Abstract
The aim of the present study was to investigate the preemptive analgesic effects of intraperitoneally administrated midazolam and diclofenac, before acute and inflammatory induced pain in rat model.
One hundred twenty-eight (n=8 in each group) male Sprague Dawley rats were included in the study. Paw movements in response to thermal stimulation or paw flinching in response to formalin injection were compared after midazolam (0.1, 1, 5 and 10 mg/kg) and diclofenac (10 mg/kg), intraperitoneal administration. Saline was used as a control.
Preemptive analgesic effect was significant in both tests when diclofenac and midazolam was administrated before the pain stimuli (p<0.01 and p<0.001). Intraperitoneal injection of midazolam in doses 5 and 10 mg/kg, increase the response time in hot plate test and decrease the number of flinches in formalin test (p<0.01 vs. p<0.001). ED50 of midazolam (with diclofenac) in hot plate test was 2.02 mg/kg (CI95% =-3.47-5.03 mg); and, 0.9 mg/kg (CI95% =-0.87-4.09 mg) in phase I and 0.7 mg/kg (CI95% = 0.48-6.63 mg) in phase II, in formalin test.
Intraperitoneally administered midazolam and diclofenac had preemptive analgesic effects on acute thermal, and inflammatory induced pain in rats.
KEY WORDS: midazolam, diclofenac, preemptive analgesia, hot plate test, formalin test
INTRODUCTION
Preemptive analgesia is an antinociceptive treatment that prevents the establishment of altered processing of afferent input that amplifies pain [1]. Pain associated with tissue damage results in prolonged modulation of the somatosensory system, with increased responsiveness of both peripheral and central pain pathways [2]. Experimental evidence proposes that to ‘prevent’ or ‘preempt’ the noxious input to the CNS, may be more effective than treatment. The idea of preemptive analgesia was first introduced into clinical practice by Crile in 1913 [3] and further developed by Wall [4] and Woolf [5]. The definition of preemptive analgesia was formed by Kissin [6]. According to him, preemptive analgesia is “treatment that prevents establishment of central sensitization caused by incisional and inflammatory injuries; it starts before incision and covers both the period of surgery and the initial postoperative period. Preemptive analgesia prevents pathologic pain that is different from physiologic pain”, which means: prevention or reversal of central and peripheral sensitization.
Midazolam, a benzodiazepine, effects mediated primarily via the central benzodiazepine receptors located in the central nervous system. Central benzodiazepine receptors are part of a macromolecular complex that also contains a γ-amino butyric acid (GABA) receptor site and a chloride ion channel. Midazolam potentiate the effects of GABA A receptors [7]. It is widely used during general anesthesia and decrease the requirement to other anesthetics. Analgesic effects of intrathecally given midazolam are well known [8-11]. However, the antinociceptive effects of midazolam administered systematically are actually becoming well known in recent years. Nishiyama [12] and Chiba et al. [13] demonstrated the anti-nociceptive effect of systemically administrated midazolam in acute thermal and inflammatory induced pain in animals. Diclofenac sodium, 2-[(2,6-dichlorophenyl) amino] benzene acetic acid, is a non-steroidal anti-inflammatory drug (NSAID) with an approximate relative COX-1/COX-2 specificity ratio of one [14]. NSAIDs inhibit the cyclo-oxygenase enzymes (COX) and decrease peripheral and central prostaglandin production. To reduce the inflammation that accompanies tissue injury, decreasing prostaglandin production attenuates the response of the peripheral and central components of the nervous system to noxious stimuli and reduce the pain occurred in response to following noxious stimuli [15]. These properties would seem to make NSAIDs ideal drugs to use in a pre-emptive approach. Preemptive analgesic effect of diclofenac is discussed in many studies, but the results are still controversy [16-18]. In the present study the preemptive analgesic properties of systemically administered midazolam and diclofenac sodium were investigated in a rat model of acute and inflammatory pain.
