The U.S. Centers for Disease Control and Prevention estimates the prevalence of autism spectrum disorders (ASDs) to be 1 in 68 children in the United States, yet no drugs to treat the debilitating social deficits of ASD are available. Oxytocin, a natural brain peptide produced in the hypothalamus, has received considerable attention as a potential treatment for social deficits in ASD. Acute intranasal oxytocin temporarily enhances social cognition, empathy, and reciprocity in individuals with ASD (1). However, recent clinical trials have yielded mixed results, leaving the field questioning whether oxytocin can live up to the hype.
Oxytocin increases the salience of social stimuli and promotes parental nurturing and social bonds. Recent studies in mice and humans have come to conflicting conclusions about its effects on social behaviors associated with autism (e.g., a lack of social interest or reciprocity). The findings of Peñagarikano et al. (2) have added exciting support for using oxytocin in ASD. The authors observed that mice with a mutation in Cntnap2 (the gene that encodes contactin-associated protein-like 2), which in humans results in ASD, display robust social deficits and reduced amounts of brain oxytocin. Remarkably, daily intranasal oxytocin treatment over development improved later social engagement, suggesting that early exposure to oxytocin restructures neural circuits to permanently rescue social impairments in this ASD model.
Numerous clinical trials of oxytocin in ASD are ongoing (clinicaltrials.gov), but completed trials have produced inconclusive results (1). Several trials in adults and children show modest improvements in social function in response to oxytocin treatment, with no adverse effects. Still, other studies failed to yield positive outcomes (1, 3).
What accounts for the variable findings in human studies? It may be attributed to variation in doses, study duration, age, and small, heterogeneous study samples. Context is likely critical to the success of oxytocin therapies. As oxytocin enhances salience and the reinforcing value of social stimuli, oxytocin therapies should be most effective when combined with behavioral therapies.
To move forward, parallel animal and human studies are needed to elucidate conserved effects of oxytocin on brain communication in response to social stimuli. Animal research is critical for understanding precise mechanisms of action. For example, oxytocin receptors are localized to cholinergic regions in the primate brain that modulate visual and auditory attention, suggesting an important role in social sensory processing (4). Oxytocin also regulates the signal-to-noise ratio in the hippocampus to tune attention toward socially relevant stimuli in mice (5). Is this a possible mechanism by which intranasal oxytocin enhances neural responses to social stimuli, while suppressing responses to nonsocial stimuli, in ASD (6)? Another link to autism is suggested by the developmental switch in the neurotransmitter γ-aminobutyric acid from excitatory to inhibitory neurotransmission at birth in mice. This switch is impaired in ASD-like mice, and oxytocin-mediated restoration of this process alleviates social deficits (7).
Parallel animal and human studies are also needed to determine the developmental consequences of chronic oxytocin exposure. For these investigations, care must be taken to avoid detrimentally affecting brain maturation, as early chronic intranasal administration apparently reduces oxytocin receptor expression and social behavior in normal mice (8). An intermittent oxytocin treatment regimen may be preferable over chronic exposure (9).
Whether a compromised oxytocin system contributes to ASDs is unclear. A meta-analysis found that variation in the oxytocin receptor gene associates with ASD (10), although individual studies fail to report associations (11, 12). Rather, it appears that variation in the oxytocin system contributes to social phenotypes, regardless of diagnosis. Blood oxytocin concentration and oxytocin receptor genotype strongly associate with social cognition but not with ASD diagnosis, which suggests that circulating oxytocin and oxytocin receptor sequence may be useful biomarkers to identify individuals most responsive to oxytocin therapies (12). The heterogeneity of ASD makes identification of therapies based on diagnoses difficult. That oxytocin was effective in Cntnap2 mutant mice with compromised oxytocin signaling does not imply that oxytocin will be effective for all ASD populations.
The U.S. National Institute of Mental Health (NIMH) is encouraging a classification system based on Research Domain Criteria, which stratifies clinical populations based on behavioral dimensions and biological mechanisms. NIMH is also requiring evidence of target engagement for clinical trial funding involving oxytocin. As an example, oxytocin improved social emotion detection in ASD males and modulated activity in the brain’s insular cortex, thus demonstrating a functional impact on an impaired neural system (13). Incorporating these suggestions will likely lead to improved oxytocin-based therapies in ASD.
Efficient brain penetration and activation of oxytocin receptors throughout the social neural network, as occurs after endogenous oxytocin release, is potentially an important limitation to the current oxytocin therapies. The next generation of therapeutics may bypass this obstacle (14). Pharmacologically enhancing endogenous oxytocin release, or developing small-molecule agonists and positive allosteric modulators, may be most effective for engaging the brain’s natural oxytocin system to improve social cognition. For example, acute melanocortin-4 receptor (MC4R) agonist treatment potentiates oxytocin release in brain reward centers (including the ventral striatum) and facilitates oxytocin-mediated social attachment in voles (14 Activating MC4Rs during the first postnatal week enhances later social relationships in these animals as well (15). Acute stimulation of MC4R also rescues social deficits in the Cntnap2 mutant mouse (2). Further, social impairment in this mouse model was rescued by a gene therapy strategy involving the expression of designer receptors exclusively activated by designer drugs (DREADDs) in oxytocin neurons, an approach that may one day be feasible in humans.
