SUMMARY
In eukaryotic cells, DNA replication proceeds with continuous synthesis of leading-strand DNA and discontinuous synthesis of lagging-strand DNA. Here we describe a method, eSPAN (enrichment and sequencing of protein-associated nascent DNA), which reveals the genome-wide association of proteins with leading and lagging strands of DNA replication forks. Using this approach in budding yeast, we confirm the strand specificities of DNA polymerases delta and epsilon and show that the PCNA clamp is enriched at lagging strands compared with leading-strand replication. Surprisingly, at stalled forks, PCNA is unloaded specifically from lagging strands. PCNA unloading depends on the Elg1-containing alternative RFC complex, ubiquitination of PCNA, and the checkpoint kinases Mec1 and Rad53. Cells deficient in PCNA unloading exhibit increased chromosome breaks. Our studies provide a tool for studying replication-related processes and reveal a mechanism whereby checkpoint kinases regulate strand-specific unloading of PCNA from stalled replication forks to maintain genome stability.
INTRODUCTION
S phase cells are particularly vulnerable to insults from DNA-damaging agents. To meet these challenges, cells have evolved sophisticated systems to regulate DNA replication and to maintain fork stability under replication stress (Bell and Dutta, 2002; Branzei and Foiani, 2010; Zou, 2013). Alterations in these regulations can result in genome instability and human cancers (Harper and Elledge, 2007; Huen et al., 2010; Kastan and Bartek, 2004). Therefore, it is important to understand how the DNA replication process is regulated under normal and replication stress conditions.
In eukaryotic cells, DNA replication initiates from multiple replication origins. Once initiated, DNA synthesis proceeds bidirectionally, with continuous synthesis of leading-strand DNA and discontinuous synthesis of the lagging strands via Okazaki fragments (Bell and Dutta, 2002; Waga and Stillman, 1998). Many proteins are involved in chromatin replication, some of which have been implicated in the synthesis of leading or lagging strands specifically. For instance, using Polε and Polδ mutants with altered specificities, it has been deduced that Polε and Polδ synthesize leading and lagging strands, respectively (Nick McElhinny et al., 2008; Pursell et al., 2007; Stillman, 2008). Furthermore, the replicative helicase MCM proteins can traverse blocks placed on the lagging, but not on the leading strands of DNA replication forks, supporting the idea that the MCM helicase travels with leading strands (Fu et al., 2011). Together, these studies support the idea that different proteins may bind and regulate the synthesis of leading or lagging strands distinctly. However, no method is currently available to discern whether a protein binds directly to leading or lagging strands of replication forks, which hinders our further understanding of the regulation of DNA replication and its related processes.
Proliferating cell nuclear antigen (PCNA) is essential for DNA replication and DNA repair (Majka and Burgers, 2004; Moldovan et al., 2007). PCNA forms a trimeric ring and is loaded onto a primer-template junction by replication factor C (RFC) that consists of Rfc1-5. PCNA serves as the sliding clamp for DNA polymerases ε and δ (O’Donnell et al., 2013; Waga and Stillman, 1998) and interacts with other proteins involved in DNA replication. For instance, PCNA recruits Fen1 endonuclease and Cdc9 DNA ligase, two enzymes required for Okazaki fragment processing (Waga and Stillman, 1998). Inactivation of Cdc9 activates PCNA ubiquitylation at lysine 107 (Das-Bradoo et al., 2010; Nguyen et al., 2013). PCNA also functions in translesion synthesis. In this case, PCNA mono-ubiquitylation at lysine 164 catalyzed by Rad6 (E2) and Rad18 (E3) promotes the error-prone repair pathway, whereas SUMOylation of PCNA K164 and K127 by Siz1 E3 SUMO ligase helps prevent uncontrolled recombination (Hoege et al., 2002; Papouli et al., 2005; Pfander et al., 2005). Therefore, PCNA is a master regulator of DNA replication and repair.
After DNA synthesis, PCNA is proposed to be unloaded from chromatin for recycling because it has been observed that chromatin-bound PCNA and SUMOylated species increase dramatically during the S phase of the cell cycle in cells lacking Elg1, a Rfc1 homolog that forms an alternative RFC complex with Rfc2-5 (Bellaoui et al., 2003; Kubota et al., 2013). However, it is currently unknown whether PCNA is regulated at stalled forks in response to DNA replication stress.
In response to DNA replication stress, checkpoint kinases are activated to maintain cell viability in yeast and human cells (Huen et al., 2010; Zou, 2013). Budding yeast cells treated with hydroxyurea (HU, an inhibitor of ribonucleotide reductase) activate Mec1 (equivalent to human ATR kinase), which in turn phosphorylates downstream kinases including Rad53. Activated Rad53 inhibits the firing of late replication origins (Santocanale and Diffley, 1998), maintains fork stability (Lopes et al., 2001), and regulates replication fork restart (Szyjka et al., 2008). It is known that Rad53 inhibits firing of late replication origins through phosphorylation of Sld3, a protein involved in initiation of DNA replication and Dbf4 (a subunit of Cdc7/Dbf4 kinase) (Lopez-Mosqueda et al., 2010; Zegerman and Diffley, 2010). However, it remains poorly understood how checkpoint kinases maintain fork stability in response to replication stress.
Herein we describe a method termed enrichment and sequencing of protein-associated nascent DNA (eSPAN), which can discern whether a protein is enriched at the leading and lagging strands of DNA replication forks. Using this method, we show that Polε and Polδ bind preferentially to leading and lagging strands, respectively, supporting their division of labor in DNA synthesis. Remarkably, we observed that PCNA is enriched at lagging strands of active forks and at leading strands of HU-stalled forks because of unloading of PCNA from lagging strands. The PCNA unloading depends on Elg1 and checkpoint kinases. Finally, we report that cells deficient in PCNA unloading exhibit increased spontaneous chromosome breaks. Together, these studies describe a method to study DNA replication-linked processes and suggest that checkpoint kinases regulate PCNA unloading from lagging strands of stalled forks.
RESULTS
eSPAN Design
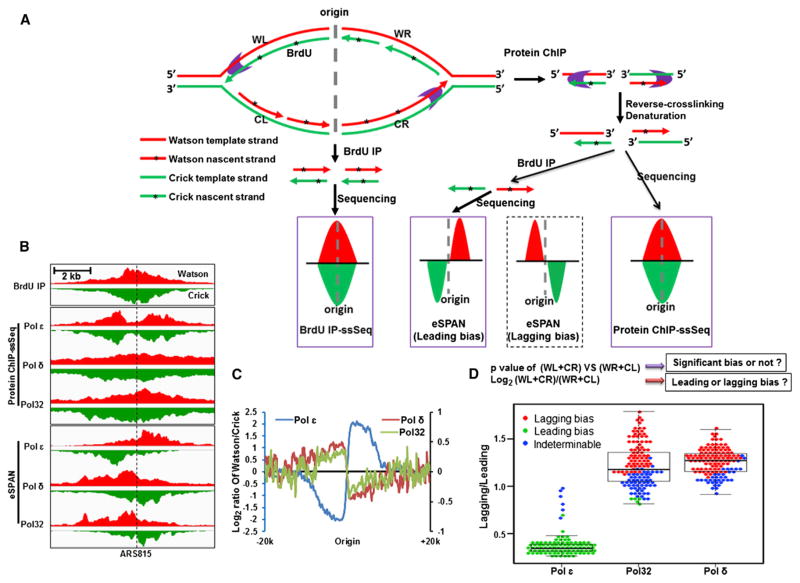
We have designed a method, eSPAN (enrichment and sequencing of protein-associated nascent strand DNA), which can discern whether a protein is enriched at leading or lagging strands of DNA replication forks (Figure 1A, middle panel). The eSPAN procedure started with chromatin immunoprecipitation (ChIP) of the protein of interest from synchronized early S phase cells. The ChIP DNA was denatured into single-stranded DNA (ssDNA). The protein bound-nascent ssDNA marked by the nucleotide analog BrdU (Knott et al., 2009) was then enriched by immunoprecipitation using anti-BrdU antibodies. The resulting ssDNA was ligated at the 3′ end with an oligo before conversion to dsDNA for adaptor ligation (Meyer et al., 2012). In this way, the strand information of each isolated ssDNA is retained. The sequencing tags were mapped to both Watson (red) and Crick (green) strands of the reference genome (Figure 1A). If a protein of interest preferentially binds to leading or lagging strands of DNA replication forks, the eSPAN peaks will exhibit a leading- or lagging-strand bias pattern (Figure 1A, middle panel).
