Abstract
The aim of the study was to improve corneal penetration and bioavailability of ofloxacin (OFX) eye preparations. OFX was incorporated in poly (lactide-co-glycolide) as biodegradable microspheres using oil in oil emulsion solvent evaporation technique. The prepared OFX microspheres were then incorporated in Gelrite® in situ gel preparation. In addition, OFX Gelrite-based in situ gel formulations were prepared. OFX formulations were characterized for gelling capacity, viscosity, and rheological properties. Release studies for OFX microspheres, OFX in situ gel, and OFX-loaded microspheres in situ gel formulations were carried out to investigate release characteristics of the drug. The prepared OFX formulations were then investigated in vivo compared with commercially available OFX eyedrops. Results showed that the optimum Gelrite concentration was at 0.4%–0.7% w/v; the prepared formulations were viscous liquid transformed into a pourable gel immediately after the addition of simulated tear fluid with a gelling factor of 27–35. Incorporation of OFX-loaded microspheres in Gelrite solution (0.4% w/v) significantly altered the release profiles of OFX-loaded microspheres in situ gel formula compared with the corresponding OFX gels and OFX microspheres. In vivo results in rabbits showed that OFX-loaded microspheres in situ gel formula improved the relative bioavailability by 11.7-fold compared with the commercially available OFX eyedrops. In addition, the longer duration of action of OFX-loaded microspheres in situ gel formula preparations is thought to avoid frequent instillations, which improves patient tolerability and compliance.
Keywords: corneal delivery, fluoroquinolones, gellan gum, Gelrite, simulated tear fluid
Introduction
Ofloxacin (OFX) is a second-generation fluoroquinolone with a broad spectrum of action against gram-positive and gram-negative bacteria.1,2 It is a potent agent against various ocular species, including Staphylococcus aureus, Staphylococcus epidermidis, Streptococcus pneumonia, chlamydial species, and various anaerobes.3 Reports showed that the mean concentration of OFX in the aqueous humor exceeded the minimum inhibitory concentration for 90% (MIC90) of the frequently occurring gram-positive and gram-negative bacteria after topical application of 0.3% OFX eye drops.4,5 Of the topically applied fluoroquinolones, OFX achieved the highest aqueous humor concentration.4 Corneal precipitations of topically applied fluoroquinolones frequently occur due to the formation of the zwitterionic form near the physiologic pH. This lowers their solubility and hence precipitation can occur at this pH. Although precipitation has not yet been reported for OFX eye drops, deposits may occur.6
Topical drug delivery is the most common method of administration for treatment of diseases of the anterior segment of the eye as it is simple, convenient, and painless.7–9 However, less than 10% bioavailability is achieved for topical ocular drug application. The low bioavailability is attributed to the ocular protecting biological barriers such as the impermeable corneal epithelium, which represents a barrier for drugs to penetrate into the eye.7–9 Moreover, rapid elimination of instilled ophthalmic drug solution occurs from the precorneal area as a result of the lacrimal secretion and nasolacrimal drainage. Consequently, frequent instillation of ophthalmic solutions is required to achieve the required therapeutic effects.10 Accordingly, efficient topical delivery of drugs to ocular tissues is a challenge to formulation scientists.9
Novel technologies were developed to improve the bioavailability of ophthalmic drugs. Mucoadhesive systems, in situ gelling systems, microparticulate systems, microemulsions, vesicular systems, implants, prodrugs, penetration enhancers, and dendrimers are examples of the applied technologies.11–21 Pharmaceutical particulate technology for the delivery of ophthalmic drugs is used to extend drug release and improve patient compliance.22 The technology can also improve the residence time of the encapsulated drug.14 Ophthalmic microparticles should not exceed 10 μm in size, otherwise, a scratching feeling in the eye can occur that could lead to corneal abrasion and injury.15 Bioadhesive ocular microspheres are novel delivery systems that remain adherent to the ocular surface for a prolonged time. As a result, the bioavailability of the encapsulated drug is improved.12,23,24
In situ-forming hydrogels are liquids which undergo phase transition upon instillation to a viscoelastic gel in response to physiological conditions in the cul-de-sac.12,14 Three types of physiological changes can induce phase transition; they are: change in temperature, change in pH, or change in electrolyte composition.14 The increase in the viscosity upon instillation results in prolonged ocular residence time.14,25 In situ gels are preferred because they are liquids that can be easily dropped in the eye. In addition, they allow for reproducible instillation of accurate doses in comparison with instillation of preformed gels.12 Low acylated gellan gum (commercially available as Gelrite®) is a polysaccharide that undergoes phase transition to clear gel in the presence of mono- or divalent cations.26 Gelrite-based formulations have been tested for different types of drugs such as timolol maleate, ciprofloxacin hydrochloride, indomethacin, pefloxacin mesylate, carteolol, and gatifloxacin.26–31
In the present study, OFX was incorporated in poly (lactide-co-glycolide) (PLGA) biodegradable microspheres, and then loaded into in situ gel formula. The incorporation of OFX microspheres in the in situ gel preparation aimed to improve OFX corneal penetration and consequently enhance its efficacy against ocular infections. In addition, the longer duration of action of this formula preparations is thought to avoid frequent instillations, which improves patient tolerability and compliance.
