Abstract
The aim of this article was to investigate the correlation between expression of O6-methylguanine-DNA methyltransferase (MGMT) in osteosarcoma and the curative effect of alkylating agent (Cis-diaminodichloroplatinum, CDDP). 42 male patients and 34 female patients with a median age of 17 years (9 to 43 years) were eligible for this study. According to histopathological types, there were 3 cases of telangiectatic osteogenic sarcoma, 22 cases of osteoblastic, 11 cases of chondroblastic and 16 cases of fibroblastic sarcoma. Immunohistochemical method was used to detect the expression of MGMT protein. The correlations between MGMT expression and the curative effect of CDDP on osteosarcoma have been investigated. It was shown by immunohistochemical staining that among 76 osteosarcoma biopsy specimens, 52 (68%) cases were positive, 27 (35%) cases were weak positive, 18 (24%), cases were moderate positive, and 7 (9%) cases were strong positive. There were no significant differences in MGMT expression among different pathological types of tumors (p>0.5). After CDDP chemotherapy, among pathologic specimens in which MGMT expression was positive, necrosis rates were as follows: grade I, 5 cases (38%); grade II, 7 cases (25%); grade III, 15 cases (21%); grade IV, 2 cases (23%). Osteosarcoma necrosis rate was low when the expression of MGMT protein was positive, whereas necrosis rate was high when there was a low level of MGMT expression (p<0.01). There was a significant negative correlation between the level of MGMT expression in osteosarcoma tissue and osteosarcoma necrosis rate after cisplatin chemotherapy.
KEY WORDS: osteosarcoma, MGMT protein, CDDP
INTRODUCTION
Chemotherapy plays a key role in the comprehensive treatment of osteosarcoma. It has significantly increased the 5-year survival rate of osteosarcoma patients as well as the success rate of limb salvage surgeries. Nowadays, Cis-diaminodichloroplatinum (CDDP) has been widely used in chemotherapy in treatment for bone and soft tissue malignancy, but the problem of drug resistance has not yet been completely solved via this protocol. Results of intensive study on cell and molecular biology indicate that, to a large extent, the drug resistance of tumor cell are caused by two aspects. On the one hand, gene MDRL encoding protein P-gp can actively transport the chemotherapeutic agents outside the cell, thus reducing the intracellular effective concentration, which makes the tumor cell escape the cytotoxicity of drugs. On the other hand, the strong repair function of tumor cell can significantly weaken the therapeutic effect of drugs. How to predict and conquer drug resistance of tumor cell is of great importance in tumor therapy. Many studies have demonstrated that MGMT can rapidly repair alkylating damage of DNA, which is the main reason of tumor cell to develop drug tolerance [1-2]. Researchers around the world have conducted individualized clinical experiments based on inhibiting MGMT activity as well as the rational use of alkylating agent [3-5]. MGMT can repair methylation and alkylation damage of guanine base in DNA, thus counteracting the killing effect of alkylating agent on tumor cells, which is a main determinant of resistance of tumor cells to chemotherapeutic alkylating agents. It was found that there was a negative correlation between high level of MGMT expression in tumor tissue and curative effect of alkylating agents [7]. Therefore, in this comparative study, we have investigated the relationship between MGMT expression and the curative effect of CDDP on osteosarcoma.
MATERIALS AND METHODS
Patients
Between January 2002 and April 2008, 76 patients with osteosarcoma (aged between 9 to 43 years old, no other pathological types of tumors were found in these patients) were eligible for this study. After admission, pre-treatment pathological tissue samples were collected, MGMT protein detections were conducted, and they were classified according to histopathological types of osteosarcoma. All patients were administrated with CDDP (120 mg/m2) three times or more than three times. Evaluation of osteosarcoma necrosis rate after chemotherapy was performed. The formula to calculate tumor cell necrosis rate was TCNR=(1-N/M)×100%. The grade of TCNR was divided to four degree: GradeI TCNR≤50%, GradeII 50%<TCNR≤90%, GradeIII 90%<TCNR≤99%, Grade IV=100%[6],
Reagents
Special blocking buffer, mouse anti-human MGMT monoclonal antibody (prepared by Field blood transfusion institute of Academy of Military Medical Sciences of China), goat anti-mouse IgG second antibody, APPAP enzyme complex, substrate, substrate buffer solution, hematoxylin complex dyeing solution, mounting medium, etc. The reagents were all prepared by Field blood transfusion institute of Academy of Military Medical Sciences of China.
