Abstract
Ultrasound-targeted microbubble destruction(UTMD) has been utilized to deliver naked siRNA into cells in in vitro settings. But whether UTMD can safely deliver naked siRNA into in vivo cells have remained unknown. This work was performed to investigate the feasibility of UTMD-enhanced naked siRNA transduction (or combined with Lipofectamine 2000) in vivo retinal cells and compare the performance between UTMD and ultrasonic irradiation alone in this enhancing effect. A dose of Cy3-labeled siRNA was injected into the vitreous cavity of rat eyes under the different conditions of Lipofectamine 2000 or/and UTMD. Transduction efficiency was assessed by fluorescence microscopy and flow cytometry. Cell and tissue damage was assessed by trypan blue exclusion test and hematoxylineosin staining, respectively. The quantity and the density of transducted cells in the group received Lipofectamine 2000 and UTMD was far more than that in other groups. The number of transducted cells in the group received Lipofectamine 2000 and ultrasonic irradiation alone was slightly more than that in the group received Lipofectamine 2000. Cy3-siRNA-positive cells can also seen in the group received UTMD alone, although the transduction efficiency is extremely low. Cell viability in each group was more than 90%, and retinal architecture in each group was well preserved. These results indicated that UTMD, with a significantly higher performance than ultrasonic irradiation alone, can effectively enhance the Lipofectamine 2000-mediated naked siRNA transduction in vivo reinal cells without any cell or tissue damage. This method can serve as a novel approach to treat the diseases of eye ground.
KEY WORDS: retina, ultrasound-targeted microbubble destruction, Lipofectamine 2000, siRNA
INTRODUCTION
RNA interference (RNAi) mediated by small interfering RNAs (siRNAs) is a powerful gene technology allowing the silencing of mammalian genes with great specificity and potency, which has been widely utilized to down-regulate sequence-specific gene expression for the treatments of various diseases [1-4]. But siRNAs do not readily penetrate the cell membrane. Moreover, they are easily degradable when exposing to nuclease in vivo [5-7]. Therefore, clinical applications of siRNA largely depend on the development of delivery systems that can bring intact siRNA into the cytoplasm of the target cells. For delivery and prolonged expression in vivo, the siRNA genes are usually constructed on vectors, such as adenovirus, recombinant adeno-associated virus, lentivirus and plasmid [8-11]. This is costly and time-consuming. In terms of the virus vectors, they often bring about some issues about safety due to their pathogenic nature [12, 13]. Although naked plasmids are free of virus-associated adverse effects, their transduction efficiency is low and their transgene expression is relatively poor [14]. Commercially available cationic lipid formulations, such as Lipofectamine (Invitrogen), RNAifect (Qiagen), have been investigated as potential enhancers of siRNA delivery in vitro. Although they are also effective when delivered systemically, cationic lipid–mediated cellular toxicity, elicited inadvertent gene expression and enhanced immune response to siRNA maybe become the obstacles to their widespread use clinically [15]. Better methods for siRNA delivery are needed. Ultrasound-targeted microbubble destruction (UTMD) is a potential site-specific gene transfer modality that has been developed over the past two decades. Recent studies confirmed that UTMD can effectively deliver naked siRNA into B-cell lymphoma, endothelial cells and mesenchymal stem cells in in vitro settings [2, 16, 17]. But to our knowledge, whether UTMD can safely deliver naked siRNA into in vivo cells have remained unknown. Moreover, our in vitro study confirmed that UTMD alone can not deliver naked siRNA into human and rat retinal pigment epithelial cells, and it is ultrasonic irradiation (not UTMD) that significantly enhanced Lipofectamine 2000-mediated naked siRNA transduction [18]. In the present study, we injected naked siRNA into the vitreous cavity of Wistar rat eyes under the different conditions of Lipofectamine 2000 or/and UTMD. By the quantification of transduction efficiency of naked siRNAs in retina, we hoped to answer the following questions as: 1) whether UTMD can effectively enhance naked siRNA or Lipofectamine 2000-mediated naked siRNA transduction to in vivo cells? 2) which one indeed enhances the Lipofectamine 2000-mediated naked siRNA transduction to in vivo retinal cells between ultrasonic irradiation alone and UTMD ?
