Abstract
The possibility to measure binding of small molecule drugs to desired targets in live cells could provide a better understanding of drug action. However, current approaches mostly yield static data, require lysis or rely on indirect assays and thus often provide an incomplete understanding of drug action. Here, we present a multiphoton fluorescence anisotropy microscopy live cell imaging technique to measure and map drug-target interaction in real time at subcellular resolution. This approach is generally applicable using any fluorescently labeled drug and enables high resolution spatial and temporal mapping of bound and unbound drug distribution. To illustrate our approach we measure intracellular target engagement of the chemotherapeutic Olaparib, a poly(ADP-ribose) polymerase inhibitor, in live cells and within a tumor in vivo. These results are the first generalizable approach to directly measure drug-target binding in vivo and present a promising tool to enhance understanding of drug activity.
Introduction
Small molecule therapeutic drugs typically exert their effects through binding to one or a few protein targets. This critical interaction - a prerequisite of therapeutic drug efficacy - is often poorly understood and can generally not be visualized in live cells or entire organisms due to the lack of methods to directly measure drug target engagement in a biological setting. As a result, most of our knowledge is incomplete, as it relies on target extraction assay systems1,2 or indirect measurements where critical spatiotemporal information is lost, which further complicates drug development3.
Recent advances in chemical techniques have allowed the creation of fluorescent drugs, prodrugs and activity based probes to interrogate target engagement4-6. To date, most of these compounds have been used in vitro while a select few have been used in vivo for imaging drug distribution (pharmacokinetics)7, or tumor detection8. However, to realize the full potential of intravital imaging with fluorescently labeled compounds determination of target engagement with subcellular resolution is needed2,9. We hypothesized that fluorescence polarization (FP) could be used to accurately measure drug binding in vitro and in vivo through multiphoton microscopy.
Fluorescence polarization10 quantifies the degree of fluorescence depolarization with respect to the polarization excitation plane, providing insight into the state or environment of the excited fluorescent molecule. FP has been extensively used in non-imaging, plate reader and kinetic in vitro assays to measure numerous fluorescent molecule and molecular drug interactions including target engagement11,12. Extending FP to optical microscopy imaging modalities could provide spatially- and temporally-resolved mapping, enabling live cell imaging of target engagement of small molecule drugs. However, microscopy imaging methods based on FP13 have been more commonly used to study homo-FRET in membrane dynamics14-16, structure in ordered biological systems17,18 and endogenous small molecules19 or labeled protein interactions20.
Herein we present multiphoton fluorescence anisotropy microscopy (MFAM) to image intracellular drug-target binding distribution in vivo. Specifically we demonstrate, with a Phase III drug candidate, that our approach is not only applicable to live cultured cells but also enables real-time imaging of drug-target engagement in vivo with submicron resolution.
Results
Fluorescence anisotropy and imaging setup characterization
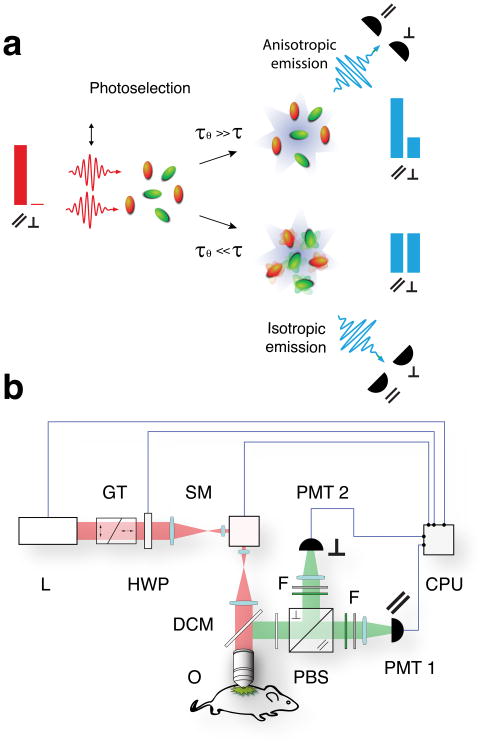
Following photoselection under polarized excitation, all excited fluorophores are aligned with the same emission dipole orientation. However, due to the presence of rotational Brownian motion, fluorophores rotate with a correlation time (τθ dependent on viscosity, molecule size and temperature21. If the excited fluorophore is free to rapidly rotate on a timescale that is shorter than its fluorescence lifetime (τθ≪ τ), emission will be isotropic (depolarized). However, when rotating slowly, the rotational correlation time will increase (τθ≫ τ) and emission will be preferentially aligned along one axis (Fig. 1a). Furthermore, a change in the fluorescence lifetime will also effect the emission polarization as molecules will have less or more time to rotate before emission. To characterize the extent of linearly polarized emission, fluorescence anisotropy (FA), a dimensionless parameter similar to FP and independent of excitation intensity (Supplementary Fig. 1, Supplementary Information: Fluorescence polarization), can be calculated. Thus, measurements of anisotropy provide insight into the rotational diffusion rate of molecules, which can be used in term to directly determine drug engagement with the target.
Figure 1. Imaging setup.
(a) Schematic representation of the two-photon photoselection process in a randomly oriented distribution of fluorophores and the resulting fluorescence emission for low (isotropic) and high (anisotropic) rotational correlation times (τθ). Blue bars indicate schematically the distribution of emission along the two orthogonal linear polarization components (‖, ⊥) as measured at the two detectors, for the two cases. Orange particles represent excited molecules. (b) The optical setup of the multiphoton fluorescence anisotropy microscope is based on a custom modified Olympus FV1000-MPE (Olympus, USA) laser scanning microscopy system equipped with an upright BX61-WI microscope (Olympus, USA). Excitation light (red beam) from a Ti:sapphire laser (L) is filtered to select a linear state of polarization and then focused onto the imaged sample. Emitted fluorescent light (green beam) is epi-collected, separated into two linearly polarized orthogonal components and spectrally filtered before non-descanned detection. GT, Glan-Thompson polarizer; HWP, half wave plate; SM, scanning mirrors; DCM, dichroic mirror; O, objective; PBS, polarization beam splitter; F, bandpass filters; PMT, photomultiplier tube; CPU, computer.
