Abstract
Antiresorptive agents are widely used to treat osteoporosis. Both reduction in bone turnover and increase in BMD may be necessary to decrease the fracture risk. The aim of the study was to evaluate the effects of aledronate on bone turnover markers and bone mineral density in postmenopausal women with osteoporosis. The study involved a group of 56 postmenopausal women with osteoporosis treated with alendronate (70 mg) weekly at the Institute of Nuclear Medicine Clinical Center University of Sarajevo during a 12-months period. Bone mineral density (BMD) at lumbar spine and proximal femur and bone turnover markers (serum β-CrossLaps, urinary N-telopeptides of type I collagen (NTx), total serum alkaline phosphatase (AP) and serum osteocalcin) were measured at baseline and after 12 months of the treatment with aledronate. BMD values significantly increased both at lumbar spine by 13.46% and proximal femur by 21.96% during the study period (-3.12±0.24 vs. -2.7±0.19 and -2.55±0.2 vs. -1.99±0.19 respectively; p<0.001). Bone turnover markers significantly decreased during the study period; C-terminal telopeptides of type I collagen fragment (β-CrossLaps) 49.0% (0.51±0.05 vs. 0.26±0.028 ng/mL), NTX 33.4% (48.3±4.9 vs. 32.15±3.25 nMBCE/mM Cr), AP 24.3% (81.1±5.2 to 61.43±5.2 IU/L) and serum osteocalcin by 29.7% (34.3±2.65 to 24.1±1.36 ng/mL)(p<0.001). Alendronate treatment increased BMD and reduced the level of bone turnover markers. Therefore, the treatment with aledronate during 12 months period can be recommended in postmenopausal women with osteoporosis.
KEY WORDS: postmenopausal osteoporosis, bone turnover markers, bone mineral density, alendronate
INTRODUCTION
Osteoporosis is defined as a skeletal disorder characterized by compromised bone strength, predisposing a person to an increased risk of fracture [1]. Properties related to bone strength include rate of bone turnover, BMD, geometry, microarchitecture, and mean degree of mineralization [2]. During the postmenopausal osteoporosis, bone resorption exceeds formation, resulting in decreased bone mass and deterioration of the microarchitecture, with consequent decreased bone strength and increased susceptibility to fracture [1]. In particular, hip fractures are an important cause of mortality and morbidity among postmenopausal women. The proximal femur of the hip is primarily composed of cortical bone. In cortical bone, high bone turnover, as observed after the menopause, is associated with decreased BMD, increased cortical porosity due to increased bone remodelling in the Haversian canal, decreased cortical thickness due to increased endocortical bone remodelling, and decreased mean degree of mineralization [3, 4]. Treatment with potent anti-resorptive drugs is a strategy for preventing hip fractures in postmenopausal women. Several therapeutic agents are currently available to treat or prevent postmenopausal osteoporosis [5, 6]. Among commercially available drugs, however, only alendronate, risedronate, intravenous zoledronate, strontium ranelate, and hormone replacement therapy (HRT) have shown anti-fracture efficacy against hip fractures in post-menopausal women [7]. Alendronate and risedronate, which are the second- and third-generation bisphosphonates, respectively, have been used as first-line drugs in the treatment of osteoporosis for many years. For example, Black et al. [8] have found that three years of treatment with alendronate increases lumbar spine bone mineral density (BMD) by about 6% and reduces vertebral fracture rate by about 50% [8]. Interestingly, alendronate treatment results in a 32% greater BMD increment in the lumbar spine than the hip, but reduces fracture risk at both sites by roughly the same degree [8]. This suggests that factors other than BMD play an important role in dictating the effects of bisphosphonates on preventing bone fractures. These factors may include the site specific biomechanical milieu, the propensity to fall or alterations in the bone tissue matrix properties. It has also been proposed that the pretreatment rate of bone turnover contributes to the ability of bone to resist fracture [9, 10]. Clinical studies have found that after adjusting for BMD, the biochemical markers of bone turnover can still predict fracture risk [9, 10]. Because elevated bone turnover markers and low bone mineral density (BMD) are independent predictors of hip fracture risk, and the risk is multiplied when both are present, bone turnover and BMD are important factors in decreasing the risk of hip fractures [11]. Both reduction in bone turnover and increase in hip BMD may be necessary to decrease the fracture risk of the hip, which is primarily composed of cortical bone and may require greater proportionate changes than trabecular bone [12, 13]. In fact, drugs with a smaller effect on bone turnover reduce the risk of only vertebral fractures, whereas those with a greater effect reduce the risk of both vertebral and nonvertebral fractures including hip fractures [14]. The aim of the study was to evaluate the effects of aledronate treatment during 12-months period on bone turnover including bone formation and bone resorption markers and bone mineral density in postmenopausal women with osteoporosis.