MATERIALS AND METHODS
Cy3-labeled siRNA preparation
Cy3-labeled siRNA(Cy3-siRNA) was purchased from RiboBio Co.,Ltd (Guangzhou, China) and was used to determine transduction efficiency of siRNA, optimize transfection condition and serve as siRNA localization. This RNAi negative control does not have homology with mammal gene and has very good pH tolerance, thus is stable in the living cell, which can be detected by flow cytometry, fluorescence microscope and laser co-focus microscope. Cy3 labeled spot was on the 5' end and this modification didn’t influence the silence activity of siRNA. A dose of 10 nmol Cy3-siRNA powder was dissolved in 150 μl diethylpyrocarbonate (Sigma, USA) treated water, then 3μL Cy3-siRNA (0.2 nmol) was drawn out and mixed with 3μL Lipofectamine2000 (L) (invitrogen, USA). The mixed solution was standing for 20 minutes before intravitreal injection, ensuring valid transduction efficiency. All the operation process had been away from light.
Microbubble contrast agents
SonoVue® microbubble contrast agent (Bracco, Milan, Italy), a composition of a core of sulfur hexafluoride (SF6) gas and an envelope of phospholipids, was reconstituted in saline solution according to the manufacturer’s protocol, and yielded a preparation containing 2-5×108 microbubbles (MBs)/mL by inversion/agitation of the unit. The average diameter of the MBs was 2.5-6.0 μm.
Animal preparation and grouping
After obtaining the approval of the local ethics committee, 84 normal adult Wistar rats (male or female, age=8-10 weeks, weight=180-200g, SLACCAS, Shanghai, China) were enrolled in this experiment. All animals were bred, maintained, and sacrificed humanely in strict compliance with the policies stated in the statement of Association for Research in Vision and Ophthalmology for the use of animals in ophthalmic and vision research. According to the content of intravitreal injection, Wistar rats were divided into six groups as follows (n=14):
Group 1 (control): 8μL sterile pyrogen-free normal saline (NS);
Group 2 (US): 3μL Cy3-siRNA (0.2 nmol), 5μL NS and an ultrasound exposure;
Group 3 (US+MBs): 3μL Cy3-siRNA (0.2 nmol), 2μL MBs, 3μLNS and an ultrasound exposure;
Group 4 (L): 6μL mixed solution of Cy3-siRNA and Lipofectamine2000 and 2μL NS;
Group 5 (L+US):6μL mixed solution of Cy3-siRNA and Lipofectamine2000, 2μL NS and an ultrasound exposure;
Group 6 (L+US+MBs): 6μL mixed solution of Cy3-siRNA and Lipofectamine2000, 2μL MBs and an ultrasound exposure.
Intravitreal injection
The method of intravitreal injection was previously described by Zheng [19,20]. Briefly, Wistar rats were anesthetized by intraperitoneal injection of 10% chloral hydrate (350mg/kg body weight). The pupils were dilated with one drop of 1% atropine sulfate and tropicamide, and the eyes were gently protruded using a rubber circle and subsequently covered with 0.3% ofloxacin eye ointment (Xingqi, Shenyang, China) to simulate a preset lens. Under a surgical microscope (SM-2000J, Eder, Shanghai, China), NS, Cy3-siRNA or a mixed solution of Cy3-siRNA and Lipofectamine 2000, with or without MBs, were injected into the left eye according the grouping using a blunt 32-gauge Hamilton syringe. The right eyes served as a control eye, and were injected with 8μL NS.
Utrasound exposure
A therapeutic ultrasound machine (Topteam161, Chattanooga, TN, USA) and a 2-cm2 probe were applied in this study. The parameters of US exposure were as follows: frequency, 1MHz, power, 2W/cm2, duty cycle, 50%, pulse recurrent freuency, 100 Hz, duration, 300 seconds. Immediately after intravitreal injection, a 2cm2 US probe placed directly onto the conjunctival surface after a small amount of coupling medium was smeared on its face, then the insonation was performed.
Retina-stretched preparation and fluorescence imaging
Six eyes of each group were harvested at 12 hour after intravitreal injection. Fundus oculi were prepared after enucleation of the globe by removing the anterior segment with a blade and carefully transferring the whole retina to a microscope slide. Six relieving incisions were made to allow the retina to be flattened. Immediately, the quantity and density of Cy3-siRNA-positive cells in retina were observed and photographed by an inverted fluorescent stereoscope (ZEISS, Stemi SV11, Jena, German). The area and mean grey of Cy3-siRNA fluorescence were analyzed and quantified using Axiovision 3.1 software (Carl Zeiss, Goettingen, Germany). Data were presented as integrity optical density (total area × mean grey of Cy3-siRNA).
