Abstract
Lung cancer is a leading cause of cancer deaths worldwide. Despite advances in chemotherapy, radiation therapy, and surgery, lung cancer continues to have a low 5-year survival rate. Thus, there is a need to better understand the molecular signaling mechanisms that drive lung tumor formation, maintenance, and progression, and to translate this knowledge into better therapeutic intervention strategies. Mouse models that recapitulate the clinical features of advanced human lung cancer are critical for testing novel therapeutic approaches. This unit describes a highly reproducible, easy-to-establish orthotopic murine model of lung cancer, provides methods for in vivo imaging and monitoring of tumor growth, and discusses the usefulness of this model for translational lung cancer research and the development of therapeutic strategies.
Keywords: non-small cell lung cancer, orthotopic model, bioluminescent in vivo imaging
INTRODUCTION
Lung cancer is a leading cause of mortality worldwide and accounts for the greatest number of deaths among cancer patients in the United States (Siegel et al., 2012). The condition is broadly classified as non-small cell lung cancer (NSCLC) and small cell lung cancer (SCLC). Approximately 80% of lung cancer cases are NSCLC, which is subdivided into adenocarcinoma (LAC), squamous cell carcinoma (LSCC), and large cell carcinoma (LCLC). The subtypes of lung cancer are characterized by distinct histology, patterns of genetic and epigenetic changes, and site of origin, all of which are likely to dictate their individual responses to therapy. Despite advances in chemotherapy, radiation therapy, and surgery, lung cancer has an overall incidence of 226,160 in the United States and continues to have a 5-year survival rate of only 15% (Siegel et al., 2012). Thus, there is a need to define more precisely the molecular signaling mechanisms that drive lung tumor formation, maintenance, and progression, and to translate this knowledge into better therapeutic intervention strategies.
Mouse studies play an integral part in determining the molecular mechanisms responsible for lung tumorigenesis and for assessing the in vivo efficacy of novel therapeutic strategies. Human tumor xenograft models established in immunologically compromised mice that are capable of supporting growth of human tumors are a mainstay of preclinical cancer models. Orthotopic murine models, where cancer cells are implanted in the originating organ, have further improved xenograft models since these allow for a study of disease progression in vivo in a more relevant microenvironment (Killion et al., 1998).
There are several methods for generating lung orthotopic tumors in immunocompromised mice. These differ mainly in the way in which the lung cancer cells are delivered. McLemore et al. (1987) described the first orthotopic model of human lung cancer in nude mice. In this instance, lung tumors were established by intrabronchial injection of lung cancer cells. Since then, additional methods using immunocompromised rodents have been developed, including intrathoracic injection of lung cancer cells into the pleural cavity (McLemore et al., 1988) or lung parenchyma (Wang et al., 1997; Doki et al., 1999; Boehle et al., 2000), and by tail vein injections (Goto et al., 2002). An orthotopic lung tumor model in which lung cancer cells are injected percutaneously into the left lung of immunocompromised mice has also been developed (Onn et al., 2003). Detailed in this unit is an orthotopic murine model of lung cancer adapted from the Onn et al. (2003) procedure for in vivo imaging and monitoring of tumor growth, and a description of how the model can be employed as a tool for translational lung cancer research.
BASIC PROTOCOL: ORTHOTOPIC NSCLC MOUSE MODEL
The experimental techniques for establishing lung orthotopic tumors from NSCLC cells are provided in this protocol. Onn et al. (2003) established optimal inoculation size, tumor growth rate and number, and frequency and timing of metastatic lesions in the contralateral lung for various cell lines encompassing the major subtypes of NSCLC. The method described in this unit utilizes H1299, a lung carcinoma cell line grown in athymic nude mice. Other suitable cell lines for establishing lung orthotopic tumor models are listed in Table 14.27.1. Although more expensive, NOD-SCID mice are more immunocompromised than athymic nude mice and display improved tumor take and growth. Syngeneic mouse lung orthotopic tumors can be established in C57BL/6 mice by implanting CMT167 or Lewis lung carcinoma (LLC) cells established from the spontaneous lung adenocarcinomas that occur in C57BL/6 mice. The use of syngeneic murine NSCLC tumor cells to generate lung orthotopic models allows for the use of an immunocompetent host to examine inflammatory and immunological factors that affect tumor growth and dissemination.
Table 14.27.1.
Generation of Lung Orthotopic Tumors Using NSCLC Cell Lines
| Cell line | Lung disease | Cell numbera | Days to morbidityb | Commercial source | References |
|---|---|---|---|---|---|
| NCI-H1299c | Carcinoma | 0.1–1 × 106 | 32–90 | ATCC | Onn et al. (2003); V.J. and A.P.F. (unpub. observ.) |
| A549c | Carcinoma | 1–3 × 106 | 50–64 | ATCC | Mordant et al. (2011); Madero-Visbal et al. (2012); V.J. and A.P.F. (unpub. observ.) |
| NCI-H358c | Bronchioloalveolar carcinoma | 0.5–1 × 106 | 42–80 | ATCC | Onn et al. (2003) |
| NCI-H1703 | Adenocarcinoma | 1 × 106 | <90 | ATCC | V.J. and A.P.F. (unpub. observ.) |
| NCI-H441 | Adenocarcinoma | 1 × 106 | 55 | ATCC | Takahashi et al. (2012) |
| NCI-H460 | Large cell carcinoma | 0.5–1 × 106 | 19–90 | ATCC | Jin (2004); Takahashi et al. (2012) |
| NCI-H226 | Squamous cell carcinoma | 1.5–2 × 106 | 63–120 | ATCC | Onn et al. (2003) |
| CMT 167c | Carcinoma from mouse | 1 × 103 | 35 | Sigma | Justilien et al. (2012) |
| Lewis lung carcinomac | Carcinoma from mouse | 1 × 103 | 21 | Life Technologies (Invitrogen) | Doki et al. (1999); V.J. and A.P.F. (unpub. observ.) |
Number of cells implanted to produce reproducible tumors.
Time in days after injection to mice showing signs of morbidity.
Metastasis to ipsilateral and contralateral lobes of the lung and the mediastinal lymph nodes.
The localization of the lungs inside the thoracic cavity makes conventional non-imaging methods for data collection, such as direct caliper measurements, unusable. This problem is circumvented by using bioluminescence imaging, computed tomography (CT), and magnetic resonance imaging (MRI) (Grossman et al., 2011; Mordant et al., 2011; Justilien et al., 2012; Liu et al., 2012; Madero-Visbal et al., 2012;). Lung orthotopic tumors formed by cancer cells transduced to express the firefly luciferase gene can be monitored for progression, invasion, and metastasis in vivo using the IVIS Spectrum imaging device and associated protocols. The number of mice needed to perform studies is reduced because IVIS (in vivo imaging system) imaging allows tumor growth to be followed in the same animals over time. Furthermore, there is a direct correlation between bioluminescence signal and lung tumor volume (Madero-Visbal et al., 2012).