Materials and methods
Materials
Poly (DL-lactide-co-glycolide), Gelrite (deacylated gellan gum), and cellophane membrane were purchased from Sigma-Aldrich Co. (St Louis, MO, USA). OFX was kindly gifted by Al-Kahira Pharmaceutical Company (Cairo, Egypt). Polyvinyl alcohol was purchased from Fluka Chemie GmbH (Steinheim, Germany). Dichloromethane (DCM), acetonitrile (ACN), methanol (MeOH), petroleum ether, liquid paraffin, sodium bicarbonate, calcium chloride dihydrate, sodium chloride, polyethylene glycol 6,000, and isopropyl myristate were purchased from El-Nasr Pharmaceutical Co. (Cairo, Egypt). Ketamine 50 mg/mL was supplied by Sigma-Tec Pharmaceutical Industries (Cairo, Egypt), and ACN and MeOH for high performance liquid chromatography (HPLC) was supplied by EMD Millipore (Billerica, MA, USA). All other solvents and chemicals were of analytical grade.
Methods
Preparation of OFX microspheres using oil in oil emulsion technique
OFX microspheres were prepared using oil in oil (O/O) emulsion solvent evaporation technique. Briefly, poly (DL-lactide-co-glycolide) was dissolved in a mixture of ACN, DCM, and MeOH in a ratio of 6:4:1, in screw-capped test tubes, to make 4% w/v solutions. OFX was added to the organic phase and then sonicated for 10 seconds using a probe sonicator under cooling (Heidolph MR 3001; Heidolph, Schwabach, Germany). The organic phase was then added dropwise to 50 mL of liquid paraffin containing lecithin (2% w/v) and was homogenized at 20,000 rpm (T10 basic ULTRA-TURRAX, IKA, Staufen, Germany) for 2 minutes to obtain the O/O emulsion. The formed emulsion was then stirred under a slower speed (500 rpm) overnight to allow slow evaporation of organic solvent. Microspheres were then harvested by centrifugation at 4,000 rpm for 30 minutes (Fischer Centrific® Centrifuge; Thermo Fisher Scientific, Waltham, MA, USA), washed three times with petroleum ether, and air-dried.
Encapsulation efficiency
Weighed amounts of the prepared microspheres were added to 50 mL DCM and sonicated for 30 minutes. OFX concentration was measured after appropriate dilution at wavelength of maximum absorption (λmax) 294 nm using a ultraviolet/visible spectrophotometer (Spectronic Genesys® with Winspec Software; Thermo Fisher Scientific). The percentage encapsulation efficiency (EE %) was calculated according to Equation 1:
| (1) |
Particle size analysis
Particle size and size distribution of the prepared OFX microspheres were measured using a laser scattering particle size distribution analyzer (HORIBA-LA300; Retsch Technology GmbH, Düsseldorf, Germany). Five analyses were performed for each microsphere sample to determine the mean diameter.
Morphology
Morphology and the surface characteristics of OFX microspheres were examined by scanning electron microscope (Jeol Fine-Coat JFC 1100E; Jeol JSM-5400 LV; JEOL, Tokyo, Japan).
Preparation of OFX Gelrite based in situ gel formulations
Solutions of Gelrite (0.1%–0.9% w/v) in boric–borax buffer (pH 7.4) were prepared by heating OFX dispersions to 90°C for 20 minutes while stirring. Solutions were then cooled at room temperature while stirring. The prepared formulations were stored in amber colored glass vials and were sterilized by autoclaving.
Characterization of in situ gel formulations
Gelling capacity and flow behavior
Gelling capacity was determined by addition of 7 mL of simulated tear fluid (STF) to a vial containing 25 mL of Gelrite-based formulation at 37°C, and then visually evaluating the gel formation and observing the flow behavior of the formulations at each concentration, before and after addition of STF. The gelling factor was calculated according to Equation 2:
| (2) |
Viscosity and rheological properties
Viscosity and rheological properties of the prepared formulae were carried out using a Brookfield rheometer (DV-III Ultra; Brookfield Engineering Laboratories, Inc., Middleboro, MA, USA). STF of pH 7.4 was added in increments of 25 mL to 200 mL to the formulations, and the viscosities at which gelation occurred were recorded. The angular velocity increased gradually from 0.01 to 100 rpm. The average of the two readings was used to calculate the viscosity.
Effect of autoclaving
Characteristics of the prepared in situ Gelrite-based gels were investigated before and after sterilization by autoclaving. Briefly, the formulations were packaged in amber-colored glass vials and were sterilized by terminal autoclaving at 121°C and 204, 774.292 pascals (15 psig) for 20 minutes.
Release study of OFX-loaded in situ gel formulations
OFX in vitro release from the prepared in situ gel formulations was carried out using a small cylindrical glass tube with a surface area of 1.77 cm2. Cellophane membrane was soaked overnight in STF, and then was clamped into one end of the tube. The in situ gelling formulation (1 mL) was placed over the cellophane membrane. The end of the tube was immersed in 100 mL of STF, kept at 37°C, and stirred at 40 rpm. The release experiments were designed to maintain sink conditions. Two-milliliter samples were withdrawn at the different time intervals. Each sample was replaced by fresh STF. The concentration of OFX was analyzed at λmax 294 nm. The release of OFX from the combined microsphere–gel formulations was compared to the release of OFX from corresponding microsphere formulations and in situ gels, prepared separately.
