Abstract
Background
The emerging insight into resting-state cortical networks has been important in understanding the fundamental architecture of brain organization. These networks, which were originally identified with functional MRI, are also seen in the correlation topography of the infraslow rhythms of local field potentials. Because of the fundamental nature of these networks and their independence from task-related activations, we posit that in addition to their neuroscientific relevance, these slow cortical potential (SCP) networks could also play an important role in clinical brain mapping.
Objective
We hypothesized that these networks would be useful in identifying eloquent cortex, such as sensorimotor cortex, in patients both awake and under anesthesia.
Methods
This study included eight subjects undergoing surgical treatment for intractable epilepsy. SCPs were recorded from the cortical surface while awake and under propofol anesthesia. To test brain-mapping utility, slow cortical potential networks were identified using data-driven (seed-independent) and anatomy-driven (seed-based) approaches. Using electrocortical stimulation as the gold standard for comparison, the sensitivity and specificity of these networks for identifying sensorimotor cortex was calculated.
Results
Networks identified with a data-driven approach in patients under anesthesia and awake were 90% and 93% sensitive, and 58% and 55% specific for sensorimotor cortex, respectively. Networks identified with systematic seed selection in patients under anesthesia and awake were 78% and 83% sensitive, and 67% and 60% specific, respectively.
Conclusion
Resting-state networks may be useful for tailoring stimulation mapping and could provide a means of identifying eloquent regions in patients while under anesthesia.
Keywords: slow cortical potential, sensorimotor cortex, electrocorticography, functional networks, brain mapping
Introduction
Mapping eloquent cortex prior to the resection of a brain lesion has been important in reducing the risk of a functional deficit following surgery. This is particularly true in surgical resection for the treatment of medically intractable epilepsy, tumors, or arteriovenous malformations.1-4 Due to the inter-individual variability in the anatomic location of motor and language areas, the mapping of these sites is especially critical.5 The current ‘gold standard’ for clinical mapping is electrocortical stimulation (ECS). There are, however, important limitations to this approach. ECS mapping can be limited by the induction of after-discharges that can induce seizures and introduce a source of error in mapping.6 Though effective in identifying important regions of functional cortex, ECS is also relatively inefficient – sites must be interrogated in series (pairs of electrodes are sequentially stimulated while patients perform various tasks), resulting in a prolonged screening process.
Somatosensory evoked potentials (SSEPs) are a second mapping tool that has played an important clinical role in brain mapping of sensorimotor cortex.7 This technique can be used to identify the transition between motor and sensory cortex in patients under general anesthesia, and is generally safe, fast, and easy to apply in the operating room. SSEPs appear to be altered in the presence of certain anesthetic agents and their sensitivity and specificity can sometimes be limited.8, 9,10 Although considered a gold standard for identification of sensorimotor regions, the utility of SSEPs is limited to this functional domain. Efforts to extend the mapping potential of such cortical electrophysiologic recordings to other eloquent areas and higher associative regions were advanced by Sidney Goldring in the early 1990's. 11
More recently, additional methods have been explored which rely on the use of passively recorded electrocorticographic (ECoG) changes in activity associated with a given stimulus or cognitive task. Amplitude modulation in different frequency bands has been used to identify focal areas associated with cortical activation. The brain generates oscillating electrical potentials over a broad range of frequencies that show characteristic task-related changes. Commonly, these have been described in the context of sensorimotor cortical activation. Relatively low frequencies in mu (8-12 Hz) and beta (18-26) ranges are thought to be produced by thalamocortical circuits that decrease in amplitude in association with actual or imagined movements.12-15 In general, these changes in lower frequency bands tend to have a large spatial distribution, and hence are only modestly specific to the type of function. Activity in the gamma frequency range (> 30 Hz) is thought to be produced by local cortical circuits.16 Gamma activity focally increases with cortical activation. Changes in these frequency rhythms have been used in offline analyses for brain mapping17-19 and compared to the gold standard of ECS.20-24 While these studies demonstrate the general feasibility of functional mapping without electrical stimulation and have the potential advantages of minimal risk (by not inducing seizures) and increased efficiency (by interrogating the entire electrode array at once), these approaches still require that the patient be conscious and able to participate in a cognitive motor or speech paradigm.
The requirement for awake participation excludes a large number of patients that could benefit from these mapping procedures that rely on activation-based changes in LFP or BOLD activity and spare the patient excess electrocortical stimulation. In the extraoperative setting (when a subdural electrode array has been implanted), children and patients with mental status impairments due to any cause may be unable to participate in the language or motor protocol. They also are often unable to be mapped preoperatively with task-based fMRI because this also requires patient cooperation. Resting-state fMRI, on the other hand, presents an emerging means to passively identify sensorimotor regions in such patients.25 In the intraoperative setting (i.e. awake craniotomies), language mapping may be impossible if the patient is medically unstable or has a compromised airway.