Detection of MGMT protein expression in osteosarcoma patients by immunohistochemical method
Immunohistochemical staining was conducted according to standard procedure strictly. Samples were observed under light microscope. After dewaxing and dehydration, tissues were formalin fixed and paraffin embedded, then placed into hydrogen peroxide solution (0.3%) for 10 minutes to inactivate endogenous enzyme. After washed off by PBS and blocked, samples were treated with first antibody at 4°C overnight. They were washed off using PBS the next day, and then were incubated with second antibody at 37°C for 30°C for 30 minutes. Finally, samples were washed conventionally, and then were colored using DAB chromogenic reagent. Cell nuclear and cytoplasm were stained by MGMT antibodies. Under microscope, karyoplasms that were brown colored and distinct from the background were considered as positive, conversely, they were considered as negative.
Determinant criterion of MGMT expression staining results
The positive immunohistochemical reaction was observed as light or dark reed colouring of the tumor nuclei and cytoplasm. Samples that were strongly stained, in which the proportion of positive cells was above 30% were considered as strong positive (+++); cases where the proportion of stained cells was between 20%~30% were considered as moderate positive (++); samples that were weakly stained, in which the proportion of stained cells was blow 10% were considered as weak positive (+). All detection results were evaluated via double-blind trial by researchers.
Curative effect evaluation according to detection results of post chemotherapeutic tumor tissue necrosis
Grade I: Light response. A few tumor necroses appeared. The necrosis rate was no greater than 50 %. Grade II: Moderate response. Tumors were partially necrotic. Necrosis rate was between 50% and 90%. There was sporadic loose fibrous tissue hyperplasia scattered in the sample. Regional survived tumor cells still existed. Grade III: Most of the tumor cells were necrotic. Necrosis rate was between 90% and 99%. There were only sporadic survived tumor lesions scattered in the sample. Fibrous tissue hyperplasia can be found. Grade IV: Tumor necrosis rate was 100%. Fibrous tissue hyperplasia can be found.
Statistical analysis
Statistical analyses were done by SPSS 13.0 software. Experimental data and the statistical significance were analyzed by χ2 test and variance analysis respectively. p<0.05 represented that the differences were of statistical significance.
RESULTS
Expression of MGMT in osteosarcoma tissue
Among 76 osteosarcoma biopsy specimens, 52 cases were positive. The semiquantitative analysis is calculated as the percentage of the positive cell in the relation to the total cells in the field (overall positive rate was 68%: 27 (36%) cases (+), 18 (24%) cases (++), 7 (9%) cases (+++). According to pathological types, there were 3 (6%) cases of 22 (42%) cases of osteoblast, 11 (21%) cases of chondroblast, and 16 (31%) cases of fibroblast. As shown in Table 1, χ2 test results demonstrated that there were no significant differences in MGMT expression among different histopathological types (of osteosarcoma p=0.662).
TABLE 1.
Immunohistochemical MGMT protein expression in different histopathological types osteosarcoma.
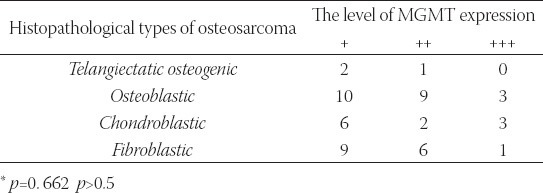
FIGURE 1.
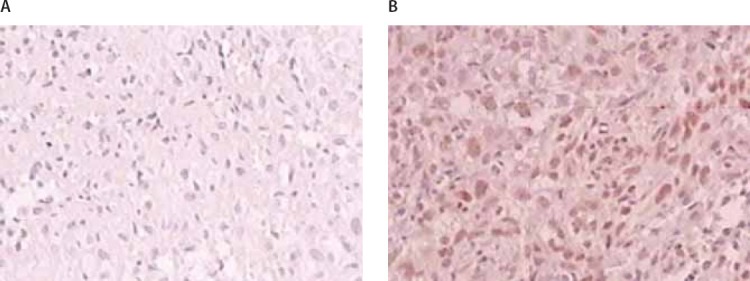
Immunohistochemical expression of MGMT in osteosarcoma tissue: (A) Negative expression (×40), (B) Positive expression (×40).