Inclusion of microspheres into the in situ gel
OFX microspheres were homogeneously dispersed into the preformed Gelrite solution in order to produce the final OFX concentration of 0.3% w/v in the microsphere-loaded in situ gel formula. The formula was prepared by dispersing OFX microspheres in 0.4% w/v Gelrite. It is important to mention that 0.4% w/v Gelrite was used to produce the in situ gelling system. The effect of OFX microspheres inclusion on gelling capacity, rheology, and in vitro release was investigated.
In vivo studies
Animals
Adult male rabbits (2.5–3.0 kg, free of ocular defects) were used. The animals were obtained from the National Research Centre, Dokky, Giza, Egypt. Animal use was approved by the Review Board for Preclinical and Clinical Research Minia University who ensured the care and use of animals conformed to the Declaration of Helsinki and the Guiding Principle in Care and Use of Animals (DHEW publication NIH 80-23). Rabbits were classified into five groups. Each group contained four rabbits. The first group served as an untreated control. The second group (positive control) received suspension of OFX microspheres in isotonic boric–borax buffer (pH 6.8). The third group received OFX in situ gel. The fourth group received OFX microspheres in 0.4% Gelrite in situ gel. The last group received OFX marketed product. All the aforementioned preparations contained 0.3% w/v OFX. The test was carried out by dropping 100 μL (corresponding to 0.3 mg OFX) of each preparation into the lower conjunctival sac of the right eye of the rabbit. The rabbits were anaesthetized by intramuscular injection of 30 mg/kg ketamine, and then 80 μL of aqueous humor were withdrawn from the anterior chamber of the eye at different time intervals. The aqueous humor samples were stored at −18°C. The relative area under the curve compared with commercial product (AUCrel), maximum OFX concentration in aqueous humor (Cmax), and the time to reach this concentration (Tmax) were calculated.
HPLC analysis of aqueous humor samples
Aqueous humor samples, after suitable dilutions, were analysed for OFX content using the previously reported HPLC method.32 An HPLC system (Agilent Technologies, Santa Clara, CA, USA) with a fluorescence detector (Model FP-2020; Jasco International Co., Ltd., Hachioji, Tokyo, Japan) was used. The analytical column was a Venusil XBP C18 (L) (250 mm ×4.6 mm, 5 μm) (Agilent Technologies) in conjunction with a pre-column module (Agilent Technologies) with a Venusil Cl8 insert. Data acquisition was performed on Young Lin Autochro-3000 software (YL Instrument Co., Ltd., Anyang-si, Gyeonggi-do, The Republic of Korea). The mobile phase was MeOH, CAN, and 0.4 M citric acid (3:1:10, v/v/v). The flow rate was 0.8 mL/min at ambient temperature. The excitation and emission wavelengths were set at 290 nm and 500 nm, respectively.
Results and discussion
Preparation and characterization of OFX-loaded PLGA microspheres
O/O emulsion solvent evaporation method was adopted for OFX incorporation in microspheres to improve the entrapment efficiency. A disadvantage for the use of microspheres suspensions in the eye is the possible drainage of the particles that leads to an incomplete drug release. The major advantage of using in situ gel formulation containing microspheres is the improved precorneal retention of the administered microspheres, provided that the gel would remain in the eye during the time for drug release for a longer period of time compared with the microspheres-alone formula.
The composition of the liquid that is dispersed in liquid paraffin consisted of ACN, DCM, and MeOH. DCM was included to lower the polarity of ACN and to ensure formation of stable emulsion.33 OFX showed poor solubility in ACN (2.5 mg/mL) as measured practically in our lab. Therefore, MeOH and DCM were used to optimize OFX encapsulation within the microspheres by increasing the solubility of OFX in the dispersed phase. The solubility of OFX in the tertiary mixture of ACN–MeOH–DCM (6:4:1 v/v/v) solvent system was 15 mg/mL. It is worth mentioning that DCM was found to be miscible with liquid paraffin in all proportions while MeOH is immiscible with liquid paraffin. OFX formula prepared using O/O method utilized 4% PLGA with an OFX:PLGA ratio of 1:3 and lecithin (emulsifier) concentration of 2% w/v.
PLGA is a synthetic, biocompatible, and biodegradable copolymer of polylactic acid and polyglycolic acid.12 PLGA is well tolerated by the human body and was previously investigated for ocular drug delivery.34 Burst release may result with the use of PLGA particles. This could be considered disadvantageous in ocular formulations and the utilization of in situ gelling systems could reduce the burst-release effect of PLGA microspheres.12
Scanning electron microscope images of OFX microspheres (Figure 1) showed rough surfaces due to presence of needle-shaped drug crystals interspersed within the polymer surface. This could have resulted from the extraction of DCM from the internal phase to the liquid paraffin when the polymer–drug solution was introduced into the external oily phase.35 DCM migration draws the drug towards the surface of the half-formed microspheres. Therefore, the drug was deposited close to the surface of the microspheres during solidification.36 Microspheres prepared using the solvent system ACN–MeOH–DCM in a volume ratio of 6:4:1 are spherical in shape with well-defined boundaries as shown in Figure 1. The particle size of the prepared microspheres was 8.5 μm with 100 % entrapment efficiency.
Figure 1.