Recent studies using resting state cortical physiology may provide an ideal means of identifying eloquent regions without the need for patient cooperation or even that they be awake. Resting state networks were first identified with functional magnetic resonance imaging (fMRI). Spontaneous BOLD fluctuations are low frequency (<0.1Hz) oscillations in neuronal activity that are anatomically correlated within distinct functional networks.26, 27 First reported by Biswal et. al., there is strong coherence which is reproducibly present between the left and right somatomotor cortices 27, 28 between language areas,29, 30 and between numerous other functional regions in the absence of task performance. Also of note, resting state network topographies have been shown to be quite similar to the topographies associated with task-evoked fMRI responses.31 These resting state networks have been used in preliminary investigations to preoperatively map motor regions adjacent to brain tumors .25 The cortical physiology that most correlates with this state invariant functional structures are cortical networks defined by very low frequency oscillations (< 0.5Hz), known as slow cortical potentials (SCP).32 It appears likely that the negative-shift of these SCPs reflects slow rhythmic depolarization of apical dendrites in superficial cortical layers 33, 34 and is thought to likely represent endogenous fluctuations of cortical excitability within functional systems. Infra-slow rhythms, more generally, have been shown to play an important role in defining the fundamental architecture and organization of cortex as it relates to both functional networks and pathologic networks.35-38 In humans, fMRI BOLD and SCP sensorimotor networks are stable throughout sleep wake cycles, and have been shown to be stable under anesthesia in both primates and humans.39-41 Investigations by He et. al. looking at ECoG frequencies ranging from <0.5 Hz to 200Hz demonstrated that the rhythms that were most closely correlated with the fMRI resting state sensorimotor network were the power envelope of gamma rhythms (during wakefulness and REM sleep) and the slow cortical potential (during wakefulness, REM sleep and slow wave sleep).32 Thus far, however, these resting state SCP networks have never been evaluated in their ability to effectively identify eloquent cortex.
The current study investigated the utility of correlated slow cortical potential fluctuations for the identification of sensorimotor cortex. These resting-state networks were compared to the current gold standard of clinical localization, namely, electrocortical stimulation. Moreover, these networks were acquired from both awake patients as well as patients under general anesthesia. Using data-driven methods as well as seed-based approaches, these SCP networks could be easily generated and used to identify functional regions. The strong concordance between resting-state networks and functional regions and the fact that they can be identified under anesthesia offer preliminary evidence for a new paradigm of brain mapping that could be very widely applied with minimal clinical risk.
Material and Methods
Subjects
Eight patients undergoing surgical treatment of intractable epilepsy participated in this study, which was approved by the Human Research and Protection Organization at Washington University School of Medicine. Before inclusion, all patients gave written informed consent. All subjects had epileptic seizures refractory to treatment with two or more anti-epileptic medications, and had previous documentation of epileptic seizures by video scalp-EEG monitoring. All subjects also underwent fluro-deoxy-glucose positron emission tomography (FDG-PET) studies. Subjects underwent intracranial EEG monitoring with a clinical indication to more precisely localize the epileptogenic zone of seizure onset, as well as perform functional mapping using ECS. Exclusion criteria included the presence of dysplastic cortex on clinical magnetic resonance imaging. Each patient underwent an initial craniotomy for the subdural placement of an electrode array that was then removed (with a second craniotomy) approximately one week later for resection of the epileptic foci.
Data Collection
Three minutes of ECoG data were recorded from each subject under two conditions: 1) under anesthesia and 2) awake. In the anesthesia condition, subjects were unresponsive to noxious stimuli secondary to a standardized regimen of intravenous propofol anesthesia. The data collection under anesthesia occurred in the operating room prior to emergence (in the first craniotomy) or after induction (in the second craniotomy) of anesthesia. Vecuronium and fentanyl were also used, but were discontinued 45 minutes prior to data collection (see reference 36 SI for detailed description of anesthetic regimen). Bispectral Index (BIS) data was also recorded as a measure of depth of anesthesia during this time and ranges can be seen in Table 1. In the awake condition, the subjects were alert, resting quietly, and motionless. Data under this condition were collected while patients were being invasively monitored in the hospital in the interval (approximately one week) between the initial implantation craniotomy and the subsequent craniotomy for grid removal and foci resection.
Table 1.
Demographic and clinical information
| Subject | Sex | Age | Seizure Type | Seizure Foci | Duration of Illness (yrs) | Antiepileptic Medications | Bispectral Index |
|---|---|---|---|---|---|---|---|
| 1 | F | 44 | CP | ATL | 36 | LMT, TPM | 30-45 |
| 2 | M | 27 | CP | MPL | 26 | CBZ, CLZ, LMT, PGB, DIA | 48-59 |
| 3 | F | 48 | CP | ATL | 47 | PHT, PGB | 24-26 |
| 4 | M | 36 | CP | ATL | 35 | VPA, TPM | 23-26 |
| 5 | F | 28 | CP | PFL | 14 | CBZ , CLZ, LMT, LRZ | 44-53 |
| 6 | F | 36 | CP | IFL | 35 | VPA, PRM, CBZ | 33-36 |
| 7 | F | 33 | CP | ATL | 19 | LMT, LEV, ZNS | 26-35 |
| 8 | F | 44 | CP | ATL | 5 | LMT, LRZ, OCB, CBZ | 40-47 |
CP = Complex Partial, ATL = Anterior Temporal Lobe, MPL = Mesial Parietal Lobe, PFL = Posterior Frontal Lobe, IFL= Inferior Frontal Lobe, LMT = lamotrigine, TPM = topiramate, ZNS = zonisamide, VPA = depakote, CBZ = carbamazepine, CLZ = clonazepam, LRZ= lorazepam, PHT = phenytoin, PGB = pregabalin, PRM = primidone, DIA = diazepam, LEV = levetiracetam, OCB = oxcarbazepine
ECoG signals were recorded and digitized from the implanted electrode array at a sampling rate of 1,200 Hz, using g.tech biosignal amplifiers in DC coupled mode with a 0.5Hz high pass filter. The data were stored by a Dell PC running BCI2000 software.42 The ECoG electrode array (AdTech or PMT) contained 48 or 64 platinum electrodes that had 2.3-mm diameter exposed surface and were spaced 10 mm apart. The electrodes were in direct contact with the cortical surface, and their location was based entirely on the presumed location of the seizure foci as determined by the clinicians. All subsequent signal analysis was performed in MATLAB.