Figure 1. Polε and Polδ Bind Leading and Lagging Strands of HU-Stalled DNA Replication Forks, Respectively.
(A) An outline of the experimental strategy for eSPAN method. Early S phase cells released from the G1 block in the presence of BrdU with/without hydroxyurea (HU) were used to perform BrdU immunoprecipitation (BrdU-IP) and protein chromatin immunoprecipitation (ChIP) of targeted protein. Protein-associated nascent single-stranded (ss) DNA was enriched with BrdU-IP. The isolated ssDNA from BrdU IP (BrdU IP-ssSeq), eSPAN, and protein ChIP (ChIP-ssSeq) was subjected to strand-specific sequencing. The sequence reads were mapped to both the Watson strand (W, red) and the Crick strand (C, green) of the reference genome.
(B) A snapshot of BrdU IP-ssSeq, protein ChIP-ssSeq and eSPAN peaks at ARS815 for Polε catalytic subunit (Polε), Polδ catalytic subunit (Polδ), and Pol32 (a subunit of Polδ subunit). The signals represent normalized sequence read densities. Red and green colors represent the Watson and Crick strand, respectively.
(C and D) Polε and Polδ associate with leading and lagging strands of replication forks, respectively. The average log2 ratios of sequence reads from Watson strands and Crick strands at 134 group I origins were calculated using a 200 bp window to obtain the average bias pattern after normalization against the corresponding BrdU-IP-ssSeq. The formula to calculate strand bias of eSPAN peaks at individual origins was shown at the top panel of (D) and was described in Experimental Procedures. Using this method, the eSPAN peaks at each of 134 origins were separated into three categories: leading-strand bias, indeterminable bias, and lagging-strand bias. The dot and box plot shows the variation of the ratio of sequence reads at lagging over leading strands of individual eSPAN peaks. The lagging/leading ratio was normalized against corresponding sequence reads of BrdU-IP. Each dot represents one eSPAN peak.
See also Figures S1 and S2.
For each eSPAN experiment, we also performed strand-specific sequencing of the corresponding protein ChIP DNA (ChIP-ssSeq, right panel of Figure 1A) as well as all nascent DNA from BrdU immunoprecipitation (BrdU-IP-ssSeq, left panel of Figure 1A). The data set information (14 independent BrdU IP-ssSeq data sets, 11 protein ChIP-ssSeq data sets, and 10 unique eSPAN data sets including biological repeats using HU-treated cells) was summarized in Figures S1A and S1B available online.
ChIP-ssSeq-Based Classification of Replication Origins
While replication origins have been identified previously using different approaches (Eaton et al., 2010; Wyrick et al., 2001), we need high-resolution mapping of origins for strand bias determination (Figures 1C and 1D). Therefore, we performed Mcm4 and Mcm6 ChIP-ssSeq using G1 cells and identified 360 Mcm4/Mcm6 peaks. Mcm4 and Mcm6 are two subunits of the replicative helicase MCM that bind to origins at G1 phase of the cell cycle. These 360 peaks also overlapped with the origins in SGD and OriDB databases (Figure S1C), suggesting that these 360 peaks, of which 310 are nontelomeric, are reliable replication origins. Because eSPAN analysis depends on whether a protein binds to the replication fork, we calculated the ChIP-ssSeq read density encompassing ± 10 Kb of the 310 nontelomeric origins for each of the 11 DNA replication proteins (Cdc45, Mcm4, Mcm6, Mcm10, PCNA, catalytic subunit of Polα, Polε and Polδ, Pol32 [a subunit of Polδ], Rfa1 [a subunit of RPA complex], and Rfc1 [a subunit of RFC complex]). Unsupervised hierarchical clustering of peak densities of these 11 proteins separated the 310 origins into two groups (Figure S1D). Group I origins (n = 134) were enriched with all 11 proteins, whereas 176 group II origins were not. In addition, the BrdU density of group I origins was significantly higher than that of group II origins (Figure S1D). Further analysis showed that the replication timing, determined in a previous report (Raghuraman et al., 2001), of group I origins was significantly earlier than that of the group II origins (Figure S1E). These results support that group I origins are early-replication origins that fire in the presence of HU and that group II origins are late replicating origins that do not fire in the presence of HU. Therefore, we analyzed each protein eSPAN by separating origins into group I and group II.
Polε and Polδ Bind Leading and Lagging Strands of DNA Replication Forks, Respectively
Inspection of eSPAN peaks at the ARS815 (Figure 1B) and ARS1021 (Figure S1A) indicated that Polε and Polδ exhibited a leading- and lagging-strand bias pattern at HU-stalled replication forks, respectively. The eSPAN peaks were distinct from the unbiased pattern of Polε and Polδ ChIP-ssSeq peaks where both nascent- and parental-strand DNA was sequenced (Figure 1B). To quantitatively evaluate the strand bias of each protein eSPAN data set at a genome-wide scale, we first calculated the average log2 ratio of the Waston:Crick strands of 134 group I origins to obtain the average bias information of group I or group II origins (Figure 1C). Second, we analyzed the bias of eS-PAN peaks at individual replication forks. To do this, we separated the total reads encompassing each origin into 4 parts (WL, WR, CL, and CR) according to Watson/Crick strands and left/right side of each ARS (Figure 1A). We then used binomial distribution to determine whether there were statistically significant differences in sequence reads of the leading strands (CR+WL) versus the lagging strands (WR+CL) using a p value < 10−5 as a cutoff. We also calculated the log2 ratio of the leading-strand reads and lagging-strand reads to determine leading or lagging bias (Figure 1D, upper panel). On the basis of p values and strand bias information, three types of eSPAN peak patterns were observed at 134 early replication origins: (1) an indeterminable bias pattern with p > 10−5, indicating inability to detect the strand bias of eSPAN peak at the origin; (2) a leading-strand bias pattern with p < 10−5 and log2[(WL+CR)/(WR+CL)] > 0; and (3) a lagging-strand bias pattern with p < 10−5 and log2[(WL+CR)/(WR+CL)] < 0. To visualize the variation in bias ratios of individual eSPAN peaks, we also used a dot-box plot to present the ratio of sequence reads of lagging over leading strands of individual eSPAN peaks (Figure 1D).
On the basis of the analysis of average profiles from group I origins, the eSPAN peaks of catalytic subunits of Polε and Polδ exhibited the leading- and lagging-strand bias pattern, respectively (Figure 1C). At individual origins, Polε eSPAN peaks showed a leading-strand bias at 96% (128/134) group I origins (Figure 1D). In contrast, Polδ eSPAN peaks exhibited a lagging-strand bias at 74% group I origins (Figure 1D). Similarly, the eSPAN peaks of Pol32, a subunit of Polδ, exhibited a lagging-strand bias pattern on the basis of the analysis of both the average bias pattern and the individual bias patterns of group I origins (Figure 1D). Analysis of input DNA as well as Mcm4 and Mcm6 ChIP-ssSeq data using G1 cells showed that neither input DNA nor Mcm4/Mcm6 ChIP-ssSeq data showed strand bias by either of the two methods (Figures S2A–S2E). These results support the idea that Polε and Polδ associate preferentially with leading and lagging strands, respectively, of replication forks originated from early origins.
