Abstract
Oxidative stress due to noise was estimated at cell level using model of growing lymphocytes. Lymphocytes were isolated and cultured using conventional methodology. Cell culture of each group was exposed to sound of frequency 1 KHz during incubation. Three groups were defined on the basis of exposure of sound with specific range of intensity and duration of exposure. Group A and Group B were exposed to sound with intensity 110 dBA for four hours per day and for eight hours per day respectively. Control group was exposed to sound less than 85 dBA. Viable cell count was performed using trypan blue. Catalase activity of each group was estimated using ELISA kit.
Viable cell count of Group A and Group B was almost same but significantly less than that of control group. Catalase activity of lymphocytes in Group B was significantly low as compared to Group A and controls (p=0.003,p< 0.05). There was no significant difference between catalase activity of Group A and control group.
Exposure of sound with frequency 1 KHz and intensity 110 dBA for 4 hours and eight hours per day may induce oxidative stress in growing lymphocytes causing the difference in viable cell count. However the catalase activity depends on duration of exposure. In case of noise exposure of 8 hours per day, it declines significantly as compared to noise exposure of 4 hours per day.
KEY WORDS: Oxidative stress, catalase activity, noise exposure, Oxidative damage in lymphocytes, Noise induced oxidative damage
INTRODUCTION
Environmental noise may be considered as the most pervasive pollutant. Due to industrial growth and progress in transportation system, it is inevitable to avoid it. Its role in changing the normal states of blood pressure, immune functions, hormone levels and respiratory systems has made it debatable during previous decades [1, 2]. Recently Moslehi et al have observed the effects of noise on digestive system of rats [3]. They found that one day to three weeks noise exposure may increase the secretion of gastric acid. However four weeks noise exposure could not raise the secretion of gastric acid that indicates the adaptation of rats according to the environment. Stansfeld et al observed the noise effects on cognitive performance of the children [4]. Effects of noise on auditory system have also been well documented [5]. Several investigations about noise induced hearing loss have led to the conclusion that noise causes excessive production of reactive oxygen species (ROS) [5, 6]. According to Samson et al, noise induced ROS production not only causes the hearing loss but also disturbs the normal physiological functions [7]. Present study aimed to investigate the minimum sound intensity and exposure duration which may induce the oxidative stress in growing lymphocytes through estimation of catalase activity.
MATERIAL AND METHODS
Samples and Procedures
All procedures were in compliance with the declaration of Helsinki, and the study protocol was approved by the ethical committee and Board of Advance studies and Research, University of the Punjab, Lahore. After taking proper consent and satisfaction of ethical criteria, 2 ml heparinated blood of a healthy individual of age 28 years old was used for the study. LSM 1077 lymphocyte separation medium (PAA, Cat# J15-004, Lot # J00409-0346), Dulbecco’s Phosphate-Buffered Saline (DPBS) (HyClone, Cat# SH30028.02, Lot # ATK33267), 2 % Fetal Bovine Serum (FBS) (PAA, Cat # A11-101, Lot # A10108-1650) and Hanks Balanced Salt Solution (HBSS) (HyClone, Cat# SH30588.01, Lot# ATL 33470) were used for the lymphocyte isolation. Viable cells were counted by using trypan blue stain. Cell culture media (10% FBS, 1X PBS, 2 mM L, glutamine, RPMI 1640) was prepared for lymphocyte. 10 ml cell culture media was poured in culture flask. 