Signal Analysis
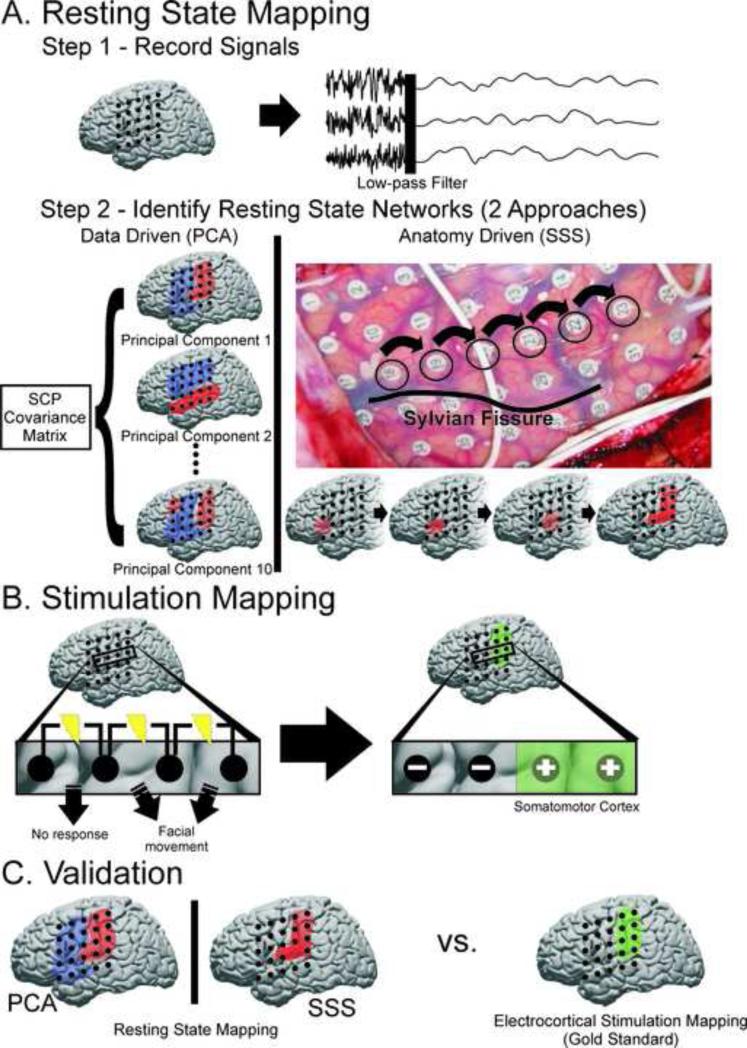
Signals from every electrode were first visually inspected and those electrodes identified as noisy (amplitude >101 times that of the majority of electrodes in the grid) were excluded from further analysis (32 of 464 electrodes discarded, 7%). Signals from the remaining electrodes were then re-referenced to the global mean and low-pass filtered using a 3rd order lowpass digital Butterworth filter (cutoff = 0.5Hz) to obtain the slow cortical potential (Figure 1 A). Because these data were initially recorded with a 0.5Hz high pass filter, the amplitude of these roll-off SCPs could not be reliably analyzed; however, the covariance between electrodes is unaffected by this recording technique. The temporal correlation of the SCP over the three minute epoch was then calculated for all electrode pairs by first computing the covariance (Eqn. 1) between each pair.
| (1) |
Here, and represent the ith of N values of the SCP signal from the mth and nth electrodes, respectively. The correlation coefficient between each electrode pair was then obtained by equation (2).
| (2) |
Fig. 1.
Schematic for identifying SCP correlation networks and testing their performance against the gold standard for delineating SM cortex. A) Step 1, resting-state electrical activity from both awake and anesthetized patients was recorded from cortical electrodes, re-referenced, and filtered for the SCP (<0.5Hz). Step 2, two approaches were taken to identifying candidate SCP correlation networks hypothesized to reflect SM cortex. The first was a data driven approach in which principal component analysis was applied to the covariance matrix of the SCP signals from each electrode. The top 10 principal components (illustrated in blue and red) were considered possible candidates for SM cortex. The second was an anatomy based approach in which seeds were selected superior to the Sylvian fissure in an anterior-posterior direction to maximize likelihood of intersecting SM cortex and identifying the corresponding SCP correlation network. B) ECS mapping was used as the “gold standard” to define SM cortex. Electrodes were labeled as ECS negative for SM cortex if they were involved in any bipolar stimulation which did not elicit a motor or sensory response. Electrodes were labeled as ECS positive for SM cortex if they were only associated with a motor or sensory response when involved in a bipolar stimulation. C) SM cortex defined by ECS mapping was used to assess the sensitivity and specificity of the new techniques based on resting state SCP correlation networks.
