Abstract
Many pharmacological agents were investigated for the prevention of renal ischemic reperfusion (I/R) injury as well as the phosphodiesterase (PDE) inhibitors. The aim of the study was to examine the possible renoprotective effect of a member in this family, tadalafil (Td) on I/R injury. Thirty-six Spraque Dawley rats were allocated to six groups as; control, sham, ischemia (I), ischemia/reperfusion (I/R), Td pretreatment ischemia (Td/I) and Td pretreatment ischemia/reperfusion (Td/IR) groups. Right nephrectomy was performed in all groups. Td was dissolved in saline solution and given as a single dose (1mg/kg) through an orogastrictube 60 min before the operation in the Td pretreatment groups. In ischemia group the left renal pedicle was occluded for 45 minutes and after than underwent left nephrectomy. In I/R group left renal pedicle was occluded for 45 minutes, reperfused for 1 hour and after then underwent nephrectomy. The left kidneys were evaluated after standard laboratory procedures with regard to tubular morphology, and leukocyte infiltration. The data were analyzed by using Kruskal–Wallis test to determine differences among the groups. A p value of < 0.05 was considered significant.
Renal tubular damage was significant increased in the ischemia and I/R group (Groups III and IV) when compared to those in the sham group (Group II), (p = 0.004, 0.004, respectively). Tubular damage, in the Td pretreatment ischemia (Td/I) (Group V) and Td pretreatment ischemia/reperfusion (Td/IR) (Group VI) were less than that in the ischemia group (Group III) (p= 0.010, p= 0.025, respectively).
Td administration prior to the renal I/R injury attenuated these morphological disarrangements, which were observed in renal I/R. Tubular necrosis, which may be considered as an important issue of the developing renal injury, was also completely prevented with Td administration.
KEY WORDS: KEY WORDS: kidney, ischemia/reperfusion injury, tadalafil, rat
INTRODUCTION
As a consequence of hemorrhage, shock, cardiac arrest, renal artery surgery, or renal transplantation, I/R injury remains a major clinical problem [1]. Preventing the renal tissue from IR injury is the main aim of the intense researches about these ischemic phenomenons [1]. PDE inhibitors are the compounds those inhibit or antagonize the biosynthesis or actions of PDEs. PDE 5 inhibitors are currently widely used for the treatment of pulmonary arterial hypertension and erectile dysfunction in men [2-5]. These vasodilator and inotropic agents have protective effects on vascular structures and myocardial muscles [5-9]. The effects of many members in this family have been studied especially on I/R injury. In the literature sildenafil citrate and vardenafil HCl are most commonly investigated PDE5 inhibitors for this subject [10-14]. Aim of our study is to determine the effects of Td on renal I/R injury by using histopathological parameters.
MATERIALS AND METHODS
All experiments in this study were performed in accordance with the guidelines for animal research from the National Institutes of Health and were approved by our university Animal Ethics Committee.
Experimental groups
Male Spraque Dawley rats weighing 250–300 g were randomly divided into six groups each with six animals. Td was dissolved in saline solution and given as a single dose (1mg/kg) through an orogastrictube 60 min before the operation in the Td pretreatment groups. The rats were anesthetized with intraperitoneal injection of ketamine. An upper abdominal midline incision was made and bilateral renal blood vessels were isolated using minimal dissection. In Group I (control), only right nephrectomy was performed. Animals in Group II (sham) were subjected to right nephrectomy and left nephrectomy was performed after waiting for 45 minutes without any ischemia. In Group III (ischemia group), rats were subjected to right nephrectomy and the left renal pedicle was occluded for 45 minutes with microvascular clamps and after than underwent left nephrectomy. In Group IV (I/R group), rats were subjected to right nephrectomy, occlusion of left renal pedicle for 45 minutes and reperfusion for 1hour and after then underwent nephrectomy. Animals in Group V (Td + Ischemia) were subjected to right nephrectomy and the left renal pedicle was occluded for 45 minutes with microvascular clamps and after than underwent left nephrectomy. In GroupVI (T+I/R) rats subjected to right nephrectomy, occlusion of left renal pedicle for 45 minutes and reperfusion for 1hour and after then underwent nephrectomy.