We noticed that 26% Polδ catalytic subunit eSPAN peaks and 46% Pol32 eSPAN peaks exhibited an indeterminable bias pattern (Figure 1D). The higher indeterminable bias of Pol32 eSPAN peaks may not due to the fact that Pol32 is also a subunit of translesion DNA polymerase ζ (Johnson et al., 2012) as Polζ may only function in G2 phase (Huang et al., 2013). Origins showing the indeterminable bias for Polδ or Pol32 eSPAN replicated less efficiently than those exhibiting lagging-strand bias (Figure S2F and Figure S2G), suggesting that origin efficiency is a factor that impacts the strand bias of eSPAN peaks. However, origin efficiency did not affect the strand bias of Polε eSPAN peaks (Figure S2H), suggesting that other factors also likely affects the strand bias of Polδ. We noticed that the Polε ChIP efficiency was significantly higher than the Polδ catalytic subunit or Pol32 (Figure S2I). Therefore, we suggest that both inefficient ChIP and weak origin firing contribute to the inability to discern the strand bias of Polδ eSPAN peaks at a high percentage of origins.
To analyze the association of Polε and Polδ with forks from group II replication origins, we released yeast G1 cells into media at 16°C to slow down cell cycle progression and performed Polε and Polδ eSPAN using early S phase cells (72, 84, and 96 min after release; Figure 2A). Polε eSPAN results indicated that Polε was enriched at the leading strands (Figures 2B and 2C), whereas Polδ eSPAN peaks exhibited lagging-strand bias at both group I and II origins (Figures 2D and 2E). We also observed that the eSPAN peaks moved forward from 1 kb (72 min after release) to ~15 kb (96 min), consistent with the idea that Polε and Polδ move bidirectionally from origins during cell cycle progression. Furthermore, Polε and Polδ eSPAN peaks exhibited a leading- and lagging-strand bias pattern, respectively, at the majority of individual origins (Figure 2F), with significantly less Polδ eSPAN peaks exhibiting indeterminable strand bias origins than those at HU (Figures 2F and 1D). In summary, Polε and Polδ eSPAN results provide direct evidence that Polε and Polδ physically bind leading and lagging strands of both active and stalled DNA replication forks, respectively, on a genome-wide scale (Figure 2G), supporting their division of labor during DNA synthesis.
Figure 2. Polε and Polδ Are Enriched at Leading and Lagging Strands of All Active Replication Forks, Respectively.
(A) Fluorescence-activated cell sorting analysis of cell cycle progression of yeast cells released from G1 block using alpha factor into S phase without HU at 16°C.
(B and C) The eSPAN peaks of the Polε catalytic subunit exhibit a leading-strand bias pattern at both group I (B) and group II origins (C) during normal cell cycle progression (72, 84, and 96 min after release).
(D and E) The eSPAN peaks of the Polδ catalytic subunit exhibit a lagging-strand bias pattern at both group I (D) and group II (E) origins during normal cell cycle progression. The average bias of eSPAN peaks at group I and group II origins in (B)–(E) was analyzed as described in Figure 1C.
(F) Polε and Polδ eSPAN peaks exhibit leading- and lagging-strand bias at almost all individual group I origins. The eSPAN data using cells 72 min after release were used for the analysis as described in Figure 1D.
(G) A model for the association of Polε and Polδ with replication forks.
See also Figure S2.
Association of Selected DNA Replication Proteins with HU-Stalled Replication Forks
Next, using eSPAN we determined how selected components of the DNA replication machinery associated with HU-stalled replication forks. The eSPAN peaks of Cdc45, Mcm6, and Mcm10 exhibited leading-strand bias on the basis of the analysis of the average bias pattern of eSPAN peaks at group I origins (Figures 3A and 3B) and also on the basis of the analysis of individual peaks at these origins (Figure 3E). These results indicate that these three proteins associate preferentially with the leading strands of DNA replication forks originated from early replication origins. Cdc45 and Mcm6 are subunits of the active replicative helicase CMG (Cdc45-MCM-GINS). The preferential association with leading strands of Cdc45 and Mcm6 strongly supports the idea the CMG complex travels with the leading strands of DNA replication forks (Fu et al., 2011; Moyer et al., 2006).
Figure 3. Analysis of Seven Proteins at HU-Stalled Replication Forks Using eSPAN.
(A) Cdc45, Mcm6, and Mcm10 are enriched at leading strands of HU-stalled replication forks.
(B) A model explaining Cdc45 and Mcm6 data in (A).
(C) Polα, Rfa1, and Rfc1 bind preferentially to lagging strands of HU-stalled replication forks.
(D) A model explaining Polα in (C).
(E) Analysis of the strand-bias pattern of eSPAN peaks of six proteins in (A) and (B) at individual origins as described in Figure 1D.
(F) An example of PCNA eSPAN peaks at origins ARS606 and ARS607 for HU-stalled forks (+HU) and active forks (−HU).
(G) Analysis of the average bias pattern of the PCNA eSPAN peaks at HU-stalled replication forks as well as active forks.
See also Figure S3.
How Mcm10 functions in DNA replication is controversial (Thu and Bielinsky, 2013). The fact that Mcm10 binds to DNA replication forks with a slight enrichment at leading strands is consistent with the idea that in addition to its role in initiation (Ricke and Bie-linsky, 2004), Mcm10 has a role regulating synthesis of both leading and lagging strands of DNA replication forks (Thu and Bielinsky, 2013).
The catalytic subunits of Polα, Rfa1, and Rfc1 were enriched at lagging strands compared with the corresponding leading strands on the basis of the analysis of both the average bias pattern of eSPAN peaks at group I origins (Figures 3C and 3D) and eSPAN peaks at individual origins (Figure 3E). Polα has the primase activity synthesizing an RNA primer to initiate DNA synthesis at the leading strand as well as each Okazaki fragment of the lagging strand. Rfa1 is a subunit of single-stranded DNA-binding protein RPA, which coats ssDNA at both leading and lagging strands. Rfc1 is the large subunit of the RFC complex involved in loading PCNA onto a primer-template junction at the leading strand and at each Okazaki fragment on the lagging strand. Therefore, while these proteins function in leading- and lagging-strand synthesis, each of these proteins are proposed to be enriched on lagging strands compared with leading strands of replication forks (Waga and Stillman, 1998). Our eSPAN results provide physical evidence supporting this notion.
PCNA Is Enriched at Leading Strands of HU-Stalled Forks, but at Lagging Strands of Nonstalled Forks
Surprisingly, PCNA eSPAN peaks showed a leading-strand bias at HU-stalled replication forks (Figures 3F, 3G, S3A, and S3B), suggesting that PCNA is enriched at leading strands of HU-stalled forks. PCNA is a clamp for DNA polymerases, Polε and Polδ. According to the current DNA replication models (Waga and Stillman, 1998), one would expect PCNA to be enriched at lagging strands because one PCNA trimer is needed for leading-strand synthesis, whereas multiple PCNA trimers are needed for lagging-strand synthesis, one for each Okazaki fragment. The enrichment of PCNA at leading strand of HU-stalled replication forks was unlikely due to the inability to ChIP PCNA on lagging strands because PCNA eSPAN repeats using two different antibodies yielded identical results (a polyclonal PCNA antibody (Zhang et al., 2000) and an antibody against the Flag epitope fused to PCNA; data not shown). Therefore, we conclude that PCNA is enriched on leading strands compared with the corresponding lagging strands of HU-stalled replication forks.
To determine whether the preferential association of PCNA with leading strands of HU-stalled forks is due to the presence of HU, we analyzed how PCNA bound active replication forks without HU during normal cell cycle progression. In contrast to the leading-strand bias observed at stalled forks, PCNA eSPAN peaks at nonstalled forks showed a lagging-strand bias at each of the three time points analyzed (Figures 3F, 3G, S3A, and S3B), consistent with the current replication model that more PCNA molecules bind lagging strands than the corresponding leading strands. Thus, the association of PCNA with leading and lagging strands of DNA replication forks is altered dramatically when DNA replication forks stall in the presence of HU.