Defining Sensorimotor (SM) Cortex with the Gold Standard of ECS Mapping
Electrodes were labeled as being over SM cortex or not over SM cortex using the gold standard of ECS mapping (Figure 1 B). The ECS mapping was performed independently by the neurologist in the extra-operative setting for the clinical purpose of localizing motor, sensory, and language cortex prior to subsequent resection of seizure foci. This mapping took place late in the telemetry, after the successful identification of seizure foci and prior to the second craniotomy. ECS was performed using cortical stimulation with bipolar stimulation of pairs of adjacent electrodes at a frequency of 60 Hz and pulse width of 500 ms. The current was ramped up from 1 mA gradually to 10 mA or until afterdischarge threshold was reached. Stimulation duration for motor mapping was three to five seconds. For each stimulated electrode pair, the presence or absence of induced involuntary motor movements was noted when mapping the motor regions. The presence or absence of induced sensations, as reported by the patient, was noted when mapping the sensory regions. All electrodes that did not evoke a motor or sensory response when stimulated were labeled as “negative” (not over SM cortex). Electrodes that were associated with a motor or sensory response when stimulated were labeled as “positive” (over SM cortex) provided that they were not also involved in a stimulated pair that did not evoke a response, in which case they were still labeled as “negative.” By defining SM cortex in this way we were more restrictive in the number of electrodes that were labeled as “positive” for SM cortex.
Intra-SM versus Extra-SM Network SCP Correlation
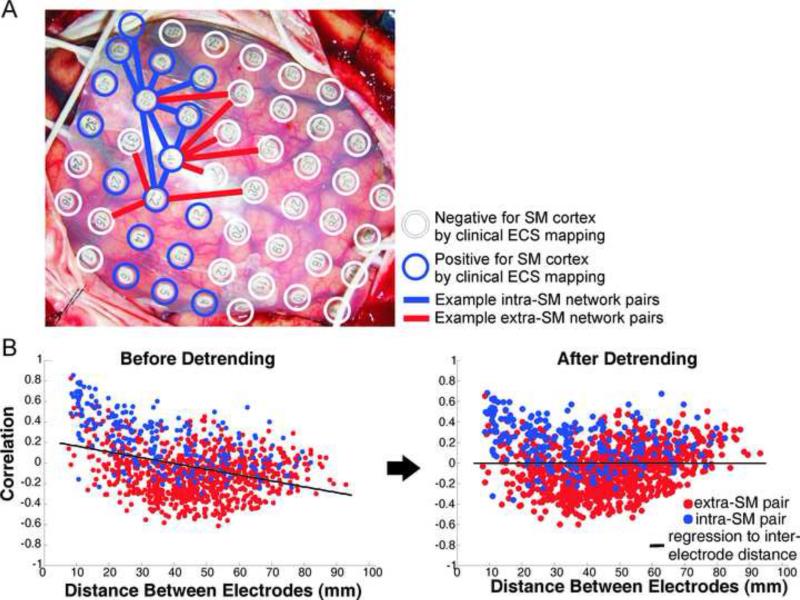
We first tested the hypothesis that slow cortical potential (<0.5Hz) correlations would be stronger for electrode pairs overlying SM cortex (as determined by stimulation mapping) as compared to other electrode pairs (Figure 2A). Before evaluating this hypothesis the influence of inter-electrode distance was removed by linear regression (Figure 2B, see ref 28 SI for methods). This was important to ensure that subsequently identified functional connectivity networks were not due purely to local volume conduction effects, as SM areas tend to be close to other SM areas.43 For each subject the distributions of intra-SM (electrode pairs overlying SM cortex) and extra-SM (electrode pairs with only one member of the pair over SM cortex) correlations were compared using a two-sample KS test. The two distributions were also compared across all 8 subjects.
Fig 2.
A) Example electrode array on cortex with ECS positive (blue) and ECS negative (white) electrodes identified. Pairwise correlations of the SCP were computed for “intra-SM” network electrode pairs (blue lines) and “extra-SM” network pairs (red lines). B) Detrending to remove dependence of SCP correlations on inter-electrode spacing.
SCP Network Identification
We next tested the hypothesis that the spatial correlation structure of SCP's could be used to identify SM cortex (as determined by stimulation). Two approaches were taken to generating SCP spatial correlation structures or networks. 1) A data-driven, seed-independent approach was taken in which principal component analysis (PCA) was applied to the SCP covariance matrix (obtained by equation (1)) as has been used previously for functional connectivity network identification in optical imaging studies.44 To accomplish this, the built-in pcacov() MATLAB function was used to generate ‘candidate’ networks (the top 10 principal components) for SM cortex (Figure 1 A). 2) An anatomy-driven approach was taken, in which electrode seeds were systematically selected and the correlation values with all other electrodes determined the ‘candidate’ network. Systematic seed selection (SSS) was a method created to utilize easily identifiable anatomic landmarks to aid in seed selection (Figure 1 A and Supplemental Video 1). It began with identification of the Sylvian fissure and selection of seed electrodes just superior to the fissure. These seeds were selected in an anterior to posterior direction such that their alignment was approximately parallel to the Sylvian fissure to maximize likelihood of intersecting SM cortex and identifying the corresponding SCP correlation network.
Once ‘candidate’ networks had been generated, the single PCA and SSS networks determined most likely to represent SM cortex were selected. In an effort to limit selection bias, this determination was based on predefined spatial features that are characteristic of SM cortex in general. Specifically, a candidate network was determined to likely reflect SM cortex if it contained 1) a large grouping of positively correlated electrodes, 2) superior to the posterior aspect Sylvian fissure, 3) longer in the ventral-dorsal direction than the rostral-caudal direction, and 4) forming a 90° to 120° angle with the Sylvian fissure. For both the PCA and SSS approaches, the ‘candidate’ network that best fit this description was selected.
Comparison of SCP Networks to ECS Mapping
After networks for SM cortex had been selected, the sensitivity and specificity was calculated for each of these networks, using ECS as defined above (Figure 1 C). For the networks identified by PCA and SSS, electrodes were labeled as “positive” for SM cortex if the strength of correlation in the network at that electrode was greater than zero. Correspondingly, electrodes with correlation values less than zero were labeled as “negative.” The sensitivities and specificities were calculated for the 8 subjects individually, as well as across all subjects. Finally, tradeoffs in sensitivity and specificity were explored for these selected networks using Receiver-Operating Characteristic (ROC) analysis. Overall sensitivity and specificity of the PCA and SSS methods were computed for a range of correlation value thresholds [-0.5 to 1].
