Abstract
Introduction
Mastocytosis is a disorder characterized by abnormal mast cell (MC) accumulation in skin and internal organs such as bone marrow. The disease follows a benign course in most patients with cutaneous and indolent systemic mastocytosis (SM); however, advanced variants associated with decreased life expectancy also exist. Pharmacotherapy of mastocytosis is aimed at the control of symptoms caused by MC mediator release, treatment of comorbidities and cytoreductive therapies in advanced variants.
Areas covered
This article will cover the general treatment principles of anti-MC mediator and cytoreductive therapies of mastocytosis. The literature discussed was retrieved with PubMed using the search terms ‘treatment of mastocytosis,’ ‘mastocytosis antimediator therapy’ and looking for important cross-references.
Expert opinion
Pharmacotherapy of mastocytosis should be individualized for each patient considering the category of disease, reduction of risk of anaphylaxis, constitutional symptoms and comorbidities including osteoporosis. Cytoreductive therapies are generally reserved for patients with aggressive mastocytosis (ASM), MC leukemia (MCL) and MC sarcoma (MCS); however, some patients with indolent disease and recurrent anaphylactic episodes not responsive to antimediator therapies may also be considered for cytoreduction on a case-by-case basis.
Keywords: antihistamines, mastocytosis, tyrosine kinase inhibitors
1. Introduction
Mastocytosis is a group of disorders characterized by the presence of abnormal numbers of mast cells (MCs) in skin and internal organs such as bone marrow, liver, spleen, gastrointestinal (GI) tract and lymph nodes. It can be seen in any age group. Mastocytosis seen in children is generally limited to skin without any internal organ involvement, and many patients exhibit spontaneous resolution by adolescence. In contrast, mastocytosis in adults is a persistent disease almost always involving the bone marrow [systemic mastocytosis (SM)]. MCs in systemic mastocytosis have an abnormal spindle-shaped and hypogranular morphology which tend to form clusters around blood vessels and paratrabecular and interstitial areas in the bone marrow and express aberrant surface markers such as CD25 and CD2 which are not found in normal MCs. Patients with systemic mastocytosis generally have elevated tryptase levels > 20 ng/ml. More than 95% of patients with adult onset SM and approximately 40% of pediatric patients with cutaneous mastocytosis have the D816V c-kit gain of function mutation. This mutation renders KIT resistant to the currently available tyrosine kinase inhibitors (TKIs).
The World Health Organization (WHO) classification of mastocytosis includes 7 categories (Table 1) [1,2]. There is no curative therapy for mastocytosis, and therefore a comprehensive management strategy should be implemented, and is applicable to all mastocytosis disease categories. The aims of this strategy include i) counseling of patients, ii) avoidance of common triggers of MC degranula-tion (such as heat, temperature changes, strenuous exercise, and alcohol), iii) prevention of MC degranulation in high-risk situations (anesthesia, use of radiocontrast media and certain drugs) and with exposure to stinging insect venom, iv) treatment of acute MC mediator-release symptoms (i.e., anaphylaxis), v) treatment of chronic MC mediator symptoms and vi) treatment of organ infiltration by MC, i.e., cytoreductive therapies, which are generally indicated in patients with advanced forms of disease. Mastocytosis is also associated with a number of comorbidities such as osteoporosis, gastric and duodenal ulcers, recurrent anaphylaxis and hematologic disorders such as myeloproliferative or myelodysplastic disorders and rarely lymphoproliferative disorders. These comorbidities should be treated according to the established guidelines for that condition. This article will focus on anti-MC mediator and cytoreductive therapy options.
Table 1.
WHO Classification of mastocytosis [110].
| Category | Prognosis | Therapeutic considerations |
|---|---|---|
| Cutaneous mastocytosis | Good | Antimediator therapy, phototherapy and topical glucocorticoids in select few cases |
| Indolent SM | Good | Antimediator therapy. Cytoreductive therapy in few selected cases with recurrent vascular instability episodes due to MC mediator release |
| SM-AHNMD | Variable depending on AHNMD | Therapy of AHNMD regardless of mastocytosis |
| ASM | Poor | Cytoreductive therapy + antimediator therapy |
| MCL | Poor | Cytoreductive therapy + antimediator therapy. Consider bone marrow transplantation. |
| MSC | Poor | Cytoreductive therapy |
| Extracutaneous mastocytomas | Good | Very rare. Optimum therapy unknown, but surgical excision should be considered if feasible. |
ASM: Aggressive systemic mastocytosis; MC: Mast cell; MCL: Mast cell leukemia; MSC: Mast cell sarcoma; SM: Systemic mastocytosis.
2. Antimediator therapy
A core component in the treatment of all categories of mastocytosis is symptom control [3,4]. Despite extensive differences in the natural history and prognosis between variants in the mastocytosis disease spectrum, there is considerable symptom overlap between indolent and aggressive disease, so that the treatment armamentarium for symptom palliation pertains to most disease variants [1,5]. Treatment regimens should be individualized because patients with mastocytosis will not necessarily experience the entire constellation of symptoms associated with this heterogeneous disease [6–8]. Although targeting the molecular aberration (c-kit D816V mutation) associated with the disease appears to be an attractive strategy, remarkable heterogeneity on clinical presentation and prognosis in patients carrying this mutation suggest that not all disease manifestations can be explained by this mutation, and the mutation confers resistance to the currently approved TKIs (such as imatinib) that target c-kit [9]. Moreover, there is limited data on the long-term toxicity of c-kit mutation-targeting therapies, giving these drugs unacceptably high risk-to-benefit ratios in most cases of cutaneous mastocytosis and symptomatically well controlled indolent SM [10], which are usually associated with a good prognosis. In these categories of mastocytosis, symptom palliation suffices without need for more aggressive therapy. For that same reason, cytoreductive therapy is not indicated for either of these two disease categories, with the exception of patients with recurrent and potentially life-threatening MC degranulation episodes [2]. Drugs used for symptom control mostly work by interfering with the receptors or receptor signaling for these mediators, and sometimes by reducing the production of MC mediators or preventing the release of mediators from MCs. A review of the available literature on these drugs follows, mostly consisting of case reports and series, with few placebo-controlled trials. This limitation is largely secondary to the infrequency of mastocytosis in the general population. It should also be noted that most of the studies on antimediator therapy precede the advent of the technologies that have facilitated today’s standards for categorizing mastocytosis [11], namely, the assays for detecting mature and total tryptase, the D816V c-kit mutation, urinary 11β-PGF2a, staining for CD2 and CD25, among others. It is likely that the application of today’s more precise diagnostic methods would lead to a different selection of patients, but it is uncertain whether it would significantly alter the essence of the results.
2.1 Antihistamines
Both sedating and nonsedating H1 antihistamines are useful for the treatment of pruritus, flushing, tachycardia [5] and reduction of symptom severity of anaphylaxis [12], with expert opinion endorsing the daytime use of nonsedating antihistamines (including cetirizine, levocetirizine, fexofenadine, loratidine and desloratadine), and nighttime use of sedating ones (such as diphenhydramine, hydroxyzine, chlorpheniramine, cyproheptadine, among others) [7]. As per expert opinion, the use of antihistamines can be adjusted according to symptom severity, ranging from ‘as needed’ use only of non-sedating antihistamines for mild disease, to scheduled doses of nonsedating histamines in combination to ‘as needed’ use of sedating or nonsedating antihistamines for breakthrough symptoms for severe disease. Many of the abovementioned symptoms result from the agonism of histamine (released in large quantities during MC degranulation) on the H1 receptor, a G protein-coupled receptor that signals through a Gq subunit. H1 antihistamines encompass a large and diverse class of compounds that act as inverse agonists on this receptor [13]. Friedman et al. conducted a double-blind, placebo-controlled (DBPC) triple-crossover trial comparing chlorpheniramine vs. low- and high-dose azelastine PO in 15 patients with tissue evidence of mastocytosis, and evaluated pruritus, flushing, fatigue, abdominal and bone pain, headaches and number of stools [14]. They concluded that these two antihistaminics were equally efficacious for the treatment of these symptoms, providing grounds for today’s lack of preference for any particular antihistaminic. In a DBPC double-crossover trial comparing cromolyn vs. cimetidine plus chlorpheniramine in eight patients with SM, Frieri et al. concluded that there was no advantage of one drug regimen over another, but that cromolyn was more beneficial in the treatment of nausea, while the antihistamine combination was more valuable for pruritus and urticaria [15]. This study supported the tailoring of drug regimens to specific symptoms. Other case reports highlight the usefulness of H1 blockers, particularly for pruritus and urtication, more so when given in combination with H2 blockers [16]. Finally, although doxepin is classified as a tricyclic antidepressant, it has greater potency antagonizing H1 receptors than most H1 blockers, and may be used in cases where nonsedating antihistamines are ineffective [4,17]. It should be noted that both doxepin and first-generation antihistamines have a dose-dependent cardiac effect of QT interval prolongation, and that in overdose may cause torsades de pointes and other potentially fatal ventricular arrhythmias [13]. This risk is greater in patients with underlying arrhythmias, hepatic or renal disease, electrolytic disturbances, those taking either other QT-prolonging drugs or drugs that inhibit the metabolism of H1 antagonists, those with low body mass and females. The evidence suggests that later generation antihistamines do not share this cardiotoxic profile [13,18].
