Abstract
The aims of this study are to determine anticardiolipin antibodies in patients with Sy Behcet and to determine correlation between the levels of anticardiolipin antibodies in serum in patients with clinic systemic and ocular manifestations. The study was conducted on 11 patients with Behcet disease (group I), and on 11 healthy subjects (group II). Anticardiolipin antibodies –aCL were determined by the standard ELISA method, where 1GPL= 1 microgram/ml IgG aCL and 1 MPL= 1 microgram/ml IgM, and were considered negative < 10 GPL or MPL, low positive (10-40 GPL and MPL), or high positive (>40 GPL and MPL). In the group of 11 patients with the diagnosis Sy Behcet, 6 of them were (54.5%) with values of anticardiolipin antibodies over 10 positive. In the control group of the healthy examinees aCl were positive in 2 cases (18.2%). There are no statistically significant differences in the presence of systemic clinic characteristics between aCl positive and negative patients. All the patients with SY Behcet in whom anticardiolipn antibodies were found have extremely severe visual damage which is not present in the group of those patients where the values of aCl were low. The difference is statistically significant. The level of anticardiolipin antibodies is increased in the patients with Behcet. There are no statistically significant differences in the presence of systemic clinical characteristics between aCL positive and negative patients. Visual acuity in patients with SY Behcet is statistically significantly much lower in patients who had increased values of aCL.
KEY WORDS: anticardiolipin antibodies, Behcet’s disease, vasculitis retinae
INTRODUCTION
Behcet’s disease is a multi - system disease named after the Turkish dermatologist Dr. Hulusi Behcet (1889-1948), who described the triad of symptoms in 1937, such as eye inflammation, oral and genital ulcers [1, 2]. The diagnosis of this disease is mostly based on clinical findings. The criteria for diagnosis were established by The Behcet’s Disease Research Committee of the Ministry of Health and Welfare of Japan. According to this classification there are major and minor criteria. Major criteria are: eye inflammation, recurrent oral aphthous mucosa, genital ulcerations and skin lesions: erythema nodosum, thrombophlebitis and cutaneous hypersensitivity. Minor criteria are: arthritis, digestive duct ulceration, epididymitis, and vascular diseases: obliteration, vessel occlusion, aneurisma and neuropsychiatric symptoms. Depending on the criteria present, there are complete, incomplete, suspect and possible types. The complete type has four major features occurring either simultaneously or at different times. The incomplete type involves three major features, or typical ocular disease with one major criterion. The suspect type is diagnosed when two major features without ocular manifestation are present. The possible type involves one major feature and possibility to develop the other ones [1, 3]. Ocular manifestations – eye involvement in 70 – 85% of these patients is the most dreaded complication [2, 3]. The disease can affect both posterior and anterior eye pole, with certain characteristic signs. A very common finding is anterior uveitis or iridocyclitis, with hypopyon and can be seen in 1/3 of these patients. Damage of the retina is the most difficult aspect of the disease and, as a consequence, can lead to severe visual damage. Basically, recurrent occlusions of retinal vessels lead to irreversible changes. Other ocular manifestations include episcleritis, filamentous keratitis, conjunctivitis and subconjunctival haemorrhage. Paralysis of extraocular muscles can be the result of neuro-Behcet’s [4,5]. The basis of histopathological lesions in Behcet’s disease is perivasculitis with tissue destruction [6]. It was thought that the basic mechanism leading to histological changes was immune complex deposition, because deposition of complement components was proved in some lesions [7,8]. Also, the role of T cells, as a basic mechanism of cell damage in Sy Behcet, has been considered [7]. In recent years many authors’ attention has been drawn to the presence of anticardiolipin antibodies in patients with Behcet’s disease. Since recurrent thrombosis, thrombocytopenia and frequent vasculitis are common manifestations of Behcet’s disease, there is a need to search for anticardiolipin antibodies in serum in these patients [9, 10]. They paid special attention to the connection between clinical manifestations and titra anticardiolipin antibodies in serum [6, 9, 11]. Anticardiolipin antibodies belong to a subgroup of antiphospholipid antibodies [5, 12, 13]. The occurrence of these antibodies has been associated with arterial and venous thrombosis, fetal loss, thrombocytopenia, a number of neurological disorders (Guillain-Barre Syndrome, migraine, chorea) and several systemic diseases (SLE, Lyme disease, Syphilis) [14, 15]. It seems likely that the major clinical features associated with raised levels of anticardiolipin antibodies are more commonly found when the antibodies are of the IgG isotype. Anticardiolipin antibodies of the IgM type are not very informative, since they were also present in ten percent of the healthy controls [10, 12]. The aims of the study were to determine the presence of anticardiolipin antibodies in patients with Sy Behcet and to determine the correlation between the level of anticardiolipin antibodies in serum of the patients and retinal vascular complications.