Results
Demographic and clinical information for the 8 subjects can be seen in Table 1.
Intra-SM versus Extra-SM Network SCP Correlation
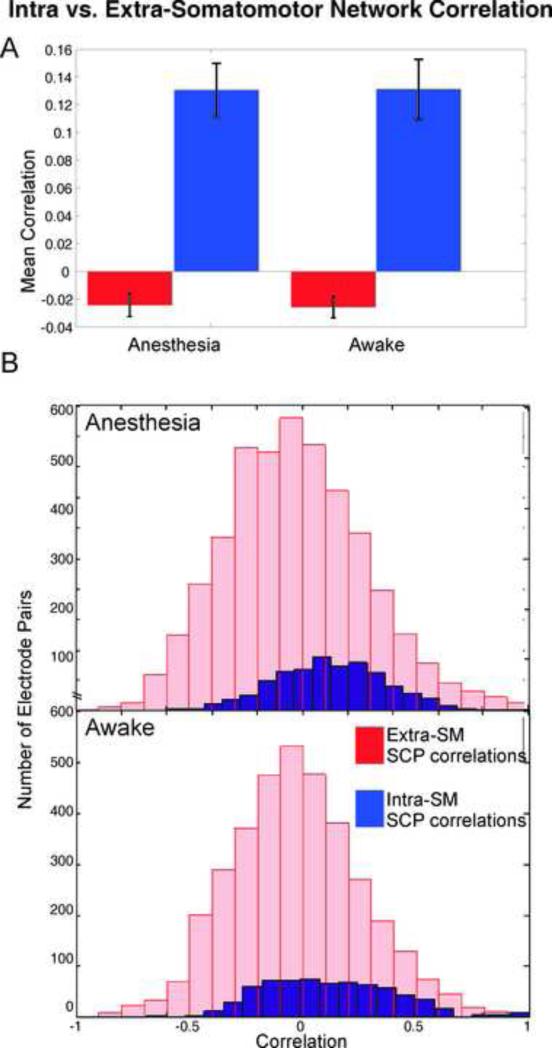
Comparison of intra-SM versus extra-SM network correlation distributions showed significantly stronger correlation of SCP fluctuations within SM cortex than with other cortical areas by two-sample KS test (p <0.0001), after de-trending for distance (Figure 3). In fact, SCP fluctuations at electrodes on SM cortex and at electrodes not on SM cortex were significantly anti-correlated (p <0.05). This was true in both the awake and anesthetized states.
Fig. 3.
Comparison of SCP correlation distributions for intra-SM network electrode pairs versus extra-SM network pairs. A) Mean correlation and 95%CI across 8 subjects. Anesthesia: intra-SM 0.12±0.02; extra-SM -0.03 ±0.01. Awake: intra-SM 0.12 ±0.02 extra-SM -0.03 ±0.01. B) Distributions of correlation scores across 8 subjects were significantly different (p <0.0001) for both under anesthesia and awake states.
Performance of SCP Networks in Identification of SM Cortex
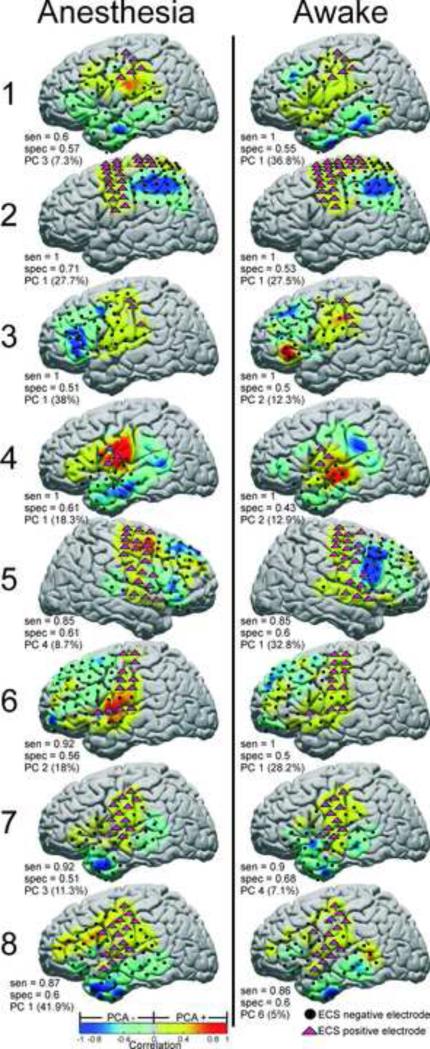
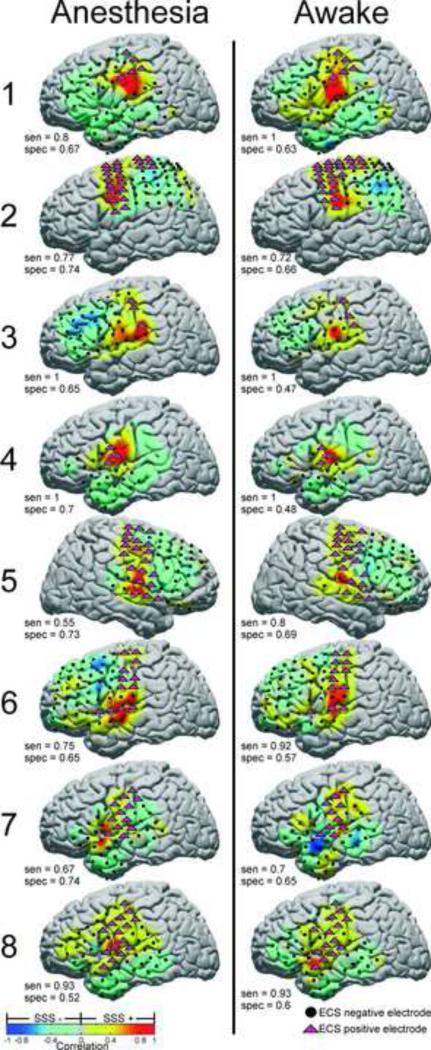
The principal component identified for localizing SM cortex is shown for each subject with its corresponding sensitivity and specificity in Figure 4. Sensitivities ranged from 60% to 100% in the anesthetized state, and from 85% to 100% in the awake state. Specificities ranged from 51% to 71% in the anesthetized state, and from 43% to 68% in the awake state.
