Abstract
The objective of this study was to investigate the ability of biofilm formation among mutans and non mutans oral streptococci and to determine the effect of Lactobacillus acidophilus DSM 20079 as a probiotic strain on the adhesion of selected streptococcal strains on the surfaces. The sample comprised 40 isolates of oral streptococci from dental plaque and caries of volunteer persons. Streptococcus mutans ATCC35668 (no24) was as an standard strain. The probiotic strain was Lactobacillus acidophilus DSM 20079. The ability of biofilm formation was investigated with colorimetric method and the strongest isolates were selected. Then the effect of probiotic strain on the adhesion of streptococci isolates was determined in polystyrene microtiter plate simultaneously and 30 minutes before streptococci entrance to the system. The results showed that 42% of mutans streptococci were strongly adherent (SA) and in non mutans streptococci, only 23.5% of isolates were found strongly adherent. The strong biofilm forming bacterium isolated was Streptococcus mutans strain22. In the next step, in the presence of probiotic strain the streptococcal adhesion were reduced, and this reduction was non significantly stronger if the probiotic strain was inoculated to the system before the oral bacteria. The Lactobacillus acidophilus had more effect on adherence of mutans streptococci than non mutans streptococci with significant difference (p < 0.05). Adhesion reduction is likely due to bacterial interactions and colonization of adhesion sites with probiotic strain before the presence of streptococci. Adhesion reduction can be an effective way on decreasing cariogenic potential of oral streptococci.
KEY WORDS: adhesion, dental caries, Lactobacillus acidophilus, probiotic, Streptococci
INTRODUCTION
Dental caries and periodontal disease are major public health problems that disturb all countries in the world [1]. Industrialized nations have controlled the problem with fluoride enriched water and personal hygiene products since early in the 1960s, but cariogenicity remains a crisis that economically burdens the health care system. Dental disease remains a “silent epidemic” in the world that threatens children and adults [1, 2]. The oral streptococci especially mutans streptococci are related with the development of caries in humans and animals [3]. The adhesion of oral streptococci such as Streptococcus mutans to tooth surfaces has the major role in their pathogenicity. Going along with the increasing antibiotic resistance of bacteria, new methods such as whole bacteria replacement therapy for decreasing of oral cavity pathogens must be investigated [4, 5]. In general, a probiotic, is a live microorganism which beneficially affects the host animal by improving its intestinal microbial balance. It’s valuable effects may be mediated by direct antagonistic effect against specific groups of organisms, resulting in a decrease in numbers or by an effect on their metabolism or by stimulation of immunity [1, 6-8]. Lactobacillus acidophilus is a widely studied probiotic bacterium. So, in this study we have determined the effect of Lactobacillus acidophilus DSM 20079 as a probiotic strain on the adhesion of oral streptococci to the surfaces.
MATERIAL AND METHODS
Strains and culture condition
In this study we isolated 40 strains of streptococci from dental plaque and caries of applicants with mean age of 25 years old with the help of dentistry sterile curettes. The standard strain was Streptococcus mutans ATCC 35668. All strains were cultured on Blood agar and mitis salivarius agar media and incubated in CO2 enriched, 37°C atmosphere. Strain’s identification was done with usual biochemical tests and Rapid identification kit of Streptococci (Rap ID STR kit). Lactobacillus acidophilus DSM 20079 was cultured in MRS broth or Agar.
Biofilm production assay by microtiter plate test
To assess the biofilm formation potential of the isolates, an overnight culture of each was grown in tryptic soy broth supplemented with 1% sucrose (TSB, Merck, Germany) for 18 - 20 h at 37 °C. The suspensions were adjusted with TSB to 0.5 on the McFarland turbidity standard as measured by absorbance (0.08 - 0.1 at 625 nm) in a spectrophotometer, corresponding to approximately 108 cfu/ml. Then, from each culture, 250 micro liter (μl) volumes were transferred into eight wells of a microtiter plate (25×106 cfu/well). Blank wells contained broth, only. Plates were made in duplicate, covered, and incubated for 24 h at 37°C. At 24 h, the planktonic suspension and nutrient solutions were aspirated and each well was washed three times with 300 μl of sterile physiological saline. The plates were vigorously shaken in order to remove all non adherent bacteria. The remaining attached bacteria were fixed with 250 μl of 96% ethanol per well and, after 15 min, plates were emptied and left to dry. Each well was then stained for 5 min with 0.2 ml of 2% crystal violet. Excess stain was rinsed off by placing the plates under running tap water. Crystal violet (CV) stain is suitable for determining the amount of biofilm. After drying the stained plates, biofilms were visible as purple rings formed on the sides of each well. The quantitative analysis of biofilm production was performed by adding 200 μl of 33% (v/v) glacial acetic acid (Merck, Germany) per well. Then the optical density (OD) of the stain was measured at 492 nm by an ELISA reader (STAT-FAX 2100) as described previously [9]. Based on the OD of the bacterial films, strains were classified into the following categories, as previously described by Stepanovic et al. [9]: OD≤ODc: not a biofilm producer (Non adherent); ODc<OD≤ 2ODc: a weak biofilm producer (Weakly adherent); 2ODc < OD≤ 4ODc: a moderate biofilm producer (Moderately adherent); 4ODc < OD: a strong biofilm producer (Strongly adherent). ODc and OD were defined as the mean OD of the blank wells and wells with biofilm, respectively.