Histopathological evaluation
Left kidneys were fixed with formalin, embeded in paraffin and stained with hematoxylin eosin. All were evaluated by the same pathologist with respect to glomerular, tubular, and interstitial morphology. Sclerosis of glomeruli was noted. About one hundred tubules of the kidney were evaluated randomly and were scored for each section. The mean score was calculated for each of the six groups. Tubular morphology was determined in four groups as: 0 (normal morphology), 1 (mild brush border abnormality such as flattening, or irregularity in shape A and size of the cells), 2 (moderate brush border abnormality, involving more than 50% of the tubular profiles), 3 (loss of microvilli in the epithelium of whole tubules and tubular necrosis). In addition, leukocyte infiltration was evaluated in four areas of the sections as 4-point scoring system: 0 = normal, 1 = infiltration less than 25%, 2 = infiltration between 25% and 50%, 3 = infiltration more than 50%.
Statistical analysis
Statistical analysis was done by using the Statistical Package for Social Sciences, version 13.0 for Windows, (SPSS, Chicago, IL). Histological damage among the groups was compared using the Kruskal-Wallis test. The value of p < 0.05 was considered statistically significant.
RESULTS
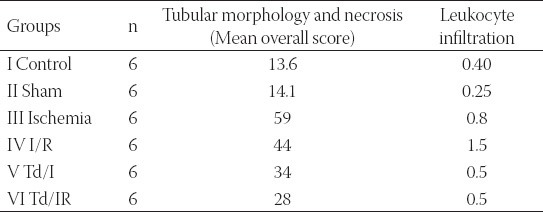
Microscopic evaluation of the control and sham groups (Group I -II) revealed a regular morphology of renal parenchyma (Figure 1). The kidneys in ischemia group (Group III) showed sclerosis of glomeruli. Bowman’s space was enlarged. There was loss of microvilli in the epithelium of proximal tubules (Figure 2-A, B). Tubular necrosis was found in both cortical and medullar regions. Enlargement of the interstitium was seen as a result of edema (Figure 2-C). Tubular degeneration was evident in I/R group (Group IV). Necrosis in the tubular epithelium of the cortex and hyaline degeneration in some areas were observed. The loss of microvilli in epithelial cells of proximal tubule was evident (Figure 3-A). Interstitial edema and leukocyte infiltration were found in the medulla (Figure 3-B). In Td + Ischemia group (Group V), capillaries in glomeruli showed normal morphology (Figures 4-A). In some areas, loss of microvilli was obvious in sections (Figure 4-B). Interstitial edema was prominent, particularly near blood vessels (Figure 4-C). In Td + I/R group (Group- VI) the kidney preserved the normal morphology, and showed normal morphology of glomeruli and tubular cells. Most of epithelial cells lining the proximal tubules were preserving microvilli structure (Figures 5-A). Stasis, congestion and hemorrhage in some sections were found in the medulla (Figure 5-B). Renal tubular damage was significantly increased in the ischemia and I/R group (Groups III and IV) when compared to those in the sham group (Group II), (p = 0.004, 0.004, respectively). Tubular necrosis and loss of microvilli was less in Td + ischemia group (Group V) and Td pretreated I/R group (GroupVI) according to ischemia group (p = 0.010, p = 0.025 respectively). With regard to histopathological scoring, tubular damage in the Td pretreated I/R group (GroupVI) was less than in the I/R group (Group IV) (Median score 28 and 44 respectively). However, the difference between the Td pretreated I/R group (GroupVI) and I/R (Group IV) groups was not significant (p = 0,128). But tubular necrosis was significant increased in Td pretreated ischemia group (Group V) and Td pretreated I/R group (Group VI) when compared to sham group (p = 0.004, p = 0.008, respectively). According to histopathological scoring the leukocyte infiltration score in the sham group (Group I) was 0.5 (Table 1). It was increased in the ischemia (Group II) and I/R group (Group III) when compared to the sham group (Group I) (median score 0.8 and 1.5, respectively). The leukocyte infiltration score was decreased in the Td pretreated ischemia group (Group V) and Td pretreated I/R group (Group VI) when compared to I/R group (GroupIV). (Median score 0.5 and 0.5) (Table 1). However, the difference between the Td pretreated ischemia group (Group V), Td pretreated I/R group (Group VI) and I/R (Group IV) groups was not statistically significant (p = 0.173).