Using multiphoton microscopy for anisotropy22 offers several advantages over other imaging modalities. Extended light penetration depth enables relatively deep imaging in tissues in a physiologically relevant context, while a diminished scattering component in the near infrared reduces tissue scattering23. Therefore, multiphoton microscopy, with its low phototoxicity and high axial resolution, is ideally suited for high-resolution drug target interaction imaging within single cells.
MFAM imaging was developed using a custom adapted commercial unit (Fig. 1b). We first tested the imaging system by measuring the viscosity dependence of anisotropy for pentamethyl-BODIPY (Me5-BODIPY), an ideal fluorophore for FA (Supplementary Information: Fluorescence lifetimes), in increasing concentration of aqueous glycerol (Fig. 2 and Supplementary Fig. 2). As expected, the measured anisotropy increased with increasing viscosity.. The superior photoselectivity by two-photon excitation compared to single photon absorption24 significantly increased anisotropy values, through enhanced photoselection, resulting in increased sensitivity (Supplementary Fig. 3). Although high numerical aperture objectives are well known to produce distorted anisotropy values at the periphery of an image25 (with small impact on-axis), restricting the field of view eliminates these aberrations (Supplementary Fig. 4 and 5, Supplementary Information: Loss of polarization through imaging).
Figure 2. Anisotropy measurement.
Me5-BODIPY anisotropy dependence on viscosity, as measured in glycerol with MFAM. Measurements are obtained from two photon images of sample drops of Me5-BODIPY and calculating the anisotropy of each pixel. Average ± stdev (n=6), fitted curve added for trend visualization.
The resolution of the imaging system was determined using fluorescent microspheres. Both planar and axial measurements of a microsphere point spread function (Fig. 3a) demonstrate the high optical resolution of FA, making MFAM ideal for 3D intracellular imaging. The calculated anisotropy error in each pixel increases at the edges of the microspheres, a consequence of low count rates26, resulting in some noise artifacts and loss of anisotropy (Supplementary Fig. 6, 7). However, anisotropy remained constant above a threshold that is determined by acquisition parameters and intrinsic noise (Supplementary Fig. 6). Next we exploited the excellent optical sectioning properties for tomographic MFAM imaging of an optical phantom simulating a bound/unbound 3D environment. Two highly homogeneous populations of green fluorescent microspheres with distinct anisotropy values (Supplementary Fig. 8 and 9) were suspended in a 2% agarose solution (Fig. 3b). In both the 3D FA color-coded reconstructions and the optically sectioned planes, the two populations of microspheres are distinguishable throughout the entire phantom depth (ca. 90 microns) and assigned the correct anisotropy-based color (Fig. 3b and Supplementary Fig. 8b).
Figure 3. Optical characterization of MFAM.
(a) MFAM point spread function characterization. Planar and axial microscope fluorescence anisotropy and plain fluorescence images of a fluorescent microsphere. (b) 3D reconstructions of a mixture of two fluorescent microspheres populations with high and low anisotropy (Supplementary Fig. 8) suspended in agarose, with the respective planar images obtained across the transversal plane indicated by the orange line. Anisotropy images color-coded based on anisotropy values. Right: planar images across the transversal plane indicated (orange line). Top, fluorescence. Bottom, anisotropy. Scale bar: 20 μm.
Imaging drug target engagement in live cells
FA has traditionally been used to measure binding of small fluorescent molecules to a larger target biomolecule27. When bound, the increased molecular mass of the probe-target complex will result in a higher rotation correlation time τθ limiting molecule rotation and increasing FA (Fig. 4a), while a shift in fluorescence lifetime could also change FA. Depending on its state (bound/unbound) a single fluorescent molecule can produce two values of anisotropy, and, because anisotropy is an additive property, the measured pixel value in an FA image is the fraction-weighted sum of the two possible anisotropy values within a voxel. MFAM fluorescence anisotropy measurements of Me5-BODIPY labeled Biotin (Biotin-BODIPY) indeed show an increase in anisotropy as a function of binding to NeutrAvidin (Fig. 4a) with a similar trend to single photon measurements (Supplementary Fig. 3), due to a change in τθ (Supplementary Information: Fluorescence lifetimes).
Figure 4. Live cell imaging of target engagement.
(a) The anisotropy value of Biotin-BODIPY (mw 676.62) increases as a function of binding to NeutrAvidin (mw 60kDa) (filled triangles), which is suppressed in the presence of 10x unlabeled biotin as competitor (open triangles). Shown are average ± stdev (n=3); curve fits added for trend visualization. Inset illustration: comparison between the rotation of a free fluorophore in solution and a fluorophore bound to a protein. Due to the large difference in size of the ligand and the receptor, the increase in fluorescence anisotropy following binding is large. (b) Average ± stdev anisotropy of non-specifically interacting (green) and PARP bound (red) AZD2281-BODIPY FL (n=3). (c) 3D anisotropy image and corresponding planar and axial cross sections of live HT1080 cells loaded with AZD2281-BODIPY FL. Green, corresponds to fluorescent drug molecules that are non-specifically bound. Red, to fluorescent drug molecules with high anisotropy suggesting target (PARP) binding. Normal fluorescence images are shown in Supplementary Fig. 18. Scale bar: 16 μm. (d) 3D anisotropy image and corresponding planar and axial cross sections of live HT1080 cells loaded with AZD2281-BODIPY FL and washed for 30 minutes. Scale bars: 20 μm.
While dyes presenting longer lifetimes could be considered as alternative candidates, BODIPY was chosen due to unique characteristics that allow intracellular imaging. Specifically: i) BODIPY is relatively non-polar with the chromophore presenting electrical neutrality, therefore minimizing perturbation to the modified drug; ii) the relatively long lifetime (the BODIPY we use here has a measured lifetime ∼ 4.0 nsec) makes it particularly suitable for fluorescence polarization-based assay; iii) BODIPY is highly permeant to live cells, easily passing through the plasma membrane, where it accumulates over time; iv) it has a high extinction coefficient (EC >80,000 cm-1M-1) and a high fluorescence quantum yield (often approaching 1.0, even in water); v) it presents a lack of ionic charge and spectra that are relatively insensitive to solvent polarity and pH; and, vi) finally, it has a large two photon cross section. Although most BODIPY dyes enjoy a relatively long lifetime, dyes such as Cy3 and the Alexa dyes will be inefficient for fluorescence anisotropy imaging, with their lifetimes so short that the anisotropy of the unbound probe will be near the fundamental anisotropy, and hence indistinguishable from the bound probe. Conversely, fluorophores with extremely long lifetimes, or phosphorescence emission, are also unsuitable as the increase in rotation correlation time will not be large enough to increase the anisotropy. It is therefore important to characterize the lifetime, by fluorescence lifetime imaging microscopy (FLIM), of the possible candidate dyes for drug labeling that could be potentially used for two photon fluorescence polarization imaging. Also, dyes presenting changes in their quantum yield upon binding will bias the readout value of total anisotropy affecting the measured binding isotherm.