MATERIALS AND METHODS
Patients
The study involved a group of 56 postmenopausal women (mean age was 59.5±5.2 years, mean menopause duration 12.0±4.6 years) diagnosed with osteoporosis and treated at the Institute of Nuclear Medicine Clinical Centre University of Sarajevo. Osteoporosis was diagnosed based on the bone mineral density (BMD) T-score values ≤ -2.5 at the lumbar spine and/or proximal femur measured with dual-energy x-ray absorptiometry (DEXA). None of the subjects suffered from any metabolic bone diseases, had a history of hormone (estrogen) replacement therapy, or had ever taken medication known to affect bone metabolism prior to the present study. Informed consent was obtained from each participant prior to participation in the study. Pre-existing vertebral fractures were documented in 5.8% of patients. All of patients included in the study received alendronate 70 mg weekly (Fosamax T, Merck) and were instructed to take the medication consistent with the instructions in the product insert. They received also 600 mg of calcium citrate daily and 400 IU of Vitamin D.
Measurement of Bone turnover markers
Serum C-terminal telopeptides of type I collagen fragment (β-CrossLaps) and urinary N-telopeptide of type I collagen (NTx) as markers of bone resorption and osteocalcin and serum alkaline phosphate (ALP), as a markers of bone formation, were measured in patients at baseline and at 12 months. Blood and urine specimens were collected on the same day and stored frozen (-20°C) until measurement. Serum osteocalcin and β-CrossLaps levels were measured using electrochemiluminescent immunoassay (ECLIA) on Rosche Elecsys system at Institute of Nuclear medicine. Referral values using this method for osteocalcin are 13-48 ng/mL and for β-Crosslaps <1.008 ng/mL. Urinary NTx level was determined by chemiluminescent method on Vitros EciQ Immunidiagnostic system (Orthro-Clinical Diagnostic) at the Institute for Clinical chemistry and biochemisty at Clinical Center University of Sarajevo. Urinary sample was collected in an early morning, second voided urine sample, stored at -20°C for later analysis. Referral values for urinary NTx using this method are 26-124 nMBCE/mM Cr. Serum calcium and AP were measured using standard methods.
Measurement of BMD
BMD was measured at the time of study entry (baseline) and after 12 months. BMD of the lumbar spine (L1–L4) in the posteroanterior projection and femoral neck (left side), expressed in g/cm2 and converted to T-score values, was measured with dual-energy x-ray absorptiometry (DEXA) on QDR 4000; Hologic system. All DEXA measurements were done at the Institute of Radiology at Clinical Centre University of Sarajevo.
Statistical analysis
All values were expressed as mean±SEM. The paired t test was used to compare differences in bone turnover markers (ALP, osteocalcin, β-Crosslaps and NTx) and BMD between baseline and 12 months of treatment. P value less than 0.05 was considered statistically significant. All statistical analysis were performed with the SPSS 16 software (version 16.0, SPSS Inc, Chicago, Illinois, USA).
