Abstract
Objective
Adults with Juvenile Myoclonic Epilepsy (JME) have subtle brain structural abnormalities in the fronto-thalamic-cortical network, poorer cognitive function, and worse long-term social outcomes, even when their seizures are controlled and/or remitted. The natural history of JME and development of abnormalities in brain structure and cognition from epilepsy onset has not been studied.
Methods
The maturational trajectories of cognitive and brain development were prospectively compared between 19 children with new-onset JME in the first two years after diagnosis and 57 healthy controls.
Results
Cognitive abilities of children with JME were similar to or worse than healthy controls at baseline but failed to reach the competence level of healthy controls at follow-up across most of the tested cognitive abilities. Abnormal patterns of brain development, as assessed by magnetic resonance imaging studies, were evident in children with JME and included attenuation of age-related decline in cortical volume, thickness, and surface area compared to typically developing children. The altered brain developmental trajectory in the JME group was evident in higher-association fronto-parietal-temporal brain regions (p < 0.05, corrected for multiple comparisons).
Interpretation
Children with JME have abnormal structural brain development and impaired cognitive development early in the course of their epilepsy.
INTRODUCTION
Juvenile Myoclonic Epilepsy (JME) is one of the most common childhood-onset epilepsy syndromes, constituting 26% of the Idiopathic Generalized Epilepsies (IGE)1. Although JME is presumed to have a genetic basis, its specific pathogenesis is unknown. There is now substantial evidence demonstrating that individuals with JME exhibit subtle anomalies in brain structure2–6 and cognition7 and poor long-term social outcomes when followed over 25 years8, including depression, social isolation, and underemployment. However, the natural history of these complications from the time of epilepsy onset remains to be characterized and is the focus of the current study.
Impairment of cognition is a significant comorbidity in individuals with JME and has a negative effect on activities of daily living9. Therefore, it is critical to examine the cognitive developmental trajectory in children with new-onset JME. In adults with chronic JME, cognitive impairment impacts specific components of executive function including response inhibition, cognitive flexibility, verbal fluency, and working memory7, while hippocampal dependent episodic memory is preserved10. Two aspects of cognitive maturation in JME crucial to cognitive health have not been studied and are the focus of the current investigation. First, is cognitive impairment static or progressive over the first two years after the diagnosis of epilepsy? Second, are components of executive dysfunction affected at the same rate in JME or are there vulnerabilities for specific aspects of executive function?
The second goal of this study is to determine whether the trajectory of structural brain maturation in children with JME deviates from that of typically developing children. Prior anatomical studies in JME were limited by cross-sectional designs2–6. It is now clear that the dynamics of brain development are better determined by examining changes over time rather than at a single time point11, 12, highlighting the importance of the prospective design in this study. Most of the findings of previous research were based on cortical volume (CV), which is a composite measure of cortical anatomy with two major determinants: cortical thickness (CT) and surface area (SA). These dissociable measures of cortical surface anatomy map different phylogeny; emerging evidence suggests that CT and CV reflect different aspects of cortical neuronal migration13. Whereas CT primarily depends on the number of neurons migrating along radial glial fibers within a cortical column, SA reflects the number of columns in a cortical region. Further, CT and SA have distinguishable genetic underpinnings14, 15 and developmental trajectories16–18. Therefore, establishing the contributions of CV, CT, and SA disturbances in JME will be informative for understanding the neurobiological mechanisms underlying altered brain development in JME.
SUBJECTS AND METHODS
Participants
This study was approved by the Institutional Review Boards of the University of Wisconsin-Madison and the Marshfield Clinic. Informed consent and assent were obtained from families and children with new- and recent-onset JME (N=19) and healthy first-degree cousin controls (N=57), all 8–18 years of age, at the University of Wisconsin-Madison and the Marshfield Clinic (Marshfield, WI). Entry criteria included the diagnosis of JME within the past 12 months, absence of other developmental disabilities, no other neurological disorder, normal neurological examination, normal routine MRI scan, and attendance in regular classrooms (i.e., not special education). All JME participants had: 1) EEG showing 4–6 Hz polyspike and slow-wave generalized discharges (independently reviewed by a board certified clinical neurophysiologist blinded to clinical, cognitive, and neuroimaging data), and 2) history of myoclonic jerks, with or without generalized tonic-clonic seizures (GTCS). A pediatric neurologist certified by the American Board of Psychiatry and Neurology diagnosed JME according to the international classification of epilepsy19, 20. The date of clinical diagnosis was determined by review of medical records and confirmed by a consensus conference. There was no relationship between age of onset of JME and baseline age-adjusted cognition (all p’s > 0.20). In addition, during the initial research visit, parents underwent a structured clinical interview that inquired into, among other clinical items, possible or probable seizures antecedent to the events, which led to the diagnosis of JME. There were 4 children who likely experienced unrecognized seizures antecedent to the child’s diagnosis of JME. There was no difference in cognition between these children versus the others without antecedent seizures (all p’s > 0.10). Control participants were first-degree cousins of the JME participants. Criteria for controls included no history of: 1) initial precipitating event (e.g., febrile seizures), 2) seizure or seizure-like event, 3) diagnosed neurological disorder, 4) loss of consciousness for > 5 minutes, or 5) family history of a first-degree relative with epilepsy or febrile convulsions. The 5-minute duration for loss of conscious was prospectively selected based on published guidelines for assessing severity of head injury21. However, none of the control subjects had a prior history of head injury. Control participants also performed in the average or above average range across all administered cognitive tests, after adjusting for age and education. We selected first-degree cousins to minimize shared genetic factors that might contribute to anomalies in brain structure and cognition, while at the same time controlling for potential socioeconomic confounds. There were no significant differences between JME and control families in terms of parent IQ (Wechsler Abbreviated Scale of Intelligence, controls: 109.7±12.22, JME: 110.82±15.41, p=0.80), and paternal employment status (full-time, part-time, unemployed; mother’s (Chi=4.72, p=.19), father’s (Chi=2.68, p=0.44)), indicating similar socioeconomic status between the two groups. Table 1 outlines the demographic characteristics of the study participants. The control group averaged 2.3 years younger than the JME group (p<0.01; t-test) but there was a similar gender distribution (p=1.0; Fisher’s exact test). Therefore, all analyses included age and gender as covariates.