To test the MFAM imaging approach in a relevant drug-target system, we chose to target poly(ADP-ribose) polymerase (PARP) with the small molecule inhibitor Olaparib (AZD2281) which had been modified to bear a BODIPY-FL handle7. This model system and its cellular location had previously been well validated7,28. PARP comprises a family of enzymes that are required for DNA repair29-31, and therefore present a potential chemotherapeutic target through inhibition. Due to the high molecular weight of PARP1 (∼120 kDa) a significant increase in anisotropy is observed for “target-bound” over “free” or “intracellular drug” AZD2281-BODIPY FL, respectively (Fig. 4b and Supplementary Fig. 10a). An anisotropy threshold can then be assigned to distinguish between the bound states and MFAM intracellular imaging of drug target engagement can be obtained in 3D (Fig. 4c,d: red, PARP bound; green, “intracellular drug”). When incubated with AZD2281-BODIPY FL we observed rapid accumulation throughout the entirety of each HT1080 cell. Intracellular drug was present in the cytoplasmic region, while bound drug was present in the nucleus (Fig. 4c and Supplementary Fig. 11), which co-localized with PARP immunostaining28 (Supplementary Fig. 10b). Following extended washing cycles, the cytoplasmic AZD2281-BODIPY FL is cleared, while the nuclear, bound drug remains (Fig. 4d). Similar nuclear binding of AZD2281-BODIPY FL was observed in other cell lines reported to express PARP as well (Supplementary Fig. 12), as validated previously28.
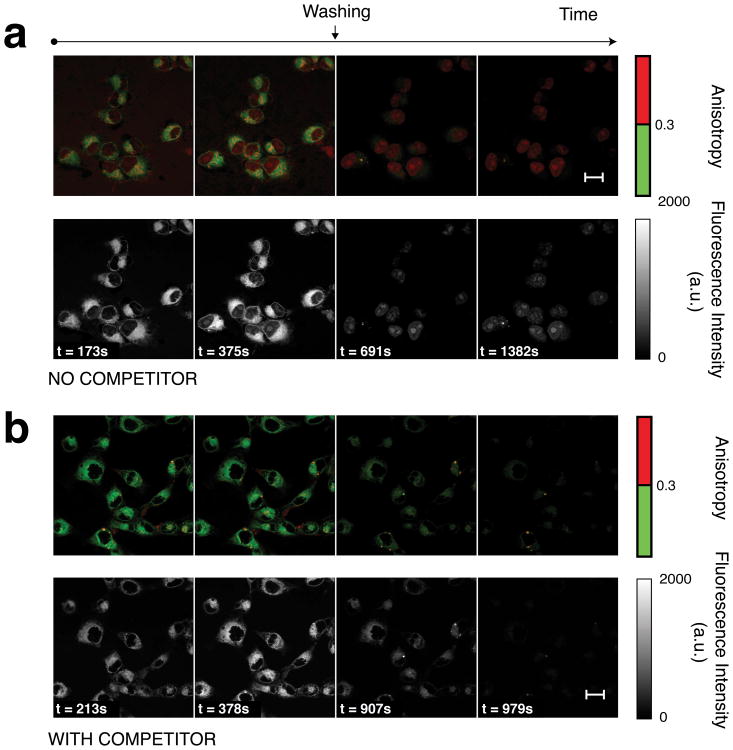
Real time in vitro measurements (Fig. 5) show AZD2281-BODIPY FL accumulated in the cytoplasm significantly more than in the nucleus, which is likely the result of interactions with intracellular membranes. Yet, only the nucleus presents high values of anisotropy, suggesting PARP binding (Fig. 5a). The high nuclear anisotropy (Fig. 5a) is not observed in the presence of unlabeled AZD2281 as competitor (5x) (Fig. 5b), which further suggests the high anisotropy measured in the nuclei was due to drug target binding and not induced by potential artifacts, such as viscosity. In addition, there was no target binding of AZD2281-BODIPY FL in the cytoplasm, as demonstrated by the significant difference between nuclear and cytoplasmic anisotropy throughout the course of loading and washing as well as the insignificant difference between cytoplasmic anisotropy in the non-competitive and competitive experiments (Fig. 6). Constant anisotropy with decreasing intensity in the cytoplasm in both non-competition and competition experiments indicates that homoFRET was not the cause of the lower anisotropy (Fig. 6). Additionally, high nuclear anisotropy is not caused by the BODIPY FL itself (Supplementary Fig. 13). Finally, there was no significant difference in fluorescence lifetime between nuclear and cytoplasmic regions in loaded HT1080 cells (Supplementary Fig. 14). Through washing and competition experiments, bound and unbound values of anisotropy in the nucleus can be determined, and the percentage of target bound AZD2281-BODIPY FL can be calculated at any point in time (Supplementary Fig. 15).
Figure 5. Imaging target engagement over time.
(a) Anisotropy and corresponding fluorescence images of AZD2281-BODIPY FL at four representative time points during drug loading and after washing. (b) Similar experiment as in (a) but in the presence of 5 fold higher concentration of unlabeled AZD2281 (competition). Scale bars: 20 μm
Figure 6. Real time imaging of drug target engagement in live cells.