The H2 histaminergic receptor is instead a Gs-coupled receptor with different physiologic properties and tissue distribution than the H1 receptor, and is particularly implicated in the regulation of gastric acid secretion [4]. The evidence behind H2 antagonists consists of case reports and small case series using cimetidine. Berg et al. reported a patient with a long history of SM whose GI symptoms were found to correlate well with serum concentrations of cimetidine, and provided early substantiation that H2 blockers do not affect serum histamine or gastrin [19]. Bredfeldt et al. used cimetidine in a 58-year-old man with SM with signs of gastric acid hypersecretion and malabsorption, and while making the caveat that malabsorption is uncommon in mastocytosis, they noted that cimetidine was helpful in normalizing a carotene absorption test, controlling abdominal pain and diarrhea, but was inefficacious in reversing steatorrhea [20]. Hirschowitz et al. described two patients with SM whose disease was uncontrolled with H1 blockers, steroids and cromolyn, and noted that cimetidine was helpful only in alleviating abdominal pain, with moderate effects on diarrhea [21]. Achord et al. pointed out the increased efficaciousness of using cimetidine along with the antimuscarinic anticholinergic propantheline to control chronic diarrhea in a patient with SM [22], while Johnson et al. observed the long-term safety (56 months) of cimetidine use [23]. In our practice, we do not see any difference between cimetidine and the newer H2 antihistamines such as ranitidine or famotidine in symptomatic control of abdominal pain, nausea, vomiting or acid reflux. Since the latter group of newer H2 blockers does not have as many drug interactions as cimetidine, we prefer their use especially in patients with multiple other medications.
2.2 Cromolyn sodium
Cromolyn is one of the mainstays in the treatment of mastocytosis [4] and is prescribed to mitigate multisystemic symptoms, including GI, cutaneous, neuropsychiatric and skeletal symptoms [8]. In vitro evidence suggests it diminishes the calcium flux necessary for degranulation following FcER1 cross-linking, and it is thus purported as a MC stabilizer. Its mechanism of action remains unclear, however, as it has minimal systemic absorption following ingestion, and negligible fat solubility plus extensive ionization at physiologic pH (which suggests an inability of the drug to enter cells, thus implicating an interaction with an as-yet-unidentified cell surface receptor for cromolyn to have biologic activity) [24]. Early case reports stressed its usefulness in treating mastocytotic bone disease while acknowledging the minimal absorption of the drug from the GI tract [25]. Soter et al. conducted a DBPC crossover trial with eight adult patients with SM, all with urticaria pigmentosa (UP), and arrived at important conclusions and observations about the use of cromolyn [26]. They found that cromolyn was useful for GI symptoms (abdominal pain and diarrhea), cutaneous (pruritus, whealing and flushing) and CNS (cognition); that amelioration in symptoms while on cromolyn (and worsening while on placebo) did not correlate with histamine levels, providing circumstantial evidence that the mechanism of action is not, or is not only, related to MC stabilization as it was speculated to be; that there is a lag period of 2 weeks for cromolyn to have an effect on GI and cutaneous symptoms and of 6 weeks for CNS symptoms; and finally, that cromolyn may work in some patients with mastocytosis with adverse reactions to ethanol ingestion and in some with malabsorption.
In support of these findings, the results from a DBPC trial with 11 patients with SM conducted by Horan et al. suggest that cromolyn significantly controls GI symptoms and improves overall disease severity even in patients on longstanding cromolyn therapy, suggesting a lack of tachyphylaxis with this drug [27]. In contrast to Soter, Horan did not find that Cromolyn yielded statistically significant improvements in “non-GI symptoms,” although it may be argued that aggregating flushing, headaches, urtication, pruritus and bone pain, as opposed to making multiple individual comparisons, could contribute to the lack of statistical significance reported. Other groups have also related a lack of efficacy with cromolyn, which may have been secondary to a therapeutic period falling short of the aforementioned 2 – 6 week lag period [28]. As mentioned earlier, one study of eight patients found that cromolyn was not superior to a combination of H1 and H2 antihistamine regimen, and did not influence blood or urine histamine levels [15]. Other noncontrolled case reports reported benefits [29–31].
Cromolyn has also been found to be useful for cutaneous mastocytosis. In a single-blind placebo-controlled trial involving 13 patients with UP, Czarnetzki et al. found that 66% of children and 70% adults experienced amelioration of their pruritus, 33% of children and 70% of adults experienced improvement in urtication and 100% of children and 66% of adults felt relief of their GI symptoms, indicating clinical usefulness, and hinting at differential effects between pediatric and adult populations [32]. Others have reported similar results in adult patients with UP [33], and pediatric patients with cutaneous bullous mastocytosis [34]. In our group’s experience, cromolyn sodium may cause worsening bloating and abdominal cramping and diarrhea when started at a full PO dose of 200 mg four times daily in some patients. In such patients, a slower and more cautious approach to introduction of the drug is recommended, such as starting at a dose of 100 mg once daily, and increased by increments of 100 mg on a weekly basis up to the desired dose (800 mg in adults, which thus takes 8 weeks to get to) in order to avoid side of effects of dyspepsia and nausea, and to improve adherence [35]. We usually assess symptomatic response in 2 – 3 months and discontinue the drug if no significant response is achieved, or if side effects impede its use. Some of these side effects include abdominal cramping, diarrhea, constipation, irritability and insomnia.
2.3 Ketotifen
Special attention has been given to ketotifen above other antihistaminics due to its ostensible MC stabilizing properties, but most experts who recommend it do not treat ketotifen as a medication unique in its class, but rather like any other classic H1 antagonist [4,6,17]. In open-label studies of small patient numbers, ketotifen was shown to be beneficial in patients with glucocorticoid dependent idiopathic anaphylaxis [36,37], although the efficacy in mastocytosis has not been conclusive. In a DBPC crossover trial where eight children with either UP or diffuse cutaneous mastocytosis were treated with either ketotifen or hydroxyzine, Kettelhut et al. found that hydroxyzine was superior to ketotifen in controlling abdominal pain and flushing [38]. Similarly, there are conflicting reports as to whether ketotifen normalizes [39,40] or does not normalize [28,38] histamine excretion. Although case reports have described in relation to ketotifen use the reversal of bone pathology in a patient with SM [40], the amelioration of neuropsychiatric, GI and cutaneous symptoms [39], and the alleviation in pruritus, ascites and rhinorrhea [41], care should be taken not to ascribe causality to a medication when these studies cannot rule out epiphenomena.
2.4 Glucocorticoids
Oral glucocorticoids have been used for various indications in mastocytosis, and are recommended for GI mal-absorption [4,8,12,17], bone disease [4] (including diffuse bone sclerosis), abdominal pain refractory to treatment with cromolyn [42], prevention of anaphylaxis [3,12], ascites [12] and diffuse cutaneous disease refractory to topical therapy [7], although the evidence supporting these indications is mostly anecdotal or limited to case reports. Free glucocorticoids traverse the cell membrane and bind the glucocorticoid receptor to modulate the transcription of numerous genes. Their effects on MCs are wide-ranging, and depending on dosage may include a decrease in the number of connective tissue MCs, the inhibition of stem cell factor production (necessary for MC growth), a decrease in the expression of FcER1 and of chemokine receptors (such as CCR3), the inhibition of the generation of several interleukins and eicosanoid mediators [43]. Friedman et al. studied the effects of tixocortol, a corticosteroid at the time purported to cause minimal adrenal suppression, on four patients with indolent SM for 8 – 15 weeks, and found that all patients experienced improvement in GI pain, stool frequency, malabsorption parameters and gross appearance of the duodenum in the two patients who had pre- and post-treatment biopsies obtained [44]. Tixocortol did appear to cause some degree of adrenal suppression, and did not remain singled out among steroids.
Topical corticosteroids are sometimes used for the treatment of cutaneous disease in mastocytosis, especially in limited skin involvement, although evidence supporting their use is either anecdotal or from small trials [45]. Cysteinyl leukotriene (cysLT) receptor 1 antagonists (LTRA).
Expert opinion supports the use of montelukast in mastocytosis [4,5], especially in the context of recalcitrant headaches, musculoskeletal pain and flushing [7]. This drug antagonizes the cysLT receptor 1,which is a G-protein coupled receptor expressed in bronchial and vascular smooth muscle cells and peripheral blood leukocytes, mediating bronchoconstriction, vascular leak and inflammation [46,47]. Antagonizing the effects of cysLTs is intuitively a sound approach considering that MCs release ample amounts of these mediators [48], and that higher levels of the end metabolite of cysLTs, LTE4, may be found in the urine of patients with SM [49]. Two case reports have pointed out the clinical utility of LTRAs, one in an infant with bullous cutaneous mastocytosis with prominent wheezing, the other in a girl with UP, highly elevated serum tryptase levels and systemic symptoms including flushing, diarrhea, abdominal pain and urinary incontinence, both cases refractory to other standard therapy for mastocytosis [50,51]. In these reports, the addition of montelukast permitted control of multisystemic and recalcitrant symptoms.