Table 1.
Demographic features of study groups
| Healthy controls | JME | |
|---|---|---|
| N | 57 | 19 |
| Baseline age (years) | 12.6 (0.40) | 14.9 (0.71T) |
| Gender (F/M) | 37/30 | 9/10 |
| Time between baseline and follow up (months) | 24.3 (0.1) | 24.4 (0.2) |
| Age of epilepsy diagnosis (years) | - | 14.0 (0.74) |
| Epilepsy duration (months) at baseline scan | - | 8.4 (0.87) |
Data are shown as mean (standard error) JME=Juvenile Myoclonic Epilepsy; F=female, M=male
Clinical outcomes and antiseizure drugs
We recorded JME participants’ seizure frequency and their antiseizure medications during the two years of follow up. We considered all types of seizures when computing seizure frequency (e.g. generalized tonic clonic, myoclonic, absence), although parental documentation of subtle seizures such as absence seizures may not be as reliable as more clinically overt seizure types. Seizure freedom was defined by congruent reports from the attending child neurologist and research interview with parent and child. Seizures discovered during research interviews were subsequently related to the attending neurologist and the child’s seizure frequency rating adjusted accordingly. For individuals treated with VPA, we summated their daily dose for each month and then calculated the average daily dose over the first two years of treatment. This calculation provided an accurate assessment of medication dose adjustments. Seizure severity was measured with the Liverpool Seizure Severity Scale for Children in those participants with active seizures (≥1 seizure in past year). The scale is based on the adult version22 and consists of 10 questions with good internal consistency (alpha 0.71–0.84) and test-retest correlation (r=0.67–0.84)23.
Neuropsychological assessment
All participants were administered a comprehensive test battery at baseline and the battery was repeated at the 2-year follow-up. General intellectual skills were assessed with the Wechsler Abbreviated Scale of Intelligence (WASI; full-scale IQ)24. Given that frontothalamic hyperexcitability is the core pathophysiology of JME25, we targeted facets of executive function and processing speed, including novel problem-solving ability (Card Sort Test from the Delis–Kaplan Executive Function System [D-KEFS]) 26, response inhibition (color word interference test from D-KEFS), and cognitive/psychomotor processing speed (Digit-Symbol Coding Test from Wechsler Intelligence Scale for Children Third Edition)27.
MRI acquisition
Two sets of T1-weighted three-dimensional spoiled gradient recalled (SPGR) images were acquired two years apart (2.02 ± 0.07 years) on the 1.5-Tesla GE Signa MR scanner at the University of Wisconsin-Madison (TR = 24 ms, TE = 5 ms, flip angle = 40°, Slices = 124, plane = coronal, slice thickness = 1.5 mm, matrix = 256×256). Longitudinal MRI data were available for analysis in 16 of the 19 JME participants and 36 of the 57 healthy controls. MRI scans (baseline and/or follow-up) were contraindicated in 15 healthy controls due to metal braces and claustrophobia. Two MRI scans in JME and 6 MRI scans in healthy controls were excluded due to movement artifacts. One JME participate and 5 healthy controls did not return for follow-up MRI scan. Subjects with and without MRI scan showed similar demographic profiles (controls: scanned vs. nonscanned; age p=0.32; gender p=0.26; JME: scanned vs. nonscanned age p=0.23; gender p=0.60).
Analyses for the possibility of scanner effect
A required change to another 1.5 Tesla GE scanner was made at the University of Wisconsin-Madison during the course of the study. The MRI scans of a comparable proportion of children with JME (63%, n=10) and healthy controls (56%, n=20) were acquired on different 1.5 Telsa GE scanners at baseline and follow-up. The remaining children underwent investigations on the same scanner at both times. Phantom studies showed less than 1% volume difference between the two GE scanners. We further examined possible scanner effects by investigating differences in the rate of prospective cortical thickness changes between healthy controls who were scanned on the same scanner (n=16) versus healthy controls who were scanned on two different scanners (n=20). Only a small area of the left posterior superior temporal gyrus (Talairach coordinates: −60, −41, 11) showed sensitivity to effects of different scanners, after controlling for age and gender. This region of interest (ROI) represented an average of 1.17% ± 0.17% of total gray matter volume (TGV) and thus was consistent with results of the phantom study (ROI = 9130 ±1603 mm3; TGV = 778828 ± 9318 mm3). When we used false discovery rate (instead of cluster correction) to correct for multiple comparisons, there were no differences in prospective cortical thickness changes between the two groups. In summary, minimal effects were associated with the MR equipment change.