Normalized intensity and anisotropy as a function of time for HT1080 cells loaded with AZD2281-BODIPY FL and washed. Values are measured in both the cytoplasmic (a) and nuclear (b) regions of the cells in the absence (black circles) and presence (gray squares) of 5 fold higher concentration of unlabeled AZD2281 (competition). Points in the graphs refer to a single experiment, average ± stdev (n=6 cells). Also shown at the right of each figure, average ± stdev at the end of the wash in the absence (black bars, n = 42 cells, 7 separate experiments) and presence (gray bars, n = 36 cells, 6 separate experiments) of unlabeled AZD2281 (5x). Bars are representative of 7 and 6 different experiments, respectively. Red arrows indicate switch from loading to washing. Fluorescence intensity refers to the sum of both perpendicular and parallel channels.
In vivo imaging of drug target engagement
Finally we used MFAM for in vivo imaging applications. In biological diffusive samples multiple scattering events limit the imaging depth by reducing the number of excitation photons in the focal area while decreasing the number of collected photons32. A decrease of the degree of polarization with resulting lower values of anisotropy is therefore present as evidenced on tissue phantom measurements (Supplementary Fig. 16, Supplementary Information: Tissue phantoms). To better characterize how diffusion and absorption limit the effective anisotropy imaging depth we first injected fluorescent microspheres into superficial tissue within a nude mouse dorsal window chamber (Fig. 7a). In vivo MFAM measurements indicated a slight depth-dependent loss of anisotropy (Fig. 7b), with a 10% loss at 100 microns, which, based on the anisotropy difference in binding measurements, does not affect target engagement measurements.
Figure 7. Imaging of AZD2281-BODIPY FL target engagement in a live mouse.
(a) In vivo fluorescence image of injected fluorescent microspheres (pink) in the vascularized (green) tissue fascia of a mouse dorsal skinfold window chamber. Scale bar: 50 μm. (b) Anisotropy of the injected fluorescent microspheres as a function of depth within the tissue fascia. Each point corresponds to a single bead measurements. (c) Confocal fluorescence image of HT1080 H2B mApple cells (red) in a mouse dorsal skinfold window chamber. After 1 -2 weeks, the tumor area is highly vascularized and, upon intravenous injection, perfused with AZD2281-BODIPY FL (green). The white square indicates the imaged area in (d). Scale bar: 100 μm. (d) In vivo anisotropy (top) and fluorescence (bottom) images of AZD2281-BODIPY FL following intravenous infusion (left) and 34 minutes later (right). Scale bar: 20 μm (e) Overall image intensity (black), nuclear intensity (gray) and nuclear anisotropy (unfilled, striped) as measured from the images in (d). Nuclear intensity and anisotropy values are average ± std error (n = 90 for image t1, n = 102 for image t1+34 min). Fluorescence intensity refers to the sum of both perpendicular and parallel channels.
After determining that our technique is viable in an in vivo setting we measured drug target engagement in a mouse in vivo. Intravenous delivery to an implanted HT1080 cell tumor showed AZD2281-BODIPY FL diffusion into the cancer cells (Fig. 7c). Cells expressing nuclear mApple-labeled H2B, which did not effect AZD2281 anisotropy measurements (Supplementary Fig. 11), were used to locate the tumor33. Binding of AZD2281-BODIPY FL to PARP in the nucleus occurred immediately upon drug infusion (Fig. 7d). The bound fraction of the drug was retained in the nucleus while the unbound extracellular and cytoplasmic drug was cleared away over time (Fig. 7d). Both the nuclear and overall fluorescence intensity decreased over time, however the nuclear anisotropy increased as unbound AZD2281-BODIPY FL was cleared (Fig. 7e).
Discussion
The ability to measure the pharmacology of drugs on a molecular level in live cells represents one of the greatest challenges in chemical biology and drug discovery9. Currently, there are no demonstrated methods for direct measurements. Subsequently, all information is based on indirect or artificial approaches that do not provide the spatiotemporal resolution and accuracy required to establish reliable models and/or do not occur in biologically relevant settings.
Here we have developed a promising novel approach utilizing multiphoton fluorescence anisotropy microscopy, which, for the first time, allows direct visualization of target bound versus unbound small molecule drugs in real time. Using a chemotherapeutic compound in Phase III clinical trials, we demonstrate that our approach is not only applicable to live cultured cells but also enables real-time imaging of drug target engagement in vivo and with submicron resolution. Our technique does not require separation between bound and free compound, is not limited to equilibrium analysis and does not affect the biological settings. As such, MFAM offers a new and fundamental imaging platform for accelerating translational drug development through insight into in vivo drug activity and inefficacy.
Methods
Cell culture
HT1080 cells (ATCC) stably expressing H2B mApple fluorescent protein28,33,34 were cultured in DMEM with 10% FBS, 1% pen-strep and 100 μg/ml geneticin (Invitrogen). HT1080 cells were cultured in DMEM with 10% FBS and 1% pen-strep. MDA-MB-436, HCC1937, and MHH-ES1 cells were cultured in RPMI with 10% FBS and 1% pen-strep. Cells were plated onto 25 mm #1 cover glass for in vitro imaging.
Tumor model
All animal experiments were performed in accordance with the Institutional Animal Care and Use Committee at Massachusetts General Hospital. Female 20-weeks old nude mice (Cox-7, Massachusetts General Hospital) were used. All surgical procedures were conducted under sterile conditions and facilitated through the use of a zoom stereomicroscope (Olympus SZ61). During all surgical procedures and imaging experiments mice were anesthetized by isofluorane vaporization (Harvard Apparatus) at a flow rate of 2 L/minute isofluorane: 2 L/minute oxygen. The body temperature of the mice was kept constant at 37°C during all imaging experiments and surgical procedures. Dorsal skinfold window chambers (DSC) were implanted one day prior to imaging following a well-established protocol. Briefly, the two layers of skin on the back of the mouse were stretched and kept in place by the DSC. One skin layer was surgically removed and replaced by a 12-mm diameter glass cover slip positioned on one side of the DSC allowing for convenient access and imaging of the tumor area. A spacer located on the DSC prevented excessive compression of both tissue and vessel guaranteeing good vascular perfusion within the tumor region.