MATERIALS AND METHODS
After Institutional Ethics Committee approval, 128 male (8 for each group) Sprague Dawley rats, weighing 250-300 g, were included in the study. The animals were housed in a cage at 20-25°C under diurnal light condition and allowed to access food and water ad libitum. All experiment was done in accordance with the Guide for the Care and Use of Laboratory Animals published by the United States National Institutes of Health (NIH Publication No. 80-23, revised 1996). Midazolam (F. Hoffmann-La Roche, Swiss) 0.1, 1, 5 and 10 mg/kg and diclofenac sodium (Proanalysis-Merck, Germany) 10 mg/kg, was dissolved in normal saline to achieve solutions for intraperitoneal (i.p.) administration. The total injected volume was adjusted to 10 ml/kg in each rat. They were used for i.p. injection at 1-ml syringe with a 23-G needle. The rats are divided in three groups; Group I, midazolam 0.1, 1, 5 and 10 mg/kg (n=8 in each group) given i.p. 15 min before the nociceptive stimulus realized with hot plate test and formalin test; Group II, diclofenac at 10 mg/kg (n=8 in each group) given i.p. 15 min before the nociceptive stimulus realized with hot plate test and formalin test, and; Group III, midazolam 0.1, 1, 5 and 10mg/kg with diclofenac 10 mg/kg (n=8 in each group) given i.p. 15 min before the nociceptive stimulus, realized with hot plate test and formalin test. Saline group (n=8), normal saline given i.p. was used as a control. The acute thermal pain was realized with hot plate test. The hot plate test was performed at 55 °C on the paw of each rat. Animals were placed on the heated smooth surface and the latency of licking, shaking of the limbs, or jumping was measured. Hot plate tests were performed 15 min or 30 min after i.p. drug injection, and repeated every 10 min during 60 min. To prevent the tissue injury the rats were removed from the hot plate test after 30 sec. Pain inhibition percentage (PIP) was calculated with following formula [19]: Pain inhibition percentage (PIP) = [(T1-T0/T0 X 100 T1 – post drug latency; T0 – pre drug latency The formalin test, model of inflammatory pain, was performed 15 or 30 minutes after drug administration. Fifty microlitres of 5% formalin was injected subcutaneously into the dorsal surface of the right hind paw with a 30-G needle. Immediately after injection, the rat was placed in an open surface, and their paw response (flinching or shaking) was observed at ten minutes intervals for a period of one hour. The number of movements was counted for one minute. Two phases were observed: phase 1 for the first six minutes after injection; and phase 2 beginning after about ten minutes. The paw movements were measured every 10 minutes during 60 minutes. A nociceptive score was determined by measuring the 4 behavioural categories: 0, the position and posture of the hind paw is indistinguishable from the contralateral paw; 1, the paw has little or no weight placed on it; 2, the paw is elevated and is not in contact with any surface; 3, the paw is licked, bitten or shaken. Behavioral side effects (agitation, allodinia, catatonic excitement and flaccidity) were observed in animals during the study.
Statistical analysis
Statistical analysis was performed using SPSS version 15. Data are expressed as the mean ± SD or 95% confidence interval (CI). The experimental groups were compared by the non-parametric Kruskal-Wallis test. Multiple comparisons after the Kruskal-Wallis test were performed using the two-tailed Dunn test. A p value less than 0.05 were considered significant.
RESULTS
Preemptive antinociceptive effects of midazolam in hot plate test were demonstrated by increase in response time compared with control group (saline). After i.p. administration of midazolam significant increase in response time were observed in doses 5 and 10 mg/kg (p <0.01 vs. p <0.001, respectively). Increase in response time in hot plate test was observed with midazolam 1 mg/kg in 10, 20 and 30 min (p <0.05) (Figure 1). Significant decrease in number of paw flinches was shown in formalin test as well, in doses 5 and 10 mg/kg midazolam (p <0.001) (Figure 2). The 50% effective dose (ED50) of the hot plate test was calculated 30 min after the midazolam i.p. administration. The ED50 2.82 mg/kg (CI95% =-1.85-5.1 mg). Dose depend antinociceptive effects were observed in both phases of formalin test; phase I and II. The 50% effective dose (ED50) of the formalin test was calculated in both phases after the midazolam i.p. administration. The ED50 1.6 mg/kg (CI95% =-0.81-4.04 mg) in phase I and 1.1 mg/kg (CI95% = 0.67-5.03 mg) in phase II. Preemptive antinociceptive effects of diclofenac sodium (10 mg/kg) administered i.p., in hot plate test and formalin test were demonstrated by increase in response time and decrease in paw flinches, compared with control group (saline) (p <0.05; p <0.01; p <0.001, respectively) (Figure 3, 4).
FIGURE 1.
