Abstract
BACKGROUND
Suppressive doses of levothyroxine therapy are reported to reduce bone mineral density (BMD) in women. Data on bone changes in premenopausal hypothyroid women with replacement therapy are limited. Hence, this study was undertaken to evaluate bone changes in this group using bone markers and BMD.
MATERIALS AND METHODS
A hospital-based case–control study including 75 premenopausal women aged 30–45 years was conducted. The subjects were categorized based on their thyroid function and history into three groups of 25 euthyroid, 25 newly diagnosed hypothyroid, and 25 hypothyroid women on 100–200 μg of levothyroxine for a minimum of 5 years. The bone changes were evaluated and compared among the groups biochemically by estimating their plasma osteocalcin and serum calcium and phosphorus and radiologically by measuring their BMD by quantitative ultrasonography. Statistical analysis was conducted by using analysis of variance, Tukey’s test, and Pearson’s correlation using IBM SPSS Statistics 20.
RESULTS
Levels of plasma osteocalcin, serum calcium, and serum phosphorus in patients on long-term levothyroxine therapy were significantly higher than those in newly diagnosed hypothyroid women and in the euthyroid group. BMD showed definite features of osteopenia (T-score: −2.26 ± 0.5) among the women in the treatment group, while it was well within the normal range in the newly diagnosed and euthyroid women. A significant correlation was found between the osteocalcin levels and T-score.
CONCLUSION
Hypothyroid women on long-term levothyroxine therapy showed signs of increased bone turnover and increased resorptive changes, though not frank osteoporosis. Hence, it may be important to evaluate the bone status of patients on levothyroxine for >5 years.
Keywords: suppressive doses, levothyroxine, premenopausal, osteocalcin
Introduction
Hypothyroidism is an endocrine disorder characterized by deficient hormone production or defective hormone action at the tissue level.1 The prevalence is 2%–15% in the general population.2 According to a population-based study conducted in South India, the prevalence of hypothyroidism in adults was found to be around 3.9%.3
The notorious effects of thyroid disorders on bone metabolism have been a topic of debate over the years. Although both osteoclastic and osteoblastic activities increase with elevated concentrations of thyroid hormones, the osteoclastic activity is predominant, with consequent bone loss.4,5
Thyroid hormones have been reported to reduce bone mineral density (BMD) in women and cause secondary osteoporotic changes.6,7 Meta-analysis of published reports has indicated that a 1% bone loss per year occurs during the postmenopausal period in women who were administered suppressive doses of thyroxine (T4).6–8
Osteoporosis is an ailment characterized by decreased bone strength. It is more prevalent among postmenopausal women, although it can also occur in men and women with underlying conditions such as hormone deficiencies and other risk factors associated with bone demineralization.6,9,10 The relation between thyroid disorders and osteoporosis was first recognized 100 years ago.9 Most of the studies on bone changes related to thyroid disorders have been carried out in postmenopausal women and post-thyroidectomy patients on suppressive doses of thyroxine.7,8,10,11 Reports on replacement doses and their effects on bone metabolism are limited.
Assessment of bone status has always been dominated by radiological diagnosis. Biomarkers of bone metabolism have always struggled to make their place, in spite of their important roles being proven over and again. Specific biochemical markers of bone turnover, such as human osteocalcin, bone alkaline phosphatase, and urinary deoxypyridinoline crosslinks and type 1 collagen-related peptide are available, with a number of applications including selection of patients for therapy, monitoring the effectiveness of therapy, prediction of bone loss, and prediction of fracture risk.1,12 Osteocalcin is a major bone matrix protein, with serum range from 5 ng/mL to 27 ng/mL. After production, it is partly incorporated into the bone matrix and the remainder is found in the blood circulation.13 A number of studies show that the circulating levels of osteocalcin reflect the rate of bone formation.6,14–16 Serum calcium and phosphorus levels are also indirect markers of bone status. There are a few studies where elevation of serum calcium and phosphorus are seen in diseases associated with bone resorption.7–9
In radiological terms, osteoporosis is defined as bone density that falls 2.5 standard deviations below the mean for young healthy adults of the same gender. It is also referred to as a T-score of −2.5.17 The advent of newer technologies such as quantitative ultrasound (QUS) has succeeded in providing solutions that avoid radiation. It has been proven to be a good radiological predictor of fracture risk.18 QUS provides information on bone density, elasticity, and structure, in addition to detecting bone loss.16,18
Data on the effect of levothyroxine replacement therapy in premenopausal women are very limited. Thus, a combination of parameters, such as serum osteocalcin, calcium, and phosphorus along with BMD assessment, were tried in our study to help assess bone remodeling in premenopausal hypothyroid women on levothyroxine therapy.