2.5 Aspirin
Roberts et al. reported, in 1980, two patients with SM and recurrent hypotension refractory to treatment with antihistaminics, in the context of elevated levels of PGD2 [52]. Noting that PGD2 is the predominant cyclooxygenase derivative in MCs, and that PGD2 is a systemic vasodilator, they reasoned that this mediator is in part responsible for hypotensive episodes in mastocytosis, and provided anecdotal evidence that aspirin normalizes levels of PGD2 metabolites while lessening the severity of anaphylactic episodes [53]. Aspirin is a nonsteroidal antiinflammatory drug (NSAID), and exerts its effects through irreversible inhibition of the cyclooxygenase (COX) isoenzymes, although it has other modes of action that distinguish it from other NSAIDs, including the acetylation of COX2 [54]. More recently, in a survey study of 20 patients with SM treated with aspirin, Butterfield noticed that the dose required to normalize urinary 11β-PGF2a, the end metabolite of PGD2 with vasoconstrictor effects, was at the most 500 mg BID [55], much lower than the dose advocated for by Roberts et al. In terms of symptom control he detailed that five patients experienced an amelioration of anaphylactic reactions, eight of them felt relief of headaches, dizziness, flushing and GI distress, while four patients said aspirin had no effect on them. While multiple confounders and biases complicate the interpretation of these results aspirin was advantageous therapy in a significant proportion of these patients. Relatedly, while studying patients with monoclonal MC activation syndrome (with comparable symptomatology and similarly elevated MC mediators or proteases as patients with SM), Butterfield et al. reported in a small case series that all the four patients had improvements in systems ranging from paroxysmal flushing, pruritus, vomiting, respiratory distress, throat swelling and abdominal pain [56]. Others have documented significant clinical improvement with aspirin use in case reports [57], while drawing attention to potentially severe adverse reactions patients with SM might develop while initiating aspirin therapy [58]. The starting doses for aspirin in Butterfield’s study ranged from 0.1 mg to 325 mg in patients without a history of adverse reactions to NSAIDs. In a roundtable discussion moderated and documented by Metcalfe, Roberts states that the rate of NSAID intolerance in patients with SM is 5% [8], a prevalence low enough to allow aspirin therapy to be initiated in the outpatient setting if patients do not have a history of NSAID intolerance and have recently tolerated these medications, but hospitalizing patients if a clear history cannot be provided, as administration of aspirin or NSAID to a patient with NSAID allergy can lead to serious and even fatal complications. Gastric intolerance may also limit the usefulness of ASA therapy in patients with mastocytosis who may have propensity for gastric irritation due to histamine induced acid hypersecretion.
2.6 Epinephrine
It is our practice to prescribe multiple units of self-injectable epinephrine to all patients diagnosed with mastocytosis, although there is lack of consensus among experts whether to follow this approach or to prescribe epinephrine only to those patients with mastocytosis with a history of anaphylaxis, or who are at increased risk of anaphylaxis. The incidence of anaphylaxis in patients with mastocytosis is approximately 30%, significantly increased when compared to general population [59], and anaphylaxis may be triggered by hymenoptera venoms, drugs or can be unprovoked. Many of the recommendations given to patients with mastocytosis with anaphylaxis are extrapolated from the literature on anaphylaxis [60]. Roberts et al. stressed the unique role epinephrine in the treatment of anaphylactic shock in a patient with SM who was refractory to vasopressor therapy with dopamine, yet was quickly responsive to epinephrine, suggesting that not every catecholamine is effective in this disease, and that epinephrine must act differently on a different set of adrenergic, or other, receptors [53]. They further supported these observations with a small case series [61]. For further details on the acute management of anaphylaxis and the best practices for discharge of patients from a health care setting after experiencing anaphylaxis, the reader is referred to the position papers on these topics from the major organizations (AAAAI, WAO, EAACI) involved with this disease [62–64].
2.7 Bisphosphonates
In a retrospective study of 75 patients with SM, Barete et al. found that nearly half of all patients had either X-ray or bone mineral densitometry evidence of some type of bone disease induced by mastocytosis, osteoporosis being the most common at 31% [65]. Moreover, only a fraction of those patients (43%) had bone pain, thus suggesting the potential for under- or delayed diagnosis of bone disease in patients with SM. They also found that all patients with osteoporosis treated with bisphosphonates (alendronate, pamidronate, risedronate and etidronate were documented) had increases in their bone mineral densitometry scores (although three patients with other bone disease types had a drop in this score), and that no patient with history of fractures re-fractured. Without proving causality, they noted that only three patients in this cohort underwent concomitant cytoreductive therapy, and that the majority had stable tryptase levels, suggesting that effects on bone were likely due to bisphosphonates and not other therapies [65]. Bisphosphonates block the bone-resorbing effect of osteoclasts and modulate the survival and function of osteoblasts, although the receptor for these drugs has not yet been identified [66]. Other case reports and small case series alert to the sometimes-insidious nature of bone disease in SM if it is not sought for, and the clinical effectiveness of bisphosphonates [67–69]. Vitamin D levels should be checked and supplemented if low, along with a sufficient daily intake of calcium. It remains to be elucidated whether newer therapies such as denosumab has efficacy in treating osteoporosis associated with mastocytosis, especially in view of the observation that MCs are capable of producing RANKL.
2.8 Other treatments for UP
In addition to the aforementioned role of topical and intralesional steroids and oral cromolyn in the treatment of UP, topical cromoglycate [3,4,42] and zinc sulfate [3,42] are recommended by some experts as available alternatives. Analyzing a cohort of 20 patients with UP, 15 of them adults, treated with 8-methoxypsoralen plus UVA (PUVA), Godt et al. found that when using oral methoxypsoralen 70% of patients report a 50% or better improvement in their clinical appearance of their lesions, 8/15 reported a decreased in pruritus, and all of them had at least a reduction in urtication, while bath PUVA was not effective in either of these three parameters [70]. Side effects over a 10-year follow-up included nausea, and vomiting in one patient, pruritus exacerbated with therapy in three, and there was one patient with a left eye, basal cell carcinoma, which has an unclear relationship to PUVA treatment. Czarnetzki et al. reported modest responses to PUVA in a trial on 10 patients with UP, and pointed out symptom recurrence after discontinuation of therapy [71]. Kinsler found PUVA useful in a 16-month-old child with diffuse cutaneous mastocytosis [72]. Case reports have called attention to polidocanol plus urea [73] and pimecrolimus [74] as potentially beneficial for UP. The benign prognosis and significant odds of spontaneous remission in childhood UP [42] should be counterpoint to the potential side effects of any therapy.
2.9 Omalizumab
A few case series and reports have suggested anti-IgE therapy as being of potential value to the treatment of mastocytosis, particularly in the context of recurrent anaphylaxis [75,76]. There are case reports pointing out the usefulness of omalizumab in other mastocytosis-related symptoms [77,78], while some warn of potential patient intolerance of this medication [79]. There has been no reported placebo controlled study with the drug.
2.10 Venom immunotherapy
The topic of Hymenoptera venom immunotherapy (HVA) in SM is complex. Currently, VIT is recommended for life in patients with SM and HVA [80], considering that sting-related fatalities have been reported in patients with SM and HVA after discontinuation of VIT [81]. SM is disproportionately represented in HVA [82,83], and VIT appears to be effective in this patient population [84].
3. Cytoreductive therapies
The WHO classification of mastocytosis includes cutaneous and indolent SM and advanced forms, such as aggressive SM (ASM), SM with an associated clonal hematological non-MC lineage disease (SM-AHNMD), MC leukemia (MCL), and MC sarcoma (MSC). The pharmacotherapy for all subtypes involves antimediator therapy for symptomatic control that was discussed in the previous section [2,11]. However, patients with advanced forms of SM with proliferation and infiltration of MCs in multiple organ systems may need treatment with TKIs and/or other cytoreductive agents to control the signs and symptoms of the MC burden. In this section, we will focus our discussion to different TKIs with emphasis on individual agent’s target, summary of selected Phase II clinical trials regarding response rate and adverse side effects. In addition, other cytoreductive therapy options, such as interferon-α and cladribine (2-CdA), will also be discussed (Table 2).
Table 2.
Tyrosine Kinase Inhibitors and IFN-α/Cladribine: focus on main KIT targets and summary of selected Phase II clinical trials.
| Agents | KIT targets | Disease category | Response rate (Measurement of response) |
|---|---|---|---|
| Imatinib [90] |
KIT WT Non-D816 mutations |
ISM, ASM, SM-AHNMD | 5% (CR) Symptomatic improvement in 30% (Valent response criteria) |
| Dasatinib [98] |
KIT WT KIT D816V Non-D816 mutations |
ISM, ASM, SM-AHNMD | 33% (OR) 6% (CR) (Valent response criteria) |
| Nilotinib [95] |
KIT WT Non-D816 mutations |
SM | 20% (OR) (Serum tryptase, BM cell counts, clinical symptoms) |
| Midostaurin [101] |
KIT WT KIT D816V Non-D816 mutations |
ASM, MCL, SM-AHNMD | 60% (OR) 52.5% (major response) (Valent response criteria) |
| Masitinib [104] |
KIT WT Non-D816 mutations |
ISM/CM with handicap | 56% (OR) (Response in symptoms: flushing, Hamilton rating, pruritus) |
| IFN-α [91] | N/A | ISM, ASM, SM-AHNMD | 53% (OR) (Skin lesions/symptoms, -MC mediator related symptoms, MC infiltrate, tryptase, organomegaly, C-Findings) |
| Cladribine [91] | N/A | ISM, ASM, SM-AHNMD | 55% (OR) (Skin lesions/symptoms, -MC mediator-related symptoms, MC infiltrate, tryptase, organomegaly, C-Findings) |
The Valent response criteria include assessment of C-findings, MC infiltrate in organs, tryptase level, and/or organomegaly. The responses can be divided into major response (MR), partial response (PR) and no response (NR). MR involves resolution of ≥ 1 C-finding without increase in other C-findings, decrease in MC infiltrate, decrease in tryptase level and organomegaly. MR has subcategories that include complete remission (CR), incomplete remission (IR), pure clinical response (PCR) [111].