Correlation between immunohistochemical expression of MGMT in osteosarcoma tissue and osteosarcoma necrosis rate after treatment
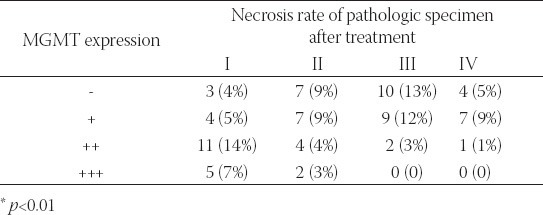
Among pathologic specimens in which MGMT expression was negative, necrosis rates were as follows: grade I, 0 cases (3/24, 12.5%); grade II, 1 case (7/24, 29.2%); grade III, 3 cases (10/24, 42%); grade IV, 1 case (4/24, 17%). Among pathologic specimens in which MGMT expression was positive, necrosis rates were as follows: grade I, 5 cases (20/52, 38%); grade II, 7 cases (13/52, 25%); grade III, 15 cases (11/52, 21%); grade IV, 2 cases (12/52, 23%). Variance analysis indicated that there were significant differences among groups (p=0.004) (Table 2). Results showed that osteosarcoma tissue with negative expression of MGMT was sensitive to CDDP. There was a negative correlation between the expression of MGMT and osteosarcoma necrosis condition after chemotherapy. The necrosis of tumor lesion was mild after chemotherapy when there was a strong positive expression of MGMT.
TABLE 2.
The correlation between tumor necrosis and MGMT immunohistochemical expression in 76 different histopathological types of osteosarcoma.
DISCUSSION
Recently, the increases of 5-year survival rate and the success rate of limb salvage surgery benefit from the important role chemotherapy played in the comprehensive treatment of osteosarcoma. These days, neo-adjuvant chemotherapy has been widely used around the world, but this treatment protocol still cannot solve completely the problem of drug resistance after chemotherapy. The MGMT expressions of the pathological samples of the osteosarcoma and the tumor necrosis rates after chemotherapy have been comprehensively analyzed. It can be concluded from our experimental results that MGMT protein expression has certain significances in the prediction of CDDP chemotherapeutic effect of the osteosarcoma patient. Study show that the antineoplastic alkylating agents could cause DNA base alkylation. The formation of O6-methylguanine is considered to have the most threatening effect on cellular activity. O6-methylguanine can cause G: C→A: T mutation, which results in cell mutation and death. MGMT is a special DNA repair enzyme universally existed in biological organisms from bacteria to mammalian. It functions as transferase and methyl acceptor simultaneously. MGMT can repair guanine damage in DNA by transferring alkylating groups from O6 position to its own cysteine residues before DNA cross-linking, and meanwhile loses its activity irreversibly. Other proteins have not been found to participate in this process so far. Therefore, MGMT is the molecular basis for cells to develop drug resistance to alkylating agents [7-10]. CDDP functions similarly to the double functional groups of alkylating agent by inhibiting DNA synthesis. Preuss et al. [10] have found that tumor cell in which MGMT has high level of activity is resistant to CDDP, whereas tumor cell is sensitive to CDDP when MGMT activity is low. Bruheim et al. [11] studied the relationship between MGMT activity and the curative effect of two chemotherapeutic drugs (cytoxan (CTX) and CDDP) on tumor xenografts. They inoculated 14 tumor cell lines into right armpits of nude mice, and then injected CTX and CDDP respectively. Results showed that the higher the MGMT activity was, the less sensitive the tumor cell to CTX. No such correlation was found with CDDP. Eichhorn et al. [12] demonstrated that drug resistance of tumor cell to chemotherapeutic agent CTX was positively correlated with MGMT expression. There are no significant differences in MGMT expression among different pathological types (p>0.5) according to our detection results. We have analyzed MGMT expressions in osteosarcoma biopsy specimens and necrosis rates that indirectly reflect curative effects of chemotherapy. We have found that MGMT expression is negatively correlated with osteosarcoma necrosis rate after chemotherapy (p<0.01). Necrosis rate of tumor samples with negative or weak positive MGMT expression is higher than that with strong positive expression. Although the mechanism of tumor cells having the potential to develop resistance to chemotherapeutic alkylating agents has not been completely understood, exciting advances have been achieved in curative effect of treatment in some tumor cell lines by inhibiting MGMT activity combined with alkylating administration. Detection of the level of MGMT activity helps to make the alkylating chemotherapy for the treatment of osteosarcoma and other carcinomatous diseases more predictably.