Flow cytometry and cell viability
Single-cell suspensions were prepared from six eyes of each group at 12 hour after intravitreal injection as previously described by Portillo [21]. Briefly, retinas were isolated and minced following by digestion in a solution containing 15 IU/ml papain and 15 μg/ml DNase (Worthington Biochemicals, Freehold, NJ) for 30 min at 37 °C. Tissue was dissociated by gentle pipetting and passed through a 40 μm cell strainer. Flow-through was mixed with Fetal bovine serum (FBS; HyClone Laboratories Inc. South Logan, UT) and washed. Tissue trapped by the strainer was digested with 1 mg/ml collagenase type I (Worthington Biochemicals) for 30 min at 37 °C to free endothelial cells. After dissociation and mixing with FBS, cells were washed once in Dulbecco’s modified Eagle’s medium(DMEM;Gibco, Grand Island, USA) with 10% FBS for 5 min at 300 g at room temperature. Cells obtained after papain-DNase and collagenase treatments were pooled. Finally, Cy3 expression of the infected cells was quantitatively examined by flow cytometry (EPICS XL, Beckman Coulter Co, Miami, USA) analysis. Data were presented as Cy3-siRNA-positive cell ratio (%, the number of infected cells per 100 retinal cells). In addition, cell viability was assessed by trypan blue exclusion test. Cells were suspended in PBS. Ten μL of cell suspension were mixed with an equal amount of 0.4% trypan blue dye (Invitrogen, USA). Blue (dead) and white (living) cells were counted microscopically in a hemocytometer (Sigma, USA).
Histopathologic examination
Two eyes of each group were harvested at 12 hour after intravitreal injection. The eyes were enucleated, and fixed in 10% formaldehyde solution at a room temperature. Thereafter, they were embedded in paraffin, and cut into 5μm-thick sections. Subsequently, the sections were stained with hematoxylin-eosin(HE) to observe retinal architecture, inflammatory cell infiltration, and proliferative membrane using light microscopy (Zeiss Axiovert S 100, Jena, Germany). All the results of histopathologic examination were confirmed by two masked expert pathologists.
Statistical analysis
Data were expressed as the means and standard deviation (mean±SD). Analysis of variance (ANOVA) was used to determine the significance of the difference in a multiple comparison. Differences were considered significant at p<0.05. All statistical analysis was performed with SPSS version 13 software for Windows (SPSS Inc, Chicago, IL).
RESULTS
Cy3-siRNA-positive cells in retina-stretched preparation are shown in Figure 1 and Figure 2. The quantity of transducted cells in Group 6 (L+US+MBs) was far more than that in other five groups (L+US, L, US+MBs, US, control), and the density of Cy3-siRNA -positive cells in this group is the highest.
FIGURE 1.

Picture showing the quantity and density of Cy3-siRNA-positive cells in retina-stretched preparation transducted by UTMD or/and Lipofectamine2000. US, ultrasound exposure (1MHz, 2W/cm2, 50%, 100Hz, 300 seconds); MBs, SonoVue®; microbubble contrast agents (2μL); L, Lipofectamine2000 (0.2 nmol) (100×magnification).
FIGURE 2.