ASM: Aggressive systemic mastocytosis; BM: Bone marrow; CR: Complete remission; ISM: Indolent systemic mastocytosis; MC: Mast cell; MCL: Mast cell leukemia; OR: Overall response (major response + partial response) if using Valent response criteria; SM-AHNMD: Systemic mastocytosis with an associated clonal hematological nonmast cell lineage disease; WT: Wild type.
3.1 Tyrosine kinase inhibitors
KIT is a transmembrane class III receptor tyrosine kinase that is involved in MC growth, proliferation and activation. The KIT mutations can affect different regions of the receptor such as extracellular, transmembrane, juxtamembrane domains or activation loop. This can lead to gain of function mutation that leads to constitutive activation of the receptor in the absence of binding of its ligand, stem cell factor [85,86]. More than 95% of patients with SM have detection of D816V KIT, a point mutation leading to substitution of valine (V) for aspartic acid (D) at the codon 816 on KIT exon 17. Therefore, agents targeting D816V KIT have been the focus of therapeutic approach in patients with SM.
Imatinib mesylate is an inhibitor of various tyrosine kinases including PDGFR and KIT. It is the only Food and Drug Administration (FDA)-approved TKI for use in patients with ASM without D816V KIT or with KIT mutation status unknown. It has been successfully used in patients with CML and chronic eosinophilic leukemia with FIP1L1-PDGFRA or PDGFRB fusion mutations. The latter can sometimes be confused with SM due to slightly elevated tryptase levels and increased numbers of aberrant MCs expressing CD25 as seen in SM. Imatinib shows activity against MCs carrying wild-type (WT), and non-codon 816 (e.g., F522C (trans-membrane) [87] and V560G (juxtamembrane)) KIT mutations (Figure 1). It is not effective in patients with SM who have D816V KIT mutation likely due to the conformational change of the activation loop that inhibits the binding of imatinib to its target domain [86,88,89]. Multiple clinical trials have supported the lack of clinical response in patients with D816V KIT mutation [90–92]. The Phase II study by Vega-Ruiz et al. included 20 patients with ASM, ISM and SM-AHNMD. There was only one patient with KITD816V negative, FIP1L1-PDGFRA negative SM-hypereosinophilic syndrome (HES) who achieved complete remission. Only six patients in this study reported symptomatic improvement with two of them being KIT-D816V positive. The side effects include rash, nausea, vomiting, headache, fatigue, edema, diarrhea and anemia and grade 4 thrombocytopenia and neutropenia [90]. In addition, a Mayo clinic report, including patients with ISM, ASM, SM-AHNMD and MCL, showed overall response rate of 17% in patients with D816V KIT versus 33% in patients without the mutation. The median time to response was 19.6 months. The side effects of imatinib included diarrhea, peripheral edema and rarely interstitial pneumonitis [91].
Figure 1.
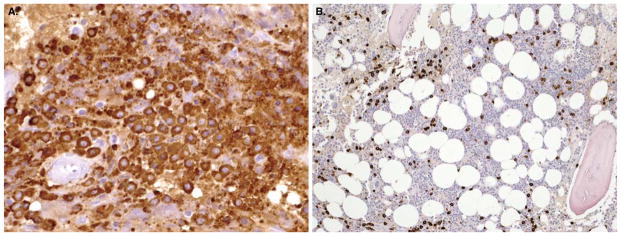
Bone marrow biopsy before (A) and after (B) cytoreductive therapy with imatinib in a patient with well-differentiated mastocytosis carrying the unusual F522C c-kit mutation.
From reference [87].
Summary: Imatinib is not effective in patients with D816V KIT mutation, which accounts for the majority of SM patients. However, it is the only FDA-approved treatment that may be effective inaggressive SM patients without exon 17 KIT mutations affecting the activation loop. These are rare cases and there are few case reports of benefit in well-differentiated SM.
Nilotinib and dasatinib are second-generation TKI agents that were also studied in Phase II clinical trials in treatment of SM patients. Nilotinib is an aminopyrimidine that has shown in vitro activity against mice with D814V KIT (equivalent to D816V mutation in human) along with WT KIT and V560G KIT mutation. In addition, it also had inhibitory activity in BCR-ABL, PDGF-R α and β [93,94]. However, the response in SM patients has not been replicated in vivo. A Phase II open-label trial by Hochhaus et al. involving 60 patients with SM showed overall response rate of 20% with 83% of these patients testing positive for D816V KIT mutation. Complete remission (CR) was reported in 3% of these patients. The most common side effects include nausea, headache, fatigue, diarrhea, rash, bone pain, pyrexia and elevated ALT. Grade 3 and 4 adverse effects of headache, thrombocytopenia and diarrhea were reported [95].
Dasatinib is a dual Src/Abl kinase inhibitor that has an inhibitory effect against WT KIT and D816V, D816F, D816Y KIT mutation, along with other kinase receptors (ABL, SRC, etc.). It has a short half-life about 3 – 5 h in vivo compared to the other TKIs [96,97]. The Phase II open-label study by Verstovsek et al., involving 33 patients with ISM, SM-AHNMD and ASM, showed complete response in only two patients (SM-CEL and SM-myelofibrosis) without D816V KIT mutation. In addition, nine SM patients (six with ISM, three with ASM) reported transient symptomatic response including rash, diarrhea, bone pain, headaches, itching, fatigue, shortness of breath, indigestion, decrease of anaphylactic reaction. Overall, approximately 33% of these patients had response with only 6% achieving complete response. Majority of the adverse events were grade 1 and 2, but grade 3 pleural effusion occurred in about 10% of these patients. Twenty-three out of 33 patients discontinued the treatment secondary to adverse events [98]. Additionally, four case reports by Purtill et al. showed variable response in symptomatic improvements with grade 1 – 2 adverse effects [99].
Summary: Dasatinib may have some activity in patients with D816V KIT mutation but may be limited by short half-life in vivo and side-effect profile. Likewise, Nilotinib has not been shown to have significant efficacy in a clinical trial.
Midostaurin (PKC412) is an investigational protein kinase C (PKC) inhibitor with in vitro activity against D816V KIT transformed cells and WT KIT cells [100]. A Phase II multicenter study is currently underway. An interim analysis of the data from this trial involved 40 patients with ASM, MCL or SM-AHNMD + ≥ C-findings (such as cytopenia, liver dysfunction, hypoalbuminemia and/or weight loss) who initially received midostaurin 100 mg BID. There was 60% overall response rate (24/40 patients) with major responses observed in 21 (52.5%) of these patients. Partial response was observed in 7.5%, stable disease in 20%, progressive disease in 7.5% and was not evaluable in 12.5% of patients. The median change in serum tryptase level was −61% in 40 patients with 40% of these patients achieving > 50% reduction. The median change in bone marrow MC infiltrate was −41% in 32 patients with 47% of patients achieving > 50% reduction. The side effects include nausea, fatigue, vomiting, diarrhea and increased lipase. Grade 3 and 4 adverse events include neutropenia (11%), anemia (3%) and thrombocytopenia (7%) [101].
Summary: Midostaurin is an investigational treatment in patients with advanced forms of SM and D816V KIT mutation with a subset of patients achieving significant reduction in serum tryptase level and MC burden. Duration of response and effects on survival has not been reported.
Masitinib (AB1010) is a KIT inhibitor that has initially been used in veterinary medicine to treat MC tumors in canines and has subsequently shown in vitro activity against WT KIT in human MCs, PDGFR and Lyn [102,103]. The Phase II study by Paul et al. involved 25 patients with ISM or CM with handicap (involving flushing, pruritus, depression, cognitive dysfunction). The endpoints were measured based on symptomatic involvement involving flushing, Hamilton rating and pruritus. Overall improvement in quality of life scores were reported in 56% of the 25 patients studied. Adverse side effects include nausea, vomiting, diarrhea, rash and edema. One patient had agranulocytosis [104].
Summary: Masitinib may provide symptomatic improvement in some patients with SM and CM with handicap but is not an effective therapy for reduction of MC burden.
3.2 Interferon-α and cladribine
Interferon-α (IFN-α) and cladribine (2-CdA) are often used in cytoreductive management of patients with aggressive SM. IFN-α has been shown to decrease MC mediator release, MC infiltration in different organ systems including bone marrow and skeletal involvement, improvement in C-findings and serum tryptase level [105,106]. IFN-α is often used in conjunction with prednisolone (initially at 1 mg/kg/dose daily) at a dose of 1 – 3 million units subcutaneously three times per week and increased as tolerated and according to response (maximum of 5 million units per day) [107]. A Mayo clinic report involving 47 patients with ISM, ASM or SM-AHNMD showed overall response rate of about 53%, which was not significantly different with or without concurrent steroid use. The response rate was superior when patients had systemic symptoms related to mediator release. The median response duration of 12 months was seen in symptomatic improvement of mediator release involving dermatological, hematological, GI and systemic manifestations. Its effect on MC was transient with relapse upon discontinuation of the therapy. The major side effects of IFN-α included flu-like symptoms, cytopenias, bone pain, fever and depression [91]. In a French study involving 20 patients with aggressive SM or ISM, 13 (65%) showed partial or minor response but none with complete response. Adverse effects included cytopenias and depression [108].