Materials and Methods
A case–control study was conducted at a tertiary-level hospital from November 2011 to October 2012. A total of 75 premeno-pausal women in the age group of 30–45 years were selected and grouped as follows: 25 hypothyroid women on levothyroxine (100–200 μg/day) for minimum 5 years (Group I), 25 newly diagnosed hypothyroid women (Group II), and 25 euthyroid women (Group III). The study was approved by the research and ethics committee of Kasturba Medical College, Manipal University. Informed consent was obtained from all the subjects. The study was carried out in accordance with the Code of Ethics of the World Medical Association (Declaration of Helsinki).
Patients excluded were post-thyroidectomy patients on suppressive doses of levothyroxine; patients with bone and joint disorders; known cases of hypertension, diabetes, and dyslipidemia; patients on steroids, oral contraceptive pills, and anticonvulsant therapy; patients on vitamin D and calcium supplements; patients with vitamin D deficiency, parathyroid disorders, long-term immobilization, and malignancy; pregnant/lactating women; and hysterectomized patients.
Blood from each subject was collected in sterile plain and ethylenediaminetetraacetic acid-containing vacutainer tubes. Serum and plasma were separated and analyzed biochemically. Estimation of serum calcium was done by the o-cresolphthalein method,19 serum phosphorus was estimated using ammonium phosphomolybdate Ultra violet method in the Roche Hitachi P.800/917 analyzer.19 Estimation of Osteocalcin was done by electrochemiluminescence immunoassay based on the sandwich principle using Elecsys N-MID-Osteocalcin kits20 (Roche Diagnostics, Mannheim, Germany) in Roche E170, e411 analyzer.
BMD of the radius bone in the forearm was measured using portable QUS. Patients’ height and weight were recorded for calculation of body mass index.
Statistical analysis
The sample size calculation was done by the statistician as per the university-approved protocol. Statistical analysis was done using analysis of variance (ANOVA) and Tukey’s method. Correlation between bone markers and thyroid hormones was determined by Pearson’s correlation.
Results
The baseline characteristics in Table 1 did not show any significant difference between the euthyroid and newly diagnosed hypothyroid groups. The data of the three groups were analyzed by ANOVA and Tukey’s test. Women between 30 years and 45 years of age were considered for selection, but the mean age of the women in groups I, II, and III was 40.4 ± 5.09, 37.88 ± 5.82, and 38.08 ± 6.06 years, respectively. Characteristics such as age, height, weight, and time since menarche showed no significant difference between the levothyroxine therapy group (Group I) and the other two groups. History of fractures was negative in all three groups. The women in the treatment group (Group I) were of average age of 40.4 ± 5.09 years, which was higher compared to that in the other two groups, but not statistically significant.
Table 1.
Baseline characteristics of the study groups (mean ± standard deviation).
| CHARACTERISTICS | ON TREATMENT GROUP I (N = 25) | NEWLY DIAGNOSED GROUP II (N = 25) | EUTHYROID GROUP III (N = 25) | P-VALUE |
|---|---|---|---|---|
| Age (years) | 40.4 ± 5.09 | 37.88 ± 5.82 | 38.08 ± 6.06 | 0.252 NS |
| Years since menarche | 35.4 ± 3.83 | 33.4 ± 3.65 | 32.1 ± 2.87 | 0.142 NS |
| Height (cm) | 154.2 ± 2.23 | 155.51 ± 3.1 | 153.2 ± 2.67 | 0.665 NS |
| Weight (kg) | 61.2 ± 5.89 | 59.30 ± 5.43 | 59.24 ± 6.20 | 0.087 NS |
| History of fractures (Y/N) | No | No | No | – |
Note: P-value <0.05 is considered significant.
Abbreviations: N, number of subjects; NS, not significant; Y/N, yes/no.
Average dosage of the group was 125 μg/day. There were two cases whose dosage was above 150 μg but their levels did not show significant elevation, probably due to the smaller duration of treatment.
The euthyroid women had a normal range (2.40 ± 0.85 international units [IU]/mL), newly diagnosed hypothyroid women had elevated TSH levels (11.96 ± 13.18 IU/mL), and the group on long-term thyroxine therapy had almost normalized TSH levels (3.93 ± 2.99 IU/mL). The TSH values showed a statistically significant difference among the three study groups (P-value: <0.001).