Prospective changes in cortical thickness
The T1 volumetric MRI scans were used for cortical reconstruction and segmentation using FreeSurfer image v5.1.0 software (http://surfer.nmr.mgh.harvard.edu/). FreeSurfer computed each participant’s CV, CT, and SA across the entire cortical mantle28, 29. The data were smoothed using a surface-based kernel of 20-mm radius. We used FreeSurfer’s longitudinal stream30 to process a set of serial MRIs from each study participant. Specifically, an unbiased within-subject template image is created using robust, inverse consistent registration31. Several processing steps, such as skull stripping, Talairach transforms, atlas registration, spherical surface maps, and parcellations are then initialized with common information from the within-subject template, substantially increasing reliability and statistical power30.
Statistical analysis
Raw cognitive test scores were obtained for each subject at baseline and follow-up. Age and gender were entered as covariates in a mixed (group, time) two-way MANCOVA. Because group by time interactions were hypothesized, simple effects were examined with one-way MANCOVAs. Statistical analyses were conducted using JMP 10 (SAS Institute Inc.). Prospective changes in CV, CT, SA over two years were examined vertex-by-vertex, using a general linear model. To estimate prospective neurodevelopmental changes more precisely, we controlled for baseline differences in cortical anatomy (CV, CT, SA). Change in cortical anatomy for the two time points was first obtained separately in each group, followed by group-wise comparisons (i.e. JME vs. controls). To control for multiple comparisons, a cluster correction was applied to all anatomical analysis using Monte Carlo stimulation with 10000 iterations (vertex-wise threshold of p<0.05)32.
RESULTS
Seizure outcome and antiseizure medication usage
At the 2-year follow-up, 5 of the 19 children with JME were seizure free for at least 12 months and were not taking an antiseizure medication; 4 of the 19 children were seizure free for at least 12 months but remained on medication. The remaining 10 children with JME had at least one seizure in the past year (with 2 of these children not on medication). At 2-year follow-up, 7 were on VPA, 1 was on both VPA and lamotrigine, 4 were on lamotrigine, and 7 were not taking antiseizure medications. No significant correlation was found between seizure severity score and daily VPA dose (p=0.88). We have continued to follow this cohort and a review of the subsequent clinical chart revealed that discontinuation of medication at the time of the 2-year follow-up was temporary in 4 of the 7 JME participants as they resumed taking seizure medications 1 to 9 years later due to seizure recurrence.
Prospective changes in cognition
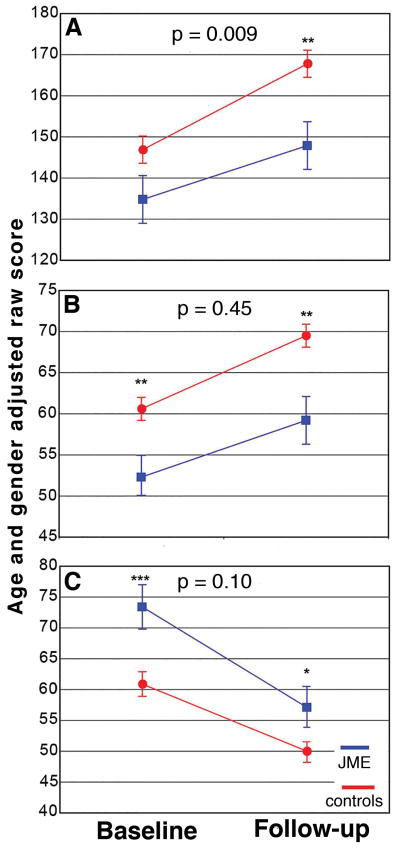
Both the JME and control groups improved their cognitive abilities over the two years, demonstrating age-dependent cognitive development. However, significant group differences existed for baseline and follow-up scores as well as the rate of improvement for some measures (Figure 1). Full scale IQ was not significantly different at baseline (p=0.30), but the JME group performed more poorly at follow-up (p=0.003) with a significant group by time interaction (p=0.009). Response inhibition and psychomotor speed scores were significantly lower at baseline (p<0.01) and follow-up in the JME group (p<0.04) with no group by time interaction (p>0.10), indicating early (baseline) cognitive impairments in JME but with interval improvement at a similar rate as control participants. Thus, children with JME did not “catch-up” to controls after two years (Figure 1B, C). Problem-solving abilities showed neither significant group differences at the two time points (Time 1, p=0.17; Time 2, p=0.31) nor group by time interaction (p=0.26).
Figure 1.
Plots of prospective changes in age- and gender-adjusted raw scores in intelligence (A), psychomotor speed (B), and response inhibition (C) for the JME group (blue line) and healthy controls (red line). P-values of group by time interactions are denoted on top of each plot. Significant group differences at baseline and follow-up are denoted by asterisks (*=p<0.05; **=p<0.01, ***=p<0.001).