NOTE: All animal procedures must be performed in accordance with an approved protocol by an Institutional Animal Care and Use Committee (IACUC). Dosage, dosing schedule, and route of drug administration should be determined prior to initiating experiments. Endpoints, such as when a response to treatment is obtained or when mice display signs of morbidity due to tumor burden, must be established and approved by the IACUC. Cells must be manipulated using aseptic techniques. Ensure all surgical instruments are sterilized prior to use. Animal procedures should be performed in pathogen-free conditions, such as in a laminar flow hood in a designated surgical suite, using proper surgical aseptic techniques.
NOTE: When evaluating compounds in vivo, preliminary pharmacokinetic studies must be performed to ensure that a known amount of compound is present in the animal and target tissue at the time of testing. For in vivo studies is it better to use measured plasma levels of the test compound, rather than the dose administered, to generate dose-response curves. For peptides, a half-life of less than 1 min is usually incompatible for a test procedure in which the effects are assessed 30 or 60 min after compound administration. However, as some agents alter gene expression, their effects can be long lasting. In these cases, the biological half-life is many times longer than the presence of the compound in the plasma. If a compound produces an effect that lasts longer than its plasma half-life, an effect on gene expression may be a component of its mechanism of action. Conversely, a pharmacological effect that parallels plasma half-life indicates a direct cause-and-effect relationship that is proportional to the plasma concentration. The lack of pharmacokinetic information on a test compound can seriously compromise the design, execution, and interpretation of a study.
Materials
H1299 lung carcinoma cell line (ATCC) or other cell line suitable for the generation of lung orthotopic tumors (see Table 14.27.1)
Complete cell culture medium: RPMI 1640 supplemented with 10% fetal bovine serum and 1% penicillin-streptomycin (all from Invitrogen)
Lipofectamine 2000 reagent (Invitrogen)
pGL3-Control Vector containing firefly luciferase gene constitutively driven by an SV40 promoter and that contains a neomycin resistance cassette (Promega)
G418 (Invitrogen)15 mg/ml D-luciferin, firefly (Caliper Life Sciences #122796)
0.05% trypsin-EDTA
0.04% trypan blue
1× phosphate-buffered saline (PBS; Invitrogen)
Matrigel, growth factor reduced (BD)
Male athymic nude mice (6 to 8 weeks old; Harlan Laboratories) or other suitable mice
Ketamine (100 mg/kg, Cardinal Health)/xylazine (10 mg/kg, Patterson Veterinary Supply) or other appropriate anesthetic
10% povidone-iodine (Betadine)
Ophthalmic ointment (containing neomycin sulfate, polymyxin B sulfate, and bacitracin zinc) (Patterson Veterinary Supply)
Buprenorphine (Cardinal Health)
Compound(s) for experimental treatment of mice, and vehicle (for control)
Isoflurane (100%) (Abbott)
10% buffered formalin (for paraffin embedding) or OCT freezing medium (for storage in liquid nitrogen)
Tissue culture flasks and plates [T150 (150 cm2) flasks, 35-mm plates]
In vivo bioluminescent imaging system including inhalational anesthesia vaporizer system with induction chamber (e.g., Xenogen IVIS 200, Caliper Life Sciences)
Living Image software for IVIS 100 (Caliper Life Sciences)
15-ml conical centrifuge tubes
Sterile surgical towels
Heating pad
Ear punch
Sterile gloves
Sterile alcohol prep pads (70% isopropyl alcohol)
3/10-cc insulin syringe with 30-G needle (BD)
Sterile surgical scissors and forceps
Sterile cotton-tipped applicators
Wound-closing surgical metal clips, stapler, and remover (e.g., AutoClip applier, AutoClip remover, and 9-mm EZ Clip; BD Medical)
Heating lamp
25-G needle
10-ml syringes
20-G feeding needle (Roboz)
0.5-ml syringes and needles ( ; BD Medical)
Dry bead sterilizer
Additional reagents and equipment for fixation in paraffin (APPENDIX 3D) or by freezing (APPENDIX 3E)
Transfect cells with luciferase expression plasmid
-
1
Culture H1299 or other NSCLC cell line suitable for growth of orthotopic tumors (Table 14.27.1). If using H1299 cells, culture in RPMI 1640 medium containing 10% FBS, 10 U/ml penicillin/10 μg/ml streptomycin in a 37°C, 5% CO2 incubator. For other NSCLC lines, culture cells according to instructions provided by the supplier.
Cells should be maintained at low passage numbers and split before they reach 90% confluency. -
2
Using Lipofectamine 2000, transfect H1299 cells with pGL3-Control Vector containing genes for firefly luciferase and neomycin resistance.
Cells should be transfected using the Lipofectamine 2000 protocol recommended by Invitrogen. Background, guidelines, and information on transfection technique troubleshooting can be obtained from the Life Technologies-Invitrogen website (www.lifetechnologies.com). -
3
At 48 hr post-transfection, select for H1299 luciferase stable transfectants (H1299/Luc) by treating the cells with 500 μg/ml G418 as follows:
Perform a 1:10 split of the cells.
Allow the cells to adhere for several hours and add 500 μg/ml G418.
Every 3 days, replace growth medium with fresh medium containing 500 μg/ml G418.
-
Split cells when they approach confluence and continue growth in 500 μg/ml of G418-containing medium to maintain stable expression of luciferase. The cells will take approximately 2 weeks to undergo selection.
H1299 cells not expressing luciferase (parental) should be placed in G418-containing growth medium in parallel with H1299 luciferase-expressing cells to identify when they undergo selection. If using some other NSCLC cell line, it will be necessary to perform a kill curve with titrating concentrations of G418 to obtain a concentration that is suitable for selection.
Assess luciferase expression in NSCLC cells
-
4
At 10 to 14 days following the start of the G418 selection process, allow 2.5 × 105 H1299 and H1299/Luc cells to adhere to separate 35-mm tissue culture dishes overnight.
The H1299 cells without luciferase serve as a control for luciferase expression. -
5
Add firefly luciferin at a final concentration of 150 μg/ml and incubate the cells for 5 min in a 37°C, 5% CO2 incubator.
There is no need to replace the luciferin-containing medium prior to imaging. -
6
Image cells with an IVIS Spectrum Imaging system using the protocol provided by the manufacturer.
Prepare NSCLC/luciferase cells for injection
Depending on the NSCLC line being utilized, the number of cells to be injected (see Table 14.27.1), and the number of mice, several tissue culture flasks (150-cm2) may be needed to generate a sufficient number of cells to establish lung orthotopic tumor models.