CONCLUSION
In conclusion, there were no significant differences in MGMT expression among different pathological types of tumors (p>0.5). After CDDP chemotherapy, among pathologic specimens in which MGMT expression was positive, necrosis rates were as follows: grade I, 5 cases (38%); grade II, 7 cases (25%); grade III, 15 cases (21%); grade IV, 2 cases (23%). Osteosarcoma necrosis rate was low when the expression of MGMT protein was positive, whereas necrosis rate was high when there was a low level of MGMT expression (p<0.01). There was a significant negative correlation between the level of MGMT expression in osteosarcoma tissue and osteosarcoma necrosis rate after cisplatin chemotherapy.
DECLARATION OF INTEREST
There is no conflict of interest to declare by the authors.
REFERENCES
- [1].Sabharwal A, Middleton MR. Exploiting the role of O6-methylguanine DNA Methyltransferase (MGMT) in cancer therapy. Curr Opin Pharmacol. 2006;6(4):355–363. doi: 10.1016/j.coph.2006.03.011. [DOI] [PubMed] [Google Scholar]
- [2].Verbeek B, Southgate TD, Gilham DE, Margison GP. O6-Methylguanine-DNA methyltransferase inactivation and chemotherapy[J] Br Med Bull. 2008;85:17–33. doi: 10.1093/bmb/ldm036. [DOI] [PubMed] [Google Scholar]
- [3].Jacinto FV, Esteller M. MGMT hypermethylation: a prognostic foe, a predictive friend. DNA Rep. 2007;10(1):10–16. doi: 10.1016/j.dnarep.2007.03.013. [DOI] [PubMed] [Google Scholar]
- [4].Helleday T, Petermann E, Lundin C. DNA repair pathways as targets for cancer therapy. Nat Rev. 2008;8(3):193–204. doi: 10.1038/nrc2342. [DOI] [PubMed] [Google Scholar]
- [5].Ho JW. Potential and Cytotoxicity of cis-Platinum Complex with Anti-tumor Activity in Combination Therapy[J] Recent Patents on Anti-Cancer Drug Discovery. 2006;1:129–134. doi: 10.2174/157489206775246485. [DOI] [PubMed] [Google Scholar]
- [6].Rosen G, Caparros B, Huvos AG, et al. Preoperative chemotherapy for osteogenic sarcoma: selection of Postoperativea-djuvant chemotherapy based on the response of the primary tumor to preoperative chemotherapy [J] cancer. 1982;49(6):1221–1230. doi: 10.1002/1097-0142(19820315)49:6<1221::aid-cncr2820490625>3.0.co;2-e. [DOI] [PubMed] [Google Scholar]
- [7].Sabharwal A, Middleton MR. Exploiting the role of O6-methylguanine DNA. methyltransferase (MGMT) in cancer therapy[J] Curr Opin Pharmascol. 2006;6(4):355–363. doi: 10.1016/j.coph.2006.03.011. [DOI] [PubMed] [Google Scholar]
- [8].Verbeek B, Southgate TD, Gilham DE, Margison GP. O6-Methyl-guanine-DNA methyltransferase inactivation and chemotherapy[J] Br Med Bull. 2008;85:17–33. doi: 10.1093/bmb/ldm036. [DOI] [PubMed] [Google Scholar]
- [9].Voelter V, Diserens AC, Moulin A, et al. Infrequent promoter methylation of the MGMT gene in liver metastases from uveal melanoma[J] Int J Cancer. 2008;123(5):1215–8. doi: 10.1002/ijc.23632. [DOI] [PubMed] [Google Scholar]
- [10].Preuss I, Eberhagen I, Haas S, Eibl RH, Kaufmann M, von Minckwitz G, et al. O6-methylguanine-DNA methyltransferase activity in breast and brain tumors. Int J Cancer. 1995;61(3):321–326. doi: 10.1002/ijc.2910610308. [DOI] [PubMed] [Google Scholar]
- [11].Bruheim S, Bruland OS, Breistol K, Maelandsmo GM, Fodstad O. Human osteosarcoma xenografts and their sensitivity to chemotherapy. Pathol Oncol Res. 2004;10(3):133–141. doi: 10.1007/BF03033741. [DOI] [PubMed] [Google Scholar]
- [12].Mattern J, Eichhorn U, Kaina B, Volm M. O6-methylguanine-DNA methyltransferase activity and sensitivity to cyclophosphamide and cisplatin in human lung tumor xenografts. Int J Cancer. 1998;77(6):919–922. doi: 10.1002/(sici)1097-0215(19980911)77:6<919::aid-ijc20>3.0.co;2-v. [DOI] [PubMed] [Google Scholar]