RESULTS
Table 1 shows longitudinal changes in serum calcium and bone turnover markers levels. A significant reduction in bone resorption markers during the study period was observed. Serum β-CrossLaps decreased by 49.0% (0.5±10.05 vs. 0.26±0.028 ng/mL; p<0.001) and urinary NTX level by 33.4% after the 12 months of treatment (48.3±4.9 vs. 32.15±3.25 nMBCE/mM Cr; p=0.004). Also, significant reduction by 24.3% in serum alkaline phosphatase was observed which decreased from baseline values, 81.1±5.21 IU/L, to 61.43±5.24 IU/L at the end of the study period (p<0.001). Serum osteocalcin level also significantly decreased from 34.3±2.65 to 24.1±1.36 ng/mL (p=0.001), while there were no changes in serum calcium level during the 12-months period (Table 1, Figure 1).
TABLE 1.
Bone remodeling markers at baseline and after 12 months of treatment with aledronate.
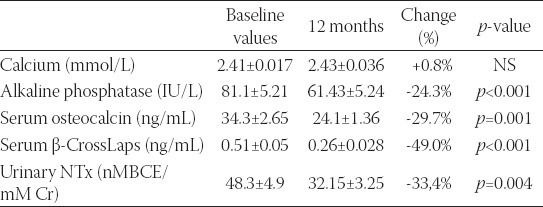
FIGURE 1.
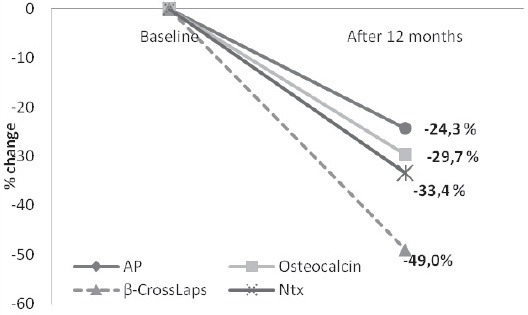
Percent changes of bone remodelling markers during 12 months treatment with aledronate.
BMD T-score values significantly increased both at lumbar spine and proximal femur by 13.46% and 21.96%, respectively (Figure 2) during the 12-months treatment period. T-score values at lumbar spine significantly increased from -3.12±0.24 to -2.7±0.19 and at proximal femur from -2.55±0.2 to -1.99±0.19 (p<0.001) (Table 2).
FIGURE 2.
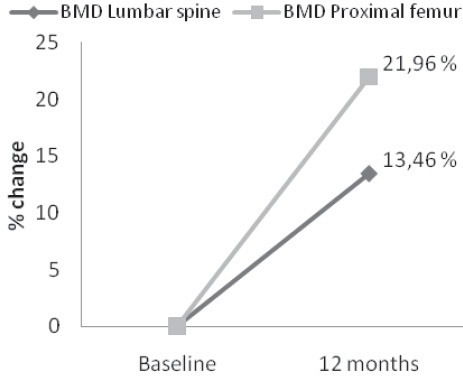
Percent changes in bone mineral density (BMD) at lumbar spine and proximal femur after one-year treatment with aledronate.
TABLE 2.
Bone mineral density at baseline and after 12 months of treatment with aledronate.