-
7
When the H1299/Luc cell cultures reach approximately 80% confluence, harvest cells via trypsinization by adding 4 ml 0.25% trypsin-EDTA and incubating at room temperature. Continuously check for cell adhesion under a microscope. After a few minutes, and when cells are detached, add 10 to 20 ml complete growth medium with 10% FBS to inactivate trypsin.
-
8
To a 100-μl aliquot of cells add 100 μl of 0.5% trypan blue and count the number of cells using a hemacytometer.
Counts of 2–5 × 105 cells/ml are optimal for accuracy of counting.Single-cell suspensions of >90% viability should be used for orthotopic injections. -
9
Pellet the remaining cells by centrifugation for 5 min at 200 to 300 × g, 4°C, and then wash cell pellet 2 times with 10 ml of 1× PBS.
-
10
Resuspend the cell pellet in ice-cold 10% growth factor–reduced Matrigel in 1× PBS to achieve the number of cells that will be injected into each mouse per 50 μl (the injections will be 50 μl per animal) (Table 14.27.1). For example, if injecting H1299 cells, prepare a concentration of 1 × 106 cells/50 μl. Maintain cells on ice until use.
Cell inoculations should be performed within 1 to 2 hr of preparing cell suspensions.
Prepare the animals for injection
-
11
Under a laminar flow hood, prepare a sterile working environment using sterile towels. Preheat the heating pad prior to surgery so normal body temperature is maintained in the anesthetized subjects during the procedure. Place a sterile towel on the heating pad.
-
12
Weigh each mouse and label it using an ear punch system.
-
13
Anesthetize the mouse with an intraperitoneal injection of ketamine/xylazine mixture (90 to 120 mg/kg ketamine, 10 mg/kg xylazine).
Purchase of ketamine requires a DEA license as it is a CIII controlled substance. Check for the appropriate depth of anesthesia by performing a toe pinch. There should be no response from the animal to the pinch if it is sufficiently anesthetized. -
14
Once the mouse is sufficiently anesthetized, place it in the lateral decubitus position on the heating pad covered by a sterile towel. Generously apply 10% betadine solution over the left thorax, above the lower rib line, just below the lower border of the scapula.
If NOD-SCID or C57BL/6 mice are used, shave the area of the left thorax before preparing the incision site with betadine and 70% isopropyl alcohol.Wear sterile gloves from this point through the end of the surgery. -
15
Remove betadine by swabbing the area with 70% isopropyl alcohol prep pads.
-
16
Ensure a single-cell suspension by gently pipetting the tumor cells up and down, and then draw up 50 μl of tumor cell suspension into a 3/10-cc insulin syringe equipped with a 30-G hypodermic needle.
-
17
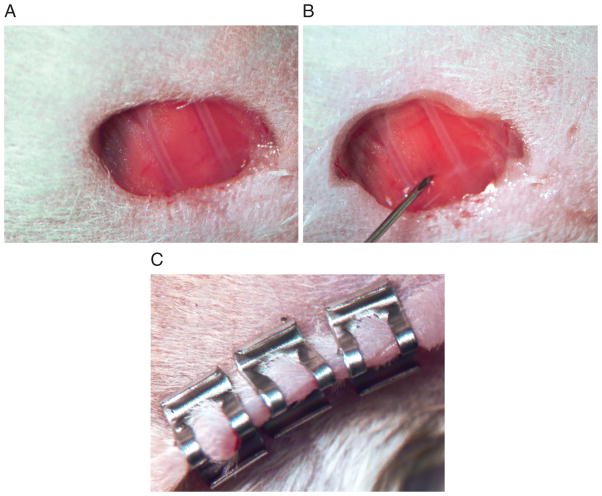
Using surgical scissors, carefully make a small incision (3 mm) in the area of the left lateral thorax. Use sterile cotton-tipped applicators to control bleeding. Remove soft tissue to expose the thoracic ribs and intercostal space to allow visualization of the left lobe of the lung (Fig. 14.27.1A).
-
18
Advance the needle quickly to a depth of approximately 5 mm into the left lobe of the lung between the 6th and 7th rib and inject the cell suspension (Fig. 14.27.1B).
Immediately after injection, the mouse respiration and heart rate will increase significantly, but should stabilize rapidly (within 2 min). -
19
Gently remove the needle and close the incision site with 2 or 3 wound clips (Fig. 14.27.1C).
-
20
Carefully apply ophthalmic ointment to the mouse’s eyes using sterile cotton-tipped applicators.
-
21
Administer buprenorphine (0.1 mg/kg) post-operatively and lay the mouse on its left side under a heating lamp. Monitor the animal until it is fully recovered from anesthesia.
-
22
Weigh and examine the mice daily for signs of infection, bleeding, loss of body weight, lethargy, and changes in food and/or water intake.
-
23
Remove surgical clips 10 days after surgery when the incision site has healed.
Figure 14.27.1.
Implantation of NSCLC cells to generate a mouse orthotopic lung tumor model. (A) A 3-mm incision is made in the area of the left lateral thorax. Soft tissue is removed to expose the thoracic ribs and intercostal space to allow visualization of the left lobe of the lung. (B) NSCLC cells are injected by quickly advancing the needle to a depth of approximate 5 mm into the left lobe of the lung between the 6th and 7th rib and injecting the cell suspension. (C) The incision site is closed with 2 or 3 sterile wound clips.
Monitor tumor growth and formation of metastases by bioluminescent imaging
The degree of tumor engraftment, growth rate, and frequency and timing of metastatic lesions are dependent on the NSCLC cell line employed. Therefore, a time kinetics curve for D-luciferin should be established for each lung orthotopic model in an initial pilot study using the protocols specified by Caliper Life Sciences. To generate a time kinetics curve, mice are administered (i.p.) D-luciferin. After 3 min of incubation, the mice are anesthetized. A left lateral luminescent image is acquired 5 min after D-luciferin injection, with subsequent images obtained every 5 to 10 min for up to 40 min. For experimental mice, images are acquired during peak luciferin emission time as determined by this kinetics curve. Acquisition times that provide a signal above background and below the saturation point of the IVIS charge-coupled device (CCD) camera range from 1 to 5 min. As the size of the tumors increases, acquisition time may need to be shortened to seconds to avoid saturated images (see Caliper Life Sciences protocol for IVIS). Acquisition times should be the same among experimental groups. Within 2 weeks post-implantation, NSCLC cells expressing luciferase can be visualized using IVIS as an initial focus at the injection site in the left lung.
-
24
Set up bioluminescent imaging system according to the manufacturer’s instructions.
-
25
Weigh mice.
-
26
Inject the mice intraperitoneally with 100 μl of luciferin solution (15 mg/ml stock) per 10 g body weight prior to isoflurane anesthesia and imaging following the manufacturer’s protocols. For H1299 cells, acquire the images 10 min following D-luciferin injection.