MATERIALS AND METHODS
Patients
The study enrolled 11 patients with Sy Behcet-group I. The patients fulfilled International criteria for Behcet’s disease and did not have any other disease at the same time. Group II enrolled 11 healthy patients.
Laboratory analysis
Anticardiolipin antibodies were performed at the Immunology Laboratory of Children’s Internal Clinic. Anticardiolipin antibodies - aCl were determined by the standard ELISA-methods. Levels for aCL-a and aG-PI-a and for IgG and IgM were measured by commercially used enzyme tubes. One GPL is defined as binding activity of 1 microgram/ml IgG aCL-a, and 1MPL is defined as binding activity of 1 microgram/ml IgM. Results of aCL-a are expressed by standard units for IgG (GPL) and for IgM (MPL) and were considered to be negative if < 10 GPL or MPL, low positive (10- 40 GPL and MPL), or high positive (> 40 GPL and MPL). Test results were considered to be positive if there were more than 20 units for IgG -aGPI and 20 standard units for IgM for GPI for IgMGPI.
Statistical analysis
To evaluate the connection between the presence of clinical features (systemic or ocular changes) and the presence of antiocardiolipin antibodies (aCL) in patients with Sy Bechet, we used Fischer’s exact test and Cohen’s kappa (κ) value.
RESULTS
The group I enrolled 11 patients, 8 male and 3 female, average age 34,6 years. Eight patients (72.7%) had changes in both eyes, and in 3 patients (27.3%) there was one side form (a total of 19 eyes). (Table 1).
TABLE 1.
Ocular changes (n=19) in patients with Sy Behcet
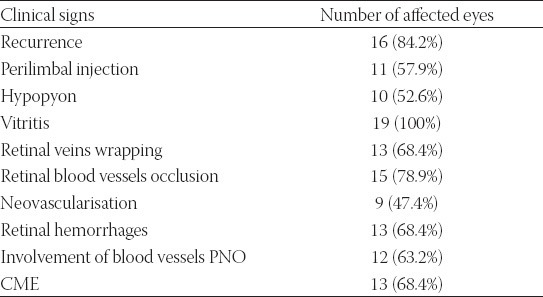
Out of 19 observed eyes, 16 (84.2%) had serious or expressed form of ocular inflammation. As for visual acuity, 7 eyes (36.8%) had acuity less than < 0.1, nine eyes (47.3%) had visual acuity <0.3, two eyes (10.5%) < 0.6 and only one eye (5.2%) had visual acuity 0.7. Hypopyon is recorded in 10 (52.6%) eyes and it is not a constant sign. Its characteristic is fast onset, but it can withdraw in one day. All 19 eyes had opacitates in vitreous part. Wrapping of retinal veins was found in 13 eyes, that is 68.4%. Occlusion of retinal veins was found in 15 (78.9%) eyes. This sign is statistically significantly the most common one in this group. Changes in blood vessels of optic disc were found in 12 (63.2%) eyes, while in two eyes in thermal stadiums there was atrophy of optic disc. Retinal haemorrhages were found in 13 eyes (68.4%), neovascularisation in 9 (47.4%) eyes, while cystoid macular edema was registered in 13 (68.4%) eyes. In the group of 11 patients with the diagnosis Sy Behcet there were 6 of them (54.5%) with anticardiolipin antibodies values over 10 (positive). In the control group, 2 out of 11 healthy subjects were aCL positive (18.2%). This difference in presentation of positive findings of aCL is not statistically significant (Fisher exact test: p=0.103; p>0.05)(Table 2).
TABLE 2.