PCNA Is Unloaded from Lagging Strands of HU-Stalled Forks
The eSPAN method measures the relative amount of a protein on leading and lagging strands of replication forks. The change in the relative amount of PCNA on leading and lagging strands of HU-stalled forks could be due either to an increase in PCNA on leading strands or a reduction of PCNA from lagging strands when active replication forks stall. To differentiate between these two possibilities, we calculated the relative amounts of PCNA on leading and lagging strands of active and HU-stalled forks. The amount of PCNA on lagging strands of HU-stalled forks was dramatically lower than that on the corresponding leading strands or that on lagging strands of active forks. In addition, PCNA on leading strands of both active and stalled forks was similar (Figure 4A). These results suggest that PCNA is preferentially unloaded from lagging strands of HU-stalled forks. We estimated that at nonstalled forks, the relative amount of PCNA on lagging strands over leading strands is about 2:1 (Figure S3C). If only one PCNA trimer associates with the leading strand of the DNA replication fork, the two PCNA trimers on the corresponding lagging strand must be all unloaded in order to generate the leading-strand bias pattern of the PCNA eSPAN peaks at HU-stalled forks (see Figure 7E).
Figure 4. PCNA Unloading Depends on Elg1 and Partially on PCNA Ubiquitylation.
(A) Box plot of relative amount of PCNA on leading and lagging strands with or without HU of group I origins. The PCNA relative amount is estimated by log2 ratio of sequence reads of PCNA eSPAN over PCNA protein ChIP.
(B) Representative PCNA eSPAN peaks at the origin ARS737 for wild-type (WT) and relevant mutations indicated on the left. Red and green colors represent the normalized read densities of the Watson and Crick strand, respectively.
(C) Average bias pattern of PCNA eSPAN peaks at group I origins in the HU-treated cells with relevant genome type shown on the right.
See also Figure S4.
Figure 7. Cells Deficient in PCNA Unloading Exhibit Genome Instability.
(A and B) Uncontrolled DNA synthesis under replication stress was observed in cells deficient in PCNA unloading. (A) An example of BrdU IP-ssSeq peaks at ARS607 in WT and mutant cells with relevant mutations shown on the right. (B) Box plot showing the distribution of BrdU peak length in the same strains as in (A). The mean and SD of the BrdU peak length are indicated in kb. Asterisks indicate p < 0.001 (two-tailed Student’s t test).
(C) BrdU-IP qPCR analysis confirms that DNA synthesis at ARS607 in the presence of HU continues further in elg1Δ and PCNA K164R mutant cells compared with wild-type cells. Cells arrested with alpha factor were released into fresh YPD with HU and BrdU for 45 min. DNA obtained from BrdU IP was analyzed by at each replication fork. In addition, the log2 ratios were calculated using the real-time PCR using primer pairs indicated in (A). Data represent mean ± SD from one BrdU-IP experiment. Similar results were obtained in an independent experiment.
(D) Cells deficient in PCNA unloading exhibit increased chromosome breaks. The percentage of cells with Rad52 foci with/without HU treatment was reported. Data represent mean ± SEM.
(E) Model summarizing the effect of different mutations on PCNA unloading from HU-stalled forks. This model was based on the idea that the ratio of PCNA trimers on lagging stranding over leading strands at normal forks is 2:1.
See also Figure S7.
PCNA Unloading Depends on Elg1 and Partially on PCNA Ubiquitylation
Elg1, which is homologous to Rfc1, forms an alternative RFC complex with Rfc2-5. A previous study proposed that Elg1 unloads PCNA from chromatin for PCNA recycling (Kubota et al., 2013). Therefore, we tested whether Elg1 is involved in PCNA unloading from lagging strands of HU-stalled forks. Compared with the leading-strand bias pattern in wild-type cells, PCNA eSPAN peaks in elg1Δ mutant cells exhibited a lagging-strand bias pattern (Figures 4B and 4C). These results support the idea that the Elg1-containing complex is also involved in PCNA unloading from lagging strands of HU-stalled replication forks.
Next, we asked whether PCNA ubiquitylation and SUMOylation affected PCNA unloading from HU-stalled forks. PCNA is mono-ubiquitylated at K164 by Rad6 (E2)-Rad18 (E3) (Das-Bradoo et al., 2010; Hoege et al., 2002). Mono-ubiquitylated PCNA can be further polyubiquitylated in a reaction depending on Mms2. In addition, K164 and K127 can also be SUMOylated by the Siz1 E3 SUMO ligase (Hoege et al., 2002; Papouli et al., 2005; Pfander et al., 2005). On average PCNA-K164R eSPAN peaks exhibited an indeterminable bias/no-bias pattern (Figures 4B and 4C). The indeterminable bias pattern of the eSPAN peaks indicates that equal amount of PCNA-K164R mutant trimer binds to leading and lagging strands of HU-stalled replication forks, implying that only one mutant PCNA-K164R trimer is unloaded from lagging strands of HU-stalled forks compared with two in wild-type (see Figure 7E). PCNA-K127R and mms2Δ mutation had a minor effect on PCNA unloading compared with PCNA-K164R, while PCNA-K164R/K127R had a similar effect on PCNA unloading as PCNA-K164R mutation. Mutations in RAD6 and RAD18 had a similar effect on PCNA unloading as PCNA-K164R mutant. In contrast, the siz1Δ or PCNA-K107R mutation had no apparent effect (Figures 4B and 4C). At individual origins, it appeared that the extent of PCNA unloading at different origins was distinct in wild-type and in these mutant cells (Figure S4A). These differences did not correlate with replication timing and/or PCNA ChIP efficiency (data not shown), suggesting that local environments such as chromatin may also affect PCNA unloading. These results suggest that mono-ubiquitylation and possibly polyubiquitylation regulates PCNA unloading from lagging strands of HU-stalled forks and that PCNA unloading efficiency may be origin-specific.
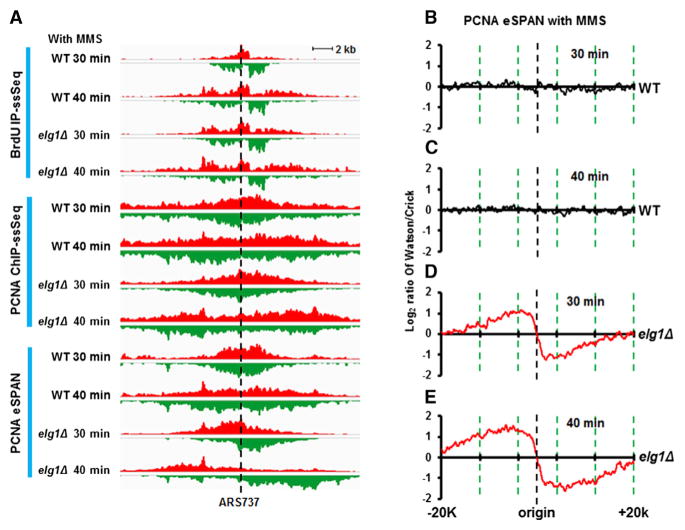
PCNA Is Unloaded from Lagging Strands of MMS-Stalled Forks
To test whether PCNA unloading is a general response to replication stress, we tested whether cells treated with methyl methanesulfonate (MMS), a DNA alkylating agent and a carcinogen, also display PCNA unloading. Wild-type or elg1Δ mutant cells were released from G1 into media containing MMS for 30 and 40 min. PCNA eSPAN peaks in wild-type cells treated with MMS exhibited an indeterminable bias pattern (Figures 5A–5C), whereas PCNA eSPAN peaks exhibited a lagging-strand bias pattern in elg1Δ mutant cells (Figures 5A, 5D, and 5E). One explanation is that one of two PCNA trimers is unloaded from the lagging strand of each MMS-stalled fork. Alternatively, the unbiased pattern of eSPAN peaks observed in MMS treated cells reflects the average of mixed forks including ongoing forks and stalled forks. Supporting the latter interpretation, replication still proceeded in the MMS-treated cell population (comparing BrdU-IP-ssSeq data at 30 min and 40 min). Moreover, PCNA eSPAN peaks in unsynchronized cells still exhibited a lagging-strand bias pattern (Figures S4B and S4C). Thus, we suggest that the PCNA indeterminable bias pattern observed in MMS treated cells is due to PCNA unloading from MMS-stalled forks.