Experimental treatment of mice
The timing for initiating therapeutic treatment of mice bearing lung orthotopic tumors depends on the goals of the individual experiment and the stage of tumorigenesis being targeted (effect on early-stage tumor versus later stage tumor, anti-metastatic and/or anti-proliferative therapy). For example, potential anti-metastatic drugs can be administered in H1299 orthotopic lung tumor models at 3 weeks after implantation, at which time the primary tumor has become established but metastatic spread has not yet begun. To reduce inter-animal variability, all mice should be imaged 7 days after injection prior to enrolling in studies. Only mice that exhibit a distinct, compact, focal tumor mass at the injection site with no signs of leakage of cells into the pleural cavity (diffuse bioluminescence within the chest) should be used for the studies. Dosage, dosing schedule, and route of drug administration should be selected on the basis of results obtained from previous preclinical pharmacokinetic and pharmacodynamic studies.
-
27
Segregate mice into treatment groups once tumor take has been confirmed by IVIS (approximately 7 to 14 days after implantation).
Treatment groups should have similar levels of bioluminescence signal.A vehicle treatment group should be included as a control to assess the effects of a test agent. -
28
Administer known therapeutic or test compound to mice using a predetermined dose, schedule, and route (i.e., p.o., i.v., s.c., or i.p.).
-
29
Monitor tumor growth over time by IVIS.
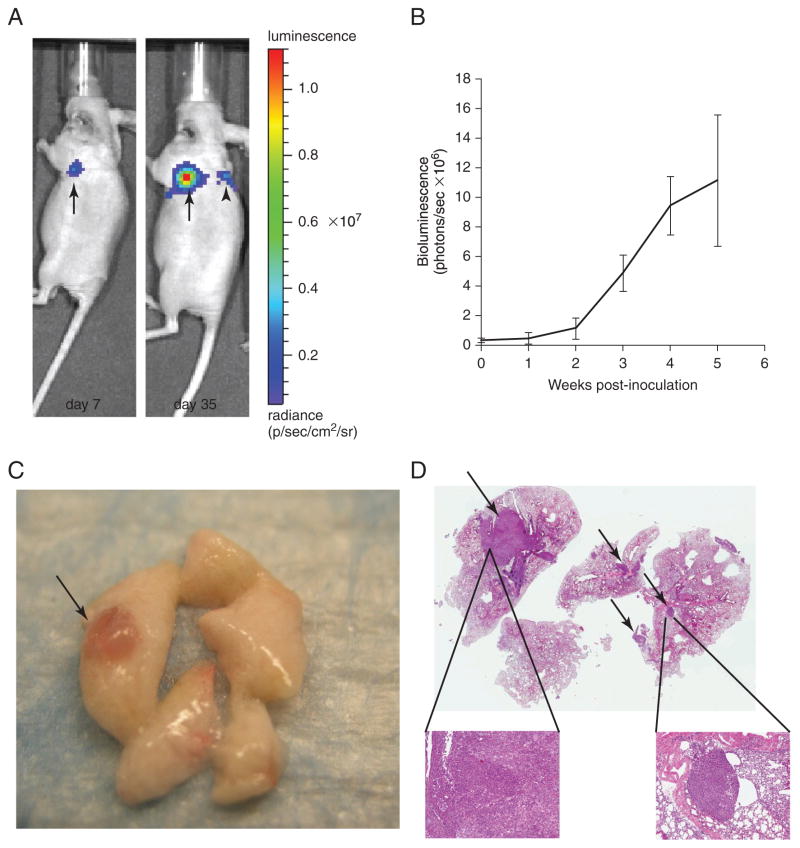
The frequency of IVIS imaging depends on the tumorigenicity of the NSCLC cell line used to establish the lung orthotopic tumor model. For example, IVIS may be performed once a week for a cell line that requires a longer period of time to cause morbidity. For more tumorigenic cell lines, IVIS should be performed twice a week to allow for a sufficient number of time points to assess therapeutic efficacy (see Table 14.27.1). At approximately 1 week after implantation, it is possible to visualize H1299 cells expressing luciferase using IVIS as an initial focus at the injection site in the left lung (Fig. 14.27.2A). Once established, there is local growth of the primary tumor. Approximately 4 weeks after implantation, metastatic spread to the ipsilateral and contralateral lobes of the lung and the mediastinal lymph nodes becomes visible (Fig. 14.27.2A). Tumor-bearing mice survive for approximately 7 weeks when inoculated with H1299 cells. They succumb to metastatic thoracic disease. -
30
Using the Caliper/Xenogen Living Image Software, quantify the bioluminescence in weekly images. Plot the quantified data as mean bioluminescence signal expressed as total flux of photons/sec (Fig. 14.27.2B).
The region of interest (ROI) tool in the software is used to measure the total photon flux emission (photons/sec) in regions over the thoracic space that emit a bioluminescence signal.See Anticipated Results for an example study that demonstrates how these data are plotted.
Figure 14.27.2.
Monitoring of tumor growth in orthotopic murine model of NSCLC by in vivo bioluminescence imaging. (A) Lateral view of bioluminescent images of mouse bearing lung orthotopic tumor of H1299. The H1299/Luc cells (1 × 106) were inoculated into the left lung of nude mice. (Left) Image of a primary H1299 tumor in the left lung at 7 days after implantation. (Right) Both primary lung tumor growth and presence of a metastatic lesion in the contralateral lung are evident 35 days after implantation. (B) Quantitative analysis of luciferase bioluminescence images taken at the indicated time points. Data are mean total bioluminescence flux (photons/sec) ± SEM. (C) Macroscopic view of H1299/Luc tumor (arrow) on lung parenchyma. (D) Hematoxylin and eosin staining of harvested mouse lung demonstrate primary tumor in left lobe (thick arrow) and multiple metastases in contralateral lobes (thin arrows) (1× magnification). Insets: Higher (5×) magnification of primary tumor and metastasis.
Euthanasia and tissue processing for pathological and histological examinations
-
31
Euthanize mice using methods approved by IACUC (such as CO2 asphyxiation).
-
32
Perform cardiac perfusion and dissection as follows:
-
Make a lower abdominal incision to expose the organs. Exsanguinate mice by cutting the inferior vena cava. Cut up along both sides of the mouse up to the neck. Make an incision into the diaphragm. Make lateral cuts into ribcage and then carefully remove it to expose the heart and lungs. Insert a 25-G needle attached to a syringe containing 10 ml of cold PBS into the right ventricle of the heart and flush out the blood using 5 to 10 ml of PBS.