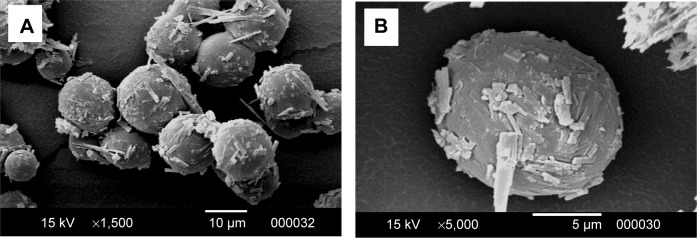
SEM images of OFX microspheres formulation at different magnifications.
Notes: (A) Low-magnification SEM image; (B) high-magnification SEM image.
Abbreviations: OFX, ofloxacin; SEM, scanning electron microscope.
Characterization of OFX Gelrite in situ gel
Table 1 shows flow behavior of the prepared in situ gel formulations. All formulations exhibited an increase in viscosity after addition of STF even at the lowest Gelrite concentration of 0.1% w/v, but with different gelling factors. The gelling factor was increased by the increase in the Gelrite concentration. Gelling factor values were two to three for formulations prepared at concentrations up to 0.3% w/v, after addition of STF. However, a significant increase in gelling factor resulted when the Gelrite concentration was increased above 0.3%. The gelling factor increased from three to 27 when the Gelrite concentration was increased from 0.3% to 0.4% w/v. At 0.4%–0.7% w/v Gelrite concentration, the formulations were viscous liquid transformed into a pourable gel immediately after the addition of STF with a gelling factor of 27–35. Using Gelrite in concentrations greater than 0.7% w/v, the formulations were gel before addition of STF. The gelling capacity is determined in order to identify Gelrite concentrations that would have an optimum viscosity to facilitate instillation into the eye as a liquid, and then undergo rapid gelation.28 The rapid gelation in the eye improves drug residence time.
Table 1.
Gelling capacity and viscosity of OFX in situ gel formulations
| Gelrite concentration (% w/v) | Physical properties | Gelling capacity | V1, cP | V2, cP |
|---|---|---|---|---|
| 0.1 | Clear and transparent solution | + | 166.7 | 333 |
| 0.2 | Clear and transparent solution | + | 1,667 | 3,333 |
| 0.3 | Clear and transparent solution | ++ | 2,500 | 8,334 |
| 0.4 | Clear, transparent, and viscous solution | +++ | 3,333 | 90,000 |
| 0.5 | Clear, transparent, and viscous solution | +++ | 6,200 | 170,000 |
| 0.6 | Clear, transparent, and viscous solution | +++ | 8,334 | 240,000 |
| 0.7 | Clear, viscous, easily pourable solution of gel-like consistency | +++ | 10,000 | 350,000 |
| 0.8 | Clear, more viscous solution of gel-like consistency | ++++ | 13,333 | 460,000 |
| 0.9 | Slightly turbid formed gel | ++++ | 33,333 | 850,000 |
Notes: +: no gelation; ++: gelation occurred after few minutes with flowable gel-like liquid; +++: immediate gelation with formation of easily flowable gel with good consistency; ++++ : non-flowable, consistent, and thick gel.
Abbreviations: OFX, ofloxacin; STF, simulated tear fluid; V1, viscosity before addition of STF; V2, viscosity after addition of STF.
The gelation of Gelrite after addition of STF is attributed to the cross-linking of Gelrite helices with STF solution cations. Similarly, gelation of Gelrite happened when it was administered to ocular mucosa due to contact with tear fluid cations, resulting in the formation of a clear gel.14,37,38 The sharp increase in gelling factor with the increase in Gelrite concentration from 0.3% to 0.4 % could be attributed to the significant increase in Gelrite cross-linking with STF cations and the interaction between polymer helices that leads to the formation of a denser structure.
Gelrite-based formulations exhibited pseudoplastic flow. The prepared gels, after addition of STF, showed thixotropic behavior where the gel was thinned when shearing force was increased, and then thickened when the stress was removed. For example, the viscosity of 0.4% Gelrite was decreased from 90,000 cP to 10,622 cP and finally to 5,000 cP when the shear rate increased from 0.01 rpm to 0.3 rpm then to 1.0 rpm, respectively (Figure 2). Similar results were reported previously.27–30 Accordingly, the increased viscosity of the formulation at low shear rates will maintain long contact between the corneal surface and the gel. During blinking, when shear rate is increased, the gel will be thinned and will be well distributed over the eye surface.39
Figure 2.
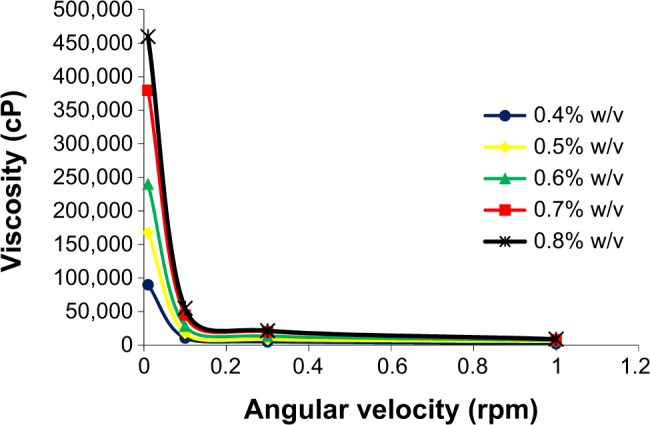
Effect of different Gelrite concentrations on rheology behavior of Gelrite-based in situ gels.
Note: As a result of overlapping, error bars were omitted for clarity.