FIGURE 1.
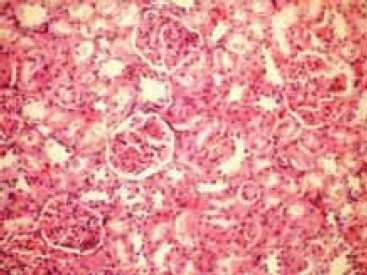
Regular morphology of renal parenchyma (Hematoxylin–eosin × 100)
FIGURE 2.
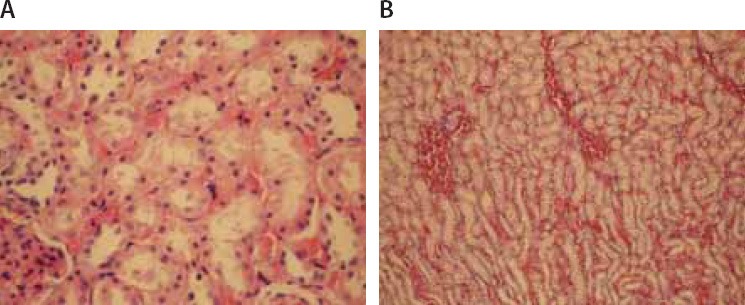
(A) Sclerosis of glomeruli and enlargement of Bowman space (Hematoxylin–eosin × 200). (B) Loss of microvilli and flattened cells in the epithelium of proximal tubules (Hematoxylin–eosin × 400). (C) Interstitial edema (Hematoxylin–eosin × 200).
FIGURE 3.
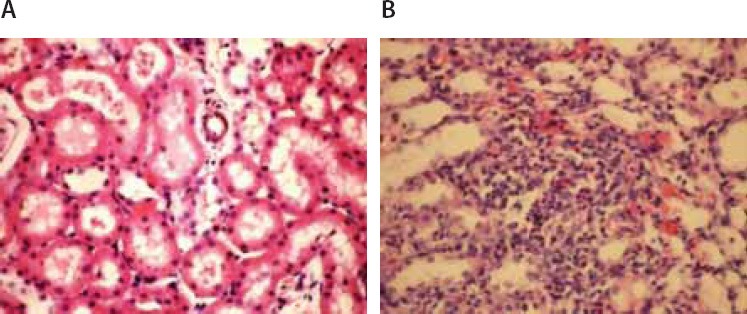
(A) Loss of microvilli in the epithelium of proximal tubules (Hematoxylin–eosin × 400). (B) Leukocyte infiltration between tubules and around blood vessel (Hematoxylin–eosin × 400)
FIGURE 4.
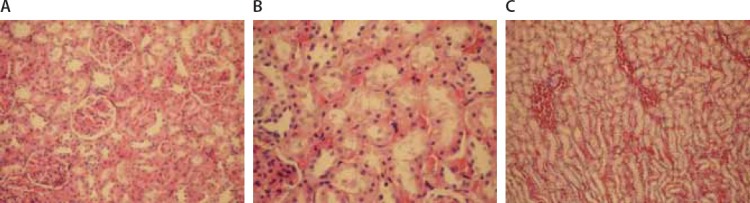
(A) Normal morphology of capillaries in glomeruli (Hematoxylin–eosin × 200). (B) Obvious loss of microvilli in some areas (Hematoxylin–eosin × 400). (C) Prominent interstitial edema near blood vessels (Hematoxylin–eosin × 200).
FIGURE 5.