Cardiac perfusion inflates the lungs and removes red blood cells. The lung tissue will turn white as the lungs are perfused. Expose the trachea by removing tissue around neck. (The trachea can be identified by its rings of cartilage.) Using forceps, gently separate the trachea from the esophagus. Make a small nick in the trachea and insert a 20-G feeding needle attached to a syringe containing 10 ml of cold PBS. Flush lungs with 5 to 10 ml PBS by way of the trachea; this will fill and expand the lung lobes significantly. Cut the trachea and connective tissue along the backside to remove the lungs, heart, and thymus. Carefully dissect the lobes of the lung away from the heart and thymus tissue.
-
-
33
Grossly examine lungs, thoracic cavity, and all abdominal organs for tumor nodules (Fig. 14.27.2C). Record findings.
-
34
Fix and process the lungs and periaortic lymph nodes for further histological analyses (e.g., immunohistochemistry or hematoxylin and eosin staining) (Fig. 14.27.2D).
The method of fixation depends on the type of analyses. Tissues can be fixed in 10% buffered formalin followed by embedding in paraffin (see APPENDIX 3D). Alternatively, fresh tissues may be snap-frozen in OCT freezing medium in liquid nitrogen (see APPENDIX 3E).
COMMENTARY
Background Information
Lung cancer is the leading cause of cancer death in the United States, with the majority of cases classified as NSCLC (~80%) and the remainder as SCLC. While surgery and radiotherapy are used to treat early-stage NSCLC, patients with unresectable metastatic disease often receive combination chemotherapy. The standard care for patients with advanced NSCLC is regimens of platinum-based chemotherapies in combination with other agents, such as taxanes, pemetrexed, and gemcitabine (Ramalingam and Belani, 2008). The development of FDA-approved targeted therapies such as erlotinib, gefitinib, and crizotinib allow for an individualized approach for treating NSCLC patients having tumors that contain specific genetic and molecular alterations, such as mutations in the epidermal growth factor receptor (EGFR) and the anaplastic lymphoma kinase (ALK) fusion gene. In addition, bevacizumab, a monoclonal antibody against vascular endothelial growth factor, is approved for use in combination with chemotherapy. Unfortunately, NSCLCs often become resistant to chemotherapy, resulting in relapse and death. As current therapies provide only a modest survival benefit, more must be done to define more fully the mechanisms that drive NSCLC formation and maintenance, and to translate this information into more effective treatments for NSCLC.
The identification of novel therapeutics depends on the availability of biologically relevant in vivo preclinical animal models. Several mouse models are employed for studying the biology of NSCLC and the efficacy of experimental agents. Genetically engineered, spontaneous, chemically induced, and xenograft mouse models are the major types of models used for NSCLC preclinical efficacy studies. Genetically engineered mouse models of NSCLC recapitulate many aspects of the disease and have the advantage of the tumor arising in the host as the result of a well-defined oncogenic insult. Unlike human lung cancer, genetically engineered, spontaneous, and chemically induced lung tumors in mice often result in adenomas (Shimkin and Stoner, 1975) and infrequently progress to adenocarcinomas or metastases. In addition, unlike human NSCLC that develop as one or two solitary tumors, these animal models of NSCLC develop many pulmonary lesions. Thus, most current genetically engineered, spontaneous, and chemical models are generally more useful for studying the biology of NSCLC than for drug discovery. Intravenous injection of tumor cells is used to study metastatic spread. While some NSCLC cells display a pattern of hematogenous metastases in solid organs, a defined primary tumor as a source for metastatic spread is missing.
Most of the research and development of novel therapeutics for NSCLC still relies on subcutaneous tumor models. Although subcutaneous tumors provide facilitate tumor establishment and measurement, and are reproducible, there are some disadvantages of these models. Because the subcutaneous microenvironment differs from the lungs, these models may not be useful for testing NSCLC therapies that either directly target or influence the tumor microenvironment. In addition, because subcutaneous tumors rarely progress to local and distant metastastatic disease, they cannot be used to accurately predict the efficacy of a compound on advanced-stage NSCLC. The superiority of orthotopic over ectopic implantation of tumor cells is demonstrated by the growth and progression of NSCLC tumors in vivo. Orthotopic lung tumor models have been particularly useful in defining the biology of the various genetically and molecularly altered subtypes of lung cancer in their native microenvironment. Furthermore, orthotopic models more accurately recapitulate the human disease than ectopic models by, for example, displaying metastatic dissemination from the primary tumor site. Moreover, orthotopic models more reliably predict the clinical response to a test agent and are more efficient for compound screening than ectopic models (Killion et al., 1998). Although orthotopic models of NSCLC have advantages over subcutaneous xenograft and genetically engineered mouse (GEM) models, they do have some disadvantages. Orthotopic models of NSCLC are more technically challenging and expensive than subcutaneous xenograft models. In addition, the growth of orthotopic tumors is more difficult to follow than that of subcutaneous tumors and it requires access to expensive equipment such as MRI or IVIS. Depending on the NSCLC cell line implanted, some orthotopic tumors may not demonstrate the natural progression of lung cancer. Although with orthotopic models tumor cells are placed in their organ of origin, questions remain about whether the tumor vasculature and tumor-stroma interactions in immunocompromised mice are similar to those that occur with human tumors. This raises questions about the suitability of the orthotopic models for tumor microenvironment studies. Furthermore, in contrast to tumors grown subcutaneously, tumors established in the lung are less susceptible to treatment with paclitaxel, suggesting that orthotopic models are more appropriate for evaluating chemotherapeutics and other therapies for human NSCLC (Onn et al., 2003). On balance, the findings indicate that orthotopic models are generally best for evaluating treatment modalities for human lung cancer.
Critical Parameters
The use of proper aseptic techniques and adherence to IACUC policies are essential for properly exploiting this model. In addition, several critical steps are needed to achieve consistency in the establishment and growth of NSCLC cells as orthotopic lung tumors. Although there are unlikely to be differences, the growth rate and drug sensitivity of luciferase-expressing cells should be compared to the parental cell line to ensure that selection for luciferase expression has not altered these parameters. The NSCLC cells to be injected should be >90% viable as determined by trypan blue exclusion. As the viability of cell suspensions decreases over time, cell inoculations should be performed within 1 to 2 hr of preparing cell suspensions. The injection volume and number of cells administered should be kept constant. If possible, all mice in a particular experiment should be injected by a single investigator. Statistical power analysis should be performed prior to initiating experiments to determine the minimum size of experimental groups needed to observe statistically significant differences. For example, a statistical analysis software package such as Sigma Plot can be used to calculate the number of mice that will be sufficient to detect a 50% reduction or 2-fold increase in tumor size, tumor burden, number of metastatic lesions, or size of metastatic lesions, with 95% confidence between experimental groups. Mice should be randomized into the various test groups once the implantation of NSCLC cells has been confirmed by IVIS imaging. Ideally, the luciferase bioluminescence of an experimental mouse tumor should not exceed more than 10% of the average at the start of test compound administration. A single stock of D-luciferin should be used throughout the experiment. It may be prepared in advance and kept for up to 1 month at −20°C.