HT1080 H2B mApple cells were harvested by trypsinization (0.25% trypsin:EDTA) and resuspended in PBS. Mice were anesthetized and approximately 106 cells (100 l 1× PBS) were injected subcutaneously into the back of female Nu/Nu mice (Cox-7, Massachusetts General Hospital, Boston, MA) aged 20 – 25 weeks in a 1:1 mixture of Matrigel (BD Biosciences). Cells were injected using a 0.5-mL insulin syringe with the needle bent at 90 degrees to better control the position of the injection site. In order to allow for the tumor to be established and neovascularization to occur, the tumors were allowed to grow for 1 - 2 weeks before DSC implantation.
Microscope configuration
The optical setup is based on a custom modified Olympus FV1000-MPE (Olympus, USA) laser scanning microscopy system equipped with an upright BX61-WI microscope (Olympus, USA) and is illustrated in details in Fig. 1B. Excitation light (red beam) from a Ti:sapphire laser (L) is filtered to select a linear state of polarization and then focused onto the imaged sample. Emitted fluorescent light (green beam) is epi-collected, separated into two linearly polarized orthogonal components and spectrally filtered before non-descanned detection. GT, Glan-Thompson polarizer; HWP, half wave plate; SM, scanning mirrors; DM, dichroic mirror; O, objective; PBS, polarization beam splitter; F, bandpass filters; PMT, photomultiplier tube; CPU, computer. The MaiTai DeepSee Ti:sapphire pulsed laser (Spectra Physics) had a pulse-width of 110 fs and a repetition rate of 80 MHz. Laser was tuned at 910 nm for two photon excitation of pentamethyl (Me5)-BODIPY and BODIPY FL. A Glan-Thompson polarizer (Newport) and a half-wave plate (Thor Labs) were inserted in the laser path toward the objective in order to create a linear state of polarization aligned along a fixed predetermined axis. Light was then focused onto the sample with a 25x 1.05 NA water immersion objective (XLPlan N, 2 mm working distance, Olympus). Fluorescence emission was detected in epi-collection mode through the same focusing objective. A dichroic filter (690 nm) diverted the fluorescent light toward a non-descanned detection path, followed by a low pass filter (685 nm). Along the detection path a polarizing beam splitter (Edmund optics) was inserted to separate the light in two orthogonal states of polarization, each one followed by a bandpass filters (490-540 nm, Chroma). Orthogonal and parallel linear polarized light was then focused and detected by two separate photomultiplier tubes (I‖ , I┴). The excitation light was linearly polarized to be parallel and perpendicular aligned to the two PMTs. Dual detector acquisition is recommended to avoid severe anisotropy artifacts induced by intensity fluctuations.
The imaging system was also operated in confocal modality. Me5-BODIPY (Exc: 493nm; Em: 503nm), BODIPY-FL (Exc: 503nm, Em: 512nm), Fluorescein (Exc: 494nm, Em: 521), and H2B-mApple (Exc: 568nm, Em: 592nm) were scanned and excited sequentially using a 473 and a 559-nm diode laser, respectively, in combination with a DM488/559-nm dichroic beam splitter. Emitted light was then separated and collected using an SDM560 beam splitter and BA490-540 and BA575-675 band-pass filters (Olympus, USA). Confocal large field-of-view images were acquired using a 2x air objective (XL Fluor 2x/340 NA 0.14) and a water-immersion objective with a high numerical aperture (NA) and large working distance (XLPlan N 25x, NA 1.05, w.d. 2mm, Olympus) were utilized.
3D multichannel serial imaging was obtained through the use of a built-in Z-axis motor with a 0.01μm step size. Different areas along the entire size of the dorsal window chamber were sequentially imaged over time using a microscope-controlled long-range XY-axis translation stage.
Optical characterization of the system
All polarizer, optical filters, polarization beamsplitter, half-wave plate, and Glan-Thompson polarizer were tested and characterized. Light from the laser was first linearly polarized using a Glan-Thompson polarizer and then aligned along a defined arbitrary axis with the use of a half waveplate. Light at the entry of the objective was measured using a polarizer and a photodetector to confirm the state of polarization remained linear along its path to the objective. Photodetectors were tested for any polarization dependence. The path from the objective to the photodetectors was also tested to assure that equal distribution of power is present between the two detectors. Voltage of the two photodiodes was slightly adjusted in order to fine tune equal signal detection. The noise contribution of the two detectors was equal for all in vitro and in vivo measurement conditions. The two detectors responded with the same linear curve along the measurement range. Calibration of the multiphoton fluorescence anisotropy microscopy systems was performed using a set of angle-adjustable linear polarizer placed in front of the detectors, and at the entry of the objective. Fluorescein in water at room temperature was used to fine tune the voltage gains on the two individual PMT sensors. 5 μl of solution were placed between a microscope slide and a cover glass and imaged. Settings were regulated such that 2 μM fluorescein solution produced an anisotropy of 0.004 after correction of the G factor. The gains settings were then maintained throughout the entirety of all measurements.
To check reproducibility over days, fluorescence slides containing uniformly distributed fluorophores were measured before each imaging sessions. Images of three different slides (each one with a different fluorophore) were taken during each imaging session to confirm that the measured anisotropy during the session matched the previous measurements. Images of the slides were taken over various time periods and at varying excitation intensity for system characterization.
Thermal variation can cause slight difference on a day-to-day basis. To compensate for them the microscope is located within a thermally stable isolating cage, mounted on an aluminum frame. Measurements over time within the same day and over several days indicate strong reproducibility in FA measurements (Supplementary Fig. 19).
Polarization distortions due to dichroic beamsplitter reflections and the objective's high numerical aperture35, such is the requirement for multiphoton microscopy, can lead to anisotropy artifacts in particular when imaging over the entire objective field of view36. While compensation could be used through different calibration methods, images collected over a restricted field of view eliminate any edge artifact (Supplementary Fig. 4 and 5, Supplementary Information: Loss of polarization through imaging).
Me5-BODIPY was brought up in DMSO (Sigma) to a 1mM stock solution. Solutions of a final concentration of 20 μM Me5-BODIPY in DMSO were mixed with glycerol (Sigma) to create varying concentrations of glycerol. Images of 5 μl drops of solution inserted between the cover glass were taken at each glycerol concentration in triplicate.