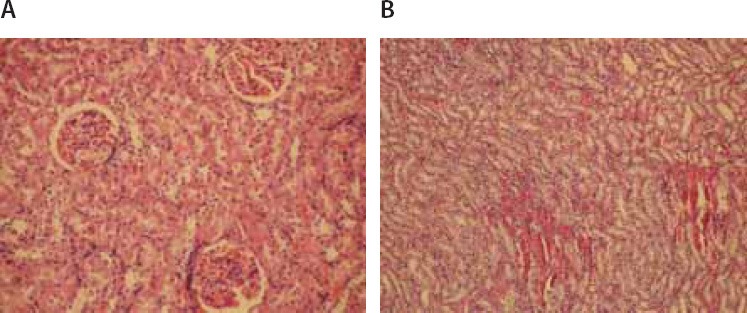
(A) Glomeruli and tubular cells showed normal appearance (Hematoxylin–eosin × 200). (B) Stasis, congestion and hemorrhage in some sections of medulla (Hematoxylin–eosin × 100).
TABLE 1.
Median tubular morphology and neutrophil infiltration scores.
DISCUSSION
PDE is a family of enzymes that regulate the cellular levels of second messengers, cyclic adenosine monophosphate (cAMP) and cyclic guanosine monophosphate (cGMP) [15]. Eleven types of different PDE families were defined in the literature [16]. Every type with its own properties modulates distinct regulatory pathways in the cell; thus, targeting specific PDE offers a fine way to treat a disease [16, 17]. PDE 5 is found in high concentration in vascular smooth muscle cells of the corpora cavernosa of the penis, in smooth muscle cells of the peripheral arterial and venous vessels as well as coronary and pulmonary circulation, and in platelets [15]. It is specific for the hydrolysis of cGMP [6]. cGMP plays an important role in regulation of intracellular calcium levels, modulation of platelet function and induces vasodilatation in I/R injury [17]. During ischemia on a kidney leads to vascular endothelial injury [18, 19]. Increased vascular resistance, produced by the loss of the ability of renal vascular endothelium to release nitric oxide (NO), or an imbalance between NO and endothelin-1 production [19]. NO has been shown to act via a variety of second-messenger cascades, although most of its effects are mediated by cGMP [20, 21]. At this point, PDE5 inhibitors may enhance the production of cGMP by removing away PDE. They increase the release of NO and improve endothelial dysfunction [19, 22]. In the literature few studies examined the effects of PDE5 inhibitors on renal I/R injury. Chintala etal. [23] found that a selective cGMP PDE5 inhibitor, Zaprinast, had anti-platelet effects following I/R in rats. According to Guan et al, the same drug enhanced renal blood flow by stimulating intracellular cGMP in ischemic acute renal failure in rats [24]. Lledo-Garcia et al. [20] reported that sildenafil has improved the outcome of warm-ischemic kidneys on the immediate posttransplant period. Oruc et al. [25] revealed that preischemic treatment with sildenafil citrate can significantly attenuate I/R-induced renal injury by decreasing leukocyte infiltration. Although the protective effects of Sildenafil and vardenafil in renal I/R injury have been examined and accepted in several studies, there is only one study in the literature about the effect of Td in renal I/R injury [26]. Td is a class of mild vasoactive drugs developed for the treatment of erectile dysfunction ED [5]. It has a different chemical structure from sildenafil and vardenafil with a mean time to maximum plasma concentration of 2 h which is two times longer than other molecules [2, 3, 27, 28]. The half-lives of sildenafil and vardenafil are 4 h and that of tadalafil is 17.5 h [28]. These advanced features make this molecule a good option in the treatment of regulation of decreased vascular resistance and ischemia. There are several studies with Td investigating effects on different organs. According to Sesti et al. [5] Td was cardioprotective in the setting of an experimental myocardial infarction. Sawamura et al. [3] suggested that Td relaxes pulmonary arteries in lungs and treatment occurs in the late phase of pulmonary arterial hypertension. According to the only study in the literature, the effect of Td can be attributed to its protective effects on renal tubular cells and inhibition of leukocyte infiltration in renal tissue [26]. Renal tubular epithelium damage plays an active role in initiating the inflammatory response [29, 30]. During ischemic periods, renal leukocyte infiltration is activated and increased [31, 32]. Activated neutrophils can release cytokines, reactive oxygen species, proteases, myeloperoxidase and other enzymes to trigger further damage [33, 34]. In the present study, tubular morphology in Td pretreated ischemia group and Td pretreated I/R group were significantly better than that in ischemia group. Although there was no statistical significance, leukocyte infiltration was decreased in Td pretreated ischemia and Td pretreated I/R group when compared to the I/R group. Moreover, further studies will be necessary to determine the role of cGMP levels and renal blood flow in ischemia-reperfusion injury.