The NSCLC cell lines selected for study are determined by the aim of the experiment. Several issues should be taken into consideration in choosing a NSCLC cell line, including the fact that these cell lines contain different oncogenic mutations and exhibit phenotypic variations in vitro and in vivo. In addition, the growth pattern of NSCLC cell lines as subcutaneous tumors may differ from their growth pattern as lung orthotopic tumors, and NSCLC cell lines display different growth rates, levels of tumorigenicity, and times to morbidity after implantation.
Troubleshooting
Potential problems that may be encountered when using this NSCLC lung orthotopic model, as well as troubleshooting advice, are summarized in Table 14.27.2.
Table 14.27.2.
Troubleshooting the Lung Orthotopic Model of NSCLC
| Problem | Recommended action |
|---|---|
| Poor tumor take/variable tumor sizes |
|
| High surgical mortality |
|
| Mice develop infection |
|
Anticipated Results
The orthotopic lung tumor model recapitulates the clinical features of advanced human lung cancer. The NSCLC lung orthotopic model is well suited for determining the effects of a drug candidate on tumor growth and survival (Table 14.27.3). While this model can be used for measuring the effects of a therapy on metastatic spread, the tumors must first be allowed to establish and the tumors in the experimental groups must display similar levels of bioluminescence at the onset of treatment.
Table 14.27.3.
Pre-Clinical Therapeutic Targeting of NSCLC Using Lung Orthotopic Tumor Models
| Therapeutic agent | Cell line(s) | Effects | Reference |
|---|---|---|---|
| Cis-diamine dichloro platinum | Lewis lung carcinoma | Inhibits primary tumor growth and metastasis to the mediastinal lymph nodes | Doki et al. (1999) |
| Selumetinib | NCI-H441 and NCI-H460 | Inhibits primary lung tumor growth and, to a lesser degree, lymph node metastases. Enhanced tumor activity when used in combination with cediranib | Takahashi et al. (2012) |
| Cediranib | NCI-H441 and NCI-H460 | Inhibits primary lung tumor growth and, to a lesser degree, lymph node metastases. Enhanced tumor activity when used in combination with selumetinib | Takahashi et al. (2012) |
| Paclitaxel | NCI-H358 and PC14PE6 | Decreases lung tumor burden | Onn et al. (2003) |
| Auranofin | H1703 and H1299 | Inhibits primary lung tumor growth | V.J. and A.P.F. (unpub. observ.; see Example study) |
Example study
The response to protein kinase Cι targeting therapy in the NSCLC lung orthotopic model
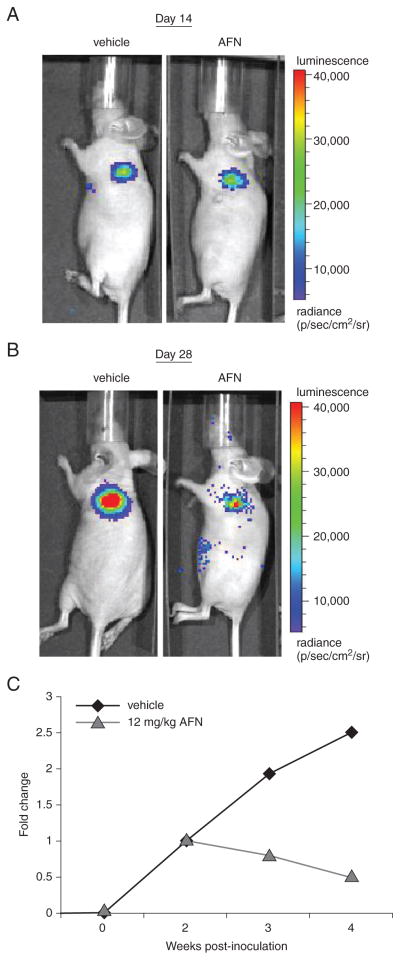
Protein kinase Cι (PKCι) is an oncogene in NSCLC (Regala, 2005), being frequently overexpressed in NSCLC cells and primary human tumors as a result of tumor-specific amplification of the PKCι gene (PRKCI). Furthermore, tumor PKCι expression is predictive of poor clinical outcome. The PKCι forms an oncogenic complex with Par6 and the Rho guanine nucleotide exchange factor, ECT2, which activates a Rac1-MEK-ERK signaling cascade that drives NSCLC cell invasion and transformed growth in vitro and tumor growth in vivo (Frederick et al., 2008; Justilien and Fields, 2009). Using a high-throughput screen, several FDA-approved antiarthritic gold compounds, including aurothiomalate and Auranofin (AFN), were identified as potent inhibitors of oncogenic PKCι-Par6 complex formation and signaling (Erdogan et al., 2006; Stallings-Mann et al., 2006). In this study, the antitumor activity of AFN was assessed in vivo in a preclinical NSCLC lung orthotopic tumor model. Lung orthotopic tumors were established in athymic nude mice by injection with 1 × 106 NCI-H1703 cells harboring PRKCI amplification and transfected to express luciferase using the protocol detailed in this unit. The IVIS was performed at 7 and 14 days to establish tumor take. Two weeks after tumor cell implantation, the mice were divided into two groups. One group received only i.p. injections of vehicle control (3% ethanol), while the other was administered AFN (12 mg/kg/day). The vehicle or AFN was injected 6 days a week for 2 weeks, with tissue harvested 4 weeks after the start or end of injection. Mice were imaged weekly using IVIS to measure tumor bioluminescence.
The data in Figure 14.27.3 demonstrate how lung orthotopic models can be used to assess the efficacy of the PKCι inhibitor AFN on tumor growth. The H1703 lung orthotopic tumors were allowed to establish for 2 weeks prior to the start of treatment. In this example, mice receiving either vehicle control or AFN displayed tumors with similar levels of tumor cell luminescence (Fig. 14.27.3A). The mouse treated with AFN emitted a lower bioluminescence signal than the vehicle-control animals, indicating a positive response to the drug with respect to tumor growth (Figs. 14.27.3B,C). The data in Figure 14.27.3 demonstrate a key advantage of using lung orthotopic models with cells expressing luciferase. By using IVIS imaging, it is possible to study the effects of an agent on the growth of individual lung orthotopic tumors in individual mice over time. This capability reduces the need to sacrifice mice at various times to assess the effects of an agent over time, reducing the number of mice needed for a study. The ability to serially image tumor-bearing mice allows for monitoring of tumor progression and/or response, facilitating the selection of appropriate experimental endpoints in individual mice. Serial tumor imaging for response also makes possible the use of therapeutic study designs in which treatment choices, doses, dosing regimens, and combinations can be altered during the experiment to assess their relative efficacies.
Figure 14.27.3.
