Abstract
In previous studies undertaken by our group, a series of 1-(1H-1,2,4-triazole-1-yl)-2-(2,4-difluorophenyl)-3-substituted-2-propanols (1a–r), which were analogs of fluconazole, was designed and synthesized by click chemistry. In the study reported here, the in vitro antifungal activities of all the target compounds were evaluated against eight human pathogenic fungi. Compounds 1a, 1q, and 1r showed the more antifungal activity than the others.
Keywords: triazole, synthesis, antifungal activity, CYP51
Introduction
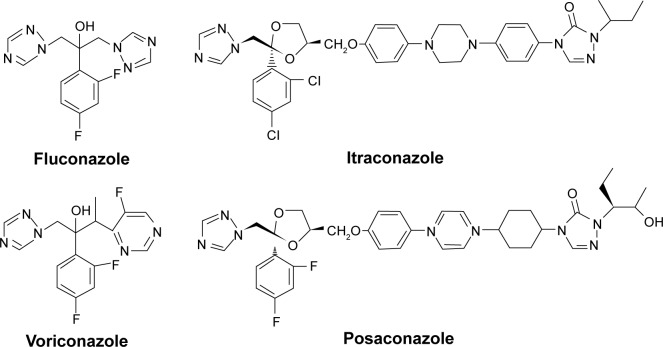
In the past three decades, deep fungal infections have sharply escalated due to the employment of clinical antitumor drugs and immunosuppressants; the widespread application of broad-spectrum antibiotics, cancer chemotherapy, radiotherapy, peritoneal dialysis, organ transplantation; and immune deficiency disorders, especially AIDS.1,2 Currently, aspergillosis, cryptococcosis, and candidiasis are three major clinical fungal infections in immunocompromised individuals.3,4 Azole nitrogen compounds have been progressively getting people’s attention, mainly because of their superiority in antifungal therapy but also for their contribution in the treatment of various microbes.5 Azoles (fluconazole, itraconazole, voriconazole, and posaconazole, Figure 1) are one very significant class of compounds for treating deep fungal infections in the clinical context.6 One of the principal problems in the treatment of Candida albicans infections is the spread of antifungal drug resistance, mainly in patients chronically subjected to antimycotic therapy such as HIV-infected people.7,8
Figure 1.
Triazole antifungal agents used in clinical therapy.
More recently, there has been a development in the number of antifungal drugs available. Five major classes of antifungal compounds are currently in clinical use: polyenes, azole derivatives, allylamines, thiocarbamates, and fluoropyrimidines.9–12 In spite of this growing list of antifungal agents in the process of being studied, treatment of fungal diseases remains unsatisfactory. The limitations of current antifungal drugs, increased incidence of systemic fungal infections, and rapid development of drug resistance have emphasized the need for the discovery of new antifungal agents with a new mode of action and fewer side effects.4,10,13–15
In particular, azole drugs are very important antifungal agents widely used in the clinical context.16 Azoles exert antifungal activity through the inhibition of cytochrome P450 14α-demethylase (CYP51), which is crucial in the process of biosynthesis of ergosterol. The CYP51 enzyme contains an iron protoporphyrin unit located in its active site, which catalyzes the oxidative removal of the 14α-methyl group of lanosterol by typical monooxygenase activity.17 Azole antifungal agents bind to the iron of the porphyrin and cause blockade of the fungal ergosterol biosynthesis pathway by preventing the access of the natural substrate lanosterol to the active site of the enzyme.18
In previous research by our group,19–27 numerous studies on the structure–activity relationships (SAR) of antifungal azoles were undertaken, and these studies led to new compounds endowed with better biological and pharmacological properties. These studies indicated that the triazole ring, the difluorophenyl group, and the hydroxyl group are the pharmacophores of antifungal agents. We focused our attention on installing various substituted benzyl groups of the side chain by click chemistry.
According to the results of these studies, we designed a series of 1-(1H-1,2,4-triazole-1-yl)-2-(2,4-difluorophenyl)-3-substituted-2-propanols (1a–r, Figure 2) containing a triazole ring, a difluorophenyl group, a hydroxyl group, and a side chain containing a piperazine group. In our design, we systematically altered the structure of fluconazole as a platform and tried to insert a 1,2,3-triazole group into the side chain.
Figure 2.
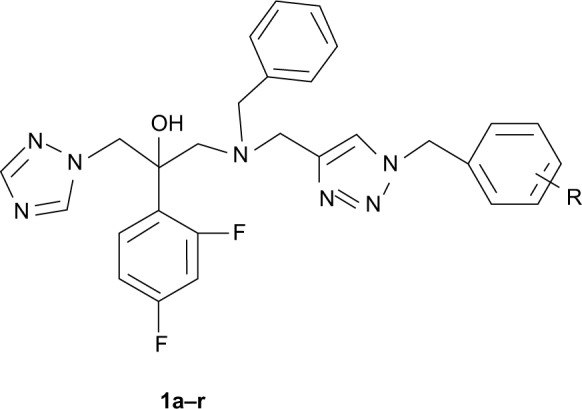
Generic structure of the designed fluconazole analogs.
Chemistry
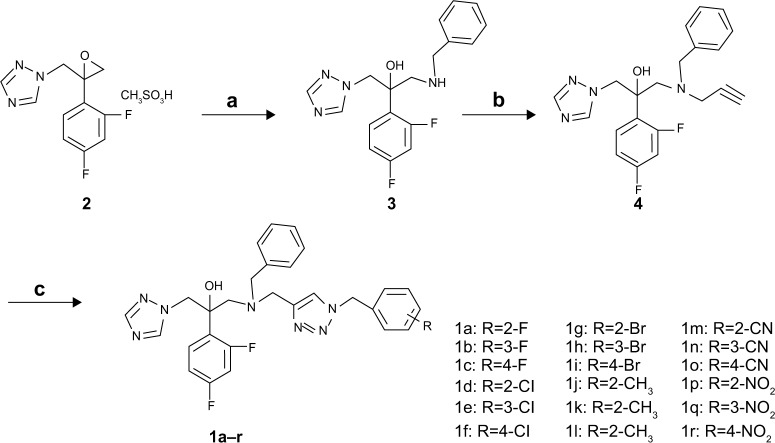
The general synthetic methodology for the preparation of the title compounds (1a–r) is outlined in Figure 3. Compound 3 was synthesized by ring-open reaction of oxirane 2 with benzylamine. Then, compound 3 was transformed into compound 4 by reacting with propargyl bromide in the presence of KI and K2CO3 in acetonitrile. The target compounds were obtained by using click chemistry28 with various substituted benzyl azides.