The effect of autoclaving on the properties of polymer solutions is considered an important parameter for ocular preparations. Figure 3 shows the viscosity profiles of in situ gel before and after autoclaving. The results showed no significant change of Gelrite-based formulation properties before and after autoclaving. This finding is in accordance with previously published reports.29,31 Gelrite is able to better withstand the elevated temperatures of autoclaving compared to other polymers.31 So, autoclaving was used to sterilize Gelrite-based formulations in order to avoid microbial contamination.
Figure 3.
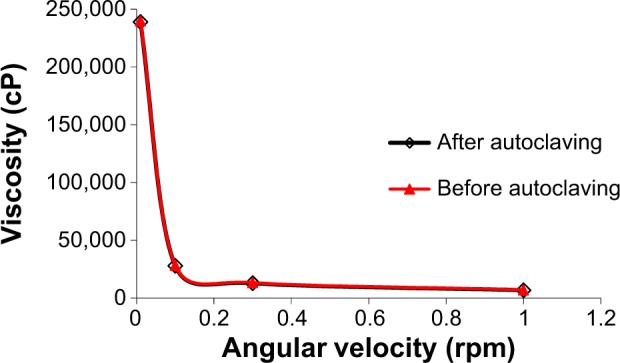
Effect of autoclaving on rheology of Gelrite-based in situ gel formula.
Note: As a result of overlapping, error bars were omitted for clarity.
Figure 4 shows the cumulative percent of OFX released as a function of time for various Gelrite formulations. All the investigated formulations showed a linear release profile for the first 12 hours of the study. The in situ gel formula with Gelrite concentration of 0.1% w/v released more than 80% of the drug after 12 hours of the experiment. The release of OFX, after 12 hours, from Gelrite in situ gel formulation was decreased with the increase in Gelrite concentration; the Gelrite in situ gel formula with 0.6% w/v Gelrite showed about 54% cumulative OFX release after 12 hours (Figure 4). The decrease in the release of OFX from in situ gel formulations is attributed to the increase of the polymer concentration, as by increasing the polymer concentration, a denser polymeric chain structure is produced. Thus, the diffusion of OFX through the denser formulations is reduced. In addition, the entrance of water into these formulations was reduced; thus, the rates of both dissolution and erosion were reduced.
Figure 4.
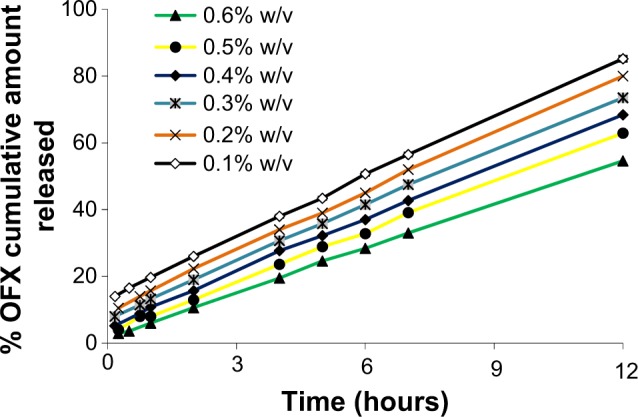
Effect of different Gelrite concentrations on the release of OFX from in situ gels.
Note: As a result of overlapping, error bars were omitted for clarity.
Abbreviation: OFX, ofloxacin.
In vitro release study of OFX microspheres loaded in situ gels
Hydrogel is used to incorporate microspheres in order to prolong the precorneal residence time of OFX microparticulate formula, and hence improve the bioavailability of the ocular drug.40 It is worth reporting that inclusion of microspheres in in situ gels did not change the viscosity, gelling capacity, or rheogram of the prepared Gelrite-based in situ gel formulations (data not shown). Incorporation of OFX-loaded microspheres in Gelrite solution (0.4% w/v) significantly altered the release profile of OFX-loaded microspheres in situ gel formula compared with the corresponding OFX gels and OFX microspheres (Figure 5).
Figure 5.
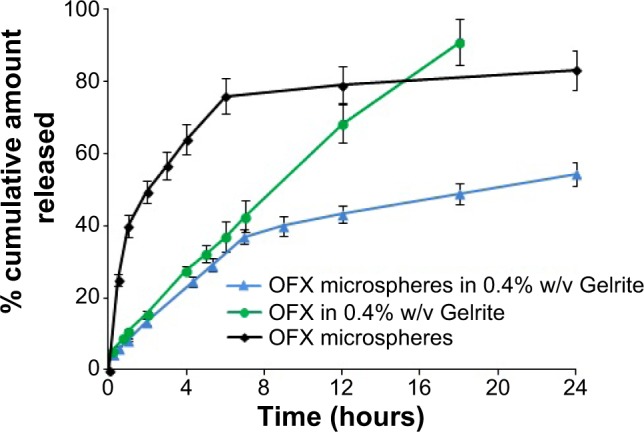
Release profile of OFX from microspheres, Gelrite in situ gel, and microsphere-loaded Gelrite in situ gel.
Abbreviation: OFX, ofloxacin.