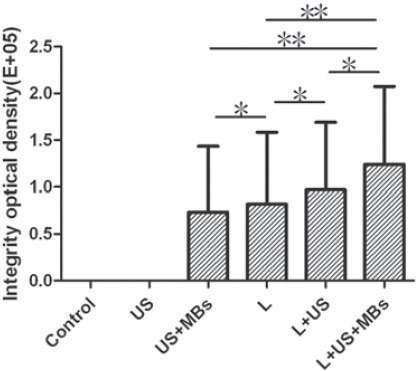
Quantitative analysis of the density of Cy3-siRNA in retina-stretched preparation transducted by UTMD or/and Lipofectamine2000. Data are presented as integrity optical density (total area×mean density of Cy3-siRNA). US, ultrasound exposure (1MHz, 2W/cm2, 50%, 100Hz, 300 seconds); MBs, SonoVue® microbubble contrast agents (2μL); L, Lipofectamine2000 (0.2 nmol) (ANOVA, *p <0.05, **p <0.01)
The number of transducted cells in Group5 (L+US) was slightly more than that in Group 4 (L). In addition, the quantity of Cy3-siRNA-positive cells in Group 4 (L) is significantly more than that in Group 3 (US+MBs). As for Group 2 (US) and Group 1 (control), none of the fluorescence can be found. Transduction efficiency of Cy3-siRNA in retinal cells under different conditions is shown in Figure 3. The ratio of Cy3-siRNA-positive cells in Group 6 (L+US+MBs) is the highest (57.8±12.5%), which is far more than that in other five groups (L+US, L, US+MBs, US, control). From Group 5 to Group 3, the ratios of Cy3-siRNA-positive cells decreased in order (19.7 ± 7.4%, 12.6 ± 5.9%, 4.3 ± 1.2%), and there is no Cy3-siRNA expression in the retinal cells of Group 2 (US) and Group 1(control) detected by flow cytometry. The effects of Lipofectamine2000,US and US plus MBs on cell viability assessed by trypan blue exclusion test are shown in Figure 4. The retinal cell viability 12 hours after transfection in Group 1(control),Group 2 (US) and Group 3 (US+MBs) was 95.65 ± 3.32%, 95.45 ± 3.75%, and 92.53 ±2.97%, respectively, and it was 95.15 ± 3.27%, 95.56 ± 3.64% and 92.27 ± 2.76% in Group 4 (L), Group5 (L+US) and Group 6 (L+US+MBs), respectively. Histological observation of each group using HE-staining of retinal architecture in Wistar rats at 12 hour after intravitreal injection is shown in Figure 5. Apparently, all layers of the retina were well preserved without photoreceptor loss, nuclear layer vacuolation, or inflammation under this condition.
FIGURE 3.

Transduction efficiency of Cy3-siRNA in retina under different conditions. US, ultrasound exposure (1MHz, 2W/cm2, 50%, 100Hz, 300 seconds); MBs, SonoVue® microbubble contrast agents (2μL); L, Lipofectamine2000 (0.2 nmol) (ANOVA, *p <0.05, **p <0.01).
FIGURE 4.

The effects of Lipofectamine2000, US and US plus MBs on retinal cell viability assessed by trypan blue exclusion test at 12 hour after intravitreal injection. US, ultrasound exposure (1MHz, 2W/cm2, 50%, 100Hz, 300 seconds); MBs, SonoVue® microbubble contrast agents (2μL); L, Lipofectamine2000 (0.2 nmol).
FIGURE 5.

Histological image using HE-staining of retinal architecture in Wistar rats 12 hours after intravitreal injection. US, ultrasound exposure (1MHz, 2W/cm2, 50%, 100Hz, 300 seconds); MBs, SonoVue® microbubble contrast agents (2μL); L, Lipofectamine2000 (0.2 nmol) (400 × magnification).
DISCUSSION
The results presented here indicate that UTMD can effectively enhance the Lipofectamine 2000-mediated naked siRNA transduction to in vivo reinal cells without any cell or tissue damage. It is UTMD that indeed enhances Lipofectamine 2000-mediated naked siRNA transduction to in vivo retinal cells, with a significantly higher performance than ultrasonic irradiation alone in this enhancing effect. UTMD alone can also slightly enhance naked siRNA transduction to in vivo cells, although the transduction efficiency is extremely low. The detailed mechanism of UTMD-enhanced naked or liposome-mediated siRNA transduction has not yet been fully explained. It is considered that bioeffects of UTMD, such as cavitation, thermal effect, radiation force, and chemical effect together may result in permeability changes of the cell membrane [22,23], and hence an increased uptake of siRNA. In the sonification zone, cavitation also creates small shock waves that increase cell permeability by disruption of the membrane barrier [24]. Ultrasound irradiation triggers two liposomal siRNA-release mechanisms: the predominant one is diffusion through liposome membrane, and the less significant one is liposome disintegration [25]. Compared to our in vitro study [18], this in vivo study produce different results as mentioned above. Although the results could be simply explained by varied degrees of physical impacts, the subtle mechanisms underlying these phenomena require further study. In addition, the transduction efficiency of siRNA in this in vivo study is relatively low, which may result from the different characteristics of contrast agents (shell gas properties and parcels), the different cells and the different target genes used in this study. As one of conventional diagnostic techniques of clinical imaging, the safety of ultrasound has been established. But the investigation about the safety of UTMD in the therapy of ophthalmological diseases has not yet been fully finished. In our study, we referred to the condition previously described by Zheng [26,27]: frequency, 1MHz; power, 2W/cm2; duty cycle,50%; pulse recurrent frequency,100Hz; duration,300 seconds; MBs concentration, 25%. Because this UTMD condition have no obvious tissue damage to the retina assessed by histopathologic examination. In the present study, cell viability in each group was more than 90%, and retinal architecture in each group was well preserved, which indicated that this dose of Lipofectamine2000 (0.2 nmol) and this UTMD condition mentioned above have no significant adverse effects on retinal cell viability and retinal architecture.