Figure 3.
Synthesis of the target compounds 1a–r.
Notes: Conditions: (a) Et3N, benzylamine, EtOH, Et3N, reflux, 5 hours, 72%; (b) propargyl bromide, KI, K2CO3, CH3CN, rt, 5–6 hours, 81%; (c) NaN3, substituted benzyl bromide, dimethyl sulfoxide, CuSO4·5H2O, sodium ascorbate, rt, 12 hours, 60%–70%.
Pharmacology
The in vitro antifungal activities of all the target compounds were evaluated against eight human pathogenic fungi – C. albicans 14053, C. albicans 20352, Candida parapsilosis, Cryptococcus neoformans, Candida glabrata, Aspergillus fumigatus, Trichophyton rubrum, and Microsporum gypseum – which are often encountered clinically, and were compared with itraconazole (ICZ), voriconazole (VCZ), and fluconazole (FCZ). All eight human pathogenic fungi were provided by Shanghai Changzheng Hospital; FCZ, ICZ, and VCZ, which served as the positive control, were obtained from their respective manufacturers.
The in vitro minimal inhibitory concentrations (MICs) of the compounds were determined by the micro-broth dilution method in 96-well micro test plates according to the methods defined by the National Committee for Clinical Laboratory Standards.29 The MIC80 was defined as the first well containing an approximate 80% reduction in growth compared with growth in the drug-free well. For assays, the title compounds to be tested were dissolved in dimethyl sulfoxide (DMSO), serially diluted in growth medium, inoculated, and incubated at 35°C. The growth MIC was determined at 24 hours for C. albicans and at 72 hours for C. neoformans.
Materials and methods
Melting points (MPs) were measured on a YRT-3 Melting Point Tester (Tianda Tianfa Technology Co., LTD, Tianjin, People’s Republic of China) and are presented uncorrected. Proton nuclear magnetic resonance (1H NMR) spectra are recorded in CDCl3, unless otherwise indicated, with an Avance II 300 spectrometer (Bruker Corporation, Billerica, MA, USA), using tetramethlysilane as the internal standard. Electrospray ionization-mass spectrometry (ESI-MS) spectra were obtained using an API 3000 liquid chromatography–mass spectrometry spectrometer (Thermo Fisher Scientific, Waltham, MA, USA). Thin-layer chromatography analysis was carried out on GF254 silica gel plates (Qingdao Haiyang Chemical Co Ltd, Qingdao, People’s Republic of China). Column chromatography was performed with silica gel 60 G (Qingdao Haiyang Chemical Co Ltd). The solvents and reagents were used as received or dried prior to use, as needed.
Compound 3: 1-(1H-1,2,4-triazol-1-yl)-2-(2,4-difluorophenyl)-3-(benzylamino)-2-propanol
To a stirred mixture of 1-[2-(2,4-difluorophenyl)-2,3-epoxypropyl]-1H-1,2,4-triazole methanesulfonate (2) (16.5 g, 0.05 mol), C2H5OH (200 mL) and N(C2H5)3 (30 mL), benzylamine (6.42 g, 0.06 mol) were added and heated at 70°C–80°C for 5 hours. The reaction was monitored by thin layer chromatography. After filtration, the filtrate was evaporated under reduced pressure. Water was added to the residue, which was then extracted with ethyl acetate. The extract was washed with saturated NaCl solution, dried over anhydrous Na2SO4, and evaporated. The residue was separated and purified readily by chromatography on silica gel to afford Compound 3 (12.4 g, 72% yield).
Compound 4: 1-(1H-1,2,4-triazol-1-yl)-2-(2,4-difluorophenyl)-3-(N-benzyl-N-propargyl amino)-2-propanol
To a stirred mixture, Compound 3 (3.44 g, 0.01 mol), propargyl bromide (2.36 g, 0.02 mol), KI (166 mg, 0.001 mol), K2CO3 (3.45 g, 0.025 mol), and CH3CN (50 mL) was stirred at room temperature for 6 hours. The reaction was monitored by thin layer chromatography. When the reaction was completed, the solid was filtrated, washed with CH3CN, then the filtrate was concentrated in a vacuum. Column chromatography of the residue afforded Compound 4 as an oil (3.09 g, 81% yield).
General procedure for the preparation of Compounds 1a–r
A mixture of NaN3 (100 mg, 1.4 mmol), 2-fluorobenzyl bromide (200 mg, 1.2 mmol), and DMSO (15 mL) was stirred at room temperature for 6 hours. Then, to this was added Compound 4 (229 mg, 0.6 mmol), sodium ascorbate (20 mg), CuSO4.5H2O (25 mg), and H2O (1 mL). The mixture was stirred at room temperature for 2 hours, then NH3·H2O was added carefully, then extracted with ethyl acetate. The organic layer was acidified with dilute hydrochloric acid, then the pH of the aqueous layer was adjusted to about 7.0 by saturation with sodium bicarbonate, then the solution was extracted with ethyl acetate, washed with water, NaHCO3 and NaCl solutions, dried with Na2SO4, and concentrated in a vacuum to afford Compound 1a (212 mg, 69% yield; MP, 92.0°C–94.0°C; 1H NMR [300 MHz, CDCl3] δ: 8.06 [1H, s, triazole-H], 7.72 [1H, s, triazole-H], 7.63–7.56 [1H, m, Ar], 7.39–7.12 [8H, m, Ar-H, triazole-H], 6.83–6.69 [2H, m, Ar-H], 5.60 [2H, s, Ar-CH2-], 4.50–4.36 [2H, m, CH2], 3.72–3.38 [4H, m, -CH2-N-CH2-], 3.28–2.90 [2H, m, CH2], 13C NMR [75 MHz, CDCl3] δ: 163.2, 159.0, 151.1, 130.2, 130.0, 129.9, 129.9, 129.8, 129.8, 129.8, 129.7, 129.6, 129.6, 129.5, 129.5, 129.4, 129.3, 128.5, 128.0, 116.2, 111.8, 104.4, 73.0, 59.7, 57.5, 56.0, 35.6, 49.2; ESI-MS, m/z calculated for C28H26F3N7O, 533.2, found [M+H]+ 534.5). (The characterization of Compounds b–r is presented in the “Supplementary materials” section).
