonal antibody. Cancer Res. 1994;54:1561–5. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.