In Table 2 and Figure 1, osteocalcin levels in the patients on long-term levothyroxine therapy were significantly higher (26.5 ± 7.8 ng/mL) than the levels in the newly diagnosed hypothyroid women and the euthyroid group. The serum levels of calcium and phosphorus were significantly higher in the treatment group compared to the other two groups. BMD showed definite features of osteopenia (T-score = −2.26 ± 0.5) among the women in the treatment group, while it was within the normal range for the newly diagnosed and euthyroid groups, as shown in Table 2. The other observation evident in Table 3 was the effect of duration of treatment on the marker levels; osteocalcin and calcium levels, T-score, and Z-score were significantly higher in the women of the treatment group who were taking levothyroxine for >10 years than the same in cases undergoing treatment for <10 years.
Table 2.
Comparison of bone markers among the three groups.
| VARIABLES | ON TREATMENT (GROUP I) N = 25 |
NEWLY DIAGNOSED (GROUP II) N = 25 |
EUTHYROID (GROUP III) N = 25 |
P-VALUE |
|---|---|---|---|---|
| Osteocalcin, (ng/mL) | 26.45 ± 7.87 | 19.75 ± 8.1 | 18.21 ± 5.3 | <0.0001** |
| Calcium, (mg/dL) | 11.14 ± 0.41 | 10.15 ± 0.66 | 10.52 ± 0.60 | <0.0001** |
| Phosphorus, (mg/dL) | 4.94 ± 0.95 | 4.18 ± 0.66 | 4.29 ± 0.86 | 0.004* |
| T-score | −2.27 ± 0.50 | 0.02 ± 1.07 | 0.16 ± 1.29 | <0.0001** |
| Z-score | −1.79 ± 0.47 | 1.17 ± 1.05 | 0.95 ± 0.93 | <0.0001** |
Notes: N, number of subjects in the group; T-score and Z-score are expressed as standard deviations (SDs) below mean;
P-value <0.001 is very significant.
Figure 1.
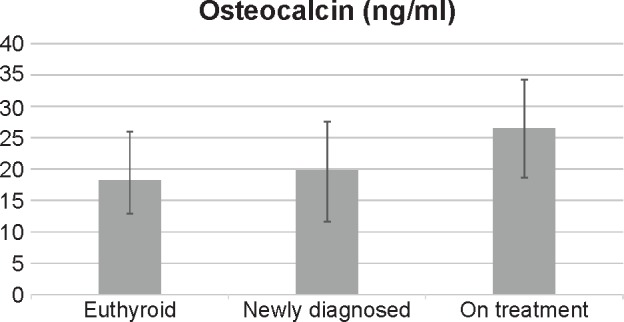
Distribution of osteocalcin among the study groups.
Table 3.
Effect of duration of treatment on bone markers and BMD scores.
| VARIABLES | ON LT- n4 SINCE 5–10 YEARS N = 16 |
ON LT-4 SINCE 11–15 YEARS N = 9 |
P-VALUE |
|---|---|---|---|
| Osteocalcin (ng/mL) | 24.62 ± 6.97 | 32.64 ± 8.81 | 0.03 |
| Calcium (mg/dl) | 10.96 ± 0.46 | 11.6 ± 0.25 | 0.007 |
| Phosphorus (mg/dl) | 4.99 ± 0.98 | 4.64 ± 0.71 | 0.465 NS |
| T-score | −2.10 ± 0.41 | −2.88 ± 0.27 | 0.001 |
| Z-score | −1.64 ± 0.38 | −2.34 ± 0.32 | 0.001 |
Notes: N, number of subjects in the group; T-score and Z-score are expressed as standard deviations (SDs) below mean; **P-value <0.001 is very significant.
In the current study, there was no significant correlation between thyroid hormones and osteocalcin.
Discussion
The objective of this study was to analyze the bone changes in premenopausal women and compare these changes among the euthyroid, newly diagnosed hypothyroid, and hypothyroid on levothyroxine therapy groups by estimation and comparison of their plasma osteocalcin, serum calcium and phosphorus levels, and BMD. As deducted from Table 1, the subjects did not show significant difference in their age, height, and weight and were therefore well matched. None of the subjects included in the study had a history of fractures (Table 1), implying that none of them had significant weak bones that could cause pathological fractures.