The prepared OFX microspheres showed a 40% burst release after 1 hour that was reduced by the incorporation of the microspheres in 0.4% w/v Gelrite to 8.4%. On the other hand, microspheres released 80% of OFX content within the first 12 hours (Figure 5). While OFX-loaded microspheres in situ gel formula released 54.4% of OFX content after 24 hours. These results showed that incorporation of microspheres in the in situ gel successfully prevented the burst release and resulted in an improved sustained-release OFX profile. A disadvantage of the use of microsphere suspensions in the eye might be drainage of the particles, leading to an incomplete drug release. The major advantage of using in situ gel formulations loaded with OFX microspheres is the improved precorneal residence time compared with the corresponding OFX microspheres.35
In vivo study
Aqueous humor drug concentrations following various times of instillation of 0.3 mg of OFX as microspheres, Gelrite-loaded microspheres in situ gel, and the commercially available eye drops are shown in Figure 6. Compared with the other investigated formulations, OFX microspheres showed the highest Cmax and shortest Tmax values (Figure 6, inset table). This indicates the highest rate of absorption. Commercial OFX eye drops showed the lowest Cmax, which could possibly be attributed to the rapid drainage, and hence reduced residence time, of the applied OFX dose from the commercial eye drops. OFX microspheres in Gelrite in situ gel formula showed improved Cmax data compared with the commercial product and delayed action compared to all investigated formulations. This result indicates a prolonged pattern of release and absorption from OFX microspheres in Gelrite in situ gel formula.
Figure 6.
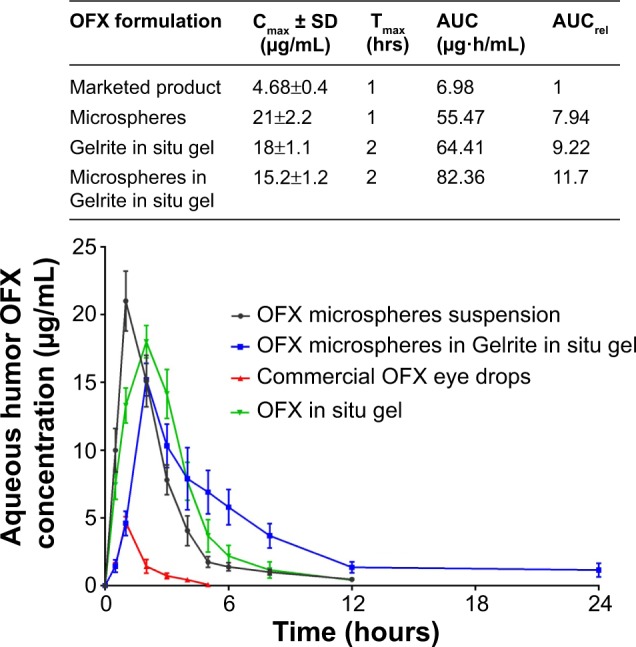
Concentration time profiles in aqueous humor of rabbits, following topical administration of different OFX formulations.
Notes: (Inset) In vivo pharmacokinetic parameters of OFX from the studied groups. *P<0.05 compared with commercial product.
Abbreviations: AUC, area under the curve maximum; AUCrel, the relative area under the curve compared with commercial product; Cmax, maximum OFX concentration in aqueous humor; OFX, ofloxacin; SD, standard deviation; Tmax, the time to reach this concentration.
Regarding the area under the curve value for the first 24 hours after OFX administration, microspheres in Gelrite in situ gel showed the highest value of area under the curve, with relative bioavailability (AUCrel) of 11.7 compared to commercial OFX product (Figure 6, inset table). This result indicates the greatest extent of drug absorption as a result of the improved residence time of OFX microspheres in Gelrite in situ gel formula.
The reported MIC90 of test organisms of OFX against 190 gram-negative and 229 gram-positive organisms from ocular sources is 2 μg/mL.5 Figure 6 shows that the commercial OFX eye drops only achieve a concentration equal to or higher than the MIC90 for the first hour after instillation. However, OFX microspheres sustained this level for approximately 4 hours. On the other hand, OFX microspheres in the in situ gel formula sustained concentrations higher than the MIC90 for approximately 8 hours (Figure 6). These results indicate that formulation of OFX as microspheres in the in situ gel of Gelrite successfully achieve the sought objectives of the present study. The formulation effectively maintains the drug concentration in the aqueous humor of rabbits above MIC90 for up to 8 hours. This will eliminate the need for frequent instillation of the drug and enhance patient compliance.
Conclusion
In this study, in situ gel formula loaded with OFX microspheres showed a prolonged pattern of release and absorption compared with OFX microspheres and with OFX in situ gel formulations. OFX microspheres were prepared by O/O emulsion solvent evaporation method utilizing PLGA as a biodegradable polymer. The prepared microspheres were added to Gelrite in situ gelling system. The improved release pattern and bioavailability of the prepared OFX-loaded microspheres in situ gel formula is attributed to improved precorneal residence time. These results support the potential use of in situ gel formulation loaded with OFX microspheres as a novel ocular drug delivery system.
Footnotes
Disclosure
The authors report no conflicts of interest in this work.