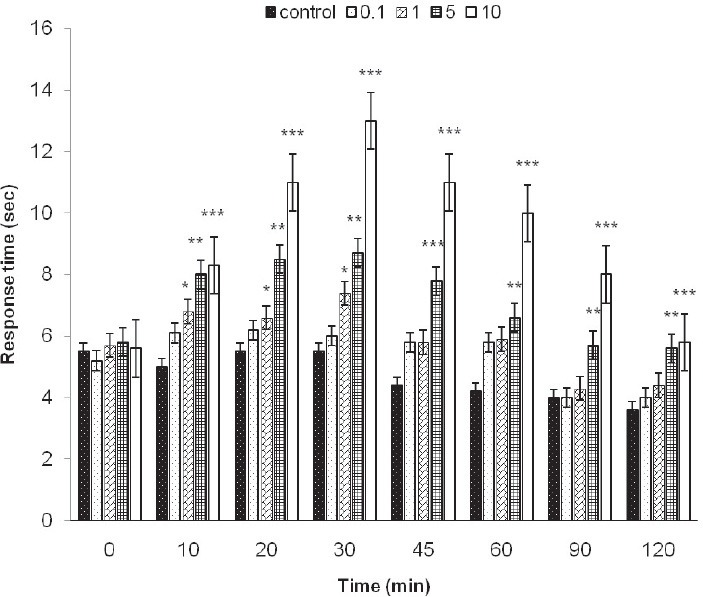
Hot plate test. Response time in the hot plate test with control group (saline) and different doses of midazolam (0.1, 1, 5 and 10 mg/kg) given during 120 min, as indicated. Data are presented as the mean ± SD (8 mice in each dose). *p<0.05; **p<0.01; ***p<0.001.
FIGURE 2.
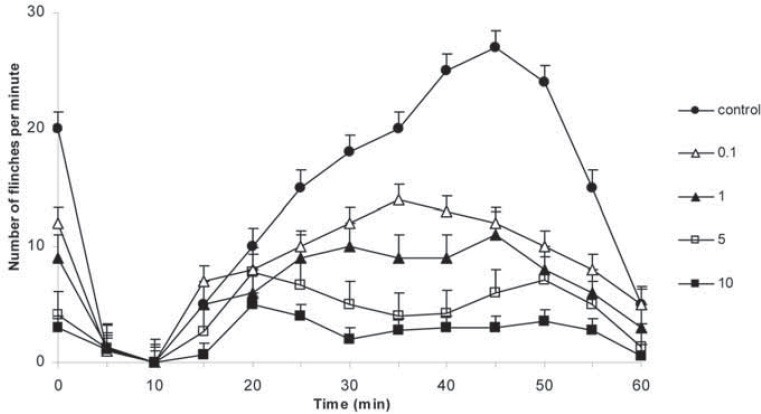
Formalin test. Number of flinches in the formalin test with control (saline) and different doses of midazolam (0.1, 1, 5 and 10 mg/kg) given during 120 min, as indicated. Data are presented as the mean ± SD (8 mice in each dose).
FIGURE 3.
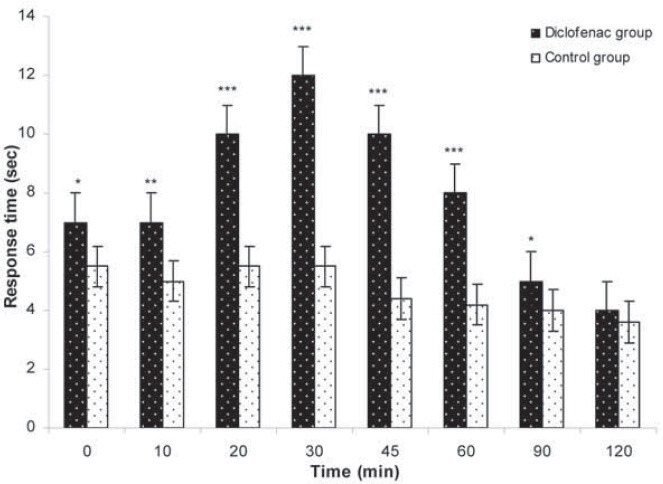
Hot plate test. Response time after i.p. administration of diclofenac sodium 10 mg/kg in the hot plate test with control (saline) given during 120 min, as indicated. Data are presented as the mean ± SD (8 mice in each dose). *p<0.05; **p<0.01; ***p<0.001.
FIGURE 4.