The effect of Lactobacillus acidophilus on biofilm formation
To assess the effect of Lactobacillus acidophilus on biofilm formation, an overnight culture of each isolate was grown in tryptic soy broth supplemented with 1% sucrose. An overnight culture of the probiotic strain in MRS broth was also prepared. The suspensions were adjusted with the same broth to 0.5 on the McFarland turbidity standard as measured by absorbance (0.08 – 0.1 at 625 nm) in a spectrophotometer, corresponding to approximately 108 cfu/ml. Biofilm formation was done according to description above with and without probiotic strain. In brief, adding mixed suspension of probiotic and streptococci (1:1) simultaneously and alternatively a suspenssion of probiotic strain was inoculated into the wells 30 min before the streptococcal suspension. Blank wells contained PBS instead of probiotic strains. After 24 hours incubation and washing 3 times with PBS, staining with CV was done. The Optical density was read with ELISA reader [9].
Statistical analysis
The data were analyzed using Duncan’s test of SAS software (Version 9.1).
RESULTS
Isolated strains were tested using the PVC microtiter biofilm assay for biofilm production. Biofilm formation at 24 h incubation varied greatly. According to the OD values, 42%, 37% and 21% of Mutans streptococci were Strongly Adherent (SA), Modereately Adherent (MA) and Weakly Adherent (WA), respectively. In non mutans streptococci, only 23.5% of isolates were found strongly adherent and 35.3% of isolates were weakly adherent; these data showed significant differences (p<0.05) with the same Mutans streptococci group (Figure 1).
FIGURE 1.
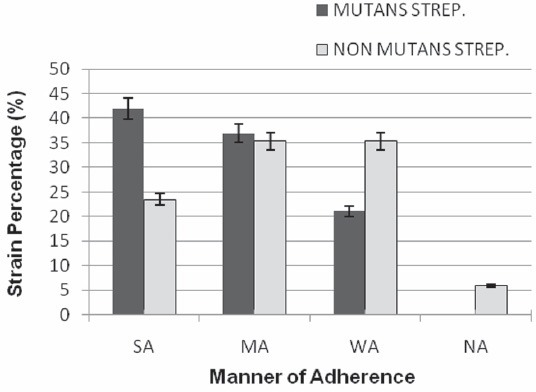
The ability of biofilm formation among Mutans and Non Mutans streptococcal isolates. (The percentages of SA and WA isolates in two groups (mutans and non mutans streptococci) have significant differences (p < 0.05 ). SA: Strongly Adherent, MA: Moderatly Adherent, WA: Weakly Adherent, NA: Non Adherent
According to the OD values again, Streptococcus mutans 22 with highest OD were scored as highly strong adherent (strong biofilm forming bacterium) and Streptococcus mutans ATCC 35668 (no 24) was also strongly adherent. Based on the microtiter plate test results, the streptococcal isolates with the strong ability of biofilm formation were selected. Then the effect of Lactobacillus acidophilus on the adherence of streptococci to microtiter plate was assessed by 2 methods (1: Mixed suspension of streptococci and probiotic, 2: Adding streptococci isolates 30 min after probiotic strain). The results showed the adherence reduction in the presence of Lactobacillus acidophilus (Figures 2 and 3). The adherence reduction of Streptococcus mutans ATCC 35668 (strain 24) to poly styrene wells in the presence of probiotic by two used methods showed significant differences (33.3% and 43.1% respectively (p<0.05)). This adherence reduction for strong biofilm forming bacterium (Streptococcus mutans 22) was significantly (p<0.05) lower than about standard strain (figure 2 A) and there were not significant differences between 2 used methods in the case of this bacterium. The mean of adherence reduction in 2 methods was 19.4 and 21.5 % respectively. So, there is no significant difference between the 2 methods (Figure 3A) but in the case of each bacterium it could be different. In general, the Lactobacillus acidophilus had more effect on adherence of mutans streptococci than non mutans streptococci with significant difference (p <0.05) (Figure 3B).