The patients were selected and categorized based on their thyroid profiles and thus showed a significant difference in their TSH levels (Table 4), as per the selection criteria. The patients on long-term levothyroxine therapy (100–200 μg/day) had TSH levels in the normal range, as they could have been restored to the euthyroid state.
Table 4.
Thyroid profiles of the three groups.
| VARIABLE | ON TREATMENT GROUP I N = 25 |
NEWLY DIAGNOSED GROUP II N = 25 |
EUTHYROID GROUP GROUP III N = 25 |
P-VALUE | |
|---|---|---|---|---|---|
| TSH (0.4–4.2 μIU/mL) | 3.93 ± 2.99 | 11.96 ± 13.18 | 2.40 ± 0.85 | <0.0001** | |
| T3 (0.7–2.04 ng/mL) | 1.22 ± 0.33 | 1.19 ± 0.32 | 1.46 ± 0.52 | 0.01* | |
| T4 (4.6–10.5 μg/dL) | 8.27 ± 2.3 | 7.28 ± 2.07 | 8.53 ± 2.00 | 0.07 | |
Notes:
P-value <0.05 is considered significant;
P-value <0.001 is very significant.
Abbreviations: N, number of subjects; TSH, thyroid-stimulating hormone; T3, triiodothyronine; T4, tetraiodothyronine.
In the current study, cases of hypothyroidism on levothyroxine treatment were found to show changes in their bone turnover, as reflected by their T-score, plasma osteocalcin levels, and serum calcium and phosphorus levels, in comparison to the euthyroid and newly diagnosed cases, as seen in Table 2.
Many hypotheses have been put forth to explain the deteriorating effects of thyroid disorders on bone metabolism. Some researchers believe that TSH may also have a direct effect on bone formation and bone resorption, mediated via the TSH receptor on osteoblast and osteoclast precursors, blaming the hypothyroidism itself as a cause of bone loss,21,22 while a larger group believes that not TSH, but thyroid hormones directly stimulate bone resorption. This action may be mediated by nuclear triiodothyronine (T3) receptors, which have been found in rat and human osteoblast cell lines and in osteoclasts derived from an osteoclastoma. Thyroid hormone indirectly promotes osteoclast formation and activation by inducing the expression of cytokines, prostaglandins, and the receptor activator of nuclear factor NF-kB ligand.23–25 Experimental studies in mice lacking either the thyroid receptor-α or thyroid receptor-β suggest that bone loss is mediated by the thyroid receptor.26–28
The third theory is that bone could be responding to the higher serum T4 concentrations achieved with T4 replacement, as a meta-analysis demonstrated reduced bone density in premenopausal women receiving replacement T4 therapy but not in postmenopausal women.26 In the current study, the T3 and T4 levels in the treated group were found to be in the normal range, which contradicts the finding of this meta-analysis. However, because this was not a randomized controlled trial and we included patients on treatment for >5 years, possibilities of normalization of thyroid status may not be negated. In such cases, initial dramatic sequence of events in the initial years of therapy may have led to resorptive changes. Meir et al,29 in their randomized controlled trial, concluded with a hypothesis that may explain our findings. They evaluated the effect of L-thyroxine (L-T4) treatment on bone metabolism in patients with subclinical hypothyroidism by measuring 24- and 48-week changes in markers of bone formation and resorption and BMD by dual-energy x-ray absorptiometry. They concluded that physiological L-thyroxine therapy accelerates bone turnover, reflecting early activation of bone remodeling units in the initial replacement of subclinical hypothyroidism. They also concluded that the observed bone loss could be an adaptive mechanism to the decreased bone turnover in preexistent hypothyroidism, and not as L-thyroxine-induced bone loss. There are other studies that support the same findings.30,31 BMD values indicate osteopenia (T-score = −2.26 ± 0.5) among the women in the treatment group, while the values were within the normal range for the newly diagnosed and euthyroid groups. Terri et al32 stated in their study that women treated with L-thyroxine at supraphysiologic dosages over a long term may be predisposed to decreased bone density in the hip, which may increase the risk of age-related bone loss. However, a study by Franklin et al9 did not show any significant changes in the BMD values in premenopausal women on replacement doses of levothryoxine.