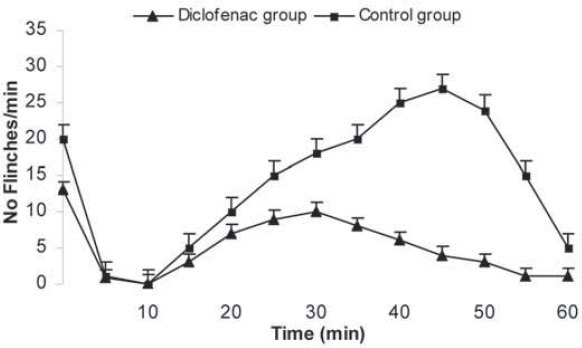
Formalin test. Number of flinches in the formalin test with control (saline) and 10 mg/kg diclofenac sodium given during 60 min, as indicated. Data are presented as the mean ± SD (8 mice in each dose).
Preemptive antinociceptive effects of diclofenac sodium and midazolam in hot plate test were demonstrated by increase in response time compared with control group (saline). After i.p. administration of diclofenac 10 mg/kg and midazolam significant increase in response time were observed in doses 0.1, 1, 5 and 10 mg/kg (p<0.01; p<0.001, respectively). Dose depend antinociceptive effects were observed 10, 20, 30, 45, 60, 90 and 120 min. after i.p. 0.1 and 1 mg/kg midazolam and diclofenac. The increase in response time, in doses 5 and 10 mg/kg midazolam and diclofenac, started in 0 min and continue to increase during 120 min (Figure 5). ED50 of midazolam (with diclofenac) in hot plate test was 2.02 mg/kg (CI95% =-3.47-5.03 mg).
FIGURE 5.
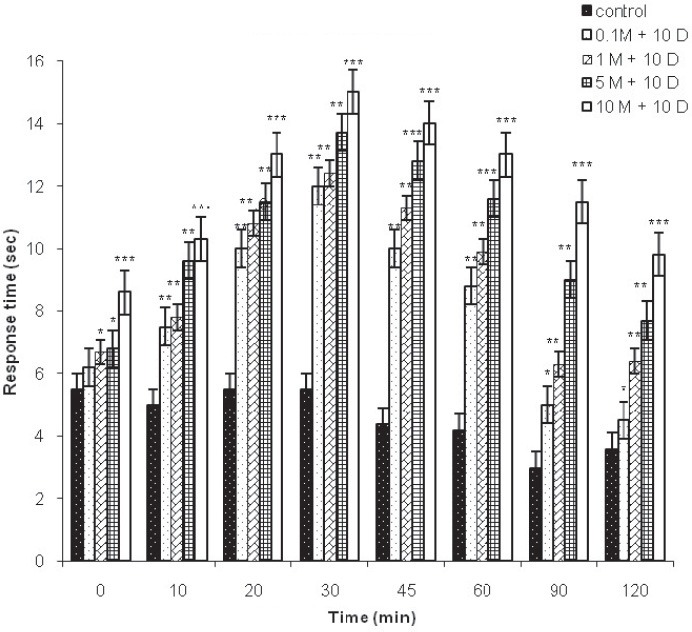
Hot plate test. Response time in the hot plate test with control group (saline) and different doses of midazolam (0.1, 1, 5 and 10 mg/kg) and diclofenac (10 mg/kg) given during 120 min, as indicated. Data are presented as the mean ± SD (8 mice in each dose). *p<0.05; **p<0.01; ***p<0.001.
Preemptive antinociceptive effects of midazolam and diclofenac sodium in formalin test were demonstrated by observation of flinching or shaking of paw after formalin injection. The paw response observed at five minutes intervals during a period of 60 minutes were compared with control group (saline). The number of paw flinches decreased significantly, when diclofenac sodium was administrated i.p. in dose 10 mg/kg and midazolam in doses 0.1, 1, 5 and 10 mg/kg (p<0.05; p<0.01 and p<0.001, respectively). (Figure 6). Dose depend antinociceptive effects were observed in both phases of formalin test; phase I and II. The 50% effective dose (ED50) of the formalin test was calculated in both phases after the midazolam and diclofenac i.p. administration. The ED50 of midazolam (with diclofenac) 0.9 mg/kg (CI95% =-0.87-4.09 mg) in phase I and 0.7 mg/kg (CI95% = 0.48-6.63 mg) in phase II. No behavioural side effects were observed in any animal.
FIGURE 6.