Figure 5. PCNA Is Unloaded from Lagging Strands of MMS-Stalled DNA Replication Forks.
(A) Two representative BrdU IP-ssSeq, PCNA ChIP-ssSeq, and PCNA eSPAN peaks at the ARS737 origin in WT and elg1Δ cells.
(B–E) Average bias profiles of PCNA eSPAN peaks in WT (B and C) and elg1Δ strains (D and E). Yeast cells arrested at G1 using alpha factor were released into S phase with 0.035% MMS. Cells were collected at 30 min (B and D) and 40 min (C and E) for eSPAN, BrdU IP-ssSeq, and PCNA protein ChIP-ssSeq.
See also Figure S4.
PCNA Unloading from HU-Stalled Forks Depends on the Activation of Checkpoint Kinases
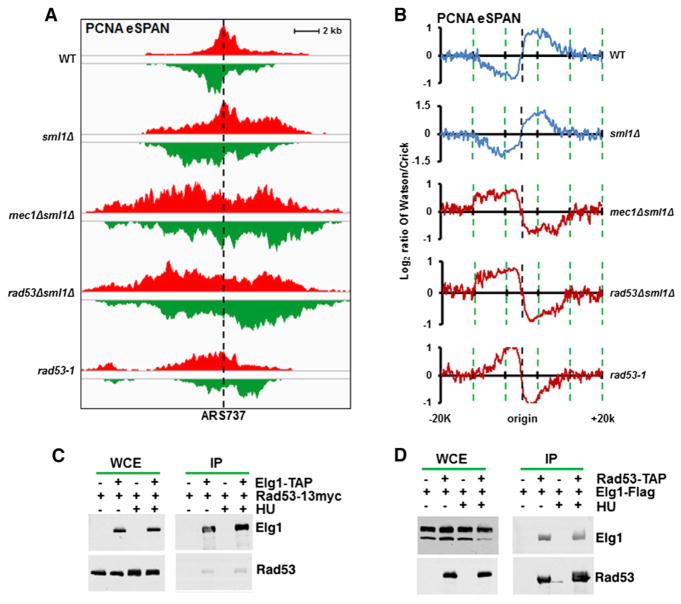
In response to HU- and MMS-induced stress, checkpoint kinases Mec1 and Rad53 are activated to maintain fork stability and to inhibit firing of late replication origins (Branzei and Foiani, 2010). Therefore, we asked whether the unloading of PCNA from HU-stalled replication forks depends on the checkpoint kinases, Mec1 and Rad53. Cells lacking Rad53 and Mec1 are not viable, but deletion of SML1, which encodes a ribonucleotide reductase inhibitor, suppresses this effect (Zhao et al., 1998). In mec1Δ sml1Δ, rad53Δ sml1Δ, and rad53-1 mutant cells, late replication origins fired in the presence of HU due to checkpoint inactivation (data not shown, but see Figure S5). Remarkably, in contrast to the leading-strand bias observed in wild-type and sml1Δ mutant cells treated with HU, PCNA eSPAN peaks exhibited lagging-strand bias at HU-stalled replication forks in mec1Δ sml1Δ, rad53Δ sml1Δ, and rad53-1 mutant cells (Figures 6A and 6B), suggesting that Mec1 and Rad53 regulate PCNA unloading from lagging strands of stalled replication forks. Consistent with this idea, inspection of PCNA eSPAN peak bias patterns at individual origins indicated that PCNA eSPAN peak bias patterns in rad53-1, mec1Δ sml1Δ, and elg1Δ mutant cells were quite similar (Figure S6A). When group I origins were separated into two subgroups on the basis of the BrdU track length in sml1Δ and rad53Δ sml1Δ mutant cells, the average bias of PCNA eSPAN peaks at these two subgroups was similar (Figure S6B), arguing against the idea that the potential ongoing DNA synthesis observed in checkpoint mutant cells contributes to the lagging-strand bias pattern of PCNA eSPAN peaks. Furthermore, we observed that Rad53 coimmunoprecipitated with Elg1 and Elg1 also coprecipitated with Rad53 in the reciprocal experiment (Figures 6C and 6D), suggesting that Elg1 interacts with Rad53 in vivo. Interestingly, HU treatment did not appear to affect the Rad53-Elg1 interaction (Figures 6C and 6D). Therefore, our results are consistent with the idea that checkpoint kinases regulate PCNA unloading, possibly through Rad53-Elg1 interaction.
Figure 6. The Checkpoint Kinases Mec1 and Rad53 Regulate PCNA Unloading from Lagging Strands of HU-Stalled Replication Forks.
(A and B) PCNA eSPAN peaks exhibit the lagging-strand pattern at HU-stalled forks in mec1Δ sml1Δ, rad53Δ sml1Δ, and rad53-1 mutant cells in contrast to the leading-strand bias pattern in wild-type cells. Average bias pattern of eSPAN peaks at group I origins was shown.
(C and D) Elg1 physically interacts with Rad53. Elg1 (C) or Rad53 (D) was immunoprecipitated (IP) from cells with or without HU treatment. Proteins in whole-cell extract (WCE) and IP were detected by western blotting using antibodies against the indicated proteins.
See also Figures S5 and S6.
Cells Deficient in PCNA Unloading Exhibited Genome Instability
Deletion of ELG1 or expression of PCNA-K164R mutation had no apparent effect on the firing of late replication origins such as ARS313 and ARS314 (Figures S5A–S5C). Remarkably, BrdU-IP-ssSeq tracks were much longer in the HU-treated elg1Δ cells than those in HU-treated wild-type strain (Figures 7A and 7B), consistent with published results showing that replication fork rate increases in elg1Δ cells under replication stress (Davidson et al., 2012). The enhanced DNA synthesis in elg1Δ cells compared with wild-type cells was confirmed using BrdU-IP and real-time PCR when these cells were treated with HU (Figure 7C). DNA synthesis also proceeded further in elg1Δ mutant cells compared with wild-type cells, both of which treated with MMS (Figures S7A and S7B). The uncontrolled DNA synthesis in the presence of HU was also observed in rad6Δ, rad18Δ and PCNA-K164R and K127R mutant cells that are defective in PCNA unloading, but not in siz1Δ or PCNA-K107R mutant cells in which PCNA unloading is not affected (Figures 7A and 7B). The elevated dNTP levels were used to explain increased DNA synthesis in elg1Δ cells. Because the dNTP levels were also elevated in PCNA-K107R cells, while to a lesser degree than PCNA-K164R cells (Figure S7C), we suggest that in addition to elevated levels of dNTPs, the inability to unload PCNA in elg1Δ and PCNA-K164R mutant cells may also contribute to the uncontrolled DNA synthesis observed in these mutant cells.
Uncontrolled DNA synthesis under replication stress results in genome instability. Therefore, we tested whether elg1Δ, PCNA-K164R, and PCNA-K127R mutant cells exhibited increased chromosome breaks using the Rad52-focus assay. Rad52 is involved in homologous recombination and forms foci at chromosome breaks during the S phase (Lisby et al., 2001). In untreated cells, PCNA-K164R mutant cells, but not elg1Δ or PCNA-K127R cells, exhibited a marked increase in cells with Rad52 foci compared with wild-type cells. When challenged with 100 mM HU, each of the three mutants (PCNA-K164R, elg1Δ, and PCNA-K127R) had more cells with Rad52 foci than wild-type cells (Figure 7D), suggesting that Elg1 and PCNA ubiquitylation are required to maintain genome stability under replication stress.