Prospective changes in cortical volume: within group comparison
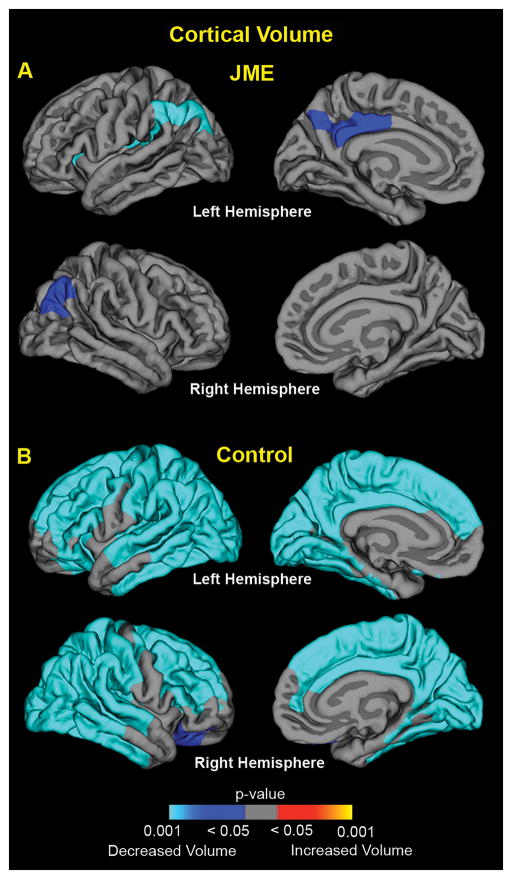
Figure 2A shows changes in cortical volume over the first two years following JME diagnosis. In the JME group, there were small regions of decreased cortical volume located in bilateral parietal cortex as well as left posterior cingulate and insular cortex (blue; areas; Figure 2A). In contrast, prospective thinning of the neocortex occurred in the healthy control group with gray matter volume loss in bilateral cerebral hemispheres (Figure 2B). All regions survived cluster correction for multiple comparisons (2-tailed, p<0.05). Note that neither group showed increased cortical volumes.
Figure 2.
Prospective cortical volume changes in the new-onset JME group (A) and the healthy control group (B). The significance of changes is shown as p-value maps (corrected for multiple comparisons) of increased (yellow/orange regions) and decreased (blue regions) cortical volume over the two-years.
Prospective changes in cortical surface anatomy: between group comparisons
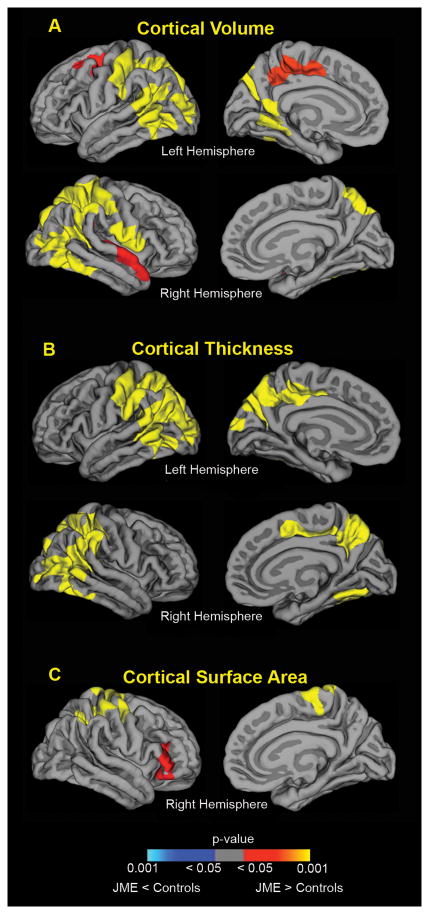
As can be seen in Figure 3A, over the two years following epilepsy diagnosis, the JME group demonstrated significantly greater CV, relative to healthy controls, in the bilateral fronto-parietal and posterior temporal association network (2 tailed, p<0.05, cluster corrected). Specifically, the JME group exhibited significantly higher volume compared to controls in the superior frontal gyrus, sensorimotor, temporal, parieto-occipital, fusiform, retrosplenial, and posterior cingulate cortex. We then examined the underlying contribution of CT and SA to the observed differences in CV. We again found greater CT in the JME group relative to controls, with spatial distribution largely overlapping with those found with CV in bilateral fronto-parietal and posterior temporal regions. Higher SA in the right superior parietal and inferior frontal cortex provided additional contribution to the increased cortical volumes observed in JME. In summary, the JME group demonstrated higher CV relative to controls over the prospective 2 years with most of the variance accounted for by increased CT and smaller but distinct contributions from the expansion of SA.
Figure 3.
Differences in cortical development between JME and healthy controls. JME group demonstrated higher cortical volume and cortical thickness in the bilateral fronto-parietal-temporal association regions over the prospective 2 years. JME group also showed greater expansion of surface area in right parietal and frontal regions, compared to controls. Color maps indicate significant p-values (corrected for multiple comparisons), with yellow/orange colors denoting JME group had higher values (JME > controls) and blue areas denoting lower values (JME < controls), relative to controls.