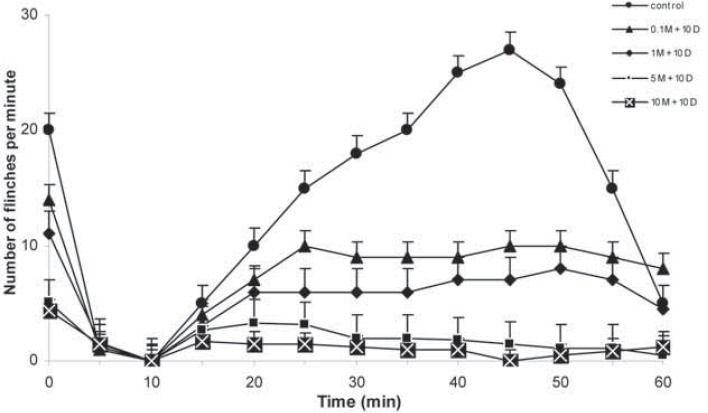
Formalin test. Number of flinches in the formalin test with control (saline) and diclofenac sodium 10 mg/kg and different doses of midazolam (0.1, 1, 5 and 10 mg/kg) given during 60 min, as indicated. Data are presented as the mean ± SD (8 mice in each dose).
DISCUSSION
In this recent study, we demonstrated that intraperitoneally administered diclofenac and midazolam had preemptive analgesic effects in the hot plate test and formalin test in rats. Preemptive analgesia can reduce both the acute and inflammatory pain and in this way can reduce peripheral and central sensitization. In our study, we used before (pre) versus placebo (no) design; we applied diclofenac and midazolam before pain stimuli. Several studies have addressed midazolam as an analgesic during intrathecal or epidural administration. Naguib et al. [20] examined the analgesic efficacy of caudal administration of midazolam, bupivacaine, or a mixture of both drugs in 45 children, undergoing inguinal herniotomy. They found that caudal midazolam in a dose of 50 μg kg-1 provides equivalent analgesia to bupivacaine 0.25%. Nishijama [12] studied the antinociceptive properties of systemically vs. inthratecally administered midazolam in a rat model of thermal and inflammatory pain. They observed systemically administered midazolam induced antinociception for inflammatory pain only, while intrathecal administration elicited antinociceptive effects on both acute thermal and inflammatory-induced pain. In 2009 Chiba et al. [13] reported the other study which was performed to investigate antinociceptive effects of different types of nociception in mice. They concluded that systemically administered midazolam had antinociceptive effects on acute thermal, acute mechanical and acute inflammatory-induced nociception in mice. The antinociceptive potency of midazolam was the same for both acute thermal-induced nociception and mechanical-induced nociception. Ong et al. [21] found that IV midazolam treatment (0.09 mg/kg) in humans has a pain-reducing effect after third molar surgery, thus improving postoperative pain management. However, it has been shown that midazolam by bolus and continuous infusion resulted in a reduction in morphine consumption and lower pain scores in 50 patients undergoing hysterectomy [22]. Anxyolitic, muscle relaxing, and sedative effects of midazolam may modify the responses in nociceptive tests. In our study we didn’t use any test to evaluate sensorimotor impairment induced by midazolam. Chiba et al. [13] used the running wheel test and the balance beam test in their experiment and showed that with midazolam in dose 10 mg/kg 10 and 30 min after i.p. drug administration, mice were still able to run on the wheel (13). As well Orii et al. [23] reported that midazolam (1, 5, and 10 mg/kg) did not induce any detectable reduction in motor response in rats. Preemptive analgesic effect was obvious with NSAIDs due to their mode of action, competing with arachidonic acid for binding to cyclooxygenase and decreasing the formation of prostaglandins [24]. Treatment with NSAIDs should be started as early as possible and should be initiated before the input of nociceptive stimuli. However, the clinical value of this technique remains still uncertain. Instrumentation of the uterus and Fallopian tubes during laparoscopy or surgery leads to prostaglandin release and, the prostaglandins released play a role in pain following laparoscopy [25]. Inhibition of prostaglandin production by the NSAIDs both peripherally and centrally should, therefore, decrease postoperative discomfort and reduce opioid requirement [26]. Woolf et al. [27] showed no difference with preoperative diclofenac from postoperative diclofenac in patients undergoing laparoscopic tubal ligation. However, Buggy et al. [28] and Gillberg