Results
The in vitro antifungal activities are summarized in Table 1, along with the MIC values (in μg/mL) against different pathogenic fungi, in comparison with ICZ, VCZ, and FCZ. The results of the study of the antifungal activities in vitro show that all 18 target compounds (1a–r) were active against nearly all fungi tested to some extent, except against A. fumigatus and C. glabrata. The MIC80 value of Compounds 1a and 1h was four times lower than that of FCZ against C. albicans 14053 in vitro (with an MIC80 value of 0.25 μg/mL). The MIC80 value of Compounds 1a, 1q, and 1r was 256 times lower than that of FCZ against M. gypseum in vitro, and the same as VCZ against M. gypseum in vitro (with an MIC80 value of 0.25 μg/mL). The MIC80 value of most target compounds against C. neoformans was the same as that of the control drugs. However, most of the target compounds’ antifungal activities were not as good as those of ICZ and VCZ.
Table 1.
Antifungal activities of the title compounds in vitro (80% minimal inhibitory concentration μg/mL)
| Compound | -R | Candida albicans 14053 | C. albicans 20352 | Candida parapsilosis | Cryptococcus neoformans | Candida glabrata | Aspergillus fumigatus | Trichophyton rubrum | Microsporum gypseum |
|---|---|---|---|---|---|---|---|---|---|
| 1a | 2-F | 2.00000 | 4.0000 | 16.00000 | 2.0 | >64.0 | >64.00 | 32.0000 | 0.50 |
| 1b | 3-F | 0.25000 | 0.5000 | 1.00000 | 0.5 | 64.0 | >64.00 | 1.0000 | 0.25 |
| 1c | 4-F | 0.50000 | 1.0000 | 8.00000 | 4.0 | 64.0 | >64.00 | 16.0000 | 2.00 |
| 1d | 2-Cl | 1.00000 | 0.2500 | 8.00000 | 8.0 | 32.0 | >64.00 | 16.0000 | 0.50 |
| 1e | 3-Cl | 2.00000 | 8.0000 | >64.00000 | 16.0 | >64.0 | >64.00 | 32.0000 | 0.25 |
| 1f | 4-Cl | 16.00000 | 32.0000 | 64.00000 | 8.0 | >64.0 | >64.00 | 64.0000 | 1.00 |
| 1g | 2-Br | 2.00000 | 1.0000 | 8.00000 | 4.0 | 64.0 | >64.00 | 16.0000 | 2.00 |
| 1h | 3-Br | 2.00000 | 4.0000 | 32.00000 | 8.0 | >64.0 | >64.00 | 32.0000 | 0.25 |
| 1i | 4-Br | 4.00000 | 8.0000 | 32.00000 | 8.0 | >64.0 | >64.00 | 64.0000 | 16.00 |
| 1j | 2-CH3 | 32.00000 | >64.0000 | >64.00000 | >64.0 | >64.0 | >64.00 | 64.0000 | 64.00 |
| 1k | 3-CH3 | 1.00000 | 1.0000 | 4.00000 | 1.0 | 4.0 | >64.00 | 8.0000 | 8.00 |
| 1l | 4-CH3 | 1.00000 | 1.0000 | 8.00000 | 2.0 | >64.0 | >64.00 | 32.0000 | 8.00 |
| 1m | 2-CN | 2.00000 | 8.0000 | 32.00000 | 16.0 | >64.0 | >64.00 | 32.0000 | 16.00 |
| 1n | 3-CN | 0.12500 | 0.5000 | 4.00000 | 0.5 | 16.0 | >64.00 | 16.0000 | 1.00 |
| 1o | 4-CN | 8.00000 | 32.0000 | >64.00000 | 4.0 | >64.0 | >64.00 | >64.0000 | 4.00 |
| 1p | 2-NO2 | 0.50000 | 4.0000 | 32.00000 | 8.0 | >64.0 | >64.00 | 64.0000 | 8.00 |
| 1q | 3-NO2 | 2.00000 | 2.0000 | 16.00000 | 8.0 | 64.0 | >64.00 | 32.0000 | 64.00 |
| 1r | 4-NO2 | 0.50000 | 2.0000 | 32.00000 | 4.0 | >64.0 | >64.00 | 32.0000 | 1.00 |
| Fluconazole | – | 1.00000 | 0.5000 | 0.50000 | 2.0 | 2.0 | >64.00 | 4.0000 | 64.00 |
| Itraconazole | – | 0.06250 | 0.0625 | 0.03125 | 2.0 | 0.5 | 2.00 | 0.1250 | 4.00 |
| Voriconazole | – | 0.03125 | 0.0625 | 0.03125 | 2.0 | 0.5 | 0.25 | 0.0625 | 0.25 |
Conclusion
A series of triazoles was successfully synthesized and characterized by ESI-MS and nuclear magnetic resonance spectroscopic analysis. In vitro antifungal activity assay indicated that most of the compounds showed antifungal activities against both systemic pathogenic fungi. The MIC80 value of the compounds in which halogen was substituted to position three against M. gypseum was better than that of the other compounds. Several compounds showed high in vitro antifungal activity that was broad spectrum, which will be valuable to future investigations.
Supplementary materials
The title compounds 1b–r were characterized as follows.