DISCUSSION
Our study shows for the first time how the dynamics of cognitive development and cortical maturation diverge between children with new-onset JME and healthy controls. The core findings include: 1) Cognitive abilities of children with JME were at times similar but generally worse than healthy controls at baseline, followed a variable temporal course over the first two years after the diagnosis of epilepsy, but failing to reach the competence level of healthy controls at follow-up across most of the tested cognitive abilities. 2) Abnormal patterns of brain structural development were evident in children with JME and were characterized by attenuation of age-related decline in CV compared to typically developing children. The altered brain developmental trajectory in the JME group was evident particularly in higher-association fronto-parietal-temporal brain regions. 3) CT dysmaturation, and to a lesser extent SA dysmaturation, appeared to drive neurodevelopmental differences between JME and typically developing children.
Our prospective investigation quantified these cognitive changes over time, revealing how early (baseline) cognitive impairment evolves from the diagnosis of epilepsy. The intellectual levels of children with new-onset JME compared to healthy controls were non-significantly lower at baseline but diverged over the following two years, with the JME group showing significantly slower gains (not progressive decline) compared to the controls. Thus, the delayed intellectual progress of children with JME appeared after the diagnosis of epilepsy, suggesting an impact of the disorder on cognitive development. However, baseline and prospective changes in components of diverse executive functions revealed trajectory patterns distinct from intellectual development. Response inhibition and psychomotor speed were significantly impaired at baseline and follow-up in the JME group compared to controls, but the two groups showed parallel rates of development over the next two years. Whereas the presence of baseline cognitive deficits suggests an antecedent impact of JME, the similar rates of cognitive development between the two groups imply that these aspects of executive function are less vulnerable to ongoing JME-related changes. Interestingly, problem solving abilities were relatively intact in our cohort during the early course of JME. This component of executive function is often impaired in adults with JME33–35, suggesting that disease-related factors may adversely impact the long term performance of this ability. Taken together, distinguishable patterns of cognitive trajectories in children with JME emerge from this study, implying both antecedent (lower baseline with parallel developmental trajectory) and modest disease-related contributions (similar baseline but slower gain over time).
In addition to altered cognitive trajectory, we also found abnormal patterns of brain maturation in children with new-onset JME. Specifically, a selective pattern of higher cortical volume relative to controls was evident in the frontal, temporal and parietal regions (Figure 1). Some of these cortical regions were part of the fronto-thalamic-cortical networks frequently emphasized in cross-sectional imaging investigations of adults with chronic JME2, 3, 5, 6 (e.g. mesial frontal, supplementary motor, posterior cingulate cortex). Other brain regions, including the temporal lobes and fusiform cortex, have also been implicated in chronic JME3, 5. Collectively, JME appeared to preferentially impact associative cortices that are crucial for higher order cognitive processing. However, as discussed below, cortical anatomy is clearly modulated by age and thus our findings are difficult to compare with findings in adult studies.
Our study demonstrates that maturational changes of CV in JME arise through a complex interplay between CT and SA. This observation is important because different cellular mechanisms govern the phylogeny of CT and SA13. The formation of the cortical SA involves radial deployment of projection neurons migrating from the ventricular zone, via glial fibers, to reach the cortical plate and form ontogenetic radial columns. Thus proliferation of progenitor cells in the ventricular zone results in increased numbers of radial ontogenetic columns and the expansion of cortical SA. In the second phase of neurogenesis, post-mitotic neurons grow linearly by dividing along the radial glial fibers, which in turn determines CT. Perturbations in both CT and SA in JME found in this study may reflect multiple phylogenetic processes; while developmental alterations in CT were more pronounced, SA was also affected, which suggests that JME impacts a more global disturbance in the mechanisms that guide cortical development. In support of this notion, preliminary evidence suggests that some of the genes associated with JME regulate cortical formation, impacting both CT and SA36. For example, mutation of EFHC1, which accounts for 3–9% of JME, is associated with disruption of both radial and tangential neuronal migration during brain development in a rodent model. Given that tangentially migrating neurons are typically inhibitory interneurons, whereas radially migrating neurons are excitatory37, aberrant cortical development may also influence cortical excitability and epileptogenesis. In summary, the neurodevelopmental differences between JME and healthy controls found in this study may reflect disruption of cortical development and thus offer new neuroimaging markers for future investigations.
Simultaneous examination of multiple dimensions of cortical anatomy (i.e. CV, CT, SA) also provides insights into the temporal trajectory of brain development. In general, cortical development follow an inverted-U trajectory, which increases during childhood, peaking around 8 to 10 years old, and then declines in adolescents11, 18 (see exception for linear and quadratic pattern16). In the age range of our participants, the adolescent controls likely have reached their developmental plateaus and were undergoing age-related reductions in cortical morphometry (Figure 2B). In contrast, delayed cortical maturation was evident in the JME group, showing significantly greater CV, CT, and SA, compared to controls. The dysmaturational pattern in CV and CT findings may be a result of reduced gray matter pruning over the first two years after JME diagnosis38. However, myelination is incomplete in children and areas of terminal myelination including gyral tips could appear relatively “dark” on T1 weighted images and thus could be classified as gray matter. Further, proliferation of myelin into margins of cortical neuropil also influences changes in cortical anatomy39. Changes in gray and white interface might reflect “neuropil” rather than exclusively gray matter morphology. Regarding surface area, a recent study demonstrated that widened sulcal width and reduced sulcal depth accounted for decreased SA in typically developing adolescents, in an age range similar to our cohort40. Whether altered sulcal morphology is the underlying mechanism of SA dysmaturation in JME will require future exploration.