250 μl of cell suspension with density of 89.33 × 106 cells was inoculated in culture media of each flask. The culture flasks were incubated in incubator (Sanyo, MCO-15 AC) with 5 % CO2 at 37 °C. Sound waves were directly exposed to growing lymphocytes. Acoustic system was developed for the exposure of sound to lymphocytes for a certain period of time. Noise effects were generated by using computer software named as NCH tone generator. This software helped in producing the sound waves of specific frequency. This software was prepared by NCH softwares, USA (http://download.cnet.com/NCH-Tone-Generator/3000-2169_4-10019045.html). Sound with frequency of 1 KHz was generated. Sound intensity was changed by using amplifier. Intensity of 110 dBA was confirmed by digital sound level meter before the start of experiment. For avoiding contamination, speakers were fitted within the lid of culture flasks. Three exposure groups were defined on the basis of exposure of specific range of sound intensity and exposure duration (in case of noise exposure). Group A was exposed to noise for 4 hours per 24 hours and group B was exposed to noise for 8 hours per 24 hours. Control group was not exposed to noise. Noise was exposed to lymphocytes after 16 hours of inoculation of culture media with lymphocytes in group A and group B. This incubation period allowed the lymphocytes to grow. After 72 hours incubation, cells were harvested in 50 ml falcon. Falcon tubes were centrifuged at 1500 rpm for 10 minutes and supernatant was discarded. 10 ml DPBS was added to culture flask and using sterile cell scraper, remaining cells attached to the surface of culture flasks were removed from the flask surface. This DPBS solution containing lymphocytes was also added to 50 ml falcon tube. Falcon tube was centrifuged again to obtain cell pellet. Supernatant was discarded. Cells were used for further analysis. Viable cells were counted by using trypan blue stain and hemocytometer. Oxidative stress was estimated by using ELISA method for Catalase assay (MERCK, catalogue # 219265). Before assay, cell lysis was performed by sonication on ice in 2 ml cold buffer (i.e., 50 mM potassium phosphate, pH 7.0, containing 1 mM EDTA). After centrifugation at 10,000 x g for 15 min at 4°C, supernatant was isolated for assay and was stored on ice. On the same day, ELISA was performed and catalase activity was estimated following the instructions of manufacturers of ELISA kit.
Statistical analysis
Viable cell count and catalase activity of different exposure groups were compared using ANOVA followed by Duncan’s test.
RESULTS
Results of viable cell count are shown in Figure 1 which indicates that the number of lymphocytes in noise exposed groups (A and B groups) was significantly smaller than that of normal sound groups (C). However in group A, viable lymphocyte count was not significantly different from that of group B. Figure 2 indicates the standard curve for the estimation of formaldehyde (micro moles). After estimation of formaldehyde concentration, catalase activity of each sample exposed to different noise groups was estimated. Results obtained for each group were compared for the assessment of noise effects on catalase activity (Figure 3). It was found that catalase activity of the samples exposed to noise for four hours was higher than that of normal sound exposed samples and noise exposed samples for eight hours. However the difference in catalase activity of 4 Hour exposed group and control group was not significant but the difference in catalase activity of 8 Hour exposed group was significantly different from that of 4 Hour exposed group. Catalase activity of 8 Hour exposed group was significantly less than that of control group.
FIGURE 1.
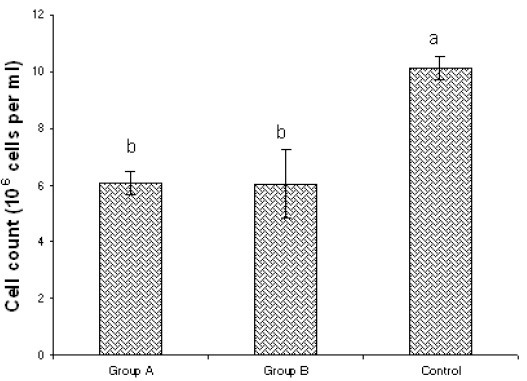
Comparison of viable cell count in different exposure groups. Bars with different letters indicate significant difference among the groups (p< 0.05).
FIGURE 2.