Compound 1b: 1-(1H-1,2,4-triazol-1-yl)-2-(2,4-difluorophenyl)-3-{N-benzyl-N-[(1-(3-fluorobenzyl)-1H-1,2,3-triazol-4-yl)methyl]amino}-2-propanol
Melting point (MP): 94.1°C–96.0°C; proton nuclear magnetic resonance (1H NMR) (300 MHz, CDCl3) δ: 8.13 (1H, s, triazole-H), 7.77 (1H, s, triazole-H), 7.64–7.55 (1H, m, Ar), 7.37–7.12 (8H, m, Ar-H, triazole-H), 6.82–6.74 (2H, m, Ar-H), 5.46 (2H, s, Ar-CH2-), 4.51–4.36 (2H, m, CH2), 3.71–3.36 (4H, m, -CH2-N-CH2-), 3.27–2.91 (2H, m, CH2); carbon-13 nuclear magnetic resonance (13C NMR) (75 MHz, CDCl3) δ: 163.3, 159.1, 151.2, 130.3, 130.1, 129.9, 129.9, 129.9, 129.7, 129.7, 129.7, 129.6, 129.6, 129.6, 129.5, 129.5, 129.4, 128.6, 128.1, 116.3, 111.9, 104.5, 73.1, 59.8, 57.6, 56.1, 35.7, 49.3; electrospray ionization-mass spectrometry (ESI-MS), m/z calculated for C28H26F3N7O, 533.2, found [M+H]+ 534.3.
Compound 1c: 1-(1H-1,2,4-triazol-1-yl)-2-(2,4-difluorophenyl)-3-{N-benzyl-N-[(1-(4-fluorobenzyl)-1H-1,2,3-triazol-4-yl)methyl]amino}-2-propanol (1c)
MP: 94.1°C–96.0°C; 1H NMR (300 MHz, CDCl3) δ: 8.11 (1H, s, triazole-H), 7.72 (1H, s, triazole-H), 7.69–7.57 (1H, m, Ar), 7.29–7.06 (8H, m, Ar-H, triazole-H), 6.84–6.69 (2H, m, Ar-H), 5.51 (2H, s, Ar-CH2-), 4.60–4.36 (2H, m, CH2), 3.67–3.34 (4H, m, -CH2-N-CH2-), 3.01–2.64 (2H, m, CH2); 13C NMR (75 MHz, CDCl3) δ: 163.1, 159.0, 151.0, 130.1, 130.0, 129.9, 129.9, 129.8, 129.8, 129.7, 129.7, 129.7, 129.6, 129.6, 129.6, 129.5, 129.4, 128.7, 128.0, 116.1, 111.7, 104.2, 73.2, 59.7, 57.5, 56.0, 35.5, 49.2; ESI-MS, m/z calculated for C28H26F3N7O, 533.2, found [M+H]+ 534.4.
Compound 1d: 1-(1H-1,2,4-triazol-1-yl)-2-(2,4-difluorophenyl)-3-{N-benzyl-N-[(1-(2-chlorobenzyl)-1H-1,2,3-triazol-4-yl)methyl]amino}-2-propanol
MP: 114.6°C–116.0°C; 1H NMR (300 MHz, CDCl3) δ: 8.01 (1H, s, triazole-H), 7.71 (1H, s, triazole-H), 7.64–7.55 (1H, m, Ar-H), 7.47–7.11 (8H, m, Ar-H, triazole–H), 6.80–6.68 (2H, m, Ar-H), 5.65 (2H, s, Ar-CH2-), 4.50–4.36 (2H, m, triazole–H), 3.68–3.39 (4H, m, -CH2-N-CH2-), 3.27–2.83 (2H, m, CH2); 13C NMR (75 MHz, CDCl3) δ: 164.5, 156.1, 151.2, 130.2, 130.1, 129.9, 129.9, 129.8, 129.8, 129.7, 129.7, 129.6, 129.6, 129.5, 129.5, 129.4, 129.4, 128.7, 128.0, 115.1, 111.5, 104.6, 73.6, 59.6, 57.3, 56.3, 35.7, 49.3; ESI-MS, m/z calculated for C28H26ClF2N7O 549.2, found [M+1]+ 550.3.
Compound 1e: 1-(1H-1,2,4-triazol-1-yl)-2-(2,4-difluorophenyl)-3-{N-benzyl-N-[(1-(3-chlorobenzyl)-1H-1,2,3-triazol-4-yl)methyl]amino}-2-propanol
MP: 112.0°C–113.8°C; 1H NMR (300 MHz, CDCl3) δ: 8.07 (1H, s, triazole-H), 7.74 (1H, s, triazole-H), 7.68–7.59 (1H, m, Ar-H), 7.36–7.10 (8H, m, Ar-H, triazole–H), 6.94–6.70 (2H, m, Ar-H), 5.52 (2H, s, Ar-CH2-), 4.54–4.36 (2H, m, CH2), 3.69–3.37 (4H, m, -CH2-N-CH2-), 3.27–2.72 (2H, m, CH2); 13C NMR (75 MHz, CDCl3) δ: 164.3, 156.2, 151.1, 130.1, 130.0, 129.9, 129.9, 129.9, 129.8, 129.8, 129.7, 129.6, 129.6, 129.5, 129.5, 129.4, 129.3, 128.6, 128.1, 115.3, 111.4, 104.2, 73.4, 59.4, 57.2, 56.2, 35.6, 49.2; ESI-MS, m/z calculated for C28H26ClF2N7O 549.2, found [M+1]+ 550.3.
Compound 1f: 1-(1H-1,2,4-triazol-1-yl)-2-(2,4-difluorophenyl)-3-{N-benzyl-N-[(1-(4-chlorobenzyl)-1H-1,2,3-triazol-4-yl)methyl]amino}-2-propanol
MP: 111.2°C–113.0°C; 1H NMR (300 MHz, CDCl3) δ: 8.07 (1H, s, triazole-H), 7.73 (1H, s, triazole-H), 7.68–7.60 (1H, m, Ar-H), 7.40–7.13 (8H, m, Ar-H, triazole–H), 6.85–6.70 (2H, m, Ar-H), 5.52 (2H, s, Ar-CH2-), 4.55–4.35 (2H, m, CH2), 3.68–3.39 (4H, m, -CH2-N-CH2-), 3.27–2.83 (2H, m, CH2); 13C NMR (75 MHz, CDCl3) δ: 164.5, 156.1, 151.3, 130.0, 130.0, 129.9, 129.9, 129.8, 129.8, 129.8, 129.7, 129.7, 129.6, 129.6, 129.5, 129.4, 129.4, 128.5, 128.3, 115.1, 111.7, 104.7, 73.1, 59.7, 57.1, 56.1, 35.7, 49.0; ESI-MS, m/z calculated for C28H26ClF2N7O 549.2, found [M+1]+ 550.4.