Our findings support a growing literature in the pediatric idiopathic epilepsies that cognitive and brain developmental abnormalities are present at the onset of epilepsy41. Results from the Childhood Absence Epilepsy (CAE) Study Group demonstrated that 36% of children have attention deficits prior to or within one week of initiating medication treatment42. Further, seizures > 20 seconds found on pretreatment EEG was associated with poor baseline attention43. Individuals with CAE showed decreased fMRI activation in the medial frontal cortex compared to controls while performing attentional vigilance tasks44. Contrasting our findings in JME, other aspects of executive function in CAE were unaffected43. Thus, syndrome specific as well as overlapping cognitive abnormalities exist across idiopathic generalized epilepsies. Whereas the presence of cognitive abnormalities at epilepsy onset has been demonstrated, our study uniquely emphasizes the natural history of JME, uncovering parallel cognitive trajectory and brain maturational alterations.
Study limitations and future directions
There are several limitations in our study. First, our JME sample size was modest but the prospective design required stringent entry criteria with a tightly timed two-year follow-up. Second, in terms of brain development, whereas gray matter abnormalities were evident in our study, we did not assess white matter and functional connectivity. Future studies using multimodal imaging techniques, including diffusion tensor imaging and resting functional imaging, will complement the current findings. Third, the influence of antiseizure medication on brain morphometry and cognition is a concern, especially for VPA, a medication that has been associated with reduced cortical thickness45. Our observational study followed the natural history of JME and thus inherent in the design was the nonrandom assignment of antiseizure medications, which precludes isolation of VPA’s impact. A related issue is whether our core cognition and imaging findings were driven by the group of children on VPA. Retrospective analysis showed that our primary findings were not impacted by this group of children. Specifically, the group of children on VPA compared to those unexposed to this medication exhibited no differences in cognitive development and very circumscribed regions of decreased CV and CT located distant from the primary imaging findings (data available from authors).
Despite these limitations, our prospective investigation of children with new-onset JME uncovers abnormal brain and cognitive development shortly after diagnosis. Understanding deviations in the trajectory of early development sets a foundation to ask questions regarding long-term developmental outcomes. First, do these developmental abnormalities represent permanent lags or temporary delays, especially in individuals with remitted epilepsy? Second, although their seizures are often controlled, individuals with JME usually require long-term antiseizure medication treatment. How medications influence long-term neurodevelopment remains an important question. Third, even when seizures are controlled, a substantial portion of individuals with JME exhibit high rates of underemployment, social isolation, impulsivity, and depression8 and it is unknown if brain developmental phenotypes could predict these long-term social outcomes. These represent critical research and clinical topics for the future.
Acknowledgments
The work was supported by grants from the National Institute of Neurological Disorders and Stroke at the National Institutes of Health (NIH) (K23 NS060993 to J.J.L. and 3RO1-44351 to B.P.H.). The project was also supported by the Clinical and Translational Science Award (CTSA) program, through the NIH National Center for Advancing Translational Sciences (NCATS), grant number UL1TR000427. J.J.L. has received speaker’s honorarium from UCB Pharma.
REFERENECE
- 1.Kobayashi E, Zifkin BG, Andermann F, EA . Juvenile Myoclonic Epilepsy. In: Jerome Engel J, Pedley Timothy A, editors. Epilepsy: A comprehensive Textbook. 2. Philadelphia: Lippincott Williams & Wilkins; 2008. [Google Scholar]
- 2.Kim JH, Lee JK, Koh SB, et al. Regional grey matter abnormalities in juvenile myoclonic epilepsy: a voxel-based morphometry study. Neuroimage. 2007 Oct 1;37(4):1132–7. doi: 10.1016/j.neuroimage.2007.06.025. [DOI] [PubMed] [Google Scholar]
- 3.Woermann FG, Free SL, Koepp MJ, Sisodiya SM, Duncan JS. Abnormal cerebral structure in juvenile myoclonic epilepsy demonstrated with voxel-based analysis of MRI. Brain. 1999 Nov;122( Pt 11):2101–8. doi: 10.1093/brain/122.11.2101. [DOI] [PubMed] [Google Scholar]