FIGURE 2.
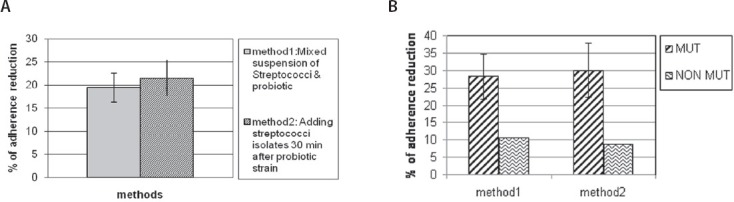
The comparison of adherence reduction in the presence of Lactobacillus acidophilus between A: 2 used methods B: Mutans and non Mutans streptococci in each 2 methods
DISCUSSION
Lactobacillus acidophilus was preferred in this study as a probiotic bacterium because of its known probiotic potential and it’s acid resistance and bile salts tolerance [8]. This bacterium such as other lactic acid bacteria produces various compounds such as organic acids, diacetyl, hydrogen peroxide, and bacteriocins or bactericidal proteins [11]. According to the results, it is cleared that the presence of Lactobacillus acidophilus DSM 20079 can cause reduction in the adherence of Streptococcal strains that it is probably related to interaction between bacteria. This adherence reduction was significantly stronger in the case of mutans streptococci while in the other study we showed that Lactobacillus fermentum reduced the adherence of non mutans streptococci more than mutans streptococci without any significant difference [12]. Inoculation of Lactobacillus acidophilus before Streptoccocal isolates (method 2) had more effect on adherence reduction without any significant difference. But in the case of Lactobacillus fementum adherence reduction was significantly stronger (p<0.05) if the probiotic strain was inoculated to the system before the oral bacteria [12]. It is thought that adhesion reduction is likely due to bacterial interactions and colonization of adhesion sites with probiotic strain before the presence of streptococci. Also, the probiotic strains were able to modify the proportion of the oral species within the biofilm. Comelli et al. [10] reported that inoculation of dairy strains before adding the oral bacteria did not increase their colonization. They also found that dairy strains and particularly Lactobacillus lactis NCC2211 were able to modify the extent of oral species within the biofilm. They also suggest that the reduction of these strains can be explained either by competition for adhesion sites or growth factors. Miller et al. [13] in their study about the effect of microbial interaction on in vitro plaque formation by Streptococcus mutans found that microbial interaction may have the potential to affect the amount and type of plaque formed, depending upon the kinds of organisms involved. They also reported that the addition of the lactobacilli to cultures of Streptococcus sanguis resulted in more inhibition of plaque formation when compared with pure cultures of Streptococcus sanguis. A 34% inhibition of plaque formation was observed when Lactobacillus casei interacted with Streptococcus mutans NCTC 10449. Furthermore Simark-Mattsson et al. [14] have shown the interference capacities of lactobacilli against strains of Streptococcus mutans and Streptococcus sobrinus. Meurman [15] showed the inhibitory activity of Lactobacillus rhamnosus GG against Streptococcus mutans in low pH and it can be useful for preventing the cariogenic effects of oral streptococci. In vivo studies have also confirmed the effects of probiotic bacteria consumption on decreasing the risk of dental caries and mutans streptococcus counts [16-18].
CONCLUSION
Adhesion reduction can be an effective way on decreasing cariogenic potential of oral streptococci and all of the evidence has shown that probiotic bacteria such as Lactobacillus spp. can affect the oral ecology.
DECLARATION OF INTEREST
Th ere is no conflict of interest in this study.