DISCUSSION
We have developed eSPAN method to measure the relative amount of a protein on the leading or lagging strands of replication forks in budding yeast. Using this eSPAN method, we provide a view into the genome-wide association with leading and lagging strands of DNA replication forks of 10 proteins in different replication machineries. We show that PCNA is unloaded from lagging strands of stalled forks under replication stress. We discuss below the advantage and limitation of the eSPAN technique and the implications of PCNA unloading when replication forks stall.
eSPAN Is a Unique Method to Study DNA Replication-Linked Processes
Many proteins involved in DNA replication have been identified. However, no method is currently available to distinguish whether a protein binds to leading or lagging strands of DNA replication forks, which hinders our understanding of the regulation of replication at DNA and chromatin levels. Here we have developed the eSPAN method and presented the following lines of evidence supporting the idea that the eSPAN method can reveal whether a protein is enriched at leading or lagging strands of DNA replication forks. First, we show that the catalytic subunits of Polε and Polδ associate preferentially with leading and lagging strands, respectively, of both HU-stalled forks and normal forks, providing direct evidence supporting the division of labor of these polymerases during DNA replication (Nick McElhinny et al., 2008; Pursell et al., 2007). Second, we show that Cdc45 and Mcm6, two subunits of active replicative helicase CMG complex, bind preferentially to leading strands of HU-stalled replication forks during the early S phase of the cell cycle. The eSPAN results provide physical evidence supporting the idea that the CMG complex travels with leading strands of DNA replication forks (Fu et al., 2011; O’Donnell et al., 2013). Third, we observed that more Polα, Rpa1, or Rfc1 molecules bind lagging-strand DNA than the corresponding leading-strand DNA. These results are consistent with the current DNA replication model on how Polα, RPA, and RFC function in DNA replication (Waga and Stillman, 1998). Taken together, the analysis of 10 key DNA replication proteins using the eSPAN method constitute direct evidence on the genome-wide association of these proteins with leading and lagging strands of DNA replication forks.
Capable of measuring relative amount of proteins at leading and lagging strands of DNA replication forks in a genome-wide scale, the eSPAN method will help address questions about DNA replication, DNA replication checkpoint, and epigenetic inheritance. For instance, how are DNA polymerases ε and δ recruited to leading and lagging strands of DNA replication forks? What factors regulate the association of CMG helicase to leading strands of DNA replication forks? In addition, it is known that DNA replication checkpoint maintains fork stability in response to DNA replication stress by unknown mechanisms (Branzei and Foiani, 2010; Kastan and Bartek, 2004; Zou, 2013). Do activated checkpoint kinases monitor alterations at leading or lagging strands? Following DNA replication, duplicated DNA is assembled into nucleosomes using both parental and newly synthesized his-tones to maintain epigenetic information (Burgess and Zhang, 2013). How is nucleosome assembly on leading and lagging strands regulated to maintain epigenetic information? We expect that the eSPAN method will help address these and other fundamental questions in the fields of DNA replication, DNA damage response, and the epigenetic inheritance in yeast and potentially in human cells.
There are several limitations for the eSPAN method. First, the eSPAN method measures the relative amount of a protein on leading and lagging strands of DNA replication forks, and it is difficult to discern whether a protein is missing from either strand using eSPAN. Second, the success of eSPAN technique depends in part on the ChIP efficiency because the first step of eSPAN method is based on chromatin immunoprecipitation. In this regard, we noticed that the bias of Polε eSPAN peak is much clearer than that of Polδ, most likely due to the fact that ChIP efficiency for Polε is significantly higher than Polδ. Third, the eSPAN method utilizes synchronized early S phase cells. Conditions that affect cell synchrony may complicate the performance and interpretation of eSPAN data sets. Finally, strand-bias determination of eSPAN peaks requires defined origins. Replication origins in higher eukaryotic cells such as humans remain insufficiently defined. Therefore, interpretation of the eSPAN data and future application and optimization of the eSPAN method in higher eukaryotic cells should consider these limitations.
PCNA Unloading from Lagging Strands of Stalled Replication Forks Is a General Response to DNA Replication Stress
Using the eSPAN method, we provide the following lines of evidence supporting the idea that PCNA is unloaded from lagging strands of stalled replication forks and that PCNA unloading is a general response to DNA replication stress. First, PCNA is enriched at lagging strands of active replication forks. However, PCNA associates preferentially with leading strands of HU-stalled replication forks because of the removal of PCNA from lagging strands. Second, PCNA is also unloaded from lagging strands of stalled forks induced by the carcinogen MMS. Third, we show that PCNA eSPAN peaks exhibit a lagging-strand bias pattern at HU stalled forks in cells deficient in Mec1 or Rad53. It has been shown that DNA replication forks collapse in cells lacking checkpoint kinases (Branzei and Foiani, 2010). The collapsed forks may explain the detection of the lagging-strand bias in checkpoint mutant cells. However, two observations argue against this interpretation. First, we show that at individual origins, the bias of PCNA eSPAN peaks in cells lacking Mec1 or Rad53 is quite similar to that in cells lacking Elg1, supporting the idea that Mec1, Rad53, and Elg1 function in the same genetic pathway to regulate PCNA unloading. Consistent with this interpretation, Rad53 physically interacts with Elg1. Second, it has been shown that DNA replication proteins bind to HU-stalled replication forks in checkpoint mutant cells (De Piccoli et al., 2012). Therefore, we suggest that PCNA unloading from lagging strands of stalled forks is regulated by checkpoint kinases.
We propose the following nonexclusive models explaining the function of PCNA unloading from lagging strands of stalled forks. First, PCNA unloading facilitates the loading of 9-1-1 complex, which also forms trimeric rings like PCNA and functions in checkpoint response, onto stalled forks for checkpoint activation. Supporting this idea, it has been shown that Elg1 and Rad24, a protein involved in loading of 9-1-1 onto damaged DNA, function redundantly in checkpoint activation (Majka and Burgers, 2004). Second, PCNA unloading helps the recruitment of translesion polymerases to the leading strands of stalled forks. Consistent with this idea, PCNA mono-ubiquitylation, which is important for translesion synthesis and recruitment of translesion polymerase, also regulates PCNA unloading from lagging strands (Moldovan et al., 2007). Third, PCNA unloading from lagging strands of stalled forks and subsequent loading of PCNA to lagging strands may mark fork restart. Finally, PCNA unloading is a mechanism whereby checkpoint kinases regulate fork stability under replication stress. Future studies are needed to test these and other possibilities to gain insight into regulation of PCNA unloading.
In human cells, ATAD5, the Elg1 homolog, is also proposed to function in PCNA unloading for recycling (Lee et al., 2013). In addition, PCNA unloading is also observed in cells deficient in new histone supplies (Mejlvang et al., 2014). Future studies are needed to determine whether PCNA is also unloaded from lagging strands under these conditions and the functional implications of PCNA unloading in human cells.
EXPERIMENTAL PROCEDURES
Yeast Strains
All yeast strains used in this study are in W303 (leu2-3, 112 ura3-1 his3-11, trp1-1, ade2-1 can1-100) genetic background and are listed in Table S1. The PCNA mutant strains, with deletion of the endogenous POL30 gene, were generated from ZGY002 by the plasmid shuffling method (Zhang et al., 2000).
eSPAN Procedure
The first step of eSPAN involves ChIP of a protein of interest. We followed a standard procedure to perform ChIP. Following ChIP, proteins were digested and protein-associated DNA was denatured. Nascent single-strand DNA was isolated using BrdU immunoprecipitation (BrdU-IP) (Viggiani et al., 2010). ssDNA library preparation used methods described previously (Meyer et al., 2012). Detailed experimental procedures are described in Supplemental Experimental Procedures.
Sequence Mapping and Data Analysis
The ssDNA libraries were sequenced using paired-end sequencing on Illumina Hi-Seq 2000 or 2500 machines. Reads were aligned to the yeast genome (sacCer3) using Bowtie2 software (Langmead and Salzberg, 2012). Only the consistent pair reads were used for the further analysis. The genome-wide read coverage of Watson and Crick strand was calculated by BEDTools (Quinlan and Hall, 2010) and in-house Perl programs. The reads of the Watson and Crick strands were merged for peak calling using the MACS software (Zhang et al., 2008).