References
- 1.Nagayama A, Nakao T, Taen H. In vitro activities of ofloxacin and four other new quinoline-carboxylic acids against Chlamydia trachomatis. Antimicrob Agents Chemother. 1988;32:1735–1737. doi: 10.1128/aac.32.11.1735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Chan KP, Chu KO, Lai WW, et al. Determination of ofloxacin and moxifloxacin and their penetration in human aqueous and vitreous humor by using high-performance liquid chromatography fluorescence detection. Anal Biochem. 2006;353:30–36. doi: 10.1016/j.ab.2006.03.016. [DOI] [PubMed] [Google Scholar]
- 3.Gwon A. Topical ofloxacin compared with gentamicin in the treatment of external ocular infection. Ofloxacin Study Group. Brit J Ophthalmol. 1992;76:714–718. doi: 10.1136/bjo.76.12.714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Beck R, van Keyserlingk J, Fischer U, Guthoff R, Drewelow B. Penetration of ciprofloxacin, norfloxacin and ofloxacin into the aqueous humor using different topical application modes. Graefes Arch Clin Exp Ophthalmol. 1999;237:89–92. doi: 10.1007/s004170050200. [DOI] [PubMed] [Google Scholar]
- 5.Richman J, Zolezio H, Tang-Liu D. Comparison of ofloxacin, gentamicin, and tobramycin concentrations in tears and in vitro MICs for 90% of test organisms. Antimicrob Agents Chemother. 1990;34:1602–1604. doi: 10.1128/aac.34.8.1602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Sinnaeve BA, Decaestecker TN, Claerhout IJ, Kestelyn P, Remon JP, Van Bocxlaer JF. Confirmation of ofloxacin precipitation in corneal deposits by microbore liquid chromatography-quadrupole time-of-flight tandem mass spectrometry. J Chromatogr B Analyt Technol Biomed Life Sci. 2003;785:193–196. doi: 10.1016/s1570-0232(02)00854-1. [DOI] [PubMed] [Google Scholar]
- 7.Järvinen K, Järvinen T, Urtti A. Ocular absorption following topical delivery. Adv Drug Deliv Rev. 1995;16:3–19. [Google Scholar]
- 8.Zhang W, Prausnitz MR, Edwards A. Model of transient drug diffusion across cornea. J Control Release. 2004;99:241–258. doi: 10.1016/j.jconrel.2004.07.001. [DOI] [PubMed] [Google Scholar]
- 9.Davies NM. Biopharmaceutical considerations in topical ocular drug delivery. Clin Exp Pharmacol Physiol. 2000;27:558–562. doi: 10.1046/j.1440-1681.2000.03288.x. [DOI] [PubMed] [Google Scholar]
- 10.Lin HR, Sung KC. Carbopol/pluronic phase change solutions for ophthalmic drug delivery. J Control Release. 2000;69:379–388. doi: 10.1016/s0168-3659(00)00329-1. [DOI] [PubMed] [Google Scholar]
- 11.Souza JG, Dias K, Pereira TA, Bernardi DS, Lopez RF. Topical delivery of ocular therapeutics: carrier systems and physical methods. J Pharm Pharmacol. 2014;66:507–530. doi: 10.1111/jphp.12132. [DOI] [PubMed] [Google Scholar]
- 12.Ludwig A. The use of mucoadhesive polymers in ocular drug delivery. Adv Drug Deliv Rev. 2005;57:1595–1639. doi: 10.1016/j.addr.2005.07.005. [DOI] [PubMed] [Google Scholar]
- 13.Hornof M, Weyenberg W, Ludwig A, Bernkop-Schnürch A. Mucoadhesive ocular insert based on thiolated poly(acrylic acid): development and in vivo evaluation in humans. J Control Release. 2003;89:419–428. doi: 10.1016/s0168-3659(03)00135-4. [DOI] [PubMed] [Google Scholar]
- 14.Nanjawade BK, Manvi FV, Manjappa AS. In situ-forming hydrogels for sustained ophthalmic drug delivery. J Control Release. 2007;122:119–134. doi: 10.1016/j.jconrel.2007.07.009. [DOI] [PubMed] [Google Scholar]
- 15.Zimmer A, Kreuter J. Microspheres and nanoparticles used in ocular delivery systems. Adv Drug Deliv Rev. 1995;16:61–73. [Google Scholar]
- 16.Radomska-Soukharev A, Wojciechowska J. Microemulsions as potential ocular drug delivery systems: phase diagrams and physical properties depending on ingredients. Acta Pol Pharm. 2005;62:465–471. [PubMed] [Google Scholar]
- 17.Carvalho EL, De la Fuente M, Seijo Rey B. Liposomes as ocular drug delivery systems. Arch Soc Esp Oftalmol. 2004;79:151–152. doi: 10.4321/s0365-66912004000400002. Spannish. [DOI] [PubMed] [Google Scholar]
- 18.Almeida H, Amaral MH, Lobão P, Lobo JM. In situ gelling systems: a strategy to improve the bioavailability of ophthalmic pharmaceutical formulations. Drug Discov Today. 2014;19:400–412. doi: 10.1016/j.drudis.2013.10.001. [DOI] [PubMed] [Google Scholar]
- 19.Järvinen T, Järvinen K. Prodrugs for improved ocular drug delivery. Adv Drug Deliv Rev. 1996;19:203–224. [Google Scholar]
- 20.Sultana Y, Aqil M, Ali A, Zafar S. Evaluation of carbopol-methyl cellulose based sustained-release ocular delivery system for pefloxacin mesylate using rabbit eye model. Pharm Dev Technol. 2006;11:313–319. doi: 10.1080/10837450600767698. [DOI] [PubMed] [Google Scholar]