Fig. 4.
Principal component analysis applied to identification of SM cortex electrodes. PC based correlation maps are shown plotted on standardized brains, with independently tested ECS positive (magenta triangles) and negative (black circles) electrodes superimposed. For each PC, SCP correlation strength is indicated by the warm/cool colormap. Electrodes with correlation greater than zero were labeled as “positive’ for SM cortex. The sensitivity, specificity, and PC number with percent variance explained are shown for the 8 subjects in both the anesthetized and awake states. Electrodes which were not tested by ECS or were discarded because of artifact are not shown.
The network identified by the anatomy based Systematic Seed Selection method is shown for each subject with the corresponding sensitivity and specificity in Figure 5. Sensitivities ranged from 55% to 100% in the anesthetized state, and from 70% to 100% in the awake state. Specificities ranged from 52% to 74% in the anesthetized state, and from 47% to 69% in the awake state.
Fig. 5.
Systematic seed selection (SSS) method applied to identification of SM cortex electrodes. Seed based correlation maps are shown plotted on standardized brains, with independently tested ECS positive (magenta triangles) and negative (black circles) electrodes superimposed. SCP correlation strength with the seed electrode is indicated by the warm/cool colormap. Electrodes with correlation greater than zero were labeled as “positive’ for SM cortex. The sensitivity and specificity are shown for each subject in both the anesthesia and awake states. Electrodes which were not tested by ECS or were discarded because of artifact are not shown.
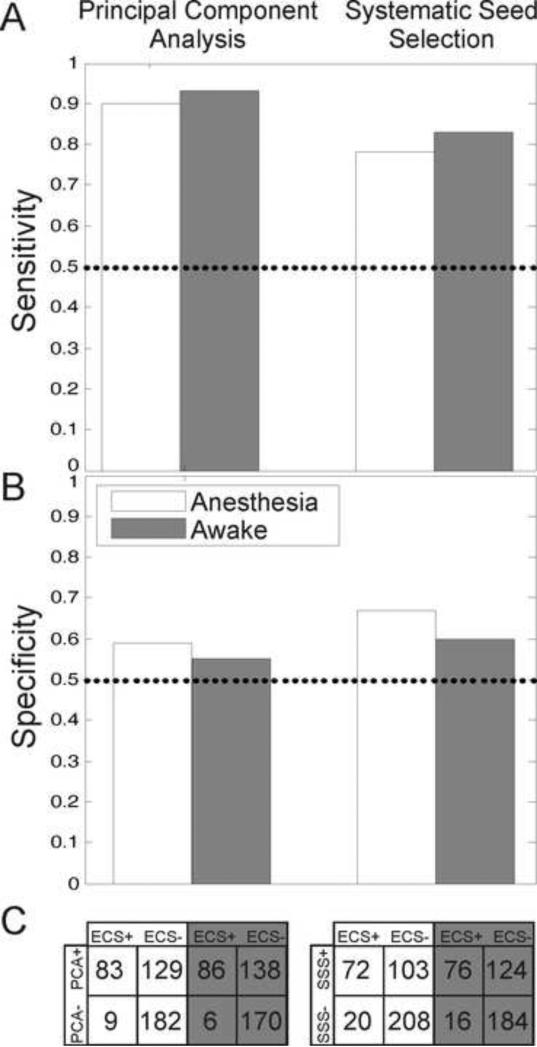
The overall sensitivity and specificity of the PCA and SSS methods in the awake and anesthetized states are shown in Figure 6. The 2x2 tables in Figure 6 C show the actual number of stimulated electrodes from the eight subjects and how they were labeled by the PCA and SSS methods in comparison to the gold standard of ECS mapping. In general, PCA had the highest sensitivity of ~90% in both the anesthetized and awake states, while the SSS method had slightly better specificity of ~60%.
Fig. 6.
Cumulative sensitivity and specificity results in the anesthetized and awake states using the principal component analysis (PCA) and systematic seed selection (SSS) methods of SCP network identification. A) Sensitivity of PCA and SSS across 8 subjects in anesthetized and awake states. See C) for corresponding electrode totals. B) Specificity of PCA and SSS across 8 subjects in anesthetized and awake states. See C) for corresponding electrode totals. C) 2x2 tables giving actual electrode totals across the eight subjects for calculating overall sensitivity and specificity. Note relatively low number of false negatives associated with the PCA method in both the awake and anesthesia conditions.