Our study showed increased resorption in the forearm, specifically the radius, which aligns with the findings of Vestergaard et al33 and Tremolliers et al34 in their studies. According to a study by Kung et al,35 primary hypothyroidism patients on levothyroxine therapy have demonstrated low values for femoral and radial BMDs. Multiple studies suggest that there is a site-dependent resorption in long-term levothyroxine therapy, and it is not generalized to the whole skeleton.5,25,27
In our study, osteocalcin levels were significantly higher (26.5 ± 7.8 ng/mL) in patients on long-term levothyroxine therapy than in the newly diagnosed hypothyroid women and the euthyroid group, indicative of an overall increase in the bone remodeling and increased activation of osteoblastic as well as indirect activation of osteoclastic activity.
Studies by Atalay et al36 and others37,38 have confirmed that osteocalcin was among the best predictors of osteoporosis. In a study by Harikumar et al,39 osteocalcin had significant correlation with BMD in postmenopausal women with osteoporosis, which was not found in our study.
In our study, there were significant differences in both the calcium and the phosphorus levels between the treatment group and the other two groups. However, the serum calcium and phosphorus levels were only slightly above the normal range in the treatment group. The possible explanation is that because osteocalcin is elevated in this group, it increases the bone turnover of calcium and phosphorus, resulting in the latter’s release into the serum.40 Serum calcium and phosphorus levels have been used routinely in the past for bone status assessment; hence, it was evaluated in this study.17,41,42 There are a few studies that have found significant hypercalcemia in suppressive thyroid therapy.5,7 Studies state that patients on replacement doses of levothyroxine do not show significant increase in serum calcium or phosphorus levels individually.6,41,42
In the current study, it was found that osteocalcin, calcium levels, T-score, and Z-score were significantly higher in the treatment group who were on levothyroxine therapy for >10 years than in the cases undergoing treatment for <10 years’ duration (Table 3).
Long-term L-thyroxine therapy for a minimum of 5 years was found to be associated with decreased hip bone density in premenopausal women, according to Terri et al32 and other studies,43,44 which agrees with our findings. According to a study by Sijanovi6 and others,27,45 women on 10 years of levothyroxine therapy show a very significant loss of bone, compared to the results in women on shorter duration of treatment, though there are changes starting from the first year of treatment. Our study showed significant bone loss in women of the treatment group of >10 years when compared to the lesser-duration group, which aligns very well with these studies.
Limitations of the study were that we did not evaluate the vitamin D and parathormone levels in the subjects, and these could have had an effect on the bone status of the subjects in case of vitamin D deficiency and hypoparathyroidism. However, because we excluded these conditions by detailed clinical history and previous laboratory reports, as well as estimated the calcium and phosphorus levels in all the subjects, the confounding effects of these factors have been minimized.
Conditions that could have a direct or indirect effect on the bone status of the patients have been excluded, thereby helping in correlating better the bone changes in the patient group with the effects after treatment. We have not only evaluated the bone turnover by using the biochemical markers but have also assessed it through radiological examination.
Estimation of markers of bone resorption help in getting a complete picture of the bone status. We intend to carry out a complete analysis of both bone formation and bone resorption markers in the treatment group. Follow-up of these subjects into menopause and evaluation of their risk for fractures can be done.
Conclusion
To summarize, hypothyroid women on long-term levothyroxine therapy showed signs of increased bone turnover and increased resorptive changes, though not frank osteoporosis. Hence, we conclude that careful monitoring of bone status in patients receiving thyroxine replacement therapy may be advocated, in an attempt to avoid possible negative outcomes of overtreatment.
Footnotes
ACADEMIC EDITOR: Marlene von Friederichs-Fitzwater, Editor in Chief
FUNDING: The funding for the research was provided by the Manipal Foundation. The authors confirm that the funder had no influence over the study design, content of the article, or selection of this journal.
COMPETING INTERESTS: Authors disclose no potential conflicts of interest.
Paper subject to independent expert blind peer review by minimum of two reviewers. All editorial decisions made by independent academic editor. Upon submission manuscript was subject to anti-plagiarism scanning. Prior to publication all authors have given signed confirmation of agreement to article publication and compliance with all applicable ethical and legal requirements, including the accuracy of author and contributor information, disclosure of competing interests and funding sources, compliance with ethical requirements relating to human and animal study participants, and compliance with any copyright requirements of third parties. This journal is a member of the Committee on Publication Ethics (COPE).