3D anisotropy phantom
Six microns green-fluorescent microspheres (InSpeck Microscope Image Intensity Calibration Kits, Invitrogen) were used for demonstrating optical sectioning capabilities. Each kit consists of seven different types of microspheres with fluorescence intensities ranging from very low to very bright (100%, 30%, 10%, 3%, 1%, 0.3%, and non-fluorescent). The fluorescence intensity of the microspheres within each vial is defined with respect to that of the microspheres with the highest fluorescence (i.e. 100%). We selected one vial containing the brightest microspheres (i.e. 100%) and another vial containing the next brightest (30%) microspheres. The fluorescence intensity of the microspheres in each vial is highly homogeneous as shown in Supplementary Fig. 8. Importantly, their value of anisotropy is not dictated by the lifetime (see Supplementary Fig. 9) or mobility of dye within the microspheres, but instead by a concentration-dependent effect (homo-FRET) (see (26) for a detailed explanation of the effect). Due to homo-FRET, the two populations of microspheres present different values of anisotropy with a highly homogenous distribution (0.274 +/- 0.008 and 0.193 +/- 0.005; see Supplementary Fig. 8). The microspheres are therefore useful for testing anisotropy distributions in phantoms26. The two populations of microspheres were mixed in equal proportion, suspended in 2% agarose and allowed it to solidify between two pieces of cover glass before imaging.
Point spread function measurements
One micron green fluorescence microspheres (Bangs Labs) on cover glass were also imaged and used for point spread function characterization.
Tissue phantoms
The tissue optical phantoms used for characterization (Supplementary Information: Tissue phantoms), contained fluorescein (20 μM) (Sigma) which brought up in 1% Intralipid (10% Solution, Baxter Healthcare) in PBS with varying concentrations of India ink following a well established protocol37. The corresponding scattering coefficient μ's was equal to 11 cm-1, a value typically considered for mouse tissue phantoms37. Optical densities of ink concentrations in PBS were determined by measuring the absorbance spectrum at 910 nm. Fluorescent images of the solution were taken at 10-micron intervals through the depth of the phantoms.
FLIM measurements
Fluorescence lifetime imaging was performed using a Zeiss 710 confocal NLO laser scanning system on an upright Zeiss Examiner stand with a 40x NA 1.1 water immersion LD C-Apochromat objective and a Becker & Hickl TCSPC system. Two-photon excitation was achieved using a Coherent Chameleon Vision II tunable laser (680-1040nm) that provided 140-femtosecond pulses at a 80-Mhz repetition rate with an output power of 3 W at the peak of the tuning curve (800 nm). Laser scanning was controlled by Zeiss Zen software and set to a pixel dwell time of 1.58 microseconds and 0.9-sec frame rate at 910nm wavelength excitation. Enhanced detection of the scattered component of the emitted (fluorescence) photons was afforded by the use of a Becker & Hickl HPM-100-40 hybrid detector, which incorporates the Hamamatsu R10467 hybrid PMT tube. Imaging was performed in the dark with blackout enclosure around the microscope to exclude external sources of light during the sensitive period of FLIM measurement. Emitted fluorescence was deflected to the non-descanned light path via a 760+ mirror and emission range was limited to 500-550nm by a Chroma filter in front of the HPM-100-40 detector. Acquisition time was typically 60 seconds with a count rate of 2-5 x 104 photons per second. Photon counting and electronic timing synchronization was controlled and measured with a Becker & Hickl TCSPC electronics (SPC-830) and SPCM software (Becker & Hickl GmbH) Lifetime decay of the fluorescence was analyzed with SPCImage software (Becker & Hickl GmbH).
Plate reader anisotropy measurements
Single photon (SP) data were collected in a plate reader set up for fluorescence polarization measurements (Tecan Sapphire 2). A G-factor for the instrument was calculated from 2μM fluorescein in water. Measurements were performed in 96 or 384 well plates.
Biotin-BODIPY FL and NeutrAvidin binding
Biotin was conjugated to Me5-BODIPY (Biotin-BODIPY) and brought to 1 mM stock solution in DMSO. Biotin-BODIPY (10 μM) was mixed with varying concentrations of NeutrAvidin (Thermo Scientific) in PBS with 1% Triton X (Sigma). Each sample was imaged in triplicate as a drop between a microscope slide and cover glass. Measurements of each sample were also performed using single photon excitation in a plate reader. Measurements were also made in the presence of 100 μM free Biotin to competitively compete with the Biotin-BODIPY.
Free molecule anisotropy
AZD2281 labeled with BODIPY FL (AZD2281-BODIPY FL) was prepared as previously described7,38. PARP1 (BioVision) was brought up in the manufactures recommended solution and added at 1.6x the concentration of AZD2281-BODIPY FL (5 μM) in imaging media containing 2.5% FBS. Free AZD2281-BODIPY FL (5 μM) (no PARP) in the same imaging media with 2.5% FBS and in DMSO solutions were also made. Images were taken of drops of solution between cover glass.
In vitro cellular imaging
Cells on 25 mm cover glass were mounted into a closed bath perfusion chamber (Warner Instruments) and perfused with a custom perfusion system that enabled solution switching in the imaging chamber. Cells were imaged in phenol red-free DMEM with 10% FBS and 1% pen-strep. AZD2281-BODIPY FL (1 μM) was perfused into the imaging chamber followed by a washout with drug free media. Images were obtained during the entire time interval at regular time points. For competition experiments, free AZD2281 (5 μM) (Selleck Chemicals) was added to the incubating solution before during and after AZD2281-BODIPY FL addition. Me5-BODIPY was used for fluorophore control experiments.
In vivo imaging
Mice were anesthetized as indicated above. When imaged for prolonged period of time, the isoflurane flow rate was reduced to ∼1 L/min. The dorsal skinfold window chamber was inserted onto a custom stabilization plate to prevent image motion artifacts and axial drifts over the time of the imaging session. Plane tracking to ensure that the same area is imaged repeatedly over the course of the drug uptake measurements was achieved through the use of a built-in Z-axis motor. Animals were warmed with a heating plate in order to keep their temperature constant.
Green fluorescent microspheres (2.5 microns) (InSpeck, Invitrogen) were dried out using an EZ-2 evaporator (Genevac) and resuspended in sterile PBS. After sonication, the microspheres were then injected into the skin tissue of a dorsal window chamber on a nude mouse. Injections were performed with a CellTram vario (Molecular Devices) through pulled glass pipettes. After the skin tissue absorbed the PBS, images of the microspheres were taken at increasing depths. The vasculature in the window chamber was imaged under brightfield with a CCD camera using a 2x objective and overlaid with a fluorescence image using the same objective.