Frequency of anticardiolipin antibodies in examined groups
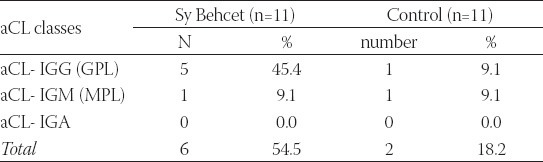
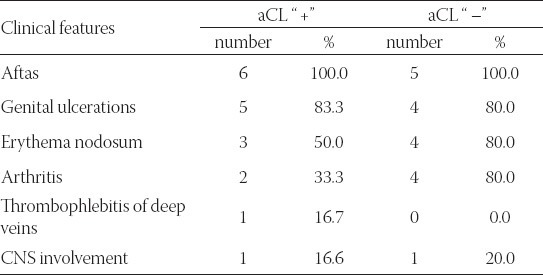
There are no statistically significant differences in the presence of systemic clinical characteristics between aCL positive and negative patients (Fisher exact test: p>0.05), that is, clinical and immunological findings do not match, or they only slightly match. All the patients in our group had aphthae, 9 of them (81.1%) had changes on the genitals. Seven patients had erythema nodosum. One patient had thrombosis of deep veins in lower extremities and high values of anticardiolipin antibodies, and at the same time he had central retinal vein occlusion, of nasal lower branch (Table 3).
TABLE 3.
Correlation of systemic clinical features in patients with Sy Behcet and the presence of anticardiolipin antibodies (aCL)
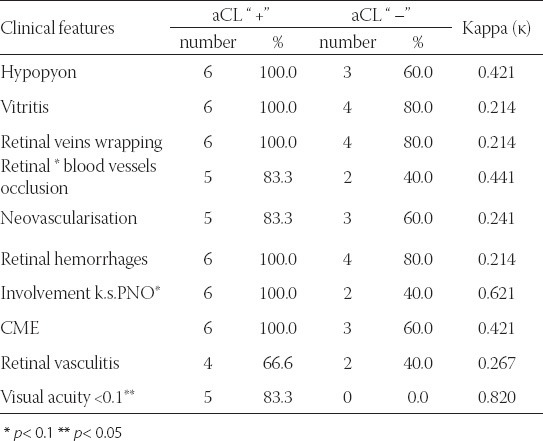
Involvement of blood vessels PNO is present as a clinical characteristic in all patients who were aCL “ +” (6 or 100%), while in the group aCL “ –” it is much less present (2 or 40%). This difference is not statistically significant for the level p< 0.05 (Fisher exact test: p=0.061 but p< 0.1 - this can be explained by small pattern). Matching of the findings (clinical and immunological) measured by Kappa values is good or very good (κ=0.621) (Table 4). Visual acuity <0.1 is present in 5 or 83.3% patients with aCL values over 10, and all aCL “–” had visual acuity over 0.1. All the patients with Sy Bechet and with anticardiolipin antibodies had severe visual damage, that was not present in the group of patients where aCL values were less than 10. This difference is statistically significant (Fisher exact test: p=0.015; p<0.05). Matching of the findings (clinical and immunological) measured by Kappa values is almost perfect (κ=0.820), (Table 4).
TABLE 4.
Correlation of clinical features in the eyes of the patients with Sy Behcet and the presence of anticardiolipin antibodies (aCL).