2-chlorodeoxyadenosine (cladribine or 2-CdA) may also be used in advanced forms of SM, especially in patients who are refractory or intolerant to IFN-α therapy. It has also been used in conjunction with other chemotherapy prior to hematopoietic stem cell transplantation and the usual dose is 5 mg/m2 or 0.13 – 0.17 mg/kg per day for 5 days. It has shown in vitro and in vivo inhibitory activity against MC [107]. The Mayo clinic study involving 26 patients with ISM, ASM or SM-AHNMD showed overall response rate of 55% with median duration of response of 11 months [91]. The French study involved 44 patients with CM, ISM, smoldering SM, ASM, SM-AHNMD or MCL who have failed to respond to other therapies including mediator-target therapies, IFN-α and/or TKIs. The major and partial response varied based on different subtypes: 7/12 patients with ASM, 3/3 with SSM, 17/19 with ISM, 2/3 with CM and 0/6 with SM-AHNMD with median duration of response of 19.5 months. The most common side effects include myelosuppression, lymphopenia with increased risk of infections [109].
Summary: Interferon-α and cladribine are effective treatments in symptomatic and advanced forms of SM. However, their effects on MC burden are transient with variable duration of response.
4. Expert opinion
Mastocytosis is a rare disease. Therapeutic trials have therefore suffered from lack of placebo controls and low patient numbers in many cases. The significant variability in patient symptoms and prognosis makes it imperative to individualize pharmacotherapy options according to each patient. When doubts about diagnosis and treatment exist, a second opinion should be sought from a referral center. The patients should be made aware that no curative therapy exists for SM, although the most common form, indolent SM, is associated with a comparable life expectancy to the general population. Patients with advanced forms of disease (such as aggressive SM) and mastocytosis associated with other non-MC hematologic disorders are usually diagnosed in the beginning due to hematologic abnormalities, and there is approximately < 5% risk of progression of indolent mastocytosis into a more advanced form of disease. Cutaneous mastocytosis seen in children is generally self-limited with approximately only 1 out of 10 cases progressing to systemic disease. In our experience, most patients with mastocytosis benefit from daily scheduled nonsedating H1 antihistamine therapy. This can be combined with other anti-mediator drugs as reviewed above depending on patient symptoms. Multiple doses of self-injectable epinephrine are prescribed to all patients with mastocytosis due to increased risk for anaphylaxis. Cytoreductive therapy should be considered in patients with ASM, SM-AHNMD, MCL and MCS. Bone marrow transplantation may offer a chance for a cure in selected patients with these advanced disease subtypes, however the experience is limited to case reports. A comprehensive management plan for the disease includes prevention of triggers of MC mediator release, prevention and treatment of anaphylaxis, pharmaco-therapy of ongoing mediator related symptoms, cytoreductive therapy when indicated and treatment of associated comorbidities. Protocols to prevent anaphylaxis in high-risk situations have been proposed, mostly consisting of the prophylactic use of antihistamines and corticosteroids, and have not been tested in randomized controlled trials. Future research into better understanding of molecular and cellular pathogenesis of disease should aid in identification of new drug targets for more effective management of all categories of mastocytosis.
4.1 Future directions
Despite advances made in diagnosis and molecular pathology of mastocytosis in the past two decades, a curative therapy for mastocytosis remains elusive. This is probably due to the incomplete understanding of molecular and cellular pathogenesis of the disease. There currently exists no approved TKI that can inhibit the D816V c-kit mutation in vivo. Further, clinical spectrum of the disease indicates that there are other yet to be identified molecular defects in addition to D816V c-kit mutation that influence the ultimate disease phenotype. Mechanisms of osteoporosis, fatigue, soft tissue pain and anaphylaxis seen in mastocytosis are incompletely understood. Identification of these pathologic mechanisms would generate new targets for more efficient therapies in the future.
Article highlights.
A significant diversity exists between the prognosis and clinical presentation of mastocytosis.
There is no curative therapy for mastocytosis. Mainstay of treatment includes control of symptoms caused by MC mediator release.
SM is associated with D816V c-kit mutation. This mutation renders the MCs resistant to imatinib. Patients with chronic eosinophilic leukemia associated with FIP1L1-PDGFRA may have increased MCs resembling mastocytosis and respond exquisitely well to imatinib. There have been no other approved TKIs to show significant benefit in clinical trials.
Cytoreductive therapies for mastocytosis include interferon-α and cladribine. These generally result in temporary and incomplete MC cytoreduction.
This box summarizes key points contained in the article.
Footnotes
Declaration of interest
C Akin has a consultancy agreement with Novartis for the PKC412 trial. Juan Carlos Cardet and Min Lee have no conflicts of interest.
Bibliography
Papers of special note have been highlighted as either of interest (•) or of considerable interest (••) to readers.
- 1••.Valent P, Horny HP, Escribano L, et al. Diagnostic criteria and classification of mastocytosis: a consensus proposal. Leuk Res. 2001;25(7):603–25. doi: 10.1016/s0145-2126(01)00038-8. International consensus report which formed the basis of the current World Health Organization’s diagnostic criteria and classification of mastocytosis. [DOI] [PubMed] [Google Scholar]
- 2••.Valent P, Akin C, Escribano L, et al. Standards and standardization in mastocytosis: consensus statements on diagnostics, treatment recommendations and response criteria. Eur J Clin Invest. 2007;37(6):435–53. doi: 10.1111/j.1365-2362.2007.01807.x. Detailed discussion and algorithms for various diagnostic techniques and recommendations for treatment of mastocytosis. [DOI] [PubMed] [Google Scholar]
- 3.Fuller SJ. New insights into the pathogenesis, diagnosis, and management of mastocytosis. Hematol Oncol Clin North Am. 2012;26(6):1143–68. doi: 10.1016/j.hoc.2012.08.008. [DOI] [PubMed] [Google Scholar]
- 4.Escribano L, Akin C, Castells M, et al. Current options in the treatment of mast cell mediator-related symptoms in mastocytosis. Inflamm Allergy Drug Targets. 2006;5(1):61–77. doi: 10.2174/187152806775269303. [DOI] [PubMed] [Google Scholar]
- 5.Escribano L, Akin C, Castells M, et al. Mastocytosis: current concepts in diagnosis and treatment. Ann Hematol. 2002;81(12):677–90. doi: 10.1007/s00277-002-0575-z. [DOI] [PubMed] [Google Scholar]
- 6.Wilson TM, Metcalfe DD, Robyn J. Treatment of systemic mastocytosis. Immunol Allergy Clin North Am. 2006;26(3):549–73. doi: 10.1016/j.iac.2006.05.009. [DOI] [PubMed] [Google Scholar]
- 7.Worobec AS. Treatment of systemic mast cell disorders. Hematol Oncol Clin North Am. 2000;14(3):659–87. vii. doi: 10.1016/s0889-8588(05)70301-4. [DOI] [PubMed] [Google Scholar]
- 8.Metcalfe DD. The treatment of mastocytosis: an overview. J Invest Dermatol. 1991;96(3 Suppl):55S–6S. doi: 10.1111/1523-1747.ep12469049. discussion 56S–59S, 60S–65S. [DOI] [PubMed] [Google Scholar]
- 9.Akin C. Molecular diagnosis of mast cell disorders: a paper from the 2005 William Beaumont Hospital Symposium on Molecular Pathology. J Mol Diagn. 2006;8(4):412–19. doi: 10.2353/jmoldx.2006.060022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Castells M, Austen KF. Mastocytosis: mediator-related signs and symptoms. Int Arch Allergy Immunol. 2002;127(2):147–52. doi: 10.1159/000048188. [DOI] [PubMed] [Google Scholar]