Strand Bias Calculation
The overlapping peaks of Mcm4 and Mcm6 ChIP-ssSeq using G1 cells were used to identify replication origins used in this study. To calculate the global strand bias profile, the log2 ratios of sequencing reads of the Watson strand over Crick strand fragments across a 200 bp window around the center of replication origins were calculated. The average bias pattern of was then normalized against the corresponding BrdU-IP-ssSeq to obtain the average bias pattern of eSPAN peaks. To calculate bias at individual origins, each eS-PAN peak region of both the Watson and Crick strands was split into left and right halves on the basis of the location of replication origin. In this way, each eSPAN peak region was separated into the following four quadrants: Watson strand at the left (WL) and the right (WR) of an origin and Crick strand at the left (CL) and the right (CR) of an origin. The number of sequence reads in each of the four quadrants was counted. The binomial distribution was used to calculate the p value to determine whether sequence reads at the leading strand (WL+CR) were different from sequence reads of the lagging strand (WR+CL) following formula: log2 (WL+CR)/(WR+CL) to determine whether an eSPAN peak exhibit a leading- or lagging-strand bias pattern.
Rad53 and Elg1 Coimmunoprecipitation Assay
To detect Rad53 and Elg1 interactions in cell extracts, a standard TAP purification procedure was performed as described previously (Han et al., 2013). Other procedures including cell synchronization and ChIP are described in Supplemental Experimental Procedures.
Supplementary Material
Acknowledgments
We thank Dr. Oscar Aparicio and Dr. Grant Brown for yeast strains and plasmids. We thank Dr. Jayme Dahlin, Dr. David MacAlpine, Dr. Robin M. Ricke, Dr. Bruce Horazdovsky, Dr. Rentian Wu, and Dr. Zhenkun Lou for critical reading of this manuscript. This work was supported by NIH grants (GM72719, GM81838 to Z.Z.). A.C. is supported by the Swedish Cancer Society. G.F. and T.O. are supported by PO1 DK068055. S.J. is a Kempe Foundation scholarship recipient, and Z.Z. is a scholar of the Leukemia and Lymphoma Society.
Footnotes
ACCESSION NUMBERS
The high-throughput sequencing raw and processed data sets have been deposited in Gene Expression Omnibus (GEO) database under the accession number GSE52614.
Supplemental Information includes Supplemental Experimental Procedures, seven figures, and two tables and can be found with this article online at http://dx.doi.org/10.1016/j.molcel.2014.09.017.
AUTHOR CONTRIBUTIONS
C.Y. and Z.Z. designed the eSPAN method. H.G. developed the strand-bias analysis methods, analyzed all the eSPAN results, and performed other bioinformatics analysis. C.Y. performed most of the eSPAN experiments. J.H. did the rad52 foci assay and other protein chemistry biochemical experiments. H.G. and Z.-X.Z. did some PCNA eSPAN experiments. S.J. and A.C. measured the dNTPs concentration in different strains. T.O. and G.F. contributed to key reagents. Z.Z., H.G., and C.Y. wrote the paper. All authors read and edited the paper.
References
- Bell SP, Dutta A. DNA replication in eukaryotic cells. Annu Rev Biochem. 2002;71:333–374. doi: 10.1146/annurev.biochem.71.110601.135425. [DOI] [PubMed] [Google Scholar]
- Bellaoui M, Chang M, Ou J, Xu H, Boone C, Brown GW. Elg1 forms an alternative RFC complex important for DNA replication and genome integrity. EMBO J. 2003;22:4304–4313. doi: 10.1093/emboj/cdg406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Branzei D, Foiani M. Maintaining genome stability at the replication fork. Nat Rev Mol Cell Biol. 2010;11:208–219. doi: 10.1038/nrm2852. [DOI] [PubMed] [Google Scholar]
- Burgess RJ, Zhang Z. Histone chaperones in nucleosome assembly and human disease. Nat Struct Mol Biol. 2013;20:14–22. doi: 10.1038/nsmb.2461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Das-Bradoo S, Nguyen HD, Wood JL, Ricke RM, Haworth JC, Bielinsky AK. Defects in DNA ligase I trigger PCNA ubiquitylation at Lys 107. Nat Cell Biol. 2010;12:74–79. doi: 10.1038/ncb2007. sup pp 1–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davidson MB, Katou Y, Keszthelyi A, Sing TL, Xia T, Ou J, Vaisica JA, Thevakumaran N, Marjavaara L, Myers CL, et al. Endogenous DNA replication stress results in expansion of dNTP pools and a mutator phenotype. EMBO J. 2012;31:895–907. doi: 10.1038/emboj.2011.485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Piccoli G, Katou Y, Itoh T, Nakato R, Shirahige K, Labib K. Replisome stability at defective DNA replication forks is independent of S phase checkpoint kinases. Mol Cell. 2012;45:696–704. doi: 10.1016/j.molcel.2012.01.007. [DOI] [PubMed] [Google Scholar]
- Eaton ML, Galani K, Kang S, Bell SP, MacAlpine DM. Conserved nucleosome positioning defines replication origins. Genes Dev. 2010;24:748–753. doi: 10.1101/gad.1913210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fu YV, Yardimci H, Long DT, Ho TV, Guainazzi A, Bermudez VP, Hurwitz J, van Oijen A, Schärer OD, Walter JC. Selective bypass of a lagging strand roadblock by the eukaryotic replicative DNA helicase. Cell. 2011;146:931–941. doi: 10.1016/j.cell.2011.07.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Han J, Zhang H, Zhang H, Wang Z, Zhou H, Zhang Z. A Cul4 E3 ubiquitin ligase regulates histone hand-off during nucleosome assembly. Cell. 2013;155:817–829. doi: 10.1016/j.cell.2013.10.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harper JW, Elledge SJ. The DNA damage response: ten years after. Mol Cell. 2007;28:739–745. doi: 10.1016/j.molcel.2007.11.015. [DOI] [PubMed] [Google Scholar]
- Hoege C, Pfander B, Moldovan GL, Pyrowolakis G, Jentsch S. RAD6-dependent DNA repair is linked to modification of PCNA by ubiquitin and SUMO. Nature. 2002;419:135–141. doi: 10.1038/nature00991. [DOI] [PubMed] [Google Scholar]
- Huang D, Piening BD, Paulovich AG. The preference for error-free or error-prone postreplication repair in Saccharomyces cerevisiae exposed to low-dose methyl methanesulfonate is cell cycle dependent. Mol Cell Biol. 2013;33:1515–1527. doi: 10.1128/MCB.01392-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huen MS, Sy SM, Chen J. BRCA1 and its toolbox for the maintenance of genome integrity. Nat Rev Mol Cell Biol. 2010;11:138–148. doi: 10.1038/nrm2831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson RE, Prakash L, Prakash S. Pol31 and Pol32 subunits of yeast DNA polymerase δ are also essential subunits of DNA polymerase ζ. Proc Natl Acad Sci USA. 2012;109:12455–12460. doi: 10.1073/pnas.1206052109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kastan MB, Bartek J. Cell-cycle checkpoints and cancer. Nature. 2004;432:316–323. doi: 10.1038/nature03097. [DOI] [PubMed] [Google Scholar]
- Knott SR, Viggiani CJ, Tavaré S, Aparicio OM. Genome-wide replication profiles indicate an expansive role for Rpd3L in regulating replication initiation timing or efficiency, and reveal genomic loci of Rpd3 function in Saccharomyces cerevisiae. Genes Dev. 2009;23:1077–1090. doi: 10.1101/gad.1784309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kubota T, Nishimura K, Kanemaki MT, Donaldson AD. The Elg1 replication factor C-like complex functions in PCNA unloading during DNA replication. Mol Cell. 2013;50:273–280. doi: 10.1016/j.molcel.2013.02.012. [DOI] [PubMed] [Google Scholar]