- 4.Betting LE, Mory SB, Li LM, et al. Voxel-based morphometry in patients with idiopathic generalized epilepsies. Neuroimage. 2006 Aug 15;32(2):498–502. doi: 10.1016/j.neuroimage.2006.04.174. [DOI] [PubMed] [Google Scholar]
- 5.Tae WS, Kim SH, Joo EY, et al. Cortical thickness abnormality in juvenile myoclonic epilepsy. J Neurol. 2008 Apr;255(4):561–6. doi: 10.1007/s00415-008-0745-6. [DOI] [PubMed] [Google Scholar]
- 6.O’Muircheartaigh J, Vollmar C, Barker GJ, et al. Focal structural changes and cognitive dysfunction in juvenile myoclonic epilepsy. Neurology. 2011 Jan 4;76(1):34–40. doi: 10.1212/WNL.0b013e318203e93d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wandschneider B, Thompson PJ, Vollmar C, Koepp MJ. Frontal lobe function and structure in juvenile myoclonic epilepsy: a comprehensive review of neuropsychological and imaging data. Epilepsia. 2012 Dec;53(12):2091–8. doi: 10.1111/epi.12003. [DOI] [PubMed] [Google Scholar]
- 8.Camfield CS, Camfield PR. Juvenile myoclonic epilepsy 25 years after seizure onset: a population-based study. Neurology. 2009 Sep 29;73(13):1041–5. doi: 10.1212/WNL.0b013e3181b9c86f. [DOI] [PubMed] [Google Scholar]
- 9.Lin JJ, Mula M, Hermann BP. Uncovering the neurobehavioural comorbidities of epilepsy over the lifespan. Lancet. 2012 Sep 29;380(9848):1180–92. doi: 10.1016/S0140-6736(12)61455-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wandschneider B, Kopp UA, Kliegel M, et al. Prospective memory in patients with juvenile myoclonic epilepsy and their healthy siblings. Neurology. 2010 Dec 14;75(24):2161–7. doi: 10.1212/WNL.0b013e318202010a. [DOI] [PubMed] [Google Scholar]
- 11.Giedd JN, Blumenthal J, Jeffries NO, et al. Brain development during childhood and adolescence: a longitudinal MRI study. Nat Neurosci. 1999 Oct;2(10):861–3. doi: 10.1038/13158. [DOI] [PubMed] [Google Scholar]
- 12.Shaw P, Kabani NJ, Lerch JP, et al. Neurodevelopmental trajectories of the human cerebral cortex. J Neurosci. 2008 Apr 2;28(14):3586–94. doi: 10.1523/JNEUROSCI.5309-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Rakic P, Ayoub AE, Breunig JJ, Dominguez MH. Decision by division: making cortical maps. Trends in neurosciences. 2009 May;32(5):291–301. doi: 10.1016/j.tins.2009.01.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Monuki ES, Walsh CA. Mechanisms of cerebral cortical patterning in mice and humans. Nat Neurosci. 2001 Nov;4( Suppl):1199–206. doi: 10.1038/nn752. [DOI] [PubMed] [Google Scholar]
- 15.Chen CH, Fiecas M, Gutierrez ED, et al. Genetic topography of brain morphology. Proc Natl Acad Sci U S A. 2013 Oct 15;110(42):17089–94. doi: 10.1073/pnas.1308091110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wierenga LM, Langen M, Oranje B, Durston S. Unique developmental trajectories of cortical thickness and surface area. Neuroimage. 2014 Feb 15;87:120–6. doi: 10.1016/j.neuroimage.2013.11.010. [DOI] [PubMed] [Google Scholar]
- 17.Lyall AE, Shi F, Geng X, et al. Dynamic Development of Regional Cortical Thickness and Surface Area in Early Childhood. Cereb Cortex. 2014 Mar 2; doi: 10.1093/cercor/bhu027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Raznahan A, Shaw P, Lalonde F, et al. How does your cortex grow? J Neurosci. 2011 May 11;31(19):7174–7. doi: 10.1523/JNEUROSCI.0054-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Engel J., Jr A proposed diagnostic scheme for people with epileptic seizures and with epilepsy: report of the ILAE Task Force on Classification and Terminology. Epilepsia. 2001 Jun;42(6):796–803. doi: 10.1046/j.1528-1157.2001.10401.x. [DOI] [PubMed] [Google Scholar]
- 20.Kasteleijn-Nolst Trenite DG, Schmitz B, Janz D, et al. Consensus on diagnosis and management of JME: From founder’s observations to current trends. Epilepsy Behav. 2013 Jul;28( Suppl 1):S87–90. doi: 10.1016/j.yebeh.2012.11.051. [DOI] [PubMed] [Google Scholar]
- 21.Cantu RC. Posttraumatic Retrograde and Anterograde Amnesia: Pathophysiology and Implications in Grading and Safe Return to Play. Journal of athletic training. 2001 Sep;36(3):244–8. [PMC free article] [PubMed] [Google Scholar]
- 22.Baker GA, Smith DF, Dewey M, Morrow J, Crawford PM, Chadwick DW. The development of a seizure severity scale as an outcome measure in epilepsy. Epilepsy Res. 1991 Apr;8(3):245–51. doi: 10.1016/0920-1211(91)90071-m. [DOI] [PubMed] [Google Scholar]
- 23.Baker G, Jacoby A, Berney T, et al. Development of an instrument to assess quality of life in children with epilepsy and learning disability. Epilepsia; European Congress of Epileptology; Oporto, Portugal. 1994. Sep, p. 47. [Google Scholar]
- 24.Wechsler D. Wechsler Abbreviated Scale of Intelligence. New York: The Psychological Corporation: Harcourt Brace & Company; 1999. [Google Scholar]