et al. [29] demonstrate that preoperative administration of ketorolac, piroxicam and diclofenac did reduce postoperative pain in patients undergoing laparoscopy. Our findings support these results as well. Bjorkman [30] studied the site and nature of the antinociceptive effect of diclofenac and paracetamol in the central nervous system. He observed the antinociceptive effect of diclofenac engage with central nervous component in different areas of central nervous system. Herrero et al. [31] studied the central antinociceptive effect of NSAID ketoprofen in two experiment models in rats and conclude that ketoprofen has central while peripheral analgesic activity. The present results suggest that intraperitoneally administered diclofenac has few effects at the level of the spinal cord and antinociceptive effects in the periphery and the brain. However the available preoperative trials of preemptive NSAID use have modest or unclear results and it may be due to controversy associated with the definition of preemptive analgesia. Even though, NSAIDs may have an ability to induce a preemptive analgesic effect. Our study suggests how the preoperative use of diclofenac was more effective. It is expected that NSAIDs will play an increasing role in preemptive analgesia and pain relief in general. The hot plate test evaluates supraspinal antinociceptive effects, and it reflects activity in thermally sensitive afferent fibers and activity of Aδ and C fibers [32]. Responses in the formalin test are mediated by both the spinal and supraspinal sites. The phase 1 response of the formalin test is caused by the direct stimulation of nociceptors by formalin or tissue damage, and is thought to be an acute pain reaction. This reflects activity that is prominent in Aβ, Aδ and high-threshold C nociceptor afferent fibers. The phase 2 response is caused by inflammation after formalin injection and central sensitization related to C activity. It reflects activity in mechanically insensitive afferent fibers and activity of Aδ and C fibers [33]. Sensory fibers respond to physical and chemical stimuli producing mediators with origin from tissue injury and inflammation. These inflammatory mediators activate or sensitize afferent fibers, and the neural impulses originated from nociceptors are transmitted via peripheral nerves to the spinal cord and with cranial nerves to cranial nerve ganglia. Prostaglandins are among the most important mediators of inflammatory pain. During inflammation prostaglandin formation is induced by COX enzymes. NSAIDs block COX enzymes production and produce analgesia [34]. Studies have highlight that NSAIDs do not increase the pain threshold in animal model such as tail-flick and hotplate tests, but they normalize the pain behavior, which is observed after tissue injury and inflammation mechanism [35, 36]. However, diclofenac cause dose dependant analgesia. The ED50 values for diclofenac are 7.20 mg/kg (3.95 ± 13.30) [37]. We assume that the inadequate administered doses of drug may decrease the concentration in peripheral nociceptive terminals and antinociceptive response may fail. The intraperitoneally administrated dose of diclofenac sodium, in our study, was 10 mg/kg, the optimal dose to cause antinociceptive response in rats.
CONCLUSION
We concluded that i.p. administered diclofenac and midazolam has preemptive and antinociceptive effects in acute thermal and inflammatory induced pain. Furthermore diclofenac added to midazolam enhance the antinociceptive effect of systemically administered midazolam.
DECLARATION OF INTEREST
The authors state that there is no conflict of interest.
REFERENCES
- [1].Kissin I. Preemtive analgesia problems with assessment of clinical significance. Methods Mol Biol. 2010;617:475–482. doi: 10.1007/978-1-60327-323-7_34. [DOI] [PubMed] [Google Scholar]