Preclinical testing of Auranofin (AFN) in lung orthotopic tumors. (A) Lateral view of representative bioluminescent images of control and AFN-treated mice bearing lung orthotopic tumors of H1703/Luc cells at 14 days after implantation. (B) Lateral view of bioluminescent images of a mouse bearing lung orthotopic tumors of H1703/Luc cells at 14 days after start of treatment. (C) Quantitative analysis of luciferase bioluminescence images taken at the indicated time points after inoculation. Data are the mean total bioluminescence flux (photons/sec).
Numerous groups have utilized a similar model to test compound efficacy as a treatment of lung cancer. Doki et al. (1999) established lung orthotopic tumors with murine LLC cells in syngeneic immunocompetent mice and assessed the effects of cis-diamine dichloro platinum (CDDP) on the growth of tumors and metastasis to the mediastinal lymph nodes. Mice were administered (i.v.) a single dose of CDDP (7 mg/kg) on day 1 or on day 10 after LLC implantation. Primary tumor volume and the weight of the mediastinal lymph nodes were assessed on day 18 after implantation. The administration of a single dose of CDDP on day 1 after implantation of LLC cells suppressed the growth of the primary tumor and inhibited metastasis to the mediastinal lymph nodes. The administration of CDDP on day 10 after tumor cell implantation caused little change in primary tumor growth, but showed partial inhibition of mediastinal lymph node metastasis.
Vascular endothelial growth factor (VEGF) signaling is an important target in anti-cancer therapy because of its role in angio-genesis, a process that is critical for tumor growth and spread. The mitogen-activated protein kinase/extracellular signal-regulated kinase (MEK), a key effector of oncogenic K-Ras signaling, is an attractive target in cancer therapy (Moodie et al., 1993; Janne et al., 2013). Takahashi et al. (2012) used lung orthotopic models of human lung adenocarcinoma and large cell lung cancer to determine the efficacy of combination therapy with selumetinib, a MEK1/2 inhibitor, and cediranib, a VEGFR inhibitor. Lung orthotopic tumors were established using 1 × 106 NCI-H441 cells or 5 × 105 NCI-H460 cells. At 10 days after implantation the mice were randomized into groups to receive vehicle control; monotherapy of selumetinib (12.5 or 25 mg/kg twice daily p.o.), cediranib (3 mg/kg once daily p.o.) or paclitaxel (200 mg per mouse once a week I.P.); or combination therapy with selumetinib (25 mg/kg twice a day p.o.) and cediranib as above. When given in combination, selumetinib was administered twice daily at 8-hr intervals and cediranib once daily, 4 hr after the first daily dose of selumetinib. Treatment was continued until the mice exhibited signs of moribund (55 days for NCI-H441–implanted mice or 19 days for NCI-H460–implanted mice), at which point the animals were harvested. The effects of treatment were assessed by measuring lung weight and primary lung tumor volume and by assessing the presence of mediastinal lymph node disease or distant metastasis. The use of selumetinib or cediranib alone was effective for the treatment of lung cancer in these models, with inhibition of primary lung tumor growth and, to a lesser degree, lymph node metastases. Combination treatment with selumetinib and cediranib enhanced the antitumor and antiangiogenic effects of these inhibitors in the lung orthotopic models.
Onn et al. (2003) investigated the effects of paclitaxel on lung orthotopic tumors established from NCI-H358 or PC14PE6 NSCLC cells. Paclitaxel treatment was initiated on day 7 after tumor cell implantation at a dose of 4 or 8 mg/kg and was administered weekly for 4 or 5 cycles, at which time control mice became moribund. Paclitaxel significantly reduced the tumor burden of lung orthotopic tumors. When comparing the effects of paclitaxel on the growth of NSCLC lung orthotopic tumors versus subcutaneous NSCLC tumors, Onn et al. (2003) found that subcutaneous tumors were more sensitive to this drug. Thus, the lung orthotopic tumor model more closely resembles human lung cancer in its partial response to chemotherapy (Perez-Soler et al., 2000). Indeed, anticancer agents that display a dramatic responses in preclinical studies that rely solely on subcutaneous tumor xenografts have been disappointing in the clinic (Killion et al., 1998). Inasmuch as the NSCLC lung orthotopic models more closely resemble the clinical condition, these models may be more relevant than conventional xenograft models when screening agents in anticipation of clinical trials.
Time Considerations
For the most accurate and efficient execution of this protocol, investigators must be organized and prepared for the surgeries well in advance. Cell transfection, selection, and growth take 2 to 3 weeks. Initially, the amount of time required to perform the surgeries will vary. A novice can expect each mouse surgery to take approximately 15 min from the time of administering anesthesia. There are several key steps that can be taken to shorten this time. It is highly recommended that the investigator perform several trial surgeries in the month leading up to the start of experiments. In addition, surgeries should be performed with one or two people assisting with the cell preparation, weighing and tagging of mice, and administration of anesthesia. A well-organized team can inject 20 mice in about 3 hr. The overall time required for a study depends on the growth rate/tumorigenicity of the NSCLC cell line employed and the endpoints of the study. In the case of H1299 cells, approximately 1 week is needed to establish primary tumors. Depending on the aim of the study, treatment and IVIS imaging can take more than 6 weeks to complete.
Acknowledgments
The authors wish to thank Dr. Kristen Hill for providing unpublished data and their colleagues in the Fields laboratory for helpful suggestions and critical review of the manuscript. The authors also wish to apologize for contributions to this area omitted in our citations due to space limitations.