CONCLUSION
This study is the second in the literature to demonstrate the protective effects of Td in experimental renal I/R injury model in the rat. The results of our study have demonstrated that given as a single dose Td administration prior to the renal I/R injury attenuated these morphological disarrangements, which were observed in renal I/R. Tubular necrosis, which may be considered as an important issue of the developing renal injury, was also completely prevented with Td administration.
DECLARATION OF INTEREST
The authors declare that they have no competing interests
REFERENCES
- [1].Pampal A, Ozen IO, Demirogullari B, Gol IH, Guclu MM, Bukan N, et al. Apart from the other members of PDE inhibitors’ family, enoximone does not enhance renal ischemic reperfusion injury: the effects of enoximone on renal ischemia reperfusion. Ren Fail. 2009;31(10):971–976. doi: 10.3109/08860220903216873. [DOI] [PubMed] [Google Scholar]
- [2].Saenz de Tejada I, Angulo J, Cuevas P, Fernández A, Moncada I, Allona A, et al. The phosphodiesterase inhibitory selectivity and the in vitro and in vivo potency of the new PDE5 inhibitor vardenafil. Int J Impot Res. 2001;13(5):282–290. doi: 10.1038/sj.ijir.3900726. [DOI] [PubMed] [Google Scholar]
- [3].Sawamura F, Kato M, Fujita K, Nakazawa T, Beardsworth A. Tadalafil, a long-acting inhibitor of PDE5, improves pulmonary hemodynamics and survival rate of monocrotaline-induced pulmonary artery hypertension in rats. J Pharmacol Sci. 2009;111(3):235–243. doi: 10.1254/jphs.09110fp. [DOI] [PubMed] [Google Scholar]
- [4].Wharton J, Strange JW, M0ller GMO, Growcott EJ, Ren X, Franklyn AP, et al. Antiproliferative effects of phosphodiesterase type 5 inhibition in human pulmonary artery cells. Am J Respir Crit Care Med. 2005;172(1):105–113. doi: 10.1164/rccm.200411-1587OC. [DOI] [PubMed] [Google Scholar]
- [5].Sesti C, Florio V, Johnson EG, Kloner RA. The phosphodiesterase-5 inhibitor tadalafil reduces myocardial infarct size. Int J Impot Res. 2007;19(1):55–61. doi: 10.1038/sj.ijir.3901497. [DOI] [PubMed] [Google Scholar]
- [6].Kukrejia RC, Ockaili R, Salloum F, Yin C, Hawkins J, Das A, et al. Cardioprotection with phosphodiesterase-5 inhibition – a novel preconditioning strategy. J Mol Cell Cardiol. 2004;36(2):165–173. doi: 10.1016/j.yjmcc.2003.11.001. [DOI] [PubMed] [Google Scholar]
- [7].Rosano GMC, Aversa A, Vitale C, Fabbri A, Fini M, Spera G. Chronic treatment with tadalafil improves endothelial function in men with increased cardiovascular risk. Eur Urol. 2005;47(2):214–220. doi: 10.1016/j.eururo.2004.10.002. [DOI] [PubMed] [Google Scholar]
- [8].Takimoto E, Champion HC, Li M, Belardi D, Ren S, Rodriguez ER, et al. Chronic inhibition of cyclic GMP phosphodiesterase 5A prevents and reverses cardiac hypertrophy. Nat Med. 2005;11(2):214–222. doi: 10.1038/nm1175. [DOI] [PubMed] [Google Scholar]
- [9].Ockaili R, Salloum F, Hawkins J, Kukreja RC. Sildenafil (Viagra) induces powerful cardioprotective effect via opening of mitochondrial KATP channels in rabbits. Am J Physiol Heart Circ Physiol. 2002;283(3):H1263–H1269. doi: 10.1152/ajpheart.00324.2002. [DOI] [PubMed] [Google Scholar]