- 25.Pulsipher DT, Seidenberg M, Guidotti L, et al. Thalamofrontal circuitry and executive dysfunction in recent-onset juvenile myoclonic epilepsy. Epilepsia. 2009 May;50(5):1210–9. doi: 10.1111/j.1528-1167.2008.01952.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Delis DC, Kramer JH, Kaplan E, Holdnack J. Reliability and validity of the Delis-Kaplan Executive Function System: an update. J Int Neuropsychol Soc. 2004 Mar;10(2):301–3. doi: 10.1017/S1355617704102191. [DOI] [PubMed] [Google Scholar]
- 27.Wechsler D. Wechsler Intelligence Scale for Children. 3. San Antonio, TX: The Psychological Corporation; 1991. [Google Scholar]
- 28.Fischl B, Sereno MI, Dale AM. Cortical surface-based analysis. II: Inflation, flattening, and a surface-based coordinate system. Neuroimage. 1999 Feb;9(2):195–207. doi: 10.1006/nimg.1998.0396. [DOI] [PubMed] [Google Scholar]
- 29.Fischl B, Dale AM. Measuring the thickness of the human cerebral cortex from magnetic resonance images. Proc Natl Acad Sci U S A. 2000;97(20):11050–5. doi: 10.1073/pnas.200033797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Reuter M, Schmansky NJ, Rosas HD, Fischl B. Within-subject template estimation for unbiased longitudinal image analysis. Neuroimage. 2012 Jul 16;61(4):1402–18. doi: 10.1016/j.neuroimage.2012.02.084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Reuter M, Rosas HD, Fischl B. Highly accurate inverse consistent registration: a robust approach. Neuroimage. 2010 Dec;53(4):1181–96. doi: 10.1016/j.neuroimage.2010.07.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Hagler DJ, Jr, Saygin AP, Sereno MI. Smoothing and cluster thresholding for cortical surface-based group analysis of fMRI data. Neuroimage. 2006 Dec;33(4):1093–103. doi: 10.1016/j.neuroimage.2006.07.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Pascalicchio TF, de Araujo Filho GM, da Silva Noffs MH, et al. Neuropsychological profile of patients with juvenile myoclonic epilepsy: a controlled study of 50 patients. Epilepsy Behav. 2007 Mar;10(2):263–7. doi: 10.1016/j.yebeh.2006.11.012. [DOI] [PubMed] [Google Scholar]
- 34.Vollmar C, O’Muircheartaigh J, Barker GJ, et al. Motor system hyperconnectivity in juvenile myoclonic epilepsy: a cognitive functional magnetic resonance imaging study. Brain. 2011 Jun;134(Pt 6):1710–9. doi: 10.1093/brain/awr098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Loring DW, Marino S, Meador KJ. Neuropsychological and behavioral effects of antiepilepsy drugs. Neuropsychol Rev. 2007 Dec;17(4):413–25. doi: 10.1007/s11065-007-9043-9. [DOI] [PubMed] [Google Scholar]
- 36.Delgado-Escueta AV, Koeleman BP, Bailey JN, Medina MT, Duron RM. The quest for juvenile myoclonic epilepsy genes. Epilepsy Behav. 2013 Jul;28( Suppl 1):S52–7. doi: 10.1016/j.yebeh.2012.06.033. [DOI] [PubMed] [Google Scholar]
- 37.Marin O. Cellular and molecular mechanisms controlling the migration of neocortical interneurons. Eur J Neurosci. 2013 Jul;38(1):2019–29. doi: 10.1111/ejn.12225. [DOI] [PubMed] [Google Scholar]
- 38.Huttenlocher PR, Dabholkar AS. Regional differences in synaptogenesis in human cerebral cortex. The Journal of comparative neurology. 1997 Oct 20;387(2):167–78. doi: 10.1002/(sici)1096-9861(19971020)387:2<167::aid-cne1>3.0.co;2-z. [DOI] [PubMed] [Google Scholar]
- 39.Yakovlev PI, Lecours AR. In: The myelogenetic cycles of regional maturation of the brain. Minkowski A, editor. Oxford: Blackwell; 1967. [Google Scholar]
- 40.Aleman-Gomez Y, Janssen J, Schnack H, et al. The human cerebral cortex flattens during adolescence. J Neurosci. 2013 Sep 18;33(38):15004–10. doi: 10.1523/JNEUROSCI.1459-13.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Lin JJ, Mula M, Hermann BP. Uncovering the neurobehavioural comorbidities of epilepsy over the lifespan. The Lancet. 2012;380:1180–92. doi: 10.1016/S0140-6736(12)61455-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Masur D, Shinnar S, Cnaan A, et al. Pretreatment cognitive deficits and treatment effects on attention in childhood absence epilepsy. Neurology. 2013 Oct 29;81(18):1572–80. doi: 10.1212/WNL.0b013e3182a9f3ca. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Dlugos D, Shinnar S, Cnaan A, et al. Pretreatment EEG in childhood absence epilepsy: associations with attention and treatment outcome. Neurology. 2013 Jul 9;81(2):150–6. doi: 10.1212/WNL.0b013e31829a3373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Killory BD, Bai X, Negishi M, et al. Impaired attention and network connectivity in childhood absence epilepsy. Neuroimage. 2011 Jun 15;56(4):2209–17. doi: 10.1016/j.neuroimage.2011.03.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Pardoe HR, Berg AT, Jackson GD. Sodium valproate use is associated with reduced parietal lobe thickness and brain volume. Neurology. 2013 May 14;80(20):1895–900. doi: 10.1212/WNL.0b013e318292a2e5. [DOI] [PMC free article] [PubMed] [Google Scholar]