Compound 1g: 1-(1H-1,2,4-triazol-1-yl)-2-(2,4-difluorophenyl)-3-{N-benzyl-N-[(1-(2-bromobenzyl)-1H-1,2,3-triazol-4-yl)methyl]amino}-2-propanol
MP: 116.8°C–118.2°C;1H NMR (300 MHz, CDCl3) δ: 8.07 (1H, s, triazole-H), 7.75 (1H, s, triazole-H), 7.63–7.61 (1H, m, Ar-H), 7.57–7.51 (2H, m, Ar-H), 7.27–7.11 (8H, m, Ar-H, triazole-H), 6.82–6.70 (2H, m, Ar-H), 5.52 (2H, s, Ar-CH2), 4.51–4.36 (2H, m, CH2), 3.73–3.40 (4H, m, -CH2-N-CH2-), 3.30–2.85 (2H, m, CH2); 13C NMR (75 MHz, CDCl3) δ: 163.5, 159.6, 151.4, 136.2, 134.8, 131.8, 131.8, 131.5, 130.5, 130.4, 130.4, 130.3, 129.8, 129.8, 129.7, 129.2, 129.2, 128.5, 128.5, 128.3, 112.2, 104.8, 73.4, 62.1, 58.2, 53.2, 52.0, 48.0; ESI-MS, m/z calculated for C28H26BrF2N7O 593.1, found [M+1]+ 594.3.
Compound 1h: 1-(1H-1,2,4-triazol-1-yl)-2-(2,4-difluorophenyl)-3-{N-benzyl-N-[(1-(3-bromobenzyl)-1H-1,2,3-triazol-4-yl)methyl]amino}-2-propanol
MP: 101.1°C–102.1°C; 1H NMR (300 MHz, CDCl3) δ: 8.03 (1H, s, triazole-H), 7.73 (1H, s, triazole-H), 7.64–7.60 (1H, m, Ar-H), 7.54–7.51 (2H, m, Ar-H), 7.29–7.14 (8H, m, Ar-H, triazole-H), 6.84–6.70 (2H, m, Ar-H), 5.54 (2H, s, Ar-CH2), 4.55–4.35 (2H, m, CH2), 3.74–3.43 (4H, m, -CH2-N-CH2-), 3.30–2.87 (2H, m, CH2); 13C NMR (75 MHz, CDCl3) δ: 163.2, 158.6, 151.0, 144.6, 133.6, 132.9, 130.7, 129.9, 129.9, 129.8, 129.8, 129.7, 129.7, 129.6, 129.5, 129.5, 129.4, 128.5, 128.2, 122.7, 111.5, 104.5, 73.8, 59.8, 57.8, 56.2, 53.5, 49.2; ESI-MS, m/z calculated for C28H26BrF2N7O 593.1, found [M+1]+ 594.5.
Compound 1i: 1-(1H-1,2,4-triazol-1-yl)-2-(2,4-difluorophenyl)-3-{N-benzyl-N-[(1-(4-bromobenzyl)-1H-1,2,3-triazol-4-yl)methyl]amino}-2-propanol
MP: 108.6°C–111.0°C; 1H NMR (300 MHz, CDCl3) δ: 8.16 (1H, s, triazole-H), 7.79 (1H, s, triazole-H), 7.64–7.62 (1H, m, Ar-H), 7.62–7.59 (2H, m, Ar-H), 7.29–7.16 (8H, m, Ar-H, triazole-H), 6.83–6.70 (2H, m, Ar-H), 5.50 (2H, s, Ar-CH2), 4.58–4.12 (2H, m, CH2), 3.70–3.49 (4H, m, -CH2-N-CH2-), 3.30–2.91 (2H, m, CH2); 13C NMR (75 MHz, CDCl3) δ: 163.7, 159.5, 151.3, 136.1, 134.7, 131.7, 131.7, 131.3, 130.1, 130.2, 130.1, 130.0, 129.9, 129.9, 129.8, 129.7, 129.6, 128.7, 128.7, 128.3, 112.1, 104.5, 73.3, 62.0, 58.1, 53.1, 52.0, 48.3; ESI-MS, m/z calculated for C28H26BrF2N7O 593.1, found [M+1]+ 594.6.
Compound 1j: 1-(1H-1,2,4-triazol-1-yl)-2-(2,4-difluorophenyl)-3-{N-benzyl-N-[(1-(2-methylbenzyl)-1H-1,2,3-triazol-4-yl)methyl]amino}-2-propanol
MP: 90.6°C–92.1°C; 1H NMR (300 MHz, CDCl3) δ: 8.08 (1H, s, triazole-H), 7.69 (1H, s, triazole-H), 7.62–7.57 (1H, m, Ar-H), 7.35–7.20 (10H, m, Ar-H, triazole-H), 6.83–6.67 (2H, m, Ar-H), 5.56 (2H, s, Ar-CH2), 4.54–4.33 (2H, m, CH2), 3.72–3.49 (4H, m, -CH2-N-CH2-), 3.30–2.91 (2H, m, CH2), 2.30 (3H, s, Ar-CH3); 13C NMR (75 MHz, CDCl3) δ: 164.1, 155.3, 138.3, 134.7, 130.1, 130.0, 129.9, 129.9, 129.8, 129.7, 129.7, 129.6, 129.6, 129.5, 129.4, 129.3, 129.2, 128.7, 128.6, 125.1, 111.8, 104.1, 72.3, 59.7, 57.3, 55.3, 54.0, 49.3, 22.3; ESI-MS, m/z calculated for C29H29F2N7O 529.6, found [M+1]+ 530.6.