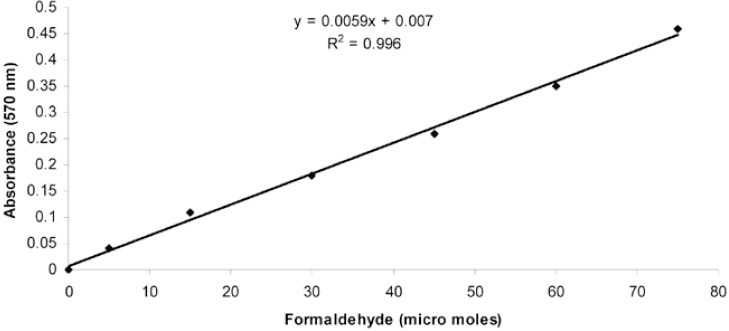
Standard curve for the estimation of formaldehyde (micro moles).
FIGURE 3.
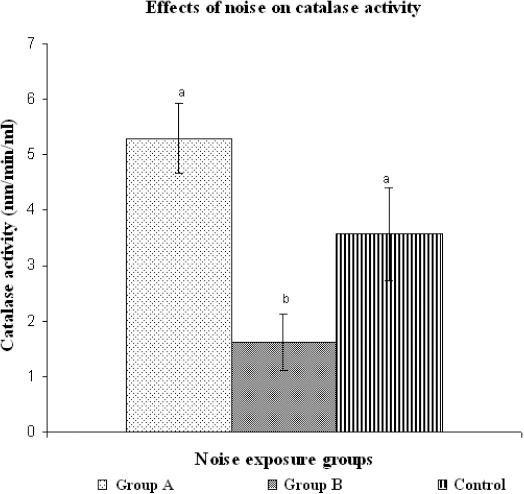
Comparison of catalase activity in different noise groups. Bars with different letters indicate significant difference among the groups (p=0.003, p< 0.05).
DISCUSSION
All cells of the human body have developed mechanisms for neutralization of reactive oxygen species. If cells fail to minimize the oxidative stress, severe cellular damage may happen. This damage may result in the development of several types of diseases like cancer, cataracts, cardiovascular diseases, aging and age related maculapathies. The studies about oxidative stress inducers may help in understanding the etiology of these health problems. Several factors have been found to induce oxidative stress. Noise exposure is one of them. Successful use of antioxidants in the treatment of noise induced hearing loss point out the role of oxidative stress due to noise. Furthermore the harmful effects of noise were reported by Van Campen et al in rats [8]. They observed that noise exposure causes the oxidative DNA damage in cochlea, brain and liver of rats. This finding helped us to build a hypothesis stating that duration of noise exposure is an important factor in increasing the oxidative stress. For confirmation of this hypothesis, lymphocytes of a healthy individual were exposed to sounds with different intensities and catalase activity of different groups was estimated. It was found that noise causes oxidative damage depending on the intensity of exposure. Catalase activity of lymphocytes was affected by the duration of noise exposure. In case of control group and A group (4 hour exposure per day), no significant difference was present between the catalase activity of two groups. However the catalase activity of B group was significantly different from control group and A group. Catalase activity was found to be decreased with the increase in noise exposure duration. Decreased catalase activity indicates high oxidative damage [9]. These findings indicate that antioxidant enzyme activity can neutralize the drastic effects of noise for a certain level. If the stress crosses the threshold value, it may result in increased production of free radicals. Depletion in activities of catalase enzyme after eight hour noise exposure indicates the enhanced radical production. Similar decline in catalase activity associated with the increased oxidative damage was observed in textile industry workers due to noise exposure [6]. Comparable effects of noise induced oxidative stress were reported by Srikumar et al during investigation of the protective effects of Triphala on cell mediated immune response [10]. Present study is a preliminary but leading investigation that has provided a model for investigation of drastic effects of noise on eukaryotic cells. Strength of the present study is the use of complete exposure control of noise with strategy to remove the variations induced by potential affecters like oxygen level, pH, temperature, radiations and oxidizing agents. Use of polymorphonuclear lymphocytes for the estimation of effects of noise on catalase activity has provided a model to study immunosuppression due to noise exposure. Use of only one oxidative damage biomarker and lack of gene expression data for claiming the stressful effects of noise on lymphocytes has restricted the study.