Author Contributions
Conceived and designed the experiments: RPB, AH. Analyzed the data: AC. Wrote the first draft of the manuscript: RPB. Contributed to the writing of the manuscript: AH, PM, VD’S. Agree with manuscript results and conclusions: RPB, AC, AH, PM and VD’S. Jointly developed the structure and arguments for the paper: RPB, AC, AH, PM and VD’S. Made critical revisions and approved final version: RPB, AC, AH, PM and VD’S. All authors reviewed and approved of the final manuscript.
REFERENCES
- 1.Demers L, Spencer C. The thyroid: pathophysiology and thyroid function testing. In: Burtis CA, Edward R, Ashwood E, David R, Bruns D, editors. Tietz Text Book of Clinical Chemistry and Molecular Diagnostics. 4th ed. Philadelphia: W.B Saunders Company; 2005. pp. 2068–2072. [Google Scholar]
- 2.Hollowell JG, Stehling NW, Flanders D, Serum TSH. T4 and thyroid antibodies in the United States population (1988 to 1994): National Health and Nutrition Examination Survey (NHANES III) J Clin Endocrinol Metab. 2002;87:489–499. doi: 10.1210/jcem.87.2.8182. [DOI] [PubMed] [Google Scholar]
- 3.Usha Menon V, Sundaram KR, Unnikrishnan AG, Jayakumar RV, Nair V, Kumar H. High prevalence of undetected thyroid disorders in an iodine sufficient adult south Indian population. J Indian Med Assoc. 2009;107:72–77. [PubMed] [Google Scholar]
- 4.Greenspan SL, Greenspan FS. The effect of thyroid hormone on skeletal integrity. Ann Intern Med. 1999;130:750–758. doi: 10.7326/0003-4819-130-9-199905040-00016. [DOI] [PubMed] [Google Scholar]
- 5.Jameson JL. Disorders of thyroid gland. In: Fauci A, Braunwald E, Kasper DL, et al., editors. Principles of Internal Medicine by Harrisons. 17th ed. USA: McGraw-Hill; 2008. pp. 2236–2237. [Google Scholar]
- 6.Sijanovi S, Karner I. Bone loss in premenopausal women on long term suppressive therapy with thyroid hormone. Med Gen Med. 2001;3(4):10–15. [PubMed] [Google Scholar]
- 7.Baqi L, Payer J, Killinger Z, et al. Thyrotropin versus thyroid hormone in regulating bone density and turnover in premenopausal women. Endocr Regul. 2010;44:57–63. doi: 10.4149/endo_2010_02_57. [DOI] [PubMed] [Google Scholar]
- 8.Baran DT. Hyperthyroidism, thyroid hormone replacement and osteoporosis. In: Favus MJ, editor. Primer on the Metabolic Bone Disease and Disorders of Mineral Metabolism. 3rd ed. Philadelphia: Lippincott Raven; 1996. pp. 286–288. [Google Scholar]
- 9.Franklyn JA, Betteridge J, Daykin J. Long term thyroxine treatment and bone mineral density. Lancet. 1992;340:9–13. doi: 10.1016/0140-6736(92)92423-d. [DOI] [PubMed] [Google Scholar]
- 10.Agrawal S, Jain A, Mahajan D, Raghunandan C. Correlation of bone mineral density with biochemical markers in postmenopausal women. Ind J Clin Biochem. 2009;24:262–265. doi: 10.1007/s12291-009-0049-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.South-Paul JE. Osteoporosis: part I. Evaluation and assessment. Am Fam Physician. 2001;63:897–904. 908. [PubMed] [Google Scholar]
- 12.Tofighi P, Shoushtarizadeh P, Hossein-nezhad A, et al. Bone markers status in Graves’ disease before and after treatment. J Publ Health. 2008;1:30–35. [Google Scholar]
- 13.Banfi G, Daverio R. In vitro stability of osteocalcin. Clin Chem. 1994;40:833–834. [PubMed] [Google Scholar]
- 14.Barsal G, Taneli F, Atay A, Hekimsoy Z, Erciyas F. Serum osteocalcin levels in hyperthyroidism before and after antithyroid therapy. Tohoku J Exp Med. 2004;203:183–188. doi: 10.1620/tjem.203.183. [DOI] [PubMed] [Google Scholar]