Compound 1k: 1-(1H-1,2,4-triazol-1-yl)-2-(2,4-difluorophenyl)-3-{N-benzyl-N-[(1-(3-methylbenzyl)-1H-1,2,3-triazol-4-yl)methyl]amino}-2-propanol
MP: 92.6°C–94.1°C; 1H NMR (300 MHz, CDCl3) δ: 8.07 (1H, s, triazole-H), 7.78 (1H, s, triazole-H), 7.70–7.58 (1H, m, Ar-H), 7.32–7.08 (10H, m, Ar-H, triazole-H), 6.83–6.69 (2H, m, Ar-H), 5.56 (2H, s, Ar-CH2), 4.69–4.35 (2H, m, CH2), 3.75–3.45 (4H, m, -CH2-N-CH2-), 3.30–2.90 (2H, m, CH2), 2.37 (3H, s, Ar-CH3); 13C NMR (75 MHz, CDCl3) δ: 163.5, 155.1, 138.1, 134.6, 130.0, 129.9, 129.9, 129.8, 129.8, 129.7, 129.7, 129.6, 129.6, 129.5, 129.5, 129.4, 129.2, 128.8, 128.6, 125.3, 111.7, 104.3, 72.5, 59.8, 57.5, 55.8, 54.1, 49.8, 22.5; ESI-MS, m/z calculated for C29H29F2N7O 529.6, found [M+1]+ 530.5.
Compound 1l: 1-(1H-1,2,4-triazol-1-yl)-2-(2,4-difluorophenyl)-3-{N-benzyl-N-[(1-(4-methylbenzyl)-1H-1,2,3-triazol-4-yl)methyl]amino}-2-propanol
MP: 91.2°C–93.0°C; 1H NMR (300 MHz, CDCl3) δ: 8.06 (1H, s, triazole-H), 7.71 (1H, s, triazole-H), 7.66–7.57 (1H, m, Ar-H), 7.29–6.97 (10H, m, Ar-H, triazole-H), 6.82–6.67 (2H, m, Ar-H), 5.50 (2H, s, Ar-CH2), 4.51–4.35 (2H, m, CH2), 3.72–3.45 (4H, m, -CH2-N-CH2-), 3.33–2.90 (2H, m, CH2), 2.38 (3H, s, Ar-CH3; 13C NMR (75 MHz, CDCl3) δ: 163.9, 155.7, 138.2, 134.5, 130.0, 130.0, 129.9, 129.9, 129.8, 129.8, 129.7, 129.7, 129.6, 129.6, 129.5, 129.3, 129.3, 128.5, 128.3, 125.3, 111.7, 104.5, 72.6, 59.5, 57.1, 55.1, 54.1, 49.7, 22.8; ESI-MS, m/z calculated for C29H29F2N7O 529.6, found [M+1]+ 530.7.
Compound 1m: 1-(1H-1,2,4-triazol-1-yl)-2-(2,4-difluorophenyl)-3-{N-benzyl-N-[(1-(2-cyanobenzyl)-1H-1,2,3-triazol-4-yl) methyl]amino}-2-propanol
MP: 79.6°C–81.4°C; 1H NMR (300 MHz, CDCl3) δ: 8.07 (1H, s, triazole-H), 7.76 (1H, s, triazole-H), 7.65–7.63 (1H, m, Ar-H), 7.54–7.21 (10H, m, Ar-H, triazole-H), 6.85–6.71 (2H, m, Ar-H), 5.76 (2H, s, Ar-CH2), 4.58–4.38 (2H, m, CH2), 3.75–3.43 (4H, m, -CH2-N-CH2-), 3.30–2.87 (2H, m, CH2), 2.38 (3H, s, Ar-CH3; 13C NMR (75 MHz, CDCl3) δ: 163.7, 158.7, 152.1, 142.2, 133.1, 131.2, 130.3, 130.1, 129.9, 129.9, 129.8, 129.8, 129.7, 129.6, 129.5, 128.9, 128.5, 127.5, 126.9, 118.2, 113.5, 111.7, 104.7, 73.5, 61.3, 58.1, 56.2, 53.1, 50.1; ESI-MS, m/z calculated for C29H26F2N8O 540.2, found [M+1]+ 541.3.
Compound 1n: 1-(1H-1,2,4-triazol-1-yl)-2-(2,4-difluorophenyl)-3-{N-benzyl-N-[(1-(3-cyanobenzyl)-1H-1,2,3-triazol-4-yl) methyl]amino}-2-propanol
MP: 82.2°C–83.5°C; 1H NMR (300 MHz, CDCl3) δ: 8.02 (1H, s, triazole-H), 7.75 (1H, s, triazole-H), 7.66–7.63 (1H, m, Ar-H), 7.53–7.15 (10H, m, Ar-H, triazole-H), 6.85–6.72 (2H, m, Ar-H), 5.59 (2H, s, Ar-CH2), 4.58–4.35 (2H, m, CH2), 3.73–3.41 (4H, m, -CH2-N-CH2-), 3.30–2.82 (2H, m, CH2); 13C NMR (75 MHz, CDCl3) δ: 163.3, 158.5, 152.3, 142.3, 133.2, 131.3, 130.1, 130.0, 129.9, 129.9, 129.8, 129.7, 129.6, 129.3, 128.9, 128.7, 127.5, 127.4, 126.3, 118.4, 113.3, 112.0, 104.5, 73.7, 61.5, 58.3, 56.3, 53.6, 50.2; ESI-MS, m/z calculated for C29H26F2N8O 540.2, found [M+1]+ 541.4.
Compound 1o: 1-(1H-1,2,4-triazol-1-yl)-2-(2,4-difluorophenyl)-3-{N-benzyl-N-[(1-(4-cyanobenzyl)-1H-1,2,3-triazol-4-yl) methyl]amino}-2-propanol
MP: 78.8°C–80.4°C; 1H NMR (300 MHz, CDCl3) δ: 8.08 (1H, s, triazole-H), 7.75 (1H, s, triazole-H), 7.71–7.63 (1H, m, Ar-H), 7.37–7.22 (10H, m, Ar-H, triazole-H), 6.86–6.72 (2H, m, Ar-H), 5.62 (2H, s, Ar-CH2), 4.62–4.34 (2H, m, CH2), 3.74–3.42 (4H, m, -CH2-N-CH2-), 3.30–2.86 (2H, m, CH2); 13C NMR (75 MHz, CDCl3) δ: 163.2, 158.7, 152.1, 142.1, 133.1, 131.1, 130.0, 130.0, 129.9, 129.8, 129.8, 129.7, 129.5, 129.0, 128.7, 128.7, 127.4, 127.3, 126.5, 118.0, 113.1, 112.1, 104.9, 73.1, 61.2, 58.1, 56.1, 53.8, 50.0; ESI-MS, m/z calculated for C29H26F2N8O 540.2, found [M+1]+ 541.5.