- Langmead B, Salzberg SL. Fast gapped-read alignment with Bowtie 2. Nat Methods. 2012;9:357–359. doi: 10.1038/nmeth.1923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee KY, Fu H, Aladjem MI, Myung K. ATAD5 regulates the lifespan of DNA replication factories by modulating PCNA level on the chromatin. J Cell Biol. 2013;200:31–44. doi: 10.1083/jcb.201206084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lisby M, Rothstein R, Mortensen UH. Rad52 forms DNA repair and recombination centers during S phase. Proc Natl Acad Sci USA. 2001;98:8276–8282. doi: 10.1073/pnas.121006298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lopes M, Cotta-Ramusino C, Pellicioli A, Liberi G, Plevani P, Muzi-Falconi M, Newlon CS, Foiani M. The DNA replication checkpoint response stabilizes stalled replication forks. Nature. 2001;412:557–561. doi: 10.1038/35087613. [DOI] [PubMed] [Google Scholar]
- Lopez-Mosqueda J, Maas NL, Jonsson ZO, Defazio-Eli LG, Wohlschlegel J, Toczyski DP. Damage-induced phosphorylation of Sld3 is important to block late origin firing. Nature. 2010;467:479–483. doi: 10.1038/nature09377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Majka J, Burgers PM. The PCNA-RFC families of DNA clamps and clamp loaders. Prog Nucleic Acid Res Mol Biol. 2004;78:227–260. doi: 10.1016/S0079-6603(04)78006-X. [DOI] [PubMed] [Google Scholar]
- Mejlvang J, Feng Y, Alabert C, Neelsen KJ, Jasencakova Z, Zhao X, Lees M, Sandelin A, Pasero P, Lopes M, Groth A. New his-tone supply regulates replication fork speed and PCNA unloading. J Cell Biol. 2014;204:29–43. doi: 10.1083/jcb.201305017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meyer M, Kircher M, Gansauge MT, Li H, Racimo F, Mallick S, Schraiber JG, Jay F, Prüfer K, de Filippo C, et al. A high-coverage genome sequence from an archaic Denisovan individual. Science. 2012;338:222–226. doi: 10.1126/science.1224344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moldovan GL, Pfander B, Jentsch S. PCNA, the maestro of the replication fork. Cell. 2007;129:665–679. doi: 10.1016/j.cell.2007.05.003. [DOI] [PubMed] [Google Scholar]
- Moyer SE, Lewis PW, Botchan MR. Isolation of the Cdc45/Mcm2-7/GINS (CMG) complex, a candidate for the eukaryotic DNA replication fork helicase. Proc Natl Acad Sci USA. 2006;103:10236–10241. doi: 10.1073/pnas.0602400103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nguyen HD, Becker J, Thu YM, Costanzo M, Koch EN, Smith S, Myung K, Myers CL, Boone C, Bielinsky AK. Unligated Okazaki fragments induce PCNA ubiquitination and a requirement for Rad59-dependent replication fork progression. PLoS ONE. 2013;8:e66379. doi: 10.1371/journal.pone.0066379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nick McElhinny SA, Gordenin DA, Stith CM, Burgers PM, Kunkel TA. Division of labor at the eukaryotic replication fork. Mol Cell. 2008;30:137–144. doi: 10.1016/j.molcel.2008.02.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Donnell M, Langston L, Stillman B. Principles and concepts of DNA replication in bacteria, archaea, and eukarya. Cold Spring Harb Perspect Biol. 2013;5(7) doi: 10.1101/cshperspect.a010108. http://dx.doi.org/10.1101/cshperspect.a010108a010108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Papouli E, Chen S, Davies AA, Huttner D, Krejci L, Sung P, Ulrich HD. Crosstalk between SUMO and ubiquitin on PCNA is mediated by recruitment of the helicase Srs2p. Mol Cell. 2005;19:123–133. doi: 10.1016/j.molcel.2005.06.001. [DOI] [PubMed] [Google Scholar]
- Pfander B, Moldovan GL, Sacher M, Hoege C, Jentsch S. SUMO-modified PCNA recruits Srs2 to prevent recombination during S phase. Nature. 2005;436:428–433. doi: 10.1038/nature03665. [DOI] [PubMed] [Google Scholar]
- Pursell ZF, Isoz I, Lundström EB, Johansson E, Kunkel TA. Yeast DNA polymerase epsilon participates in leading-strand DNA replication. Science. 2007;317:127–130. doi: 10.1126/science.1144067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quinlan AR, Hall IM. BEDTools: a flexible suite of utilities for comparing genomic features. Bioinformatics. 2010;26:841–842. doi: 10.1093/bioinformatics/btq033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raghuraman MK, Winzeler EA, Collingwood D, Hunt S, Wodicka L, Conway A, Lockhart DJ, Davis RW, Brewer BJ, Fangman WL. Replication dynamics of the yeast genome. Science. 2001;294:115–121. doi: 10.1126/science.294.5540.115. [DOI] [PubMed] [Google Scholar]
- Ricke RM, Bielinsky AK. Mcm10 regulates the stability and chromatin association of DNA polymerase-alpha. Mol Cell. 2004;16:173–185. doi: 10.1016/j.molcel.2004.09.017. [DOI] [PubMed] [Google Scholar]
- Santocanale C, Diffley JF. A Mec1- and Rad53-dependent checkpoint controls late-firing origins of DNA replication. Nature. 1998;395:615–618. doi: 10.1038/27001. [DOI] [PubMed] [Google Scholar]
- Stillman B. DNA polymerases at the replication fork in eukaryotes. Mol Cell. 2008;30:259–260. doi: 10.1016/j.molcel.2008.04.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Szyjka SJ, Aparicio JG, Viggiani CJ, Knott S, Xu W, Tavaré S, Aparicio OM. Rad53 regulates replication fork restart after DNA damage in Saccharomyces cerevisiae. Genes Dev. 2008;22:1906–1920. doi: 10.1101/gad.1660408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thu YM, Bielinsky AK. Enigmatic roles of Mcm10 in DNA replication. Trends Biochem Sci. 2013;38:184–194. doi: 10.1016/j.tibs.2012.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Viggiani CJ, Knott SR, Aparicio OM. Genome-wide analysis of DNA synthesis by BrdU immunoprecipitation on tiling microarrays (BrdU-IP-chip) in Saccharomyces cerevisiae. Cold Spring Harb Protoc. 2010;2010:256–264. doi: 10.1101/pdb.prot5385. http://dx.doi.org/10.1101/pdb.prot5385. [DOI] [PubMed] [Google Scholar]
- Waga S, Stillman B. The DNA replication fork in eukaryotic cells. Annu Rev Biochem. 1998;67:721–751. doi: 10.1146/annurev.biochem.67.1.721. [DOI] [PubMed] [Google Scholar]
- Wyrick JJ, Aparicio JG, Chen T, Barnett JD, Jennings EG, Young RA, Bell SP, Aparicio OM. Genome-wide distribution of ORC and MCM proteins in S. cerevisiae: high-resolution mapping of replication origins. Science. 2001;294:2357–2360. doi: 10.1126/science.1066101. [DOI] [PubMed] [Google Scholar]
- Zegerman P, Diffley JF. Checkpoint-dependent inhibition of DNA replication initiation by Sld3 and Dbf4 phosphorylation. Nature. 2010;467:474–478. doi: 10.1038/nature09373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Z, Shibahara K, Stillman B. PCNA connects DNA replication to epigenetic inheritance in yeast. Nature. 2000;408:221–225. doi: 10.1038/35041601. [DOI] [PubMed] [Google Scholar]
- Zhang Y, Liu T, Meyer CA, Eeckhoute J, Johnson DS, Bernstein BE, Nusbaum C, Myers RM, Brown M, Li W, Liu XS. Model-based analysis of ChIP-Seq (MACS) Genome Biol. 2008;9:R137. doi: 10.1186/gb-2008-9-9-r137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao X, Muller EG, Rothstein R. A suppressor of two essential checkpoint genes identifies a novel protein that negatively affects dNTP pools. Mol Cell. 1998;2:329–340. doi: 10.1016/s1097-2765(00)80277-4. [DOI] [PubMed] [Google Scholar]
- Zou L. Four pillars of the S-phase checkpoint. Genes Dev. 2013;27:227–233. doi: 10.1101/gad.213306.113. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.