DISCUSSION
The results of our study show that the treatment of post-menopausal osteoporosis with aledronate during the 12 months period is effective in increasing the BMD both at lumbar spine and proximal femur. In our study, BMD increased at lumbar spine by 13.46% and proximal femur by 21.96%. Our results are in concordance with the results from other studies which have observed increases in bone mineral density by 13.7 percent at the lumbar spine, 10.3 percent at the trochanter, 5.4 percent at the femoral neck and 6.7 percent at the total proximal femur as compared with base-line values [14, 15]. Meta-analysis conducted by Papapoulos et al. [16] demonstrated the efficacy of alendronate against hip fractures in postmenopausal women with osteoporosis. The rates of hip fractures were 29 per 10,000 person-years at risk (PYR) in the alendronate group and 62 PYR in the control group. The overall risk reduction rate was 55%. Reductions in risk of hip fractures with alendronate treatment were consistent across all studies. The long-term (10-year) safety of alendronate has been confirmed, and the efficacy of alendronate against hip fractures reductions in bone turnover remain stable throughout 10 years of alendronate treatment and are associated with continued gains in lumbar spine and hip BMD. Moreover, vertebral and nonvertebral fracture safety data suggest no loss of benefit [15, 17]. Riggs et al. [18] who demonstrated that suppression of bone turnover and an increase in vertebral BMD contribute equally to the propensity of bone to resist a vertebral fracture. Riggs et al. [18] proposed that the efficacy of the suppression of bone turnover rate in reducing the incidence of bone fractures could be explained by considering the pre-treatment turnover rate. The high turnover rate in the vertebra may exacerbate the effects of bone loss by accelerating the loss of trabecular connectivity and thickness [19, 20, 21]. Normalization of the bone turnover rate in the vertebra, therefore, reduces the effects of high turnover rate on the microarchitectural deterioration and improve bone’s biomechanical properties even more than expected based on BMD alone [20, 21, 22]. Furthermore, aledronate treatment during 12 months in our study was effective in reducing the level of bone turnover markers. Serum β-CrossLaps decreased by 49.0% and urinary NTX level by 33.4%. Our results are in line with the results from other studies [15, 16, 17]. Moreover, treatment with alendronate will suppress but will not abolish bone turnover. Bone turnover will be reduced to a non-zero limit and it will reach this limit regardless of the pre-treatment remodeling rate or the treatment dose. In agreement with these observations, clinical trials have demonstrated that in postmenopausal women treated up to 10 years with 5 mg or 10 mg of alendronate, bone turnover reaches a non-zero steady state after about 12 months of treatment [15]. More importantly, both doses suppress bone turnover to a similar limit [15]. Alendronate inhibits osteoclastic bone resorption, by reducing bone turnover, increasing hip BMD, decreasing cortical porosity, and produces more uniform mineralization (ie, increases the mean degree of mineralization of bone (MDMB) in cortical bone, possibly contributing to a reduction in the risk of hip fractures [23, 24]. In alendronate-treated postmenopausal women, the distribution of the degree of mineralization in cortical bone shows a clear shift toward the highest mineralization values and a decrease in the number of bone structure units having low values of mineralization [23]. The MDMB augmentation probably accounts for most of the increase in BMD seen with alendronate. According to the hypothesis proposed by Boivin et al. [23], the reduction in the activation frequency caused by the anti-resorptive effect of alendronate is followed by a prolonged secondary mineralization that increases the percentage of bone structure units having reached a maximum degree of secondary mineralization and, though this mechanism, augmentation of the MDMB. Evidence derived from the literature, stratified and based on strict EBM guidelines, suggests the efficacy of alendronate against hip fractures in postmenopausal women with osteoporosis with an overall risk reduction rate of 55%. According to the analyses of the Fracture Intervention Trial, each 1 standard deviation reduction in a 1-year change in bone-specific-alcalinee phospathase BSAP is associated with 39% fewer hip fractures in alendronate-treated postmenopausal women, and those with at least a 30% reduction in BSAP have a 74% lower risk of hip fractures relative to those with less than 30% [14]. Alendronate is effective in reducing the risk of hip fractures across a spectrum of ages. The mechanism for this anti-fracture efficacy has been clarified; alendronate suppresses bone turnover and subsequently increases hip BMD, decreases cortical porosity, improves parameters of hip structure geometry (cortical thickness, cross-sectional area, section modulus, and buckling ratio), and produces more uniform mineralization (increases the MDMB) in cortical bone. A once-weekly alendronate regimen provides better adherence (compliance and persistence) of the patients to the treatment than the once-daily dosing regimen, leading to greater efficacy against hip fractures. Thus, the efficacy of alendronate against hip fractures has been confirmed in postmenopausal women with osteoporosis, especially with once-weekly dosing regimen [12, 15, 16, 17].
CONCLUSION
Alendronate treatment during 12 months period, administered once-weekly is effective in increasing BMD both at lumbar spine and femoral neck and reducing bone turnover. Therefore, the treatment with aledronate can be recommended in postmenopausal women with osteoporosis which decreases the effects of high turnover rate on the microarchitectural deterioration and improves bone’s biomechanical properties.