- 21.Svenson S. Dendrimers as versatile platform in drug delivery applications. Eur J Pharm Biopharm. 2009;71:445–462. doi: 10.1016/j.ejpb.2008.09.023. [DOI] [PubMed] [Google Scholar]
- 22.Sultana Y, Aqil M, Ali A, Samad A. Advances in the topical ocular drug delivery. Expert Rev Ophthalmol. 2007;2:309–323. [Google Scholar]
- 23.Bin Choy Y, Park JH, Prausnitz MR. Mucoadhesive Microparticles Engineered for Ophthalmic Drug Delivery. J Phys Chem Solids. 2008;69:1533–1536. doi: 10.1016/j.jpcs.2007.10.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Patil SB, Sawant KK. Mucoadhesive microspheres: a promising tool in drug delivery. Curr Drug Deliv. 2008;5:312–318. doi: 10.2174/156720108785914970. [DOI] [PubMed] [Google Scholar]
- 25.Liu Z, Li J, Nie S, Liu H, Ding P, Pan W. Study of an alginate/HPMC-based in situ gelling ophthalmic delivery system for gatifloxacin. Int J Pharm. 2006;315:12–17. doi: 10.1016/j.ijpharm.2006.01.029. [DOI] [PubMed] [Google Scholar]
- 26.Rozier A, Mazuel C, Grove J, Plazonnet B. Gelrite®: A novel, ion-activated, in-situ gelling polymer for ophthalmic vehicles. Effect on bioavailability of timolol. Int J Pharm. 1989;57:163–168. [Google Scholar]
- 27.Balasubramaniam J, Pandit JK. Ion-activated in situ gelling systems for sustained ophthalmic delivery of ciprofloxacin hydrochloride. Drug Deliv. 2003;10:185–191. doi: 10.1080/713840402. [DOI] [PubMed] [Google Scholar]
- 28.Balasubramaniam J, Kant S, Pandit JK. In vitro and in vivo evaluation of the Gelrite gellan gum-based ocular delivery system for Indomethacin. Acta Pharm. 2003;53:251–261. [PubMed] [Google Scholar]
- 29.Sultana Y, Aqil M, Ali A. Ion-activated, Gelrite®-based in situ ophthalmic gels of pefloxacin mesylate: comparison with conventional eye drops. Drug Deliv. 2006;13:215–219. doi: 10.1080/10717540500309164. [DOI] [PubMed] [Google Scholar]
- 30.El-Kamel A, Al-Dosari H, Al-Jenoobi F. Environmentally responsive ophthalmic gel formulation of carteolol hydrochloride. Drug Deliv. 2006;13:55–59. doi: 10.1080/10717540500309073. [DOI] [PubMed] [Google Scholar]
- 31.Kalam MA, Sultana Y, Samad A, et al. Gelrite-Based In Vitro Gelation Ophthalmic Drug Delivery System of Gatifloxacin. J Dispers Sci Technol. 2008;29:89–96. [Google Scholar]
- 32.Basci NE, Hanioglu-Kargi S, Soysal H, Bozkurt A, Kayaalp SO. Determination of ofloxacin in human aqueous humour by high-performance liquid chromatography with fluorescence detection. J Pharm Biomed Anal. 1997;15:663–666. doi: 10.1016/s0731-7085(96)01889-4. [DOI] [PubMed] [Google Scholar]
- 33.Kim BK, Hwang SJ, Park JB, Park HJ. Preparation and characterization of drug-loaded polymethacrylate microspheres by an emulsion solvent evaporation method. J Microencapsul. 2002;19:811–822. doi: 10.1080/0265204021000022770. [DOI] [PubMed] [Google Scholar]
- 34.Gavini E, Chetoni P, Cossu M, Alvarez MG, Saettone MF, Giunchedi P. PLGA microspheres for the ocular delivery of a peptide drug, vancomycin using emulsification/spray-drying as the preparation method: in vitro/in vivo studies. Eur J Pharm Biopharm. 2004;57:207–212. doi: 10.1016/j.ejpb.2003.10.018. [DOI] [PubMed] [Google Scholar]
- 35.Albertsson AC, Carlfors J, Sturesson C. Preparation and characterisation of poly(adipic anhydride) microspheres for ocular drug delivery. J Appl Polym Sci. 1996;62:695–705. [Google Scholar]
- 36.Nafea EH, El-Massik MA, El-Khordagui LK, Marei M, Khalafallah NM. Alendronate PLGA microspheres with high loading efficiency for dental applications. J Microencapsul. 2007;24:525–538. doi: 10.1080/02652040701439807. [DOI] [PubMed] [Google Scholar]
- 37.Dai L, Liu X, Tong Z. Critical behavior at sol–gel transition in gellan gum aqueous solutions with KCl and CaCl2 of different concentrations. Carbohydr Polym. 2010;81:207–212. [Google Scholar]
- 38.Ogawa E, Matsuzawa H, Iwahashi M. Conformational transition of gellan gum of sodium, lithium, and potassium types in aqueous solutions. Food Hydrocoll. 2002;16:1–9. [Google Scholar]
- 39.Rupenthal ID, Green CR, Alany RG. Comparison of ion-activated in situ gelling systems for ocular drug delivery. Part 1: physicochemical characterisation and in vitro release. Int J pharm. 2011;411:69–77. doi: 10.1016/j.ijpharm.2011.03.042. [DOI] [PubMed] [Google Scholar]
- 40.Ravi Kumar MN. Nano and microparticles as controlled drug delivery devices. J Pharm Pharm Sci. 2000;3:234–258. [PubMed] [Google Scholar]