DISCUSSION
In the literature available there are not many data about the values of anticardiolipin antibodies in patients with Sy Behcet. In our study, in the group of 11 patients with diagnosed Sy Behcet, there were 6 patients and in the control group, there were 2 patients with positive anticardiolipin antibodies. This difference in presentation of positive findings aCL is not statistically significant. Also there are no statistically significant differences in the presence of systemic clinical characteristics between aCL positive and negative patients, that is, clinical and immunological findings do not match, or they slightly match. Retinal blood vessels occlusion is present as a clinical feature in great number of patients who are aCL “ +”, while in group aCL “–” it is much less present. But this difference is not statistically significant. Our results are mostly the same as the results of the others ophthalmologist, and most of them also couldn’t find statistical significance in their investigations. We only found the statistical significance in correlation of visual acuity and presence of anticardiolipin antibodies. Takay et al. [14] in their study of 128 patients with Behcet’s disease used standard ELISA method for aCL. IgG was found in 7%, IgM in 1.6% of the patients, IgA in 4.6%. They did not find statistical significance compared to the control group. Also, there were no correlations with any isotopes or any clinical manifestations. It is interesting that the same authors found great statistical significance between titra IgA aCL-a in patients with HLA-B5 negative patients and control group, while this was not found in patients who were HLA-B5 positive. Kang et al. [15] showed in his study that anticardiolipin antibodies were found in 25,5% patients with BD, 41,7% had uveitis, 25,0% vitritis, and retinal vasculitis was found in 8,3% patients. Priori et al. [16] found Ig-aCL in 30% patients with Behcet’s disease who had ocular disorders as well, while patients treated with corticosteroids had low titre anticardiolipin antibodies. Ang et al. [17] found in their study IgG-aCL in 33% patients with Behcet’s disease with ocular changes, compared to increased IgG-aCL in 30% patients who did not have ocular manifestations. Evereklioglu et al. [18] found in their study of 70 patients with Sy Behcet positive values aCL-a in 13 patients or 19%, 7 patients with IgG, 3 with IgM, and 3 with both IgG and IgM, and they emphasized significantly great relation between the presence of aCl-a and retinal vasculitis. Kim WU et al. [19] found in their study high IgM - aCL titar in patients who predominantly had skin manifestations and erythema nodosum. Apart from this study, there were no correlations with any specific manifestations of Behcet’s disease and titra antibodies, especially thrombosis, more than expected. It is interesting that there is significantly lower average frequency of aCl-9.5% in patients from Turkey, compared to other studies where it is averagely 25.5%. This can be explained by methodological differences such as 2 SD, 3 SD, 4 SD which are accepted above average in normal subjects, who are positive in different serials, what can affect obtained data. Alternatively, regional differences, whether environmental or genetics, can also have an impact on the presence of aCL-a, as they also have an impact on other antibodies [20,21]. Differences in clinical manifestations in patients with Sy Behcet in different countries were also noted. Similarly, positive test HLA B51 was more frequent among the patients from the countries where the disease itself is more frequent (Turkey, Japan), compared to patients from Europe [20]. All in all, the problem with this type of analysis is the fact that the countries the studies come from do not necessarily show the ethnicity of the patients. Different values can be a consequence of more factors: adjusting the time of investigation, therapy management. Difference in findings by different authors is probably the result of different technical approaches for measurement of aCL-a, so there is an urgent need to standardise aCl tests.
CONCLUSION
Level of anticardiolipin antibodies is increased in patients with Sy Behcet. There are no statistically significant differences in the presence of systemic clinical characteristics between aCL positive and negative patients. Visual acuity in patients with SY Behcet is statistically significantly much lower in patients who had increased values of aCL.
DECLARATION OF INTEREST
Authors declare no conflict of interest.
REFERENCES