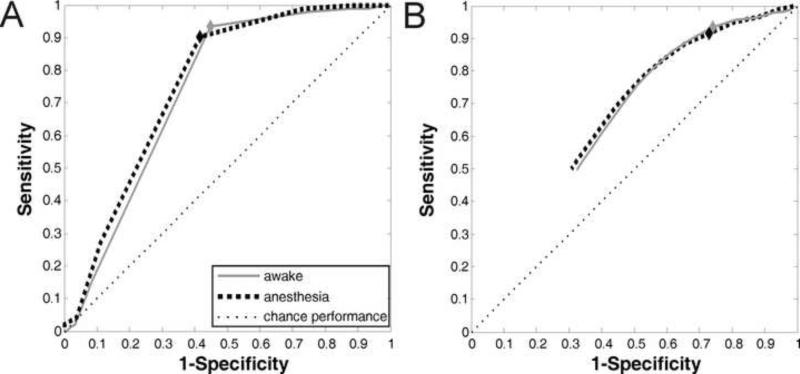
Analysis of tradeoffs in sensitivity and specificity with ROC analysis revealed that both the PCA method and the SSS method performed better than chance (Figure 7). PCA was the superior method, with a greater area under the ROC curve. The threshold correlation value of zero appeared to be an optimal cutoff for both methods. The fact that a correlation value of zero is the optimal cutoff in the ROC analysis suggests that this transition from positively correlated regions to regions of zero or negligible correlation is a non-arbitrary physiologic boundary that is relevant to functional localization, namely stimulation positive sites in the brain.
Fig. 7.
Receiver-operating characteristics (ROC) for A) principal component analysis (PCA) and B) systematic seed selection (SSS) while under anesthesia and awake. Diamonds denote where on the ROC curve results reported in this study were obtained (correlation value threshold = 0). Thin dashed line denotes chance performance.
Discussion
The brain's intrinsic functional architecture of correlated fluctuations in resting-state metabolic activity has been well established.27, 28 The electrophysiologic equivalents of these networks are represented by the correlated fluctuations of the slow cortical potentials (SCPs) which also reflect this organization of functional connectivity.32 Recent work has also shown that resting-state functional networks based on both BOLD signal and SCP can be preserved across differing states of consciousness, including total loss of responsiveness due to anesthesia.39-41 Given this, we hypothesized that resting-state SCP fluctuations might be useful for mapping eloquent cortex in both the extra-operative setting with awake patients as well as intraoperatively with patients under anesthesia. In this study, we demonstrate the high level of similarity between SCP sensorimotor networks, identified in both the awake and anesthetized state, and motor and sensory regions identified with clinical stimulation mapping. Whether using a data-driven approach or a surgically-oriented anatomy-driven approach, sensorimotor cortex could be identified with good sensitivity and modest specificity. SCP fluctuations within sensorimotor cortices were found to be highly correlated, while little correlation existed with SCP fluctuations in regions that were stimulation negative for sensorimotor response (care must be taken in interpreting anti-correlations as they can be introduced by methodology 45). Taken together, this work demonstrates the fundamental nature of sensorimotor resting state networks and their potential relevance to clinical brain mapping.
The current gold standard for mapping eloquent cortex clinically is with cortical stimulation and SSEPs. There have also been numerous studies investigating the use of task-evoked cortical activations, whether with fMRI, MEG, or ECoG, for identifying eloquent cortex.22-24, 46-48 There has also been work using task independent resting state functional MRI for preoperative mapping of motor cortex in patients with brain tumors.25, 49 To our knowledge, this is the first investigation of the use of SCP resting-state networks for clinical brain mapping. This is important, because while resting state networks can be identified preoperatively, this method would provide an additional technique to verify the network in the operating room quickly and efficiently. Further, we investigate the utility of these networks during surgical anesthesia as well as in awake patients. Our results show that resting-state networks defined by correlated spontaneous fluctuations of cortical electrical activity are highly sensitive and moderately specific for identifying sensorimotor cortex in both awake and anesthetized patients. The use of these networks could therefore complement current stimulation techniques to improve the mapping process in a number of ways. By recording from multiple cortical sites in parallel and requiring only several minutes of data, this ECoG based technique requires minimal additional time, and simultaneously allows tailoring of stimulation to enhance mapping efficiency. Because signal is passively recorded without stimulation, there is no risk of induced seizures, which can decrease the efficiency of the mapping process. In the extra-operative setting, patients with intractable epilepsy who are being invasively monitored can often have a high predisposition for developing stimulation-induced seizures. Having the added capability to predict the optimal sites for stimulation mapping can enhance efficiency for finding a motor site for a given region of brain stimulated. This may be both safer for the patient and less time consuming for the epileptologist, although further characterization of this technique is required. Relative to SSEPs, which are also fast and avoid the risks of cortical stimulation, this method is an additional means to elucidate the topography of sensorimotor cortex in anesthetized patients, however, because it is based on resting-state functional connectivity (a physiologic phenomenon also associated with other cognitive functions such as attention and language), it has the potential to be extended to identifying other eloquent areas beyond sensorimotor with further investigation.
Although we have focused on sensorimotor cortex in this study, there are a number of patient groups for whom stimulation mapping options for language cortex are limited by the need for patient participation. This includes pediatric patients or patients who are otherwise too medically tenuous to undergo an awake craniotomy for mapping (i.e. poor airway, claustrophobia, high cardiopulmonary risk factors). In these cases, a technique based on resting-state electrophysiology such as the one we introduce, which can give information about the location of eloquent cortex without requiring the patient to be awake or engaged, may have the potential to greatly improve the quality of care. It is important to point out that although the motivations for investigating a resting-state network-based mapping technique are numerous, the current indications supported by our results are limited to use of this technique only as an adjunct to current mapping modalities.