Compound 1p: 1-(1H-1,2,4-triazol-1-yl)-2-(2,4-difluorophenyl)-3-{N-benzyl-N-[(1-(2-nitrobenzyl)-1H-1,2,3-triazol-4-yl) methyl]amino}-2-propanol
MP: 80.2°C–81.5°C; 1H NMR (300 MHz, CDCl3) δ: 8.17–8.15 (1H, m, Ar-H), 8.06 (1H, s, triazole-H), 7.72 (1H, s, triazole-H), 7.65–7.59 (3H, m, Ar-H), 7.29–7.17 (7H, m, Ar-H, triazole-H), 6.83–6.72 (2H, m, Ar-H), 5.93 (2H, s, Ar-CH2), 4.58–4.38 (2H, m, CH2), 3.76–3.41 (4H, m, -CH2-N-CH2-), 3.30–2.97 (2H, m, CH2); 13C NMR (75 MHz, CDCl3) δ: 163.5, 158.7, 148.3, 136.5, 133.7, 130.1, 130.0, 129.9, 129.9, 129.8, 129.8, 129.7, 129.6, 129.6, 129.5, 129.5, 129.4, 128.7, 123.8, 122.5, 111.2, 104.7, 73.6, 59.1, 57.6, 55.3, 53.1, 49.0; ESI-MS, m/z calculated for C28H26F2N8O3 560.2, found [M+1]+ 561.4.
Compound 1q: 1-(1H-1,2,4-triazol-1-yl)-2-(2,4-difluorophenyl)-3-{N-benzyl-N-[(1-(3-nitrobenzyl)-1H-1,2,3-triazol-4-yl) methyl]amino}-2-propanol
MP: 82.0°C–83.6°C; 1H NMR (300 MHz, CDCl3) δ: 8.04 (1H, s, triazole-H), 7.73 (1H, s, triazole-H), 7.63–7.60 (1H, m, Ar-H), 7.50–7.17 (10H, m, Ar-H, triazole-H), 6.84–6.70 (2H, m, Ar-H), 5.50 (2H, s, Ar-CH2), 4.56–4.35 (2H, m, CH2), 3.71–3.41 (4H, m, -CH2-N-CH2-), 3.32–2.90 (2H, m, CH2); 13C NMR (75 MHz, CDCl3) δ: 162.5, 154.3, 136.7, 132.0, 131.1, 130.3, 129.9, 129.9, 129.8, 129.8, 129.7, 129.6, 129.6, 129.5, 129.4, 129.0, 128.6, 127.6, 126.6, 123.2, 111.7, 104.2, 73.3, 59.8, 57.6, 56.2, 53.4, 49.2; ESI-MS, m/z calculated for C28H26F2N8O3 560.2, found [M+1]+ 561.3.
Compound 1r: 1-(1H-1,2,4-triazol-1-yl)-2-(2,4-difluorophenyl)-3-{N-benzyl-N-[(1-(4-nitrobenzyl)-1H-1,2,3-triazol-4-yl) methyl]amino}-2-propanol
MP: 78.5°C–80.0°C; 1H NMR (300 MHz, CDCl3) δ: 8.26–8.15 (2H, m, Ar-H), 8.05 (1H, s, triazole-H), 7.74 (1H, s, triazole-H), 7.64–7.20 (9H, m, Ar-H, triazole-H), 6.85–6.71 (2H, m, Ar-H), 5.67 (2H, s, Ar-CH2), 4.60–4.34 (2H, m, CH2), 3.72–3.42 (4H, m, -CH2-N-CH2-), 3.36–2.91 (2H, m, CH2); 13C NMR (75 MHz, CDCl3) δ: 162.7, 158.5, 148.6, 136.7, 133.9, 130.4, 130.0, 129.9, 129.8, 129.8, 129.7, 129.7, 129.6, 129.6, 129.5, 129.4, 129.4, 128.6, 123.8, 122.7, 111.8, 104.4, 73.2, 59.8, 57.5, 55.8, 53.2, 49.2; ESI-MS, m/z calculated for C28H26F2N8O3 560.2, found [M+1]+ 561.5.
Acknowledgments
This work was supported by the National Key Basic Research Program of China (no 2013CB531602), the National Natural Science Foundation of China (no 81330083), the Creativity and Innovation Training Program of Second Military Medical University, and the Pharmaceutical Education Research Project of the Chinese Association of Higher Medical Education Professional Committee (grant no ZD201220).
Electronic supplementary information available: proton nuclear magnetic resonance and electrospray ionization-mass spectrometry spectral data of Compounds 1b–r.
Footnotes
Disclosure
The authors report no conflicts of interest in this work.