- [1].Ozkiris A. Possible future therapeutics for Behcet's Syndrom. Expert Opin Ther Patents. 2007;17(1):9–16. [Google Scholar]
- [2].Evereklioglu C. Managing the symptoms of Behcet's disease. Expert Opin Pharmacother. 2004;5(2):317–328. doi: 10.1517/14656566.5.2.317. [DOI] [PubMed] [Google Scholar]
- [3].Fresko I, Yazici H. Treatment strategies for Behcet's disease. Expert Opin Pharmacother. 2008;9(18):3211–3219. doi: 10.1517/14656560802457749. [DOI] [PubMed] [Google Scholar]
- [4].Gedik S, Akova YA, Yilmaz G, Bozbeyoglu S. Indocyanine green and fundus fluorescein angiographic finding in patients with active ocular Behcet's Disease. Ocul Immunol Inflamm. 2005;13:51–58. doi: 10.1080/09273940490518757. [DOI] [PubMed] [Google Scholar]
- [5].Cabrita FVL, Foster CS. Anticardiolipin antibodies and ocular disease. Ocul Immunol Inflamm. 2005;13:265–270. doi: 10.1080/09273940490912434. [DOI] [PubMed] [Google Scholar]
- [6].Miserocchi E, Baltatiz S, Foster CS. Ocular features associated with anticardiolipin antibodies: a descriptive study. Am J Ophthalmol. 2001;131(4):451–456. doi: 10.1016/s0002-9394(00)00884-9. [DOI] [PubMed] [Google Scholar]
- [7].Du L, Kijlstra A, Yang P. Immune response genes in uveitis. Ocul Immunol Inflamm. 2009;17:249–256. doi: 10.1080/09273940902999356. [DOI] [PubMed] [Google Scholar]
- [8].Bardak Y, Aridogan BC. The demonstration of serum interleukin6-8, tumor necrosis factor-alpha, complement and immunoglobulin levels in Behcet's disease with ocular involvement. Ocul Immunol Inflamm. 2004;12:53–58. doi: 10.1076/ocii.12.1.53.28062. [DOI] [PubMed] [Google Scholar]
- [9].Menichelli M, Nicotra GC, Minisola G. Catastrophic antiphospholipid Syndrom in a patient with Behcet's disease. Scand J Rheumatol. 2002;31:100–102. doi: 10.1080/03009740252937630. [DOI] [PubMed] [Google Scholar]
- [10].Klok AM, Geertzen R, Rothova A, Baarsma GS, Kijlstra A. Anticardiolipin antibodies in uveitis. Current Eye Res. 1992;2:209–213. doi: 10.3109/02713689208999535. [DOI] [PubMed] [Google Scholar]
- [11].Read RW, Chong LP, Rao NA. Occlusive retinal vassculitis associated with systemic lupus erythematosus. Arch Ophthalmol. 2000;118(4):588–589. doi: 10.1001/archopht.118.4.588. [DOI] [PubMed] [Google Scholar]
- [12].Dogulu CF, Kansu T, Kadayifcilar S. The role of IgM isotope anticardiolipin antibodiesin occlusive ocular vascular disease: report of two cases with primary antiphospholipid antibody syndrome. Eye. 2000;14:5789–5790. doi: 10.1038/eye.2000.208. [DOI] [PubMed] [Google Scholar]
- [13].Monterhermoso A, Cervera R, Font j, Ramos-Casalas M, Garcia-Carrasco M, Formiga F, Callejas JL, Jorfan M, Grino MC, Ingelmo M. Association of antiphospholipid antibodies with retinal vascular disease in systemic lupus erythematosus. Semin Arthritis Rheum. 1999;28(5):326–332. doi: 10.1016/s0049-0172(99)80017-1. [DOI] [PubMed] [Google Scholar]
- [14].Tokay S, Direskeneli H, Yardakul S, Akoglu T. Anticardiolipin antibodies in Behcet's diseases: a reassessment. Rheumatology. 2001;40(920):192–195. doi: 10.1093/rheumatology/40.2.192. [DOI] [PubMed] [Google Scholar]
- [15].Kang HJ, Lee YW, Han SH, Cho HC, Lee KM. Anticardiolipin and Anti-b2-Glycoprotein in Behcet's Disease. J Corean Med Sci. 1998;13:400–404. doi: 10.3346/jkms.1998.13.4.400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Priori R, Conti F, Pittoni V, Garofalo T, Sorice M, Valesini G. Is there a role for antiphospholipid-binding protein antibodies in the patogenesis of thrombosis in Behcet’ disease? Thromb Haemost. 2000;83(1):173–174. [PubMed] [Google Scholar]
- [17].Ang LP, Yap EY, Fam HB. Billateral choroidal infraction in patients with antiphospholpiid syndrome: a case report. Clin Experiment Ophthalmol. 2000;28(4):326–328. doi: 10.1046/j.1442-9071.2000.00322.x. [DOI] [PubMed] [Google Scholar]
- [18].Everekliogly C. Current concepts in the etiology and treatment of Behcet Disease. Sym Ophthalmol. 2005;50:297–350. doi: 10.1016/j.survophthal.2005.04.009. [DOI] [PubMed] [Google Scholar]
- [19].Kim WU, Chung SM, Han TW, Sah WJ, Kim MH. Elevated soluble Fas in aqueous humor of patients with Behcet's Uveitis: correlation with uveitis severity. Jap J Ophthalmol. 2002;464:18–23. doi: 10.1016/s0021-5155(01)00469-5. [DOI] [PubMed] [Google Scholar]
- [20].Durani K, Papaliodis GN. The genetics of Adamantiades-Behcet's disease. Semin Ophthalmol. 2008;23:73–79. doi: 10.1080/08820530701745264. [DOI] [PubMed] [Google Scholar]
- [21].Bonfioli AA, Orefice F. Behcet's Disease. Semin Ophthalmol. 2005;20:199–206. doi: 10.1080/08820530500231953. [DOI] [PubMed] [Google Scholar]