CONCLUSION
This study demonstrated UTMD can effectively enhance the Lipofectamine 2000-mediated naked siRNA transduction to in vivo reinal cells, which reduces the dose of liposome and the attendant adverse effects. Although some limitations present, UTMD-enhanced liposome-mediated siRNA transduction to retina can serve as a novel approach to treat the diseases of eye ground. Further studies are needed to evolve so that an optimal condition of UTMD and an appropriate dose of liposome can be obtained. Meanwhile, new types of microbubble contrast agents require further study.
ACKNOWLEDGMENTS
The authors gratefully acknowledge technical assistance and helpful discussion from the entire staff at the department of ophthalmology and pathology of Shanghai Jiaotong University Affiliated First People’s Hospital.
DECLARATION OF INTEREST
The authors have no conflict of interest to declare.
REFERENCES
- [1].Reich SJ, Fosnot J, Kuroki A, Tang W, Yang X, Maguire AM, et al. Small interfering RNA (siRNA) targeting VEGF effectively inhibits ocular neovascularization in a mouse model. Mol Vis. 2003;9:210–216. [PubMed] [Google Scholar]
- [2].Otani K, Yamahara K, Ohnishi S, Obata H, Kitamura S, Nagaya N. Nonviral delivery of siRNA into mesenchymal stem cells by a combination of ultrasound and microbubbles. J Control Release. 2009;133:146–153. doi: 10.1016/j.jconrel.2008.09.088. [DOI] [PubMed] [Google Scholar]
- [3].Davidson BL, McCray PB., Jr Current prospects for RNA interference-based therapies. Nat Rev Genet. 2011;12:329–340. doi: 10.1038/nrg2968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Angaji SA, Hedayati SS, Poor RH, Madani S, Poor SS, Panahi S. Application of RNA interference in treating human diseases. J Genet. 2010;89:527–537. doi: 10.1007/s12041-010-0073-3. [DOI] [PubMed] [Google Scholar]
- [5].Bruna S, Dapas B, Farra R, Grassi M, Pozzato G, Giansante C, et al. Improving siRNA bio-distribution and minimizing side effects. Curr Drug Metab. 2011;12:11–23. doi: 10.2174/138920011794520017. [DOI] [PubMed] [Google Scholar]
- [6].Vandenbroucke RE, Lentacker I, Demeester J, De Smedt SC, Sanders NN. Ultrasound assisted siRNA delivery using PEG-siPlex loaded microbubbles. J Control Release. 2008;126:265–273. doi: 10.1016/j.jconrel.2007.12.001. [DOI] [PubMed] [Google Scholar]
- [7].Matsuda A. Development of highly nuclease-resistant chemically-modified oligonucleotides. Yakugaku Zasshi. 2011;131:285–298. doi: 10.1248/yakushi.131.285. [DOI] [PubMed] [Google Scholar]
- [8].Sliva K, Schnierle BS. Selective gene silencing by viral delivery of short hairpin RNA. Virol J. 2010;7:248. doi: 10.1186/1743-422X-7-248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Koornneef A, Maczuga P, van Logtenstein R, Borel F, Blits B, Ritsema T, et al. Apolipoprotein B knockdown by AAV-delivered shRNA lowers plasma cholesterol in mice. Mol Ther. 2011;19:731–740. doi: 10.1038/mt.2011.6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Liu YP, Berkhout B. Lentiviral delivery of RNAi effectors against HIV-1. Curr Top Med Chem. 2009;9(12):1130–1143. doi: 10.2174/156802609789630866. [DOI] [PubMed] [Google Scholar]
- [11].Mowa MB, Crowther C, Arbuthnot P. Therapeutic potential of adenoviral vectors for delivery of expressed RNAi activators. Expert Opin Drug Deliv. 2010;7:1373–1385. doi: 10.1517/17425247.2010.533655. [DOI] [PubMed] [Google Scholar]
- [12].Alexander JJ, Hauswirth WW. Adeno-associated viral vectors and the retina. Adv Exp Med Biol. 2008;613:121–128. doi: 10.1007/978-0-387-74904-4_13. [DOI] [PubMed] [Google Scholar]
- [13].Wang QZ, Lv YH, Diao Y, Xu R. The design of vectors for RNAi delivery system. Curr Pharm Des. 2008;14:1327–1340. doi: 10.2174/138161208799316357. [DOI] [PubMed] [Google Scholar]