- 11.Valent P, Akin C, Arock M, et al. Definitions, criteria and global classification of mast cell disorders with special reference to mast cell activation syndromes: a consensus proposal. Int Arch Allergy Immunol. 2012;157(3):215–25. doi: 10.1159/000328760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Worobec AS, Metcalfe DD. Mastocytosis: current treatment concepts. Int Arch Allergy Immunol. 2002;127(2):153–5. doi: 10.1159/000048189. [DOI] [PubMed] [Google Scholar]
- 13.Simons FERA, Cezmi A. Histamine and H1 antihistamines. In: Adkinson NF, editor. Middleton’s allergy: principles and practice. 7. Mosby; China: 2009. [Google Scholar]
- 14.Friedman BS, Santiago ML, Berkebile C, et al. Comparison of azelastine and chlorpheniramine in the treatment of mastocytosis. J Allergy Clin Immunol. 1993;92(4):520–6. doi: 10.1016/0091-6749(93)90076-r. [DOI] [PubMed] [Google Scholar]
- 15.Frieri M, Alling DW, Metcalfe DD. Comparison of the therapeutic efficacy of cromolyn sodium with that of combined chlorpheniramine and cimetidine in systemic mastocytosis. Results of a double-blind clinical trial. Am J Med. 1985;78(1):9–14. doi: 10.1016/0002-9343(85)90454-1. [DOI] [PubMed] [Google Scholar]
- 16.Gasior-Chrzan B, Falk ES. Systemic mastocytosis treated with histamine H1 and H2 receptor antagonists. Dermatology. 1992;184(2):149–52. doi: 10.1159/000247526. [DOI] [PubMed] [Google Scholar]
- 17.Marone G, Spadaro G, Granata F, et al. Treatment of mastocytosis: pharmacologic basis and current concepts. Leuk Res. 2001;25(7):583–94. doi: 10.1016/s0145-2126(01)00039-x. [DOI] [PubMed] [Google Scholar]
- 18.Pratt CM, Hertz RP, Ellis BE, et al. Risk of developing life-threatening ventricular arrhythmia associated with tefenadine in comparison with over-the-counter antihistamines, ibuprofen and clemastine. Am J Cardiol. 1994;73(5):346–52. doi: 10.1016/0002-9149(94)90006-x. [DOI] [PubMed] [Google Scholar]
- 19.Berg MJ, Bernhard H, Schentag JJ. Cimetidine in systemic mastocytosis. Drug Intell Clin Pharm. 1981;15(3):180–3. doi: 10.1177/106002808101500303. [DOI] [PubMed] [Google Scholar]
- 20.Bredfeldt JE, O’Laughlin JC, Durham JB, et al. Malabsorption and gastric hyperacidity in systemic mastocytosis. Results of cimetidine therapy. Am J Gastroenterol. 1980;74(2):133–7. [PubMed] [Google Scholar]
- 21.Hirschowitz BI, Groarke JF. Effect of cimetidine on gastric hypersecretion and diarrhea in systemic mastocytosis. Ann Intern Med. 1979;90(5):769–71. doi: 10.7326/0003-4819-90-5-769. [DOI] [PubMed] [Google Scholar]
- 22.Achord JL, Langford H. The effect of cimetidine and propantheline on the symptoms of a patient with systemic mastocytosis. Am J Med. 1980;69(4):610–14. doi: 10.1016/0002-9343(80)90476-3. [DOI] [PubMed] [Google Scholar]
- 23.Johnson GJ, Silvis SE, Roitman B, et al. Long-term treatment of systemic mastocytosis with histamine H2 receptor antagonists. Am J Gastroenterol. 1980;74(6):485–9. [PubMed] [Google Scholar]
- 24.Edwards AMH, Stephen T. The Chromones: sodium cromolyn and nedocromil sodium. In: Adkinson NF, editor. Middleton’s allergy: principles and practice. 7. Mosby; China: 2009. [Google Scholar]
- 25.Alexander RR. Disodium cromoglycate in the treatment of systemic mastocytosis involving only bone. Acta Haematol. 1985;74(2):108–10. doi: 10.1159/000206179. [DOI] [PubMed] [Google Scholar]
- 26.Soter NA, Austen KF, Wasserman SI. Oral disodium cromoglycate in the treatment of systemic mastocytosis. N Engl J Med. 1979;301(9):465–9. doi: 10.1056/NEJM197908303010903. [DOI] [PubMed] [Google Scholar]
- 27.Horan RF, Sheffer AL, Austen KF. Cromolyn sodium in the management of systemic mastocytosis. J Allergy Clin Immunol. 1990;85(5):852–5. doi: 10.1016/0091-6749(90)90067-e. [DOI] [PubMed] [Google Scholar]
- 28.Mallet AI, Norris P, Rendell NB, et al. The effect of disodium cromoglycate and ketotifen on the excretion of histamine and N tau-methylimidazole acetic acid in urine of patients with mastocytosis. Br J Clin Pharmacol. 1989;27(1):88–91. doi: 10.1111/j.1365-2125.1989.tb05339.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Dolovich J, Punthakee ND, MacMillan AB, et al. Systemic mastocytosis: control of lifelong diarrhea by ingested disodium cromoglycate. Can Med Assoc J. 1974;111(7):684–5. [PMC free article] [PubMed] [Google Scholar]
- 30.Zachariae H, Herlin T, Larsen PO. Oral disodium cromoglycate in mastocytosis. Acta Derm Venereolo. 1981;61(3):272–3. [PubMed] [Google Scholar]
- 31.Businco L, Cantani A, Businco E, et al. Systemic mastocytosis in a 5-year-old child: successful treatment with disodium cromoglycate. Clin Allergy. 1984;14(2):147–52. doi: 10.1111/j.1365-2222.1984.tb02646.x. [DOI] [PubMed] [Google Scholar]
- 32.Czarnetzki BM, Behrendt H. Urticaria pigmentosa: clinical picture and response to oral disodium cromoglycate. Br J Dermatol. 1981;105(5):563–7. doi: 10.1111/j.1365-2133.1981.tb00800.x. [DOI] [PubMed] [Google Scholar]
- 33.Lindskov R, Lange Wantzin G, Knudsen L, et al. Urticaria pigmentosa treated with oral disodium cromoglycate. Dermatologica. 1984;169(1):49–52. doi: 10.1159/000249567. [DOI] [PubMed] [Google Scholar]
- 34.Welch EA, Alper JC, Bogaars H, et al. Treatment of bullous mastocytosis with disodium cromoglycate. J Am Acad Dermatol. 1983;9(3):349–53. doi: 10.1016/s0190-9622(83)70140-4. [DOI] [PubMed] [Google Scholar]
- 35.Cardet JC, Castells MC, Hamilton MJ. Immunology and clinical manifestations of non-clonal mast cell activation syndrome. Curr Allergy Asthma Rep. 2013;13(1):10–18. doi: 10.1007/s11882-012-0326-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Patterson R, Fitzsimons EJ, Choy AC, et al. Malignant and corticosteroid-dependent idiopathic anaphylaxis: successful responses to ketotifen. Ann Allergy Asthma Immunol. 1997;79(2):138–44. doi: 10.1016/S1081-1206(10)63100-6. [DOI] [PubMed] [Google Scholar]
- 37.Dykewicz MS, Wong SS, Patterson R, et al. Evaluation of ketotifen in corticosteroid-dependent idiopathic anaphylaxis. Ann Allergy. 1990;65(5):406–10. [PubMed] [Google Scholar]
- 38.Kettelhut BV, Berkebile C, Bradley D, et al. A double-blind, placebo-controlled, crossover trial of ketotifen versus hydroxyzine in the treatment of pediatric mastocytosis. J Allergy Clin Immunol. 1989;83(5):866–70. doi: 10.1016/0091-6749(89)90097-3. [DOI] [PubMed] [Google Scholar]
- 39.Ting S. Ketotifen and systemic mastocytosis. J Allergy Clin Immunol. 1990;85(4):818. doi: 10.1016/0091-6749(90)90205-i. [DOI] [PubMed] [Google Scholar]
- 40.Graves L, III, Stechschulte DJ, Morris DC, et al. Inhibition of mediator release in systemic mastocytosis is associated with reversal of bone changes. J Bone Min Res. 1990;5(11):1113–19. doi: 10.1002/jbmr.5650051104. [DOI] [PubMed] [Google Scholar]
- 41.Povoa P, Ducla-Soares J, Fernandes A, et al. A case of systemic mastocytosis; therapeutic efficacy of ketotifen. J Intern Med. 1991;229(5):475–7. doi: 10.1111/j.1365-2796.1991.tb00379.x. [DOI] [PubMed] [Google Scholar]
- 42.Castells M, Metcalfe DD, Escribano L. Diagnosis and treatment of cutaneous mastocytosis in children: practical recommendations. Am J Clin Dermatol. 2011;12(4):259–70. doi: 10.2165/11588890-000000000-00000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Schleimer RP. Pharmacology of glucocorticoids in allergic disease. In: Adkinson NF, editor. Middleton’s allergy: principles and practice. 7. Mosby; China: 2009. [Google Scholar]
- 44.Friedman BS, Metcalfe DD. Effects of tixocortol pivalate on gastrointestinal disease in systemic mastocytosis: a preliminary study. Clin Exp Allergy. 1991;21(2):183–8. doi: 10.1111/j.1365-2222.1991.tb00828.x. [DOI] [PubMed] [Google Scholar]
- 45.Barton J, Lavker RM, Schechter NM, et al. Treatment of urticaria pigmentosa with corticosteroids. Arch Dermatol. 1985;121(12):1516–23. [PubMed] [Google Scholar]
- 46.Boyce JA. Eicosanoids in asthma, allergic inflammation, and host defense. Curr Mol Med. 2008;8(5):335–49. doi: 10.2174/156652408785160989. [DOI] [PubMed] [Google Scholar]
- 47.Wenzel SE. Antileukotriene therapy in asthma. In: Adkinson NF, editor. Middleton’s allergy: principles and practice. 7. Mosby; China: 2009. [Google Scholar]