Oxytocin remains an exciting target for improving social function. However, investigations into its potential therapeutic application are still in the early stages. Some would argue that the therapeutic value of intranasal oxytocin remains tenuous. Indeed, there are insufficient data for physicians to prescribe oxytocin to patients or for parents to seek oxytocin for their autistic child. Nonetheless, increasing information on mechanisms from animal studies, optimizing current therapeutic paradigms, and developing next-generation approaches to target the oxytocin system will hopefully lead to improving social function in ASD and other psychiatric disorders.
“…approaches to target … oxytocin… will hopefully lead to improving social function in ASD…”
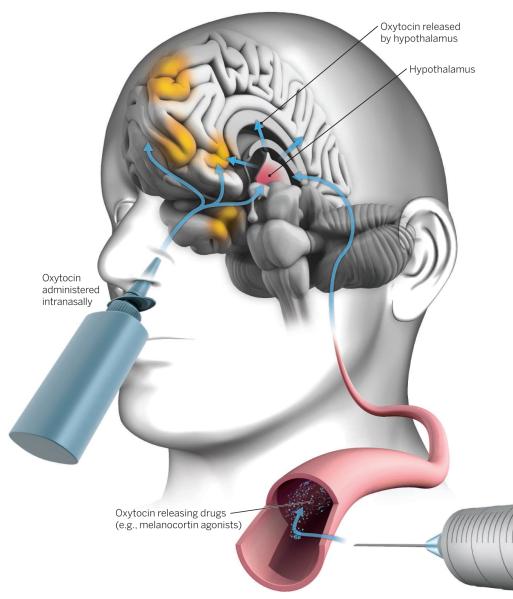
Oxytocin therapeutic strategies.
Next-generation therapies (such as MC4R agonists) that stimulate the release of endogenous oxytocin in the brain (hypothalamus) could evoke more potent and targeted effects compared to intranasal oxytocin treatment. Combining oxytocin with behavioral therapy may maximize therapeutic potential. Brain regions affected by intranasal oxytocin include the medial prefrontal cortex, anterior insula, orbitofrontal cortex, amygdala, and the ventral striatum (orange).
REFERENCES
- 1.Anagnostou E, et al. Brain Res. 2014;1580:188. doi: 10.1016/j.brainres.2014.01.049. [DOI] [PubMed] [Google Scholar]
- 2.Peñagarikano O, et al. Sci. Transl. Med. 2015;7:271ra8. doi: 10.1126/scitranslmed.3010257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Guastella AJ, et al. J. Child Psychol. Psychiatry. 2014 10.1111/jcpp.12305. [Google Scholar]
- 4.Freeman SM, Inoue K, Smith AL, Goodman MM, Young LJ. Psychoneuroendocrinology. 2014;45:128. doi: 10.1016/j.psyneuen.2014.03.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Owen SF, et al. Nature. 2013;500:458. doi: 10.1038/nature12330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Gordon I, et al. Proc. Natl. Acad. Sci. U.S.A. 2013;110:20953. doi: 10.1073/pnas.1312857110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Tyzio R, et al. Science. 2014;343:675. doi: 10.1126/science.1247190. [DOI] [PubMed] [Google Scholar]
- 8.Huang H, et al. Neuropsychopharmacology. 2014;39:1102. doi: 10.1038/npp.2013.310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Teng BL, et al. Neuropharmacology. 2013;72:187. doi: 10.1016/j.neuropharm.2013.04.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.LoParo D, Waldman ID. Mol. Psychiatry. 2014;10 doi: 10.1038/mp.2014.77. 1038/mp.2014.77. [DOI] [PubMed] [Google Scholar]
- 11.Skuse DH, et al. Proc. Natl. Acad. Sci. U.S.A. 2014;111:1987. [Google Scholar]
- 12.Parker KJ, et al. Proc. Natl. Acad. Sci. U.S.A. 2014;111:12258. [Google Scholar]
- 13.Aoki Y, et al. Brain. 2014;137:3073. doi: 10.1093/brain/awu231. [DOI] [PubMed] [Google Scholar]
- 14.Modi ME, et al. Neuropsychopharmacology. 2015 10.1038/npp.2015.35. [Google Scholar]
- 15.Barrett CE, et al. Neuropharmacology. 2014;85:357. doi: 10.1016/j.neuropharm.2014.05.041. [DOI] [PMC free article] [PubMed] [Google Scholar]