- [14].Tsunoda S, Mazda O, Oda Y, Iida Y, Akabame S, Kishida T, et al. Sonoporation using microbubble BR14 promotes pDNA/siRNA transduction to murine heart. Biochem Biophys Res Commun. 2005;336:118–127. doi: 10.1016/j.bbrc.2005.08.052. [DOI] [PubMed] [Google Scholar]
- [15].Akhtar S, Benter IF. Nonviral delivery of synthetic siRNAs in vivo. J Clin Invest. 2007;117:3623–3632. doi: 10.1172/JCI33494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Kinoshita M, Hynynen K. A novel method for the intracellular delivery of siRNA using microbubble-enhanced focused ultrasound. Biochem Biophys Res Commun. 2005;335:393–399. doi: 10.1016/j.bbrc.2005.07.101. [DOI] [PubMed] [Google Scholar]
- [17].Kinoshita M, Hynynen K. Key factors that affect sonoporation efficiency in in vitro settings: the importance of standing wave in sonoporation. Biochem Biophys Res Commun. 2007;359:860–865. doi: 10.1016/j.bbrc.2007.05.153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Li HL, Du LF, Zheng XZ, Wang HP, Gu Q, Chen XF. Ultrasound-targeted microbubble mediated liposome small interference RNA transfection to retinal pigment epithelium cells. Chin J Med Imaging Tech. 2010;1:22–24. [Google Scholar]
- [19].Zheng XZ, Li HL, Du LF, Gu Q, Wang HP. A rat model of proliferative vitreoretinopathy induced by RPE-J cells and platelet-rich plasma. Asian Biomed. 2009;3:507–515. [Google Scholar]
- [20].Zheng XZ, Du LF, Wang HP. A immunohistochemical analysis of a rat model of proliferative vitreoretinopathy and a comparison of the expression of TGF-β and PDGF among the induction methods. Bosn J Basic Med. 2010;10:204–209. doi: 10.17305/bjbms.2010.2686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Portillo JA, Okenka G, Kern TS, Subauste CS. Identification of primary retinal cells and ex vivo detection of proinflammatory molecules using flow cytometry. Mol Vis. 2009;15:1383–1389. [PMC free article] [PubMed] [Google Scholar]
- [22].Howard CM, Forsberg F, Minimo C, Liu JB, Merton DA, Claudio PP. Ultrasound guided site specific gene delivery system using adenoviral vectors and commercial ultrasound contrast agents. J Cell Physiol. 2006;209:413–421. doi: 10.1002/jcp.20736. [DOI] [PubMed] [Google Scholar]
- [23].Mukherjee D, Wong J, Griffin B, Ellis SG, Porter T, Sen S, et al. Tenfold augmentation of endothelial uptake of vascular endothelial growth factor with ultrasound after systemic administration. J Am Coll Cardiol. 2000;35:1678–1686. doi: 10.1016/s0735-1097(00)00575-1. [DOI] [PubMed] [Google Scholar]
- [24].Zheng XZ, Li HL, Du LF, Wang HP, Gu Q. Comparative analysis of gene transfer to human and rat retinal pigment epithelium cell line by a combinatorial use of recombinant adeno-associated virus and ultrasound or/and microbubbles. Bosn J Basic Med. 2009;9:174–181. doi: 10.17305/bjbms.2009.2802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Enden G, Schroeder A. A mathematical model of drug release from liposomes by low frequency ultrasound. Ann Biomed Eng. 2009;37:2640–2645. doi: 10.1007/s10439-009-9785-z. [DOI] [PubMed] [Google Scholar]
- [26].Zheng XZ, Li HL, Du LF, Wang HP, Gu Q. In vivo and in vitro effects of ultrasound or/and microbubbles on recombinant adeno-associated virus-mediated transgene expression in the retina. Asian Biomed. 2009;3:497–506. [Google Scholar]
- [27].Li HL, Zheng XZ, Wang HP, Li F, Wu Y, Du LF. Ultrasound-targeted microbubble destruction enhances AAV mediated gene transfection: human RPE cells in vitro and the rat retina in vivo. Gene Ther. 2009;16:1146–1153. doi: 10.1038/gt.2009.84. [DOI] [PubMed] [Google Scholar]