- [10].Mizutani A, Murakami K, Okajima K, Kira S, Mizutani S, Kudo K, et al. Olprinone reduces ischemia/reperfusion-induced acute renal injury in rats through enhancement of cAMP. Shock. 2005;24(3):281–287. doi: 10.1097/01.shk.0000175555.95676.34. [DOI] [PubMed] [Google Scholar]
- [11].Karabulut R, Sönmez K, Sancak B, Türkyilmaz Z, Demirogullari B, Ozen IO, et al. Effects of amrinone on bilateral renal ischemia/reperfusion injury. Urol Res. 2002;30(3):164–168. doi: 10.1007/s00240-002-0256-3. [DOI] [PubMed] [Google Scholar]
- [12].Okusa MD, Linden J, Huang L, Rosin DL, Smith DF, Sullivan G. Enhanced protection from renal ischemia reperfusion [correction of ischemia: reperfusion] injury with A(2A)-adenosine receptor activation and PDE 4 inhibition. Kidney Int. 2001;59(6):2114–2125. doi: 10.1046/j.1523-1755.2001.00726.x. [DOI] [PubMed] [Google Scholar]
- [13].Zheng X, Xie L, Qin J, Shen H, Chen Z, Jin Y. Effects of wortmannin on phosphorylation of PDK1, GSK3-beta, PTEN and expression of Skp2 mRNA after ischemia/reperfusion injury in the mouse [28] kidney. Int Urol Nephrol. 2008;40(1):185–192. doi: 10.1007/s11255-007-9215-9. [DOI] [PubMed] [Google Scholar]
- [14].Anas C, Ozaki T, Maruyama S, Yamamoto T, Zu Gotoh M, Ono Y, et al. Effects of olprinone, a phosphodiesterase III inhibitor, on ischemic acute renal failure. Int J Urol. 2007;14(3):219–225. doi: 10.1111/j.1442-2042.2007.01689.x. [DOI] [PubMed] [Google Scholar]
- [15].Reffelmann T, Kloner RA. Phosphodiesterase 5 inhibitors: are they cardioprotective? Cardiovasc Res. 2009;83(2):204–212. doi: 10.1093/cvr/cvp170. [DOI] [PubMed] [Google Scholar]
- [16].Kukreja RC, Ockaili R, Salloum F, Yin C, Hawkins J, Das A, et al. Cardioprotection with phosphodiesterase-5 inhibition--a novel preconditioning strategy. J Mol Cell Cardiol. 2004;36(2):165–173. doi: 10.1016/j.yjmcc.2003.11.001. [DOI] [PubMed] [Google Scholar]
- [17].Reffelmann T, Kloner RA. Cardiovascular effects of phosphodiesterase 5 inhibitors. Curr Pharm Des. 2006;12(27):3485–3494. doi: 10.2174/138161206778343073. [DOI] [PubMed] [Google Scholar]
- [18].Lien YH, Lai LW, Silva AL. Pathogenesis of renal ischemia/reperfusion injury: lessons from knockout mice. Life Sci. 2003;74(5):543–552. doi: 10.1016/j.lfs.2003.08.001. [DOI] [PubMed] [Google Scholar]
- [19].Willams P, Lopez H, Britt D, Chan C, Ezrin A, Hottendorf R. Characterization of renal ischemia–reperfusion injury in rats. J Pharmacol Toxicol Methods. 1997;37(1):1–7. doi: 10.1016/s1056-8719(96)00141-4. [DOI] [PubMed] [Google Scholar]
- [20].Lledo-Garcia E, Rodriguez-Martinez D, Cabello-Benavente R, Moncada-Iribarren I, Tejedor-Jorge A, Dulin E, et al. Sildenafil improves immediate posttransplant parameters in warm-ischemic kidney transplants: experimental study. Transplant Proc. 2007;39(5):1354–1356. doi: 10.1016/j.transproceed.2007.01.082. [DOI] [PubMed] [Google Scholar]
- [21].Lucas KA, Pitari GM, Kazerounian S, Ruiz-Stewart I, Park J, Schulz S, et al. Guanylyl cyclases and signalling by cyclic GMP. Pharmacol Rev. 2000;52(3):375–414. [PubMed] [Google Scholar]