AZD2281-BODIPY FL (7.5 μl in DMSO) was mixed with 30 μl of 1:1 solutol:dimethylacetimide (Sigma) and slowly added to 112.5 microliters of PBS. The drug was injected through a tail vein intravenously and imaged with MFAM using a 25x objective. Confocal images of drug infusion into the tumor were taken using a 2x objective.
Image processing
During image acquisition in two photon microscopy only a small number of photons are typically measured by the photodetectors with numbers ranging from tens to a few thousands with a statistical variation in the recorded number following a Poisson model of the noise. At lower counts per pixel, the error on the calculated anisotropy value will be then increasingly higher giving rise to images presenting severe noise artifacts (a rigorous treatment on the role of photon statistics on fluorescence polarization can be found in (26)). To account for noise induced variation we decided therefore to statistically weight every pixel anisotropy value within each image by its corresponding total intensity. Intensity weighted images were created by assigning colors based on anisotropy values, indicated by the scale bar, to each pixel in the fluorescence image. The intensity of the image is therefore dependent on the fluorescence intensity, while the color is dependent on the calculated anisotropy.
In addition a BM3D collaborative filter was applied on each image39.
Data analysis
Images were analyzed in Matlab (Mathworks) and ImageJ. All anisotropy measurements were calculated from the equation r = (I‖ - I┴)/(I‖ +2I┴). The detector noise of the two photodetectors was subtracted from the whole images before the data were processed. Fluorescence images represent the denominator of the anisotropy equation, which represents the entire fluorescence from the sample. Anisotropy values were obtained by defining a region of interest and measuring the average anisotropy within that region. Regions were extended to fluorescent images to calculate the corresponding intensity. Regions were extended to fluorescent images to calculate the corresponding intensity.
Supplementary Material
Acknowledgments
This project was funded in part by Federal funds from the National Heart, Lung, and Blood Institute, National Institutes of Health, Department of Health and Human Services (under Contract No. HHSN268201000044C), National Cancer Institute (T32CA079443 and P50CA086355), and from the Institute of Biomedical Engineering (under R01EB006432), and in part by 1R01CA164448-01.
Footnotes
Author Contributions: C.V. conceived and designed the study and built the setup. C.V. and J.M.D. performed the experiments, acquired and elaborated the data, and wrote the manuscript. P.F.F. contributed to the writing of the manuscript and together with C.V. developed the noise-processing algorithm. L.A.C., CV, and J.M.D. performed the FLIM measurements and L.A.C. contributed also to the writing of the manuscript. R.M. contributed to the experimental planning, and writing of the manuscript. R.W contributed to the experimental planning, data analysis, funding and writing of the manuscript. All authors reviewed manuscript drafts, provided input on the content and approved the final version.
Competing Financial Interests: The authors declare no competing financial interests.
References
- 1.Adibekian A, et al. Confirming target engagement for reversible inhibitors in vivo by kinetically tuned activity-based probes. J Am Chem Soc. 2012;134:10345–10348. doi: 10.1021/ja303400u. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Martinez Molina D, et al. Monitoring drug target engagement in cells and tissues using the cellular thermal shift assay. Science. 2013;341:84–87. doi: 10.1126/science.1233606. [DOI] [PubMed] [Google Scholar]
- 3.Paul SM, et al. How to improve R&D productivity: the pharmaceutical industry's grand challenge. Nat Rev Drug Discov. 2010;9:203–214. doi: 10.1038/nrd3078. [DOI] [PubMed] [Google Scholar]
- 4.Edgington LE, et al. Functional imaging of legumain in cancer using a new quenched activity-based probe. J Am Chem Soc. 2013;135:174–182. doi: 10.1021/ja307083b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Yang KS, Budin G, Reiner T, Vinegoni C, Weissleder R. Bioorthogonal imaging of aurora kinase A in live cells. Angew Chem Int Ed Engl. 2012;51:6598–6603. doi: 10.1002/anie.201200994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Yang Z, et al. Folate-based near-infrared fluorescent theranostic gemcitabine delivery. J Am Chem Soc. 2013;135:11657–11662. doi: 10.1021/ja405372k. [DOI] [PubMed] [Google Scholar]
- 7.Thurber GM, et al. Single-cell and subcellular pharmacokinetic imaging allows insight into drug action in vivo. Nat Commun. 2013;4:1504. doi: 10.1038/ncomms2506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.van Dam GM, et al. Intraoperative tumor-specific fluorescence imaging in ovarian cancer by folate receptor-alpha targeting: first in-human results. Nat Med. 2011;17:1315–1319. doi: 10.1038/nm.2472. [DOI] [PubMed] [Google Scholar]
- 9.Simon GM, Niphakis MJ, Cravatt BF. Determining target engagement in living systems. Nat Chem Biol. 2013;9:200–205. doi: 10.1038/nchembio.1211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Perrin F. The polarisation of fluorescence light. Average life of molecules in their excited state. Journal De Physique Et Le Radium. 1926;7:390–401. [Google Scholar]
- 11.Jameson DM, Ross JA. Fluorescence polarization/anisotropy in diagnostics and imaging. Chem Rev. 2010;110:2685–2708. doi: 10.1021/cr900267p. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Weber G. Polarization of the fluorescence of macromolecules. I. Theory and experimental method. Biochem J. 1952;51:145–155. doi: 10.1042/bj0510145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bigelow CE, Conover DL, Foster TH. Confocal fluorescence spectroscopy and anisotropy imaging system. Opt Lett. 2003;28:695–697. doi: 10.1364/ol.28.000695. [DOI] [PubMed] [Google Scholar]