REFERENCES
- [1].Caglar E, Kargul B, Tanbogaet I. Bacteriotherapy and probiotics role on Oral health. Oral Dis. 2005;11:1–7. doi: 10.1111/j.1601-0825.2005.01109.x. [DOI] [PubMed] [Google Scholar]
- [2].Kargul B, Caglar E, Tanbogaet I. History of water fluoridation. J Clin Pediatr Dent. 2003;27:213–217. doi: 10.17796/jcpd.27.3.lu247rq056w47466. [DOI] [PubMed] [Google Scholar]
- [3].Nakas E, Zukanovic A. The prevalence of cariogenic salivary micro organisms in children of various children. Bosnian J Basic Med Sci. 2007;7(2):168–172. doi: 10.17305/bjbms.2007.3075. [DOI] [PubMed] [Google Scholar]
- [4].Cudic M. Development of novel antibacterial peptide that kill resistant isolates. Peptides. 2002;23:2071–2083. doi: 10.1016/s0196-9781(02)00244-9. [DOI] [PubMed] [Google Scholar]
- [5].Nicolas G, Auger I, Beaudoin M, Halle F. Improved methods for mutacin detection and production. J Microbiol Meth. 2004;59:351–61. doi: 10.1016/j.mimet.2004.07.013. [DOI] [PubMed] [Google Scholar]
- [6].Meurman J.H. Probiotics: do they have a role in oral medicine and dentistry. Eur J Oral Sci. 2005;113:188–196. doi: 10.1111/j.1600-0722.2005.00191.x. [DOI] [PubMed] [Google Scholar]
- [7].Teughels W, Essche M, Sliepen I. Probiotics and oral health care. Periodontol 2000. 2008;48:111–147. doi: 10.1111/j.1600-0757.2008.00254.x. [DOI] [PubMed] [Google Scholar]
- [8].Millette M, Luquet F. M, Ruiz M. T, Lacroix M. Characterization of probiotic properties of Lactobacillus strains. Dairy Sci Technol. 2008;88(6):695–705. [Google Scholar]
- [9].Stepanovic S, Vukovic D, Dakic I, Savic B. A modified microtiter plate test for quantification of staphylococcal biofilm formation. J Microbiol Meth. 2000;40(2):175–179. doi: 10.1016/s0167-7012(00)00122-6. [DOI] [PubMed] [Google Scholar]
- [10].Comelli E.M, Guggenheim B, Stingele F, Nesser J. selection of dairy bacterial strains as probiotics for oral health. Eur J Oral Sci. 2002;110:218–224. doi: 10.1034/j.1600-0447.2002.21216.x. [DOI] [PubMed] [Google Scholar]
- [11].Lengkey HAW, Adriani L. Effects of milk fermented with Lactobacillus acidophilus and Bifidobacterium spp. on lactic acid and acetic acid content and on Staphylococcus aureus and Pseudomonas aeruginosa. Biotechnology in Animal Husbandry. 2009;25(5-6):719–724. [Google Scholar]
- [12].Tahmourespour A, Kasra Kermanshahi R, Salehi R, Nabi nejad A. The effect of L. fermentum ATCC 9338 as a probiotic on the adhesion of oral streptococci in vitro. Iran J Med Microbiol. 2008;2(1):45–51. [Google Scholar]
- [13].Miller CH, Kleinman JL. Effect of microbial interactions on in vitro plaque formation by S. mutans. J Den Res. 1974;53(2):427–434. doi: 10.1177/00220345740530024201. [DOI] [PubMed] [Google Scholar]
- [14].Simark-Mattsson C, Emilson C.G, Hakansson E.G, Jacobsson C, Roos K, Holm S. Lactobacillus-mediated interference of mutans streptococci in caries-free vs. caries-active subjects. Eur J Oral Sci. 2007;115(4):308–314. doi: 10.1111/j.1600-0722.2007.00458.x. [DOI] [PubMed] [Google Scholar]
- [15].Meurman J, Antila H, Korhonen A, Saliminen S. Effect of Lactobacillus - rhamnosus strain GG (ATCC-53103) on the growth of Streptococcus sobrinus in-vitro. Eur J Oral Sci. 1995;103(4):253–258. doi: 10.1111/j.1600-0722.1995.tb00169.x. [DOI] [PubMed] [Google Scholar]
- [16].Näse L, Hatakka K, Savilahti E, Saxelin M, Pönkä A, Poussa T, Korpela R, Meurman J. Effect of long-term consumption of a probiotic bacterium, Lactobacillus rhamnosus GG in milk on dental caries and caries risk in children. Caries Res. 2001;35:412–420. doi: 10.1159/000047484. [DOI] [PubMed] [Google Scholar]
- [17].Ahola A, Yli-Knuuttila H, Suomalainen T, Poussa T, Ahlstrom A, Meurman JH, Korpelaet R. Short term consumption of probiotic-containing cheese and effect on dental caries risk factors. Arch Oral Biol. 2002;47:799–804. doi: 10.1016/s0003-9969(02)00112-7. [DOI] [PubMed] [Google Scholar]
- [18].Nikawa H, Makihira S, Fukushima H, Nishimura H. Lactobacillus reuteri in bovine milk fermented decreases the oral carriage of mutans streptococci. Int J Food Microbiol. 2004;95(2):219–223. doi: 10.1016/j.ijfoodmicro.2004.03.006. [DOI] [PubMed] [Google Scholar]