Literature Cited
- Boehle AS, Dohrmann P, Leuschner I, Kalthoff H, Henne-Bruns D. An improved orthotopic xenotransplant procedure for human lung cancer in SCID bg mice. Ann Thorac Surg. 2000;69:1010–1015. doi: 10.1016/s0003-4975(00)01090-0. [DOI] [PubMed] [Google Scholar]
- Doki Y, Murakami K, Yamaura T, Sugiyama S, Misaki T, Saiki I. Mediastinal lymph node metastasis model by orthotopic intrapulmonary implantation of Lewis lung carcinoma cells in mice. Br J Cancer. 1999;79:1121–1126. doi: 10.1038/sj.bjc.6690178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Erdogan E, Lamark T, Stallings-Mann M, Jamieson L, Pellechia M, Thompson EA, Johansen T, Fields AP. Aurothioma-late inhibits transformed growth by targeting the PB1 domain of protein kinase Ciota. J Biol Chem. 2006;38:28450–28459. doi: 10.1074/jbc.M606054200. [DOI] [PubMed] [Google Scholar]
- Frederick LA, Matthews JA, Jamieson L, Justilien V, Thompson EA, Radisky DC, Fields AP. Matrix metalloproteinase-10 is a critical effector of protein kinase Ciota-Par6alpha-mediated lung cancer. Oncogene. 2008;27:4841–4853. doi: 10.1038/onc.2008.119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goto H, Yano S, Zhang H, Matsumori Y, Ogawa H, Blakey DC, Sone S. Activity of a new vascular targeting agent, ZD6126, in pulmonary metastases by human lung adenocarcinoma in nude mice. Cancer Res. 2002;62:3711–3715. [PubMed] [Google Scholar]
- Grossman CE, Pickup S, Durham A, Wileyto EP, Putt ME, Busch TM. Photo-dynamic therapy of disseminated non-small cell lung carcinoma in a murine model. Lasers Surg Med. 2011;43:663–675. doi: 10.1002/lsm.21102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Janne PA, Shaw AT, Pereira JR, Jeannin G, Vansteenkiste J, Barrios C, Franke FA, Grinsted L, Zazulina V, Smith P, Smith I, Crino L. Selumetinib plus docetaxel for KRAS-mutant advanced non-small-cell lung cancer: A randomised, multicentre, placebo-controlled, phase 2 study. Lancet Oncol. 2013;14:38–47. doi: 10.1016/S1470-2045(12)70489-8. [DOI] [PubMed] [Google Scholar]
- Jin 2004 [Google Scholar]
- Justilien V, Fields AP. Ect2 links the PKCι–Par6α complex to Rac1 activation and cellular transformation. Oncogene. 2009;41:3597–3607. doi: 10.1038/onc.2009.217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Justilien V, Regala RP, Tseng IC, Walsh MP, Batra J, Radisky ES, Murray NR, Fields AP. Matrix metalloproteinase-10 is required for lung cancer stem cell maintenance, tumor initiation and metastatic potential. PLoS One. 2012;7:e35040. doi: 10.1371/journal.pone.0035040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Killion JJ, Radinsky R, Fidler IJ. Orthotopic models are necessary to predict therapy of transplantable tumors in mice. Cancer Metastasis Rev. 1998;17:279–284. doi: 10.1023/a:1006140513233. [DOI] [PubMed] [Google Scholar]
- Liu X, Liu J, Guan Y, Li H, Huang L, Tang H, He J. Establishment of an orthotopic lung cancer model in nude mice and its evaluation by spiral CT. J Thorac Dis. 2012;4:141–145. doi: 10.3978/j.issn.2072-1439.2012.03.04. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Madero-Visbal RA, Colon JF, Hernandez IC, Limaye A, Smith J, Lee CM, Arlen PA, Herrera L, Baker CH. Bioluminescence imaging correlates with tumor progression in an orthotopic mouse model of lung cancer. Surg Oncol. 2012;21:23–29. doi: 10.1016/j.suronc.2010.07.008. [DOI] [PubMed] [Google Scholar]
- McLemore TL, Liu MC, Blacker PC, Gregg M, Alley MC, Abbott BJ, Shoemaker RH, Bohlman ME, Litterst CC, Hubbard WC, Brennan RH, McMahon JB, Fine DL, Eggleston JC, Mayo JG, Boyd MR. Novel intrapulmonary model for orthotopic propagation of human lung cancers in athymic nude mice. Cancer Res. 1987;47:5132–5140. [PubMed] [Google Scholar]
- McLemore TL, Eggleston JC, Shoemaker RH, Abbott BJ, Bohlman ME, Liu MC, Fine DL, Mayo JG, Boyd MR. Comparison of intrapulmonary, percutaneous intrathoracic, and subcutaneous models for the propagation of human pulmonary and nonpulmonary cancer cell lines in athymic nude mice. Cancer Res. 1988;48:2880–2886. [PubMed] [Google Scholar]
- Moodie SA, Willumsen BM, Weber MJ, Wolfman A. Complexes of Ras.GTP with Raf-1 and mitogen-activated protein kinase kinase. Science. 1993;260:1658–1661. doi: 10.1126/science.8503013. [DOI] [PubMed] [Google Scholar]
- Mordant P, Loriot Y, Lahon B, Castier Y, Leseche G, Soria JC, Vozenin MC, Decraene C, Deutsch E. Bioluminescent orthotopic mouse models of human localized non-small cell lung cancer: feasibility and identification of circulating tumour cells. PLoS One. 2011;6:e26073. doi: 10.1371/journal.pone.0026073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Onn A, Isobe T, Itasaka S, Wu W, O’Reilly MS, Ki Hong W, Fidler IJ, Herbst RS. Development of an orthotopic model to study the biology and therapy of primary human lung cancer in nude mice. Clin Cancer Res. 2003;9:5532–5539. [PubMed] [Google Scholar]
- Perez-Soler R, Kemp B, Wu QP, Mao L, Gomez J, Zeleniuch-Jacquotte A, Yee H, Lee JS, Jagirdar J, Ling YH. Response and determinants of sensitivity to paclitaxel in human non-small cell lung cancer tumors heterotransplanted in nude mice. Clin Cancer Res. 2000;6:4932–4938. [PubMed] [Google Scholar]
- Ramalingam S, Belani C. Systemic chemotherapy for advanced non-small cell lung cancer: recent advances and future directions. Oncologist. 2008;13:S5–S13. doi: 10.1634/theoncologist.13-S1-5. [DOI] [PubMed] [Google Scholar]
- Regala 2005 [Google Scholar]
- Shimkin MB, Stoner GD. Lung tumors in mice: application to carcinogenesis bioassay. Adv Cancer Res. 1975;21:1–58. doi: 10.1016/s0065-230x(08)60970-7. [DOI] [PubMed] [Google Scholar]
- Siegel R, Naishadham D, Jemal A. Cancer statistics, 2012. CA Cancer J Clin. 2012;62:10–29. doi: 10.3322/caac.20138. [DOI] [PubMed] [Google Scholar]
- Stallings-Mann M, Jamieson L, Regala RP, Weems C, Murray NR, Fields AP. Cancer Res. 2006;66:1767–1774. doi: 10.1158/0008-5472.CAN-05-3405. [DOI] [PubMed] [Google Scholar]
- Takahashi O, Komaki R, Smith PD, Jurgensmeier JM, Ryan A, Bekele BN, Wistuba II, Jacoby JJ, Korshunova MV, Biernacka A, Erez B, Hosho K, Herbst RS, O’Reilly MS. Combined MEK and VEGFR inhibition in orthotopic human lung cancer models results in enhanced inhibition of tumor angiogenesis, growth, and metastasis. Clin Cancer Res. 2012;18:1641–1654. doi: 10.1158/1078-0432.CCR-11-2324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang HY, Ross HM, Ng B, Burt ME. Establishment of an experimental intra-pulmonary tumor nodule model. Ann Thorac Surg. 1997;64:216–219. doi: 10.1016/s0003-4975(97)00343-3. [DOI] [PubMed] [Google Scholar]