- 14.Varma R, Mayor S. GPI-anchored proteins are organized in submicron domains at the cell surface. Nature. 1998;394:798–801. doi: 10.1038/29563. [DOI] [PubMed] [Google Scholar]
- 15.Sharma P, et al. Nanoscale organization of multiple GPI-anchored proteins in living cell membranes. Cell. 2004;116:577–589. doi: 10.1016/s0092-8674(04)00167-9. [DOI] [PubMed] [Google Scholar]
- 16.Weber P, Wagner M, Schneckenburger H. Fluorescence imaging of membrane dynamics in living cells. J Biomed Opt. 2010;15:046017. doi: 10.1117/1.3470446. [DOI] [PubMed] [Google Scholar]
- 17.Vrabioiu AM, Mitchison TJ. Structural insights into yeast septin organization from polarized fluorescence microscopy. Nature. 2006;443:466–469. doi: 10.1038/nature05109. [DOI] [PubMed] [Google Scholar]
- 18.Kampmann M, Atkinson CE, Mattheyses AL, Simon SM. Mapping the orientation of nuclear pore proteins in living cells with polarized fluorescence microscopy. Nat Struct Mol Biol. 2011;18:643–649. doi: 10.1038/nsmb.2056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Yu Q, Heikal AA. Two-photon autofluorescence dynamics imaging reveals sensitivity of intracellular NADH concentration and conformation to cell physiology at the single-cell level. J Photochem Photobiol, B. 2009;95:46–57. doi: 10.1016/j.jphotobiol.2008.12.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Gough AH, Taylor DL. Fluorescence anisotropy imaging microscopy maps calmodulin binding during cellular contraction and locomotion. J Cell Biol. 1993;121:1095–1107. doi: 10.1083/jcb.121.5.1095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lakowicz JR. Principles of Fluorescence Spectroscopy. 3rd. Springer; 2006. [Google Scholar]
- 22.Vishwasrao HD, Trifilieff P, Kandel ER. In vivo imaging of the actin polymerization state with two-photon fluorescence anisotropy. Biophys J. 2012;102:1204–1214. doi: 10.1016/j.bpj.2012.01.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ghosh N, Majumder SK, Gupta PK. Fluorescence depolarization in a scattering medium: effect of size parameter of a scatterer. Phys Rev E Stat Nonlin Soft Matter Phys. 2002;65:026608. doi: 10.1103/PhysRevE.65.026608. [DOI] [PubMed] [Google Scholar]
- 24.Lakowicz JR, Gryczynski I, Gryczynski Z, Danielsen E. Time-resolved fluorescence intensity and anisotropy decays of 2,5-Diphenyloxazole by two-photon excitation and frequency-domain fluorometry. J Phys Chem. 1992;96:3000–3006. doi: 10.1021/j100186a042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Axelrod D. Fluorescence polarization microscopy. Methods Cell Biol. 1989;30:333–352. [PubMed] [Google Scholar]
- 26.Lidke KA, Rieger B, Lidke DS, Jovin TM. The role of photon statistics in fluorescence anisotropy imaging. IEEE Trans Image Process. 2005;14:1237–1245. doi: 10.1109/tip.2005.852458. [DOI] [PubMed] [Google Scholar]
- 27.Rossi AM, Taylor CW. Analysis of protein-ligand interactions by fluorescence polarization. Nat Protoc. 2011;6:365–387. doi: 10.1038/nprot.2011.305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Reiner T, et al. Imaging therapeutic PARP inhibition in vivo through bioorthogonally developed companion imaging agents. Neoplasia. 2012;14:169–177. doi: 10.1593/neo.12414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Wahlberg E, et al. Family-wide chemical profiling and structural analysis of PARP and tankyrase inhibitors. Nat Biotechnol. 2012;30:283–288. doi: 10.1038/nbt.2121. [DOI] [PubMed] [Google Scholar]
- 30.Evers B, et al. Selective inhibition of BRCA2-deficient mammary tumor cell growth by AZD2281 and cisplatin. Clin Cancer Res. 2008;14:3916–3925. doi: 10.1158/1078-0432.CCR-07-4953. [DOI] [PubMed] [Google Scholar]
- 31.Gibson BA, Kraus WL. New insights into the molecular and cellular functions of poly(ADP-ribose) and PARPs. Nat Rev Mol Cell Biol. 2012;13:411–424. doi: 10.1038/nrm3376. [DOI] [PubMed] [Google Scholar]
- 32.Dunn AK, Wallace VP, Coleno M, Berns MW, Tromberg BJ. Influence of optical properties on two-photon fluorescence imaging in turbid samples. Appl Opt. 2000;39:1194–1201. doi: 10.1364/ao.39.001194. [DOI] [PubMed] [Google Scholar]
- 33.Hoffman RM, Yang M. Subcellular imaging in the live mouse. Nat Protoc. 2006;1:775–782. doi: 10.1038/nprot.2006.109. [DOI] [PubMed] [Google Scholar]
- 34.Yamamoto N, et al. Cellular dynamics visualized in live cells in vitro and in vivo by differential dual-color nuclear-cytoplasmic fluorescent-protein expression. Cancer Res. 2004;64:4251–4256. doi: 10.1158/0008-5472.CAN-04-0643. [DOI] [PubMed] [Google Scholar]
- 35.Bahlmann K, Hell SW. Electric field depolarization in high aperture focusing with emphasis on annular apertures. J Microsc. 2000;200:59–67. doi: 10.1046/j.1365-2818.2000.00739.x. [DOI] [PubMed] [Google Scholar]
- 36.Schon P, Munhoz F, Gasecka A, Brustlein S, Brasselet S. Polarization distortion effects in polarimetric two-photon microscopy. Opt Express. 2008;16:20891–20901. doi: 10.1364/oe.16.020891. [DOI] [PubMed] [Google Scholar]
- 37.Baeten J, Niedre M, Dunham J, Ntziachristos V. Development of fluorescent materials for Diffuse Fluorescence Tomography standards and phantoms. Opt Express. 2007;15:8681–8694. doi: 10.1364/oe.15.008681. [DOI] [PubMed] [Google Scholar]
- 38.Reiner T, Earley S, Turetsky A, Weissleder R. Bioorthogonal small-molecule ligands for PARP1 imaging in living cells. Chembiochem. 2010;11:2374–2377. doi: 10.1002/cbic.201000477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Dabov K, Foi A, Katkovnik V, Egiazarian K. Image denoising by sparse 3-D transform-domain collaborative filtering. IEEE Trans Image Process. 2007;16:2080–2095. doi: 10.1109/tip.2007.901238. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.