REFERENCES
- [1].NIH Consensus Development Panel on Osteoporosis Prevention, Diagnosis, and Therapy. Osteoporosis prevention, diagnosis, and therapy. JAMA. 2001;285(6):785–795. doi: 10.1001/jama.285.6.785. [DOI] [PubMed] [Google Scholar]
- [2].Epstein S. The roles of bone mineral density, bone turnover, and other properties in reducing fracture risk during antiresorptive therapy. Mayo Clin Proc. 2005;80(3):379–388. doi: 10.4065/80.3.379. [DOI] [PubMed] [Google Scholar]
- [3].Bousson V, Bergot C, Meunier A, Barbot F, Parlier-Cuau C, Laval-Jeantet AM, et al. CT of the middiaphyseal femur: cortical bone mineral density and relation to porosity. Radiology. 2000;217(1):179–187. doi: 10.1148/radiology.217.1.r00se11179. [DOI] [PubMed] [Google Scholar]
- [4].Szulc P, Seeman E, Duboeuf F, Sornay-Rendu E, Delmas PD. Bone fragility: failure of periosteal apposition to compensate for increased endocortical resorption in postmenopausal women. Bone Miner Res. 2006;21(12):1856–1863. doi: 10.1359/jbmr.060904. [DOI] [PubMed] [Google Scholar]
- [5].Iwamoto J, Takeda T, Sato Y. Efficacy and safety of alendronate and risedronate for postmenopausal osteoporosis. Curr Med Res Opin. 2006;22(5):519–928. doi: 10.1185/030079906X100276. [DOI] [PubMed] [Google Scholar]
- [6].Iwamoto J, Takeda T, Sato Y. Effects of antifracture drugs in postmenopausal, male and glucocorticoid-induced osteoporosis--use-fulness of alendronate and risedronate. Expert Opin Pharmacother. 2007;8(16):2743–2756. doi: 10.1517/14656566.8.16.2743. [DOI] [PubMed] [Google Scholar]
- [7].Delmas PD. Treatment of postmenopausal osteoporosis. Lancet. 2002;359(9322):2018–2026. doi: 10.1016/S0140-6736(02)08827-X. [DOI] [PubMed] [Google Scholar]
- [8].Black DM, Cummings SR, Karpf DB, Cauley JA, Thompson DE, Nevitt MC, et al. Randomised trial of effect of alendronate on risk of fracture in women with existing vertebral fractures. Fracture Intervention Trial Research Group. Lancet. 1996;348(9041):1535–1541. doi: 10.1016/s0140-6736(96)07088-2. [DOI] [PubMed] [Google Scholar]
- [9].Sarkar S, Reginster JY, Crans GG, Diez-Perez A, Pinette KV, Delmas PD. Relationship between changes in biochemical markers of bone turnover and BMD to predict vertebral fracture risk. J Bone Miner Res. 2004;19:394–401. doi: 10.1359/JBMR.0301243. [DOI] [PubMed] [Google Scholar]
- [10].Bauer DC, Black DM, Garnero P, Hochberg M, Ott S, Orloff J, et al. Change in bone turnover and hip, non-spine, and vertebral fracture in alendronate-treated women: the fracture intervention trial. J Bone Miner Res. 2004;19:1250–1258. doi: 10.1359/JBMR.040512. [DOI] [PubMed] [Google Scholar]
- [11].Kulenovic I, Rasic S, Kulenovic E. Osteoporosis: current trends in diagnosis and management. Bosn J Basic Med Sci. 2006 Feb;6(1):24–8. doi: 10.17305/bjbms.2006.3205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Rizzoli R, Laroche M, Krieg MA, Frieling I, Thomas T, Delmas P, et al. Strontium ranelate and alendronate have differing effects on distal tibia bone microstructure in women with osteoporosis. Rheumatol Int. 2010;30(10):1341–1348. doi: 10.1007/s00296-010-1542-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Epstein S. Is cortical bone hip? What determines cortical bone properties? Bone. 2007;41(1 Suppl 1):S3–S8. doi: 10.1016/j.bone.2007.03.006. [DOI] [PubMed] [Google Scholar]