- 15.Van de Ven AC, Erdtsieck RJ. Changes of bone mineral density, quantitative ultrasound parameters and markers of bone turnover during treatment of hyperthyroidism. Ned J Med. 2008;66:428–431. [PubMed] [Google Scholar]
- 16.Glüer CC. Quantitative ultrasound techniques for the assessment of osteoporosis: expert agreement on current status. The International Quantitative Ultrasound Consensus Group. J Bone Miner Res. 1997;12:1280–1288. doi: 10.1359/jbmr.1997.12.8.1280. [DOI] [PubMed] [Google Scholar]
- 17.Lorenzo JA, Canalis E, Raisz LG. Metabolic bone disease. In: Kronenberg HM, Melmed S, Polonsky KS, Larsen PR, Price DC, editors. Williams Textbook of Endocrinology. 11th ed. Philidelphia: W.B Saunders company; 2008. pp. 1269–1300. [Google Scholar]
- 18.Williams ED, Daymond TJ. Evaluation of calcaneus bone densitometry against hip and spine for diagnosis of osteoporosis. Br J Radiol. 2003;76:123–128. doi: 10.1259/bjr/56105358. [DOI] [PubMed] [Google Scholar]
- 19.Endres D, Rude R. Bone and mineral metabolism. In: Burtis C, Edward R, Ashwood E, Bruns D, editors. Tietz Text Book of Clinical Chemistry and Molecular Diagnostic. 4th ed. Philadelphia: W.B Saunders Company; 2005. pp. 1901–1941. [Google Scholar]
- 20.Gundberg CM. Biology, physiology, and clinical chemistry of osteocalcin. J Clin Ligand Assay. 1998;21(2):128–138. [Google Scholar]
- 21.Mazziottia G, Porcellia T, Patellia I, Vescovib P, Giustinaa A. Serum TSH values and risk of vertebral fractures in euthyroid post-menopausal women with low bone mineral density. Bone. 2010;46(3):747–751. doi: 10.1016/j.bone.2009.10.031. [DOI] [PubMed] [Google Scholar]
- 22.Laurent E, Markowicz E, Lisart J, Maertelaer V. Serum triiodothyronine, bone turnover, and bone mass changes in euthyroid pre- and postmenopausal women. Calcif Tissue Int. 1991;49(2):95–100. doi: 10.1007/BF02565128. [DOI] [PubMed] [Google Scholar]
- 23.Basset P, Okada A, Chenard MP, et al. Matrix metalloproteinases as stromal effectors of human carcinoma progression: therapeutic implications. Matrix Biol. 1997;15:535–541. doi: 10.1016/s0945-053x(97)90028-7. [DOI] [PubMed] [Google Scholar]
- 24.Kanatani M, Sugimoto T, Sowa H, Kobayashi T, Kanzawa M, Chihara K. Thyroid hormone stimulates osteoclast differentiation by a mechanism independent of RANKL–RANK interaction. J Cell Physiol. 2004;201:17–25. doi: 10.1002/jcp.20041. [DOI] [PubMed] [Google Scholar]
- 25.Miura M, Tanaka K, Komatsu Y, et al. A novel interaction between thyroid hormones and 1,25(OH)(2)D(3) in osteoclast formation. Biochem Biophys Res Commun. 2002;291:987–994. doi: 10.1006/bbrc.2002.6561. [DOI] [PubMed] [Google Scholar]
- 26.Abu EO, Bord S, Horner A. The expression of thyroid hormone receptors in human bone. Bone. 1997;21:137–142. doi: 10.1016/s8756-3282(97)00097-5. [DOI] [PubMed] [Google Scholar]
- 27.Reddy PA, Harinarayan C, Sachan A, et al. Review. Effects of thyrotoxicosis on bone. Indian J Med Res. 2012;135:277–282. [PMC free article] [PubMed] [Google Scholar]
- 28.Garton M, Reid I, Loveridge N, et al. Bone mineral density and metabolism in premenopausal women taking l-thyroxine replacement therapy. J Clin Endocrinol. 1994;41(6):747–755. doi: 10.1111/j.1365-2265.1994.tb02789.x. [DOI] [PubMed] [Google Scholar]
- 29.Meier C, Beat M, Guglielmetti M. Restoration of euthyroidism accelerates bone turnover in patients with subclinical hypothyroidism: a randomized controlled trial. Osteoporos Int. 2004;15:209–216. doi: 10.1007/s00198-003-1527-8. [DOI] [PubMed] [Google Scholar]