References
- 1.Wingard JR, Leather H. A new era of antifungal therapy. Biol Blood Marrow Transplant. 2004;10(2):73–90. doi: 10.1016/j.bbmt.2003.09.014. [DOI] [PubMed] [Google Scholar]
- 2.Fridkin SK, Jarvis WR. Epidemiology of nosocomial fungal infections. Clin Microbiol Rev. 1996;9(4):499–511. doi: 10.1128/cmr.9.4.499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Sheng CQ, Zhang WN, Ji HT, et al. Design, synthesis and antifungal activity of novel triazole derivatives. Chin Chem Lett. 2004;15(4):404–407. [Google Scholar]
- 4.Sheng C, Zhang W, Ji H, et al. Structure-based optimization of azole antifungal agents by CoMFA, CoMSIA, and molecular docking. J Med Chem. 2006;49(8):2512–2525. doi: 10.1021/jm051211n. [DOI] [PubMed] [Google Scholar]
- 5.Gadhave PP, Dighe NS, Pattan SR, Deotarse P, Musmade DS, Shete RV. Current biological and synthetic profile of triazoles: a review. Ann Biol Res. 2010;1(1):82–89. [Google Scholar]
- 6.Groll AH, Lumb J. New developments in invasive fungal disease. Future Microbiol. 2012;7(2):179–184. doi: 10.2217/fmb.11.154. [DOI] [PubMed] [Google Scholar]
- 7.Wildfeuer A, Seidl HP, Paule I, Haberreiter A. In vitro evaluation of voriconazole against clinical isolates of yeasts, moulds and dermatophytes in comparison with itraconazole, ketoconazole, amphotericin B and griseofulvin. Mycoses. 1998;41(7–8):309–319. doi: 10.1111/j.1439-0507.1998.tb00344.x. [DOI] [PubMed] [Google Scholar]
- 8.Georgopapadakou NH. Antifungals: mechanism of action and resistance, established and novel drugs. Curr Opin Microbiol. 1998;1(5):547–557. doi: 10.1016/s1369-5274(98)80087-8. [DOI] [PubMed] [Google Scholar]
- 9.Kontoyiannis DP, Mantadakis E, Samonis G. Systemic mycoses in the immunocompromised host: an update in antifungal therapy. J Hosp Infect. 2003;53(4):243–258. doi: 10.1053/jhin.2002.1278. [DOI] [PubMed] [Google Scholar]
- 10.Ghannoum MA, Rice LB. Antifungal agents: mode of action, mechanisms of resistance, and correlation of these mechanisms with bacterial resistance. Clin Microbiol Rev. 1999;12(4):501–517. doi: 10.1128/cmr.12.4.501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.White TC, Marr KA, Bowden RA. Clinical, cellular, and molecular factors that contribute to antifungal drug resistance. Clin Microbiol Rev. 1998;11(2):382–402. doi: 10.1128/cmr.11.2.382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wong-Beringer A, Kriengkauykiat J. Systemic antifungal therapy: new options, new challenges. Pharmacotherapy. 2003;23(11):1441–1462. doi: 10.1592/phco.23.14.1441.31938. [DOI] [PubMed] [Google Scholar]
- 13.Lepesheva GI, Hargrove TY, Ott RD, Nes WD, Waterman MR. Biodiversity of CYP51 in trypanosomes. Biochem Soc Trans. 2006;34(Pt 6):1161–1164. doi: 10.1042/BST0341161. [DOI] [PubMed] [Google Scholar]
- 14.Pfaller MA, Messer SA, Hollis RJ, Jones RN. In vitro activities of posaconazole (Sch 56592) compared with those of itraconazole and fluconazole against 3,685 clinical isolates of Candida spp and Cryptococcus neoformans. Antimicrob Agents Chemother. 2001;45(10):2862–2864. doi: 10.1128/AAC.45.10.2862-2864.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Sheehan DJ, Hitchcock CA, Sibley CM. Current and emerging azole antifungal agents. Clin Microbiol Rev. 1999;12(1):40–79. doi: 10.1128/cmr.12.1.40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lewis RE. Current concepts in antifungal pharmacology. Mayo Clin Proc. 2011;86(8):805–817. doi: 10.4065/mcp.2011.0247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Di Santo R, Tafi A, Costi R, et al. Antifungal agents. 11. N-substituted derivatives of 1-[(aryl)(4-aryl-1H-pyrrol-3-yl)methyl]-1H-imidazole: synthesis, anti-Candida activity, and QSAR studies. J Med Chem. 2005;48(16):5140–5153. doi: 10.1021/jm048997u. [DOI] [PubMed] [Google Scholar]
- 18.Odds FC, Brown AJ, Gow NA. Antifungal agents: mechanisms of action. Trends Microbiol. 2003;11(6):272–279. doi: 10.1016/s0966-842x(03)00117-3. [DOI] [PubMed] [Google Scholar]
- 19.Zou Y, Yu S, Li R, et al. Synthesis, antifungal activities and molecular docking studies of novel 2-(2,4-difluorophenyl)-2-hydroxy-3-(1H-1, 2,4-triazol-1-yl)propyl dithiocarbamates. Eur J Med Chem. 2014;74:366–374. doi: 10.1016/j.ejmech.2014.01.009. [DOI] [PubMed] [Google Scholar]
- 20.Yu S, Chai X, Wang N, et al. Synthesis and antifungal activity of the novel triazole compounds. Med Chem Commun. 2013;4(4):704–708. [Google Scholar]
- 21.Yu S, Wang L, Wang Y, et al. Molecular docking, design, synthesis and antifungal activity study of novel triazole derivatives containing the 1,2,3-triazole group. RSC Adv. 2013;3(32):13486–13490. [Google Scholar]
- 22.Yu S, Chai X, Hu H, et al. Synthesis and antifungal evaluation of novel triazole derivatives as inhibitors of cytochrome P450 14alpha-demethylase. Eur J Med Chem. 2010;45(10):4435–4445. doi: 10.1016/j.ejmech.2010.07.002. [DOI] [PubMed] [Google Scholar]
- 23.Wang Y, Xu K, Bai G, et al. Synthesis and antifungal activity of novel triazole compounds containing piperazine moiety. Molecules. 2014;19(8):11333–11340. doi: 10.3390/molecules190811333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wang BG, Yu SC, Chai XY, Yan YZ, Hu HG, Wu QY. Design synthesis and biological evaluation of 3-substituted triazole derivatives. Chin Chem Lett. 2011;22(5):519–522. [Google Scholar]
- 25.Wang N, Chai X, Chen Y, et al. Synthesis, antifungal activity, and molecular docking studies of novel triazole derivatives. Med Chem. 2013;9(3):384–388. doi: 10.2174/1573406411309030009. [DOI] [PubMed] [Google Scholar]
- 26.Yu S, Chai X, Wang Y, et al. Triazole derivatives with improved in vitro antifungal activity over azole drugs. Drug Des Devel Ther. 2014;8:383–390. doi: 10.2147/DDDT.S58680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Yu S, Wang Q, Zhang J, Wu Q, Guo Z. Synthesis and Evaluation of Protein Conjugates of GM3 Derivatives Carrying Modified Sialic Acids as Highly Immunogenic Cancer Vaccine Candidates. Medchemcomm. 2011;2(6):524–530. doi: 10.1039/C1MD00033K. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Zhang XJ, Li HY, You LF, Tang Y, Hsung RP. Copper salt-catalyzed azide-[3+2] cycloadditions of ynamides and bis-ynamides. Adv Synth Catal. 2006;348(16–17):2437–2442. [Google Scholar]
- 29.National Committee for Clinical Laboratory Standards . Reference Method for Broth Dilution Antifungal Susceptibility Testing of Yeasts: Approved Standard. Document M27-A2. Wayne, PA: National Committee for Clinical Laboratory Standards; 2002. [Google Scholar]