- [22].Friebe A, Koesling D. Regulation of nitric oxide-sensitive guanylyl cyclase. Circ Res. 2003;93(2):96–105. doi: 10.1161/01.RES.0000082524.34487.31. [DOI] [PubMed] [Google Scholar]
- [23].Chintala MS, Bernardino V, Chiu PJ. Cyclic GMP but not cyclic AMP prevents renal platelet accumulation after ischemia–reperfusion in anesthetized rats. J Pharmacol Exp Ther. 1994;271(3):1203–1208. [PubMed] [Google Scholar]
- [24].Guan Z, Miller SB, Greenwald JE. Zaprinast accelerates recovery from established acute renal failure in the rat. Kidney Int. 1995;47(6):1569–1575. doi: 10.1038/ki.1995.220. [DOI] [PubMed] [Google Scholar]
- [25].Oruc O, Inci K, Aki FT, Zeybek D, Muftuoglu SF, Kilinc K, et al. Sildenafil attenuates renal ischemia reperfusion injury by decreasing leukocyte infiltration. Acta Histochem. 2010;112(4):337–344. doi: 10.1016/j.acthis.2009.02.005. [DOI] [PubMed] [Google Scholar]
- [26].Guzeloglu M, Yalcinkaya F, Atmaca S, Bagriyanik A, Oktar S, Yuksel O, et al. The Beneficial effects of Tadalafil on renal ischemia-reperfusion injury in rats. Urol Int. 2011;86(2):197–203. doi: 10.1159/000321927. [DOI] [PubMed] [Google Scholar]
- [27].Wright PJ. Comparison of phosphodiesterase type 5 (PDE5) inhibitors. Int J Clin Pract. 2006;60(8):967–975. doi: 10.1111/j.1742-1241.2006.01049.x. [DOI] [PubMed] [Google Scholar]
- [28].Rosen RC, Kostis JB. Overview of phosphodiesterase 5 inhibition in erectile dysfunction. Am J Cardiol. 2003;92(9A):9M–18M. doi: 10.1016/s0002-9149(03)00824-5. [DOI] [PubMed] [Google Scholar]
- [29].Senturk H, Kabay S, Bayramoglu G, Ozden H, Yaylak F, Yucel M, et al. Silymarin attenuates the renal ischemia/reperfusion injury-induced morphological changes in the rat kidney. World J Urol. 2008;26(4):401–407. doi: 10.1007/s00345-008-0256-1. [DOI] [PubMed] [Google Scholar]
- [30].Gobe G, Johnson DW. Distal tubular epithelial cells of the kidney: Potential support for proximal tubular cell survival after renal injury. Int J Biochem Cell Biol. 2007;39(9):1551–1561. doi: 10.1016/j.biocel.2007.04.025. [DOI] [PubMed] [Google Scholar]
- [31].Riera M, Torras J, Herrero I, Valles J, Paubert-Braquet M, Cruzado JM, et al. Neutrophils accentuate renal cold ischemia–reperfusion injury. Dose-dependent protective effect of a platelet-activating factor receptor antagonist. J Pharmacol Exp Ther. 1997;280(2):786–794. [PubMed] [Google Scholar]
- [32].Sutton TA, Fisher CJ, Molitoris BA. Microvascular endothelial injury and dysfunction during ischemic acute renal failure. Kidney Int. 2002;62(5):1539–1549. doi: 10.1046/j.1523-1755.2002.00631.x. [DOI] [PubMed] [Google Scholar]
- [33].Sener G, Tugtepe H, Yuksel M, Cetinel S, Gedik N, Yegen BC. Resveratrol improves ischemia/reperfusion-induced oxidative renal injury in rats. Arch Med Res. 2006;37(7):8221–829. doi: 10.1016/j.arcmed.2006.04.003. [DOI] [PubMed] [Google Scholar]
- [34].Lauriat S, Linas SL. The role of neutrophils in acute renal failure. Semin Nephrol. 1998;18(5):498–504. [PubMed] [Google Scholar]