- [2].Woolf CJ, Chong MS. Preemptive analgesia-treating postoperative pain by preventing the establishment of central sensitization. Anesth Analg. 1993;77:362–379. doi: 10.1213/00000539-199377020-00026. [DOI] [PubMed] [Google Scholar]
- [3].Crile GW. The kinetic theory of shock and its prevention through anoci-association. Lancet. 1913;185:7–16. [Google Scholar]
- [4].Wall PD. The prevention of postoperative pain. Pain. 1988;33:289–290. doi: 10.1016/0304-3959(88)90286-2. [DOI] [PubMed] [Google Scholar]
- [5].Woolf CJ. Central mechanisms of acute pain. In: Bond MR, Charlton JE, Woolf CJ, editors. Amsterdam: Proc 6th World Congress on Pain, Elsevier; 1991. pp. 25–34. [Google Scholar]
- [6].Kissin I. Preemptive analgesia. Anesthesiology. 2000;93:1138–1143. doi: 10.1097/00000542-200010000-00040. [DOI] [PubMed] [Google Scholar]
- [7].Reves JG, Fragen RJ, Vinik HR, Greenblatt JD. Midazolam: pharmacology and uses. Anesthesiology. 1985;62:310–324. [PubMed] [Google Scholar]
- [8].Edwards M, Serrao JM, Gent JP, Goodchild CS. On the mechanism by which midazolam causes spinally mediated analgesia. Anesthesiology. 1990;73:273–277. doi: 10.1097/00000542-199008000-00015. [DOI] [PubMed] [Google Scholar]
- [9].Yanez A, Sabbe MB, Stevens CW, Yaksh TL. Interaction of midazolam and morphine in the spinal cord of the rat. Neuropharmacology. 1990;29:359–364. doi: 10.1016/0028-3908(90)90094-8. [DOI] [PubMed] [Google Scholar]
- [10].Nishiyama T. The post-operative analgesic action of midazolam following epidural administration. Eur J Anaesthesiol. 1995;12:369–374. [PubMed] [Google Scholar]
- [11].Nishiyama T, Yokoyama T, Hanaoka K. Midazolam improves postoperative epidural analgesia with continuous infusion of local anaesthetics. Can J Anaesth. 1998;45:551–555. doi: 10.1007/BF03012706. [DOI] [PubMed] [Google Scholar]
- [12].Nishiyama T. Analgesic effects of systemic midazolam: comparison with intrathecal administration. Can Journ Anesth. 2006;53:1004–1009. doi: 10.1007/BF03022529. [DOI] [PubMed] [Google Scholar]
- [13].Chiba S, Nishiyama T, Yoshikava M, Yamada Y. The antinociceptive effects of midazolam on three different types of nociception in mice. J Pharmacol Sci. 2009;109:71–77. doi: 10.1254/jphs.08094fp. [DOI] [PubMed] [Google Scholar]
- [14].Van der Marel CD, Anderson BJ, Romsing J, Jacqz-Aigrain E, Tibboel D. Diclofenac and metabolite pharmacokinetics in children. Paediatr Anaesth. 2004;14:443–451. doi: 10.1111/j.1460-9592.2004.01232.x. [DOI] [PubMed] [Google Scholar]
- [15].Kokki IH. Nonsteroidal anti-inflammatory drugs for postoperative pain: a focus on children. Paediatr Drugs. 2003;5:103–123. doi: 10.2165/00128072-200305020-00004. [DOI] [PubMed] [Google Scholar]
- [16].Riad W, Moussa A. Preoperative analgesia with rectal diclofenac and/or paracetamol in children undergoing inguinal hernia repair. Anaesthesia. 2007;62:1241–1245. doi: 10.1111/j.1365-2044.2007.05248.x. [DOI] [PubMed] [Google Scholar]
- [17].Tuzuner AM, Ucok C, Kucukyavuz Z, Alkis N, Alanoglu Z. Preoperative diclofenac sodium and tramadol for pain relief after bimaxillary osteotomy. J Oral Maxillofac Surg. 2007;65:2453–2458. doi: 10.1016/j.joms.2007.06.622. [DOI] [PubMed] [Google Scholar]
- [18].Yukawa Y, Kato F, Ito K, Terashima T, Horie Y. A prospective randomized study of preemptive analgesia for postoperative pain in the patients undergoing posterior lumbar interbody fusion continuous subcutaneous morphine, continuous epidural morphine, and diclofenac sodium. Spine. 2005;30:2357–2361. doi: 10.1097/01.brs.0000184377.31427.fa. [DOI] [PubMed] [Google Scholar]
- [19].Yin W, Wang TS, Yin FZ, Cai BC. Analgesic and anti-inflammatory properties of brucine and brucine-N-oxide extracted from seeds of Strychnos nuxvomica. J Ethnopharmacol. 2003;88:205–214. doi: 10.1016/s0378-8741(03)00224-1. [DOI] [PubMed] [Google Scholar]