- 48.Castells M. Mast cell mediators in allergic inflammation and mastocytosis. Immunol Allergy Clin North Am. 2006;26(3):465–85. doi: 10.1016/j.iac.2006.05.005. [DOI] [PubMed] [Google Scholar]
- 49.Butterfield JH. Increased leukotriene E4 excretion in systemic mastocytosis. Prostaglandins Other Lipid Mediat. 2010;92(1–4):73–6. doi: 10.1016/j.prostaglandins.2010.03.003. [DOI] [PubMed] [Google Scholar]
- 50.Tolar J, Tope WD, Neglia JP. Leukotriene-receptor inhibition for the treatment of systemic mastocytosis. N Engl J Med. 2004;350(7):735–6. doi: 10.1056/NEJM200402123500723. [DOI] [PubMed] [Google Scholar]
- 51.Turner PJ, Kemp AS, Rogers M, et al. Refractory symptoms successfully treated with leukotriene inhibition in a child with systemic mastocytosis. Pediatr Dermatol. 2012;29(2):222–3. doi: 10.1111/j.1525-1470.2011.01576.x. [DOI] [PubMed] [Google Scholar]
- 52.Roberts LJ, II, Sweetman BJ, Lewis RA, et al. Increased production of prostaglandin D2 in patients with systemic mastocytosis. N Engl J Med. 1980;303(24):1400–4. doi: 10.1056/NEJM198012113032405. [DOI] [PubMed] [Google Scholar]
- 53.Roberts LJ, II, Turk JW, Oates JA. Shock syndrome associated with mastocytosis: pharmacologic reversal of the acute episode and therapeutic prevention of recurrent attacks. Adv Shock Res. 1982;8:145–52. [PubMed] [Google Scholar]
- 54.Meade EA, Smith WL, DeWitt DL. Differential inhibition of prostaglandin endoperoxide synthase (cyclooxygenase) isozymes by aspirin and other non-steroidal anti-inflammatory drugs. J Biol Chem. 1993;268(9):6610–14. [PubMed] [Google Scholar]
- 55.Butterfield JH. Survey of aspirin administration in systemic mastocytosis. Prostaglandins Other Lipid Mediat. 2009;88(3–4):122–4. doi: 10.1016/j.prostaglandins.2009.01.001. [DOI] [PubMed] [Google Scholar]
- 56.Butterfield JH, Weiler CR. Prevention of mast cell activation disorder-associated clinical sequelae of excessive prostaglandin D(2) production. Int Arch Allergy Immunol. 2008;147(4):338–43. doi: 10.1159/000144042. [DOI] [PubMed] [Google Scholar]
- 57.Lorcerie B, Arveux I, Chauffert B, et al. Aspirin and systemic mastocytosis. Lancet. 1989;2(8672):1155. doi: 10.1016/s0140-6736(89)91516-x. [DOI] [PubMed] [Google Scholar]
- 58.Crawhall JC, Wilkinson RD. Systemic mastocytosis: management of an unusual case with histamine (H1 and H2) antagonists and cyclooxygenase inhibition. Clin Invest Med. 1987;10(1):1–4. [PubMed] [Google Scholar]
- 59.Greenhawt M, Akin C. Mastocytosis and allergy. Curr Opin Allergy Clin Immunol. 2007;7(5):387–92. doi: 10.1097/ACI.0b013e3282a6443e. [DOI] [PubMed] [Google Scholar]
- 60.Simons FE, Gu X, Silver NA, et al. EpiPen Jr versus EpiPen in young children weighing 15 to 30 kg at risk for anaphylaxis. J Allergy Clin Immunol. 2002;109(1):171–5. doi: 10.1067/mai.2002.120758. [DOI] [PubMed] [Google Scholar]
- 61.Turk J, Oates JA, Roberts LJ., II Intervention with epinephrine in hypotension associated with mastocytosis. J Allergy Clin Immunol. 1983;71(2):189–92. doi: 10.1016/0091-6749(83)90098-2. [DOI] [PubMed] [Google Scholar]
- 62.Lieberman P, Nicklas RA, Oppenheimer J, et al. The diagnosis and management of anaphylaxis practice parameter: 2010 update. J Allergy Clin Immunol. 2010;126(3):477–80. e1–42. doi: 10.1016/j.jaci.2010.06.022. [DOI] [PubMed] [Google Scholar]
- 63.Simons FE, Ardusso LR, Bilo MB, et al. World allergy organization guidelines for the assessment and management of anaphylaxis. World Allergy Organ J. 2011;4(2):13–37. doi: 10.1097/WOX.0b013e318211496c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Muraro A, Roberts G, Clark A, et al. The management of anaphylaxis in childhood: position paper of the European academy of allergology and clinical immunology. Allergy. 2007;62(8):857–71. doi: 10.1111/j.1398-9995.2007.01421.x. [DOI] [PubMed] [Google Scholar]
- 65.Barete S, Assous N, de Gennes C, et al. Systemic mastocytosis and bone involvement in a cohort of 75 patients. Ann Rheum Dis. 2010;69(10):1838–41. doi: 10.1136/ard.2009.124511. [DOI] [PubMed] [Google Scholar]
- 66.Bellido T, Plotkin LI. Novel actions of bisphosphonates in bone: preservation of osteoblast and osteocyte viability. Bone. 2011;49(1):50–5. doi: 10.1016/j.bone.2010.08.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Brumsen C, Hamdy NA, Papapoulos SE. Osteoporosis and bone marrow mastocytosis: dissociation of skeletal responses and mast cell activity during long-term bisphosphonate therapy. J Bone Min Res. 2002;17(4):567–9. doi: 10.1359/jbmr.2002.17.4.567. [DOI] [PubMed] [Google Scholar]
- 68.Cundy T, Beneton MN, Darby AJ, et al. Osteopenia in systemic mastocytosis: natural history and responses to treatment with inhibitors of bone resorption. Bone. 1987;8(3):149–55. doi: 10.1016/8756-3282(87)90014-7. [DOI] [PubMed] [Google Scholar]
- 69.Marshall A, Kavanagh RT, Crisp AJ. The effect of pamidronate on lumbar spine bone density and pain in osteoporosis secondary to systemic mastocytosis. Br J Rheumatol. 1997;36(3):393–6. doi: 10.1093/rheumatology/36.3.393. [DOI] [PubMed] [Google Scholar]
- 70.Godt O, Proksch E, Streit V, et al. Short- and long-term effectiveness of oral and bath PUVA therapy in urticaria pigmentosa and systemic mastocytosis. Dermatology. 1997;195(1):35–9. doi: 10.1159/000245681. [DOI] [PubMed] [Google Scholar]
- 71.Czarnetzki BM, Rosenbach T, Kolde G, et al. Phototherapy of urticaria pigmentosa: clinical response and changes of cutaneous reactivity, histamine and chemotactic leukotrienes. Arch Dermatol Res. 1985;277(2):105–13. doi: 10.1007/BF00414106. [DOI] [PubMed] [Google Scholar]
- 72.Kinsler VA, Hawk JL, Atherton DJ. Diffuse cutaneous mastocytosis treated with psoralen photochemotherapy: case report and review of the literature. Br J Dermatol. 2005;152(1):179–80. doi: 10.1111/j.1365-2133.2004.06300.x. [DOI] [PubMed] [Google Scholar]
- 73.Bowling J, Cork MJ. Severe pruritus in a patient with urticaria pigmentosa treated with topical 5% urea and 3% polidocanol cream. J Dermatolog Treat. 2003;14(3):190–1. doi: 10.1080/09546630310007079. [DOI] [PubMed] [Google Scholar]
- 74.Correia O, Duarte AF, Quirino P, et al. Cutaneous mastocytosis: two pediatric cases treated with topical pimecrolimus. Dermatol Online J. 2010;16(5):8. [PubMed] [Google Scholar]
- 75.Carter MC, Robyn JA, Bressler PB, et al. Omalizumab for the treatment of unprovoked anaphylaxis in patients with systemic mastocytosis. J Allergy Clin Immunol. 2007;119(6):1550–1. doi: 10.1016/j.jaci.2007.03.032. [DOI] [PubMed] [Google Scholar]
- 76.Douglass JA, Carroll K, Voskamp A, et al. Omalizumab is effective in treating systemic mastocytosis in a nonatopic patient. Allergy. 2010;65(7):926–7. doi: 10.1111/j.1398-9995.2009.02259.x. [DOI] [PubMed] [Google Scholar]
- 77.Siebenhaar F, Kuhn W, Zuberbier T, et al. Successful treatment of cutaneous mastocytosis and Meniere disease with anti-IgE therapy. J Allergy Clin Immunol. 2007;120(1):213–15. doi: 10.1016/j.jaci.2007.05.011. [DOI] [PubMed] [Google Scholar]
- 78.Kontou-Fili K. High omalizumab dose controls recurrent reactions to venom immunotherapy in indolent systemic mastocytosis. Allergy. 2008;63(3):376–8. doi: 10.1111/j.1398-9995.2007.01604.x. [DOI] [PubMed] [Google Scholar]
- 79.Molderings GJ, Raithel M, Kratz F, et al. Omalizumab treatment of systemic mast cell activation disease: experiences from four cases. Intern Med. 2011;50(6):611–15. doi: 10.2169/internalmedicine.50.4640. [DOI] [PubMed] [Google Scholar]
- 80.Bonadonna P, Zanotti R, Muller U. Mastocytosis and insect venom allergy. Curr Opin Allergy Clin Immunol. 2010;10(4):347–53. doi: 10.1097/ACI.0b013e32833b280c. [DOI] [PubMed] [Google Scholar]
- 81.Oude Elberink JN, de Monchy JG, Kors JW, et al. Fatal anaphylaxis after a yellow jacket sting, despite venom immunotherapy, in two patients with mastocytosis. J Allergy Clin Immunol. 1997;99(1 Pt 1):153–4. doi: 10.1016/s0091-6749(97)70314-2. [DOI] [PubMed] [Google Scholar]