- 30.Taleman JM, Kaufman X, Janssens H, Vandecauter A, Vermeulen A. Reduced forearm bone mineral content and biochemical evidence of increased bone Turnover in women with euthyroid goitre treated with thyroid hormone. Clin Endocrinol. 1990;33(1):107–117. doi: 10.1111/j.1365-2265.1990.tb00471.x. [DOI] [PubMed] [Google Scholar]
- 31.Chai R, Ye Z, Zhan Z, Liu W, Yu M, Liu Y. The effects of levothyroxine replacement therapy on bone and mineral metabolism in patients with hypothyroidism. Zhonghua Nei Ke Za Zhi. 1999;38(1):18–21. [PubMed] [Google Scholar]
- 32.Terri L, Kerrigan J, Kelly A, Lewis E, Baran T. Long-term L-thyroxine therapy is associated with decreased hip bone density in premenopausal women. JAMA. 1988;259(21):3137–3141. [PubMed] [Google Scholar]
- 33.Vestergaard P, Weeke J, Hoeck HC, et al. Fractures in patients with primary idiopathic hypothyroidism. Thyroid. 2000;10(4):335–340. doi: 10.1089/thy.2000.10.335. [DOI] [PubMed] [Google Scholar]
- 34.Trémollières F, Pouillès JM, Louvet JP, Ribot C. Transitory bone loss during substitution treatment for hypothyroidism. Results of a two year prospective study. Rev Rhum Mal Osteoartic. 1991;58(12):869–875. [PubMed] [Google Scholar]
- 35.Kung AWC, et al. The effect of thyroid hormone on bone metabolism and osteoporosis. J Hong Kong Med Assoc. 1994;46(3):247–248. [Google Scholar]
- 36.Atalay S, Elci A, Kayadibi H, Onde Can B, Aka N. Diagnostic Utility of osteocalcin, undercarboxylated osteocalcin, and alkaline phosphatase for osteoporosis in premenopausal and postmenopausal women. Ann Lab Med. 2012;32:23–30. doi: 10.3343/alm.2012.32.1.23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ross DS, Ardisson LJ, Nussbaum SR, Meskell MJ. Serum osteocalcin in patients taking L-Thyroxine who have subclinical hyperthyroidism. J Clin Endocrinol Metabol. 1991;72:2507–2509. doi: 10.1210/jcem-72-2-507. [DOI] [PubMed] [Google Scholar]
- 38.Ribot C, Tremollieres F, Pouilles JM, Louvet JP. Bone mineral density and thyroid hormone therapy. Clin Endocrinol. 1990;33(2):143–154. doi: 10.1111/j.1365-2265.1990.tb00477.x. [DOI] [PubMed] [Google Scholar]
- 39.Hari Kumar KV, Muthukrishnan J, Verma A, Modi KD. Correlation between bone markers and bone mineral density in postmenopausal women with osteoporosis. Endocr Pract. 2008;14(9):1102–1107. doi: 10.4158/EP.14.9.1102. [DOI] [PubMed] [Google Scholar]
- 40.Seibel MJ. Biochemical markers of bone turnover part I: biochemistry and variability. Clin Biochem Rev. 2005;26(I):97–122. [PMC free article] [PubMed] [Google Scholar]
- 41.Kisacol G, Kaya A, Gonen S, Tunc R. Bone and calcium metabolism in subclinical hyperthyroidism and hypothyroidism. Endocr J. 2003;50(6):657–661. doi: 10.1507/endocrj.50.657. [DOI] [PubMed] [Google Scholar]
- 42.Glenn M, Harris S, Sokoll L, Dawson-Hughes B. Accelerated bone loss in hypothyroid patients overtreated with L-thyroxine. Ann Intern Med. 1990;113(4):265–269. doi: 10.7326/0003-4819-113-4-265. [DOI] [PubMed] [Google Scholar]
- 43.Galliford TM, Murphy E, Williams AJ, Bassett JH, Williams GR. Effects of thyroid status on bone metabolism: a primary role for thyroid stimulating hormone or thyroid hormone? Minerva Endocrinol. 2005;30(4):237–246. [PubMed] [Google Scholar]
- 44.Gogakos AI, Bassett JHD, Williams GR. Thyroid and bone. Arch Biochem Biophys. 2010;503(1):129–136. doi: 10.1016/j.abb.2010.06.021. [DOI] [PubMed] [Google Scholar]
- 45.Adlin EV, Maurer AH, Marks AD, Channick BJ. Bone mineral density in postmenopausal women treated with L-thyroxine. Am J Med. 1991;90(3):360–366. doi: 10.1016/0002-9343(91)90577-k. [DOI] [PubMed] [Google Scholar]