- [20].Naguib M, el Gammal M, Elhattab YS, Seray M. Midazolam for caudal analgesia in children: comparison with caudal bupivacaine. Can J Anaesth. 1995;42:758–764. doi: 10.1007/BF03011172. [DOI] [PubMed] [Google Scholar]
- [21].Ong CK, Seymour RA, Tan JM. Sedation with midazolam leads to reduced pain after dental surgery. Anesth Analg. 2004;98:1289–1293. doi: 10.1213/01.ane.0000111107.18755.cc. [DOI] [PubMed] [Google Scholar]
- [22].Gilliland HEM, Prasad BK, Mirakhur RK, Fee JPH. An investigation of the potential morphine sparing effect of midazolam. Anaesthesia. 1996;51:808–811. doi: 10.1111/j.1365-2044.1996.tb12605.x. [DOI] [PubMed] [Google Scholar]
- [23].Orii R, Ohashi Y, Halder S, Giombini M, Maze M, Fujinaga M. GA-BAergic interneurons at supraspinal and spinal levels differentially modulate the antinociceptive effect of nitrous oxide in Fischer rats. Anesthesiology. 2003;98:1223–1230. doi: 10.1097/00000542-200305000-00026. [DOI] [PubMed] [Google Scholar]
- [24].Mccrory CR, Lindahl SG. Cyclooxygenase inhibition for postoperative analgesia. Anesth Analg. 2002;95:169–176. doi: 10.1097/00000539-200207000-00030. [DOI] [PubMed] [Google Scholar]
- [25].Wang ZH, Wu R, Ge X. Relationships between pelvic pain and prostaglandin levels in plasma and peritoneal fluid collected from women after sterilization. Contraception. 1992;45:67–71. doi: 10.1016/0010-7824(92)90142-g. [DOI] [PubMed] [Google Scholar]
- [26].Mccormack K. Non-steroidal anti-inflammatory drugs and spinal nociceptive processing. Pain. 1994;59:9–43. doi: 10.1016/0304-3959(94)90045-0. [DOI] [PubMed] [Google Scholar]
- [27].Woolf CJ, Chong MS. Preemptive analgesia – treating postoperative pain by preventing the establishment of central sensitization. Anesth Analg. 1993;77:362–379. doi: 10.1213/00000539-199377020-00026. [DOI] [PubMed] [Google Scholar]
- [28].Buggy DJ, Walli C, Carton EG. Preoperative or postoperative diclofenac for laparoscopic tubal ligation. Br J Anaesth. 1994;73:767–770. doi: 10.1093/bja/73.6.767. [DOI] [PubMed] [Google Scholar]
- [29].Gillberg LE, Harsten AS, Stahl LB. Preoperative diclofenac sodium reduces post-laparoscopy pain. Can J Anaesth. 1993;40:406–408. doi: 10.1007/BF03009507. [DOI] [PubMed] [Google Scholar]
- [30].Bjorkman R. Central antinociceptive effects of non-steroidal anti-inflammatory drugs and paracetamol. Experimental studies in the rat. Acta Anaesthesiol Scand Suppl s103. 1995;39:1–44. [PubMed] [Google Scholar]
- [31].Herrero JF, Parrado A, Cervero F. Central and peripheral actions of the NSAID ketoprofen on spinal cord nociceptive reflexes. Neuropharmacology. 1997;36:1425–1431. doi: 10.1016/s0028-3908(97)00120-2. [DOI] [PubMed] [Google Scholar]
- [32].Espejo EF, Mir D. Structure of the rat's behaviour in the hot plate test. Behav Brain Res. 1993;56:171–176. doi: 10.1016/0166-4328(93)90035-o. [DOI] [PubMed] [Google Scholar]
- [33].Tjolsen A, Berge OG, Hunskaar S, Rosland JH, Hole K. The formalin test: an evaluation of the method. Pain. 1992;51:5–17. doi: 10.1016/0304-3959(92)90003-T. [DOI] [PubMed] [Google Scholar]
- [34].Dray A. Inflammatory mediators of pain. Br J Anaesth. 1995;75:125–131. doi: 10.1093/bja/75.2.125. [DOI] [PubMed] [Google Scholar]
- [35].Bjorkam R, Hedner J, Hedner T, Henning M. Central, naloxone-re-versible antinociception by diclofenac in the rat. Naunyn Schmie-debergs Arch Pharmacol. 1990;342:171–176. doi: 10.1007/BF00166960. [DOI] [PubMed] [Google Scholar]
- [36].Miranda HF, Lopez J, Sierralta F, Correa A, Pinard G. NSAID antinociception measured in chemical and a thermal assay in mice. Pain Res Manage. 2001;6:190–196. doi: 10.1155/2001/701427. [DOI] [PubMed] [Google Scholar]
- [37].Miranda HF, Sierralta F, Pinard G. Neostigmine interactions with non steroidal anti-inflammatory drugs. Br J Pharmacol. 2002;135:1591–1597. doi: 10.1038/sj.bjp.0704599. [DOI] [PMC free article] [PubMed] [Google Scholar]