- [14].Iwamoto J, Sato Y, Takeda T, Matsumoto H. Hip fracture protection by alendronate treatment in postmenopausal women with osteoporosis: a review of the literature. Clin Interv Aging. 2008;3(3):483–489. doi: 10.2147/cia.s3177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Bone HG, Hosking D, Devogelaer JP, Tucci JR, Emkey RD, Tonino RP, et al. Alendronate Phase III Osteoporosis Treatment Study Group. Ten years’ experience with alendronate for osteoporosis in postmenopausal women. N Engl J Med. 2004;350(12):1189–1199. doi: 10.1056/NEJMoa030897. [DOI] [PubMed] [Google Scholar]
- [16].Papapoulos SE, Quandt SA, Liberman UA, Hochberg MC, Thompson DE. Meta-analysis of the efficacy of alendronate for the prevention of hip fractures in postmenopausal women. Osteoporos Int. 2005;16(5):468–474. doi: 10.1007/s00198-004-1725-z. [DOI] [PubMed] [Google Scholar]
- [17].Black DM, Schwartz AV, Ensrud KE, Cauley JA, Levis S, Quandt SA, et al. FLEX Research Group. Effects of continuing or stopping alendronate after 5 years of treatment: the Fracture Intervention Trial Long-term Extension (FLEX): a randomized trial. JAMA. 2006;296(24):2927–2938. doi: 10.1001/jama.296.24.2927. [DOI] [PubMed] [Google Scholar]
- [18].Riggs BL, Melton LJ, 3rd, O’Fallon WM. Drug therapy for vertebral fractures in osteoporosis: evidence that decreases in bone turnover and increases in bone mass both determine antifracture efficacy. Bone. 1996;18(3 Suppl):197S–201S. doi: 10.1016/8756-3282(95)00502-1. [DOI] [PubMed] [Google Scholar]
- [19].Diab T, Allen MR, Burr DB. Alendronate treatment results in similar levels of trabecular bone remodeling in the femoral neck and vertebra. Osteoporos Int. 2009;20(4):647–652. doi: 10.1007/s00198-008-0717-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Akhter MP, Lappe JM, Davies KM, Recker RR. Transmenopausal changes in the trabecular bone structure. Bone. 2007;41:111–116. doi: 10.1016/j.bone.2007.03.019. [DOI] [PubMed] [Google Scholar]
- [21].Chavassieux P, Seeman E, Delmas PD. Insights into material and structural basis of bone fragility from diseases associated with fractures: how determinants of the biomechanical properties of bone are compromised by disease. Endocr Rev. 2007;28:151–164. doi: 10.1210/er.2006-0029. [DOI] [PubMed] [Google Scholar]
- [22].Borah B, Dufresne TE, Ritman EL, Jorgensen SM, Liu S, Chmielewski PA, et al. Long-term risedronate treatment normalizes mineralization and continues to preserve trabecular architecture: sequential triple biopsy studies with micro-computed tomography. Bone. 2006;39:345–352. doi: 10.1016/j.bone.2006.01.161. [DOI] [PubMed] [Google Scholar]
- [23].Boivin GY, Chavassieux PM, Santora AC, Yates J, Meunier PJ. Alendronate increases bone strength by increasing the mean degree of mineralization of bone tissue in osteoporotic women. Bone. 2000;27(5):687–94. doi: 10.1016/s8756-3282(00)00376-8. [DOI] [PubMed] [Google Scholar]
- [24].Roschger P, Rinnerthaler S, Yates J, Rodan GA, Fratzl P, Klaushofer K. Alendronate increases degree and uniformity of mineralization in cancellous bone and decreases the porosity in cortical bone of osteoporotic women. Bone. 2001;29(2):185–191. doi: 10.1016/s8756-3282(01)00485-9. [DOI] [PubMed] [Google Scholar]