CONCLUSION
In conclusion, we found that duration of noise exposure have important role in changing the catalase activity of growing lymphocytes. In case of short duration of noise exposure, catalase activity increases but its difference is not significant as compared to the control group. Discordantly noise exposure for longer duration may result in the decline in catalase activity of growing lymphocytes which indirectly indicates the excessive production of reactive oxygen species molecules. So, noise exposure for long duration causes oxidative stress in growing lymphocytes.
ACKNOWLEDGEMENTS
Present research work was completed using funds for indigenous scholarship of Syed Kashif Nawaz awarded by Higher Education Commission (HEC) Pakistan. We thank HEC for providing the financial support.
DECLARATION OF INTEREST
Authors declare no conflict of interest.
REFERENCES
- [1].Babisch W. Stress hormones in the research on cardiovascular effects of noise. Noise Health. 2003;5:1–11. [PubMed] [Google Scholar]
- [2].Nawaz SK, Hasnain S. Noise induced hypertension and prehypertension in Pakistan. Bosn J Basic Med Sci. 2010;10:239–244. doi: 10.17305/bjbms.2010.2694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Moslehi A, Nabavizadeh-Rafsanjani F, Keshavarz M, Rouhbakhsh N, Sotudeh M, Salimi E. Traffic noise exposure increases gastric acid secretion in rat. Acta Med Iran. 2010;48:77–82. [PubMed] [Google Scholar]
- [4].Stansfeld S, Hygge S, Clark C, Alfred T. Night time aircraft noise exposure and children's cognitive performance. Noise Health. 2010;12:255–262. doi: 10.4103/1463-1741.70504. [DOI] [PubMed] [Google Scholar]
- [5].Fetoni AR, Garzaro M, Ralli M, Landolfo V, Sensini M, Pecorari G, Mordente A, Paludetti G, Giordano C. The monitoring role of otoacoustic emissions and oxidative stress markers in the protective effects of antioxidant administration in noise-exposed subjects: a pilot study. Med Sci Monit. 2009;15:PR1–8. [PubMed] [Google Scholar]
- [6].Yildirim I, Kilinc M, Okur E, Inanc Tolun F, Kiliç MA, Kurutas EB, Ekerbiçer HC. The effects of noise on hearing and oxidative stress in textile workers. Ind Health. 2007;45:743–749. doi: 10.2486/indhealth.45.743. [DOI] [PubMed] [Google Scholar]
- [7].Samson J, Sheeladevi R, Ravindran R. Oxidative stress in brain and antioxidant activity of Ocimum sanctum in noise exposure. Neurotoxicology. 2007;28:679–685. doi: 10.1016/j.neuro.2007.02.011. [DOI] [PubMed] [Google Scholar]
- [8].Van Campen LE, Murphy WJ, Franks JR, Mathias PI, Toraason MA. Oxidative DNA damage is associated with intense noise exposure in the rat. Hear Res. 2002;164:29–38. doi: 10.1016/s0378-5955(01)00391-4. [DOI] [PubMed] [Google Scholar]
- [9].Kumar VH, Patel A, Swartz DD, Wang H, Wynn KA, Nielsen LC, Ryan RM. Exposure to supplemental oxygen and its effects on oxidative stress and antioxidant enzyme activity in term new born lambs. Pediatr Res. 2010;67:66–71. doi: 10.1203/PDR.0b013e3181bf587f. [DOI] [PubMed] [Google Scholar]
- [10].Srikumar R, Parthasarathy NJ, Manikandan S, Narayanan GS, Sheeladevi R. Effect of Triphala on oxidative stress and on cell mediated immune response against noise stress in rats. Mol Cell Biochem. 2006;283:64–67. doi: 10.1007/s11010-006-2271-0. [DOI] [PubMed] [Google Scholar]