- 82.Haeberli G, Bronnimann M, Hunziker T, et al. Elevated basal serum tryptase and hymenoptera venom allergy: relation to severity of sting reactions and to safety and efficacy of venom immunotherapy. Clin Exp Allergy. 2003;33(9):1216–20. doi: 10.1046/j.1365-2222.2003.01755.x. [DOI] [PubMed] [Google Scholar]
- 83.Bonadonna P, Perbellini O, Passalacqua G, et al. Clonal mast cell disorders in patients with systemic reactions to Hymenoptera stings and increased serum tryptase levels. J Allergy Clin Immunol. 2009;123(3):680–6. doi: 10.1016/j.jaci.2008.11.018. [DOI] [PubMed] [Google Scholar]
- 84.Gonzalez de Olano D, Alvarez-Twose I, Esteban-Lopez MI, et al. Safety and effectiveness of immunotherapy in patients with indolent systemic mastocytosis presenting with Hymenoptera venom anaphylaxis. J Allergy Clin Immunol. 2008;121(2):519–26. doi: 10.1016/j.jaci.2007.11.010. [DOI] [PubMed] [Google Scholar]
- 85.Boissan M, Feger F, Guillosson JJ, et al. c-Kit and c-kit mutations in mastocytosis and other hematological diseases. J Leukoc Biol. 2000;67(2):135–48. doi: 10.1002/jlb.67.2.135. [DOI] [PubMed] [Google Scholar]
- 86•.Ustun C, DeRemer DL, Akin C. Tyrosine kinase inhibitors in the treatment of systemic mastocytosis. Leuk Res. 2011;35(9):1143–52. doi: 10.1016/j.leukres.2011.05.006. An extensive review of the published clinical and in vitro studies on the effects of various tyrosine kinase inhibitors on mast cells in mastocytosis and their effects on D816V c-kit mutation. [DOI] [PubMed] [Google Scholar]
- 87••.Akin C, Fumo G, Yavuz AS, et al. A novel form of mastocytosis associated with a transmembrane c-kit mutation and response to imatinib. Blood. 2004;103(8):3222–5. doi: 10.1182/blood-2003-11-3816. First report showing a clonal mast cell disease driven by a c-kit mutation can respond to the tyrosine kinase inhibitor imatinib. [DOI] [PubMed] [Google Scholar]
- 88.Akin C, Brockow K, D’Ambrosio C, et al. Effects of tyrosine kinase inhibitor STI571 on human mast cells bearing wild-type or mutated c-kit. Exp Hematol. 2003;31(8):686–92. doi: 10.1016/s0301-472x(03)00112-7. [DOI] [PubMed] [Google Scholar]
- 89.Frost MJ, Ferrao PT, Hughes TP, et al. Juxtamembrane mutant V560GKit is more sensitive to imatinib (STI571) compared with wild-type c-kit whereas the kinase domain mutant D816VKit is resistant. Mol Cancer Ther. 2002;1(12):1115–24. [PubMed] [Google Scholar]
- 90.Vega-Ruiz A, Cortes JE, Sever M, et al. Phase II study of imatinib mesylate as therapy for patients with systemic mastocytosis. Leuk Res. 2009;33(11):1481–4. doi: 10.1016/j.leukres.2008.12.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91•.Lim KH, Pardanani A, Butterfield JH, et al. Cytoreductive therapy in 108 adults with systemic mastocytosis: outcome analysis and response prediction during treatment with interferon-alpha, hydroxyurea, imatinib mesylate or 2-chlorodeoxyadenosine. Am J Hematol. 2009;84(12):790–4. doi: 10.1002/ajh.21561. Mayo clinic experience on cytoreductive therapy of advanced variants of mastocytosis. [DOI] [PubMed] [Google Scholar]
- 92.Pagano L, Valentini CG, Caira M, et al. Advanced mast cell disease: an Italian Hematological Multicenter experience. Int J Hematol. 2008;88(5):483–8. doi: 10.1007/s12185-008-0166-4. [DOI] [PubMed] [Google Scholar]
- 93.von Bubnoff N, Gorantla SH, Kancha RK, et al. The systemic mastocytosis-specific activating cKit mutation D816V can be inhibited by the tyrosine kinase inhibitor AMN107. Leukemia. 2005;19(9):1670–1. doi: 10.1038/sj.leu.2403887. [DOI] [PubMed] [Google Scholar]
- 94.Verstovsek S, Akin C, Manshouri T, et al. Effects of AMN107, a novel aminopyrimidine tyrosine kinase inhibitor, on human mast cells bearing wild-type or mutated codon 816 c-kit. Leuk Res. 2006;30(11):1365–70. doi: 10.1016/j.leukres.2006.04.005. [DOI] [PubMed] [Google Scholar]
- 95.Hochhaus A, Ottmann OG, Lauber S, et al. A phase II study of nilotinib, a novel inhibitor of c-Kit, PDGFR, and Bcr-Abl, administered to patients with systemic mastocytosis. Blood. 2006:108. [Google Scholar]
- 96.Shah NP, Lee FY, Luo R, et al. Dasatinib (BMS-354825) inhibits KITD816V, an imatinib-resistant activating mutation that triggers neoplastic growth in most patients with systemic mastocytosis. Blood. 2006;108(1):286–91. doi: 10.1182/blood-2005-10-3969. [DOI] [PubMed] [Google Scholar]
- 97.Schittenhelm MM, Shiraga S, Schroeder A, et al. Dasatinib (BMS-354825), a dual SRC/ABL kinase inhibitor, inhibits the kinase activity of wild-type, juxtamembrane, and activation loop mutant KIT isoforms associated with human malignancies. Cancer Res. 2006;66(1):473–81. doi: 10.1158/0008-5472.CAN-05-2050. [DOI] [PubMed] [Google Scholar]
- 98.Verstovsek S, Tefferi A, Cortes J, et al. Phase II study of dasatinib in Philadelphia chromosome-negative acute and chronic myeloid diseases, including systemic mastocytosis. Clin Cancer Res. 2008;14(12):3906–15. doi: 10.1158/1078-0432.CCR-08-0366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Purtill D, Cooney J, Sinniah R, et al. Dasatinib therapy for systemic mastocytosis: four cases. Eur J Haematol. 2008;80(5):456–8. doi: 10.1111/j.1600-0609.2008.01048.x. [DOI] [PubMed] [Google Scholar]
- 100.Meyer T, Regenass U, Fabbro D, et al. A derivative of staurosporine (CGP 41 251) shows selectivity for protein kinase C inhibition and in vitro anti-proliferative as well as in vivo anti-tumor activity. Int J Cancer. 1989;43(5):851–6. doi: 10.1002/ijc.2910430519. [DOI] [PubMed] [Google Scholar]
- 101.Gotlib J, Kluin-Nelemans HC, George T, et al. KIT Inhibitor midostaurin in patients with advanced systemic mastocytosis: results of a planned interim analysis of the Global CPKC412D2201 Trial. 54th ASH Annual meeting and Exposition; Atlanta, GA. 2012. [Google Scholar]
- 102.Dubreuil P, Letard S, Ciufolini M, et al. Masitinib (AB1010), a potent and selective tyrosine kinase inhibitor targeting KIT. PLoS One. 2009;4(9):e7258. doi: 10.1371/journal.pone.0007258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Hahn KA, Legendre AM, Shaw NG, et al. Evaluation of 12- and 24-month survival rates after treatment with masitinib in dogs with nonresectable mast cell tumors. Am J Vet Res. 2010;71(11):1354–61. doi: 10.2460/ajvr.71.11.1354. [DOI] [PubMed] [Google Scholar]
- 104.Paul C, Sans B, Suarez F, et al. Masitinib for the treatment of systemic and cutaneous mastocytosis with handicap: a phase 2a study. Am J Hematol. 2010;85(12):921–5. doi: 10.1002/ajh.21894. [DOI] [PubMed] [Google Scholar]
- 105.Lehmann T, Lammle B. IFNalpha treatment in systemic mastocytosis. Ann Hematol. 1999;78(10):483–4. doi: 10.1007/s002770050604. [DOI] [PubMed] [Google Scholar]
- 106.Hauswirth AW, Simonitsch-Klupp I, Uffmann M, et al. Response to therapy with interferon alpha-2b and prednisolone in aggressive systemic mastocytosis: report of five cases and review of the literature. Leuk Res. 2004;28(3):249–57. doi: 10.1016/s0145-2126(03)00259-5. [DOI] [PubMed] [Google Scholar]
- 107.Pardanani A. Systemic mastocytosis in adults: 2011 update on diagnosis, risk stratification, and management. Am J Hematol. 2011;86(4):362–71. doi: 10.1002/ajh.21982. [DOI] [PubMed] [Google Scholar]
- 108.Simon J, Lortholary O, Caillat-Vigneron N, et al. Interest of interferon alpha in systemic mastocytosis. The French experience and review of the literature. Pathol Biol. 2004;52(5):294–9. doi: 10.1016/j.patbio.2004.04.012. [DOI] [PubMed] [Google Scholar]
- 109.Hermine O, Hirsh I, Damaj G, et al. Long term efficacy and safety of cladribine in adult systemic mastocytosis: a French multicenter study of 44 patients. Blood. 2010;116:1982. [Google Scholar]
- 110.Horny HPMD, Bennett JM, et al. WHO classification of tumours of haematopoietic and lymphoid tissues. IARC Press; Lyon, France: 2008. WHO classification of mastocytosis; pp. 54–63. [Google Scholar]
- 111.Valent P, Akin C, Sperr WR, et al. Aggressive systemic mastocytosis and related mast cell disorders: current treatment options and proposed response criteria. Leuk Res. 2003;27(7):635–41. doi: 10.1016/s0145-2126(02)00168-6. [DOI] [PubMed] [Google Scholar]