The lower specificity of SCP mapping to identify stimulation positive sites speaks to an important difference in these modalities. Resting-state SCP fluctuations identify cortical areas that are functionally connected. Stimulation of cortex creates transient lesions to delineate cortical areas “essential” for function; namely, the injury to this region would compromise overt motor or sensory function. Thus, SCP mapping identifies regions of cortex that while associated with motor operation are not necessarily critical to its performance. The relatively lower specificity arises because these sites, which are functionally related and identified by SCP but are not “essential,” are thus labeled as false positives. From a clinical standpoint, as a screening methodology for further stimulation-based cortical interrogation, it is certainly better to have false positive sites that can then be invalidated with further stimulation, rather than false negatives that are never subsequently checked.
Beyond the clinical relevance of these findings, this work also reveals more on the fundamental network architecture of cortex. Several studies using general anesthesia have demonstrated that while lower level sensory regions (auditory and visual cortices) do not alter their cortical-cortical connectivity, higher order associative regions such as frontal-parietal cortices and memory and pain circuits were more significantly changed.50, 51 Our findings support and extend these findings by demonstrating that, in addition to primary sensory cortices, the topographic distribution of network connectivity for sensorimotor regions (a lower level output region of the brain) is also preserved through anesthetic suppression. The persistence of this intrinsic functional brain organization during different states of consciousness, including loss of consciousness with anesthetics, further supports the fundamental nature of these sensorimotor networks.41 The results presented here, namely that resting-state networks in awake and anesthetized patients compare similarly to sensorimotor sites identified by stimulation, provide yet more evidence of the stability of the sensorimotor network independent of conscious state.
It is important to note several limitations to the present study. First, the proposed technique identifies the entire sensorimotor network and does not distinguish between sensory and motor, or hand and face, with the subcentimeter resolution of ECS. This is an unavoidable consequence of the large-scale correlated fluctuations of the resting-state SM network. This inability to sub-parcellate the SM cortex may further limit the technique's useful clinical specificity, as it is occasionally necessary to identify and resect only sensory or face-motor regions. Observations of gyral and sulcal anatomy in conjunction with tailored stimulation are required to make these finer determinations. Secondly, the method does require surgeon input in selecting the appropriate network; however, predefined rules for guiding this choice were implemented to address this potential source of bias. We propose that the requirement for surgeon input also affects current ECS and SSEP based mapping, in which decisions about where stimulation should begin or where electrodes should be placed are based on gyral/sulcal anatomy. The presented method is thus consistent with current techniques in that the surgeon uses all available information to make the best decision.
There are limitations inherent to work done in human epilepsy patients, including limited electrode coverage and anatomic resolution. In these patients, the electrode grid location is determined entirely by clinical factors, and cannot be altered for research purposes. Thus the grid location is biased to be over presumed regions that would include sensory motor cortex. That said, these findings likely are most relevant to surgeries where a surgeon is going to be operating in and around regions that involve perisylvian and rolandic cortex. Additionally, current grid configurations are somewhat coarse (1cm spacing). Increasing the spatial resolution by using higher density grids would allow more precise delineation of network boundaries; however, it also would require more amplifiers and increase the risk of infection associated with wires exiting through the scalp. Further, epilepsy patients have altered cortical physiology.52, 53 In this study we compare functional networks to stimulation mapping results within individual subjects - there is no attempt to generalize the networks across subjects or extend them to normal subjects. Our results show that in a given epilepsy patient, resting-state networks based on correlated SCP fluctuations contain information about cortical areas identified with stimulation.
As with any mapping technique, it is important to point out that the sensitivity and specificity of this technique may vary with age, level of consciousness, and the presence of seizure foci, tumors, or other intracerebral pathology. For example, the method showed notably poor sensitivity in subject 5, the only subject with a posterior frontal lobe seizure focus. These potential sources of variability are yet to be rigorously investigated. Further, this work was done using a standardized propofol anesthetic regimen. It is not known how network-based mapping techniques will perform with alternative anesthetic agents. Finally, we have only investigated the sensorimotor network in the present study. Extension of such techniques to mapping language cortex, however, would be of great clinical utility. Whether mapping language cortex with resting-state cortical electrophysiology will be possible in patients under anesthesia is an important area for further study.
Resting-state networks defined by correlated spontaneous fluctuations of slow cortical potentials hold promise for identifying “essential” sensorimotor cortex in patients who are awake or under surgical anesthesia. While the neurosurgeon's armamentarium currently includes a number of valuable mapping tools that cannot be under emphasized, use of these networks may help tailor stimulation and make clinical brain mapping a safer and more efficient process. Going forward, this completely passive method of mapping could potentially be extended to identify other important eloquent regions, such as language and attention. Application to new networks, however, will require further study and validation.
Supplementary Material
Supplemental Video 1. Video illustrates the anatomy-based approach to systematic seed selection and resting-state SCP network identification. It also demonstrates qualitative correspondence between stimulation mapping and resting-state SCP mapping results.
Acknowledgements of Funding & Support
The authors would like to thank the generous support from the Doris Duke Charitable Foundation and the National Institute of Health (NIH R21 CA159470-01A1) which has made this research possible.
Abbreviations
- ECoG
electrocorticography
- SCP
Slow Cortical Potential
- ECS
electrocortical stimulation
- SM
sensorimotor
- BOLD
blood-oxygenation level dependent
- PCA
principal component analysis
- SSS
systematic seed selection
Footnotes
Disclosures and conflicts of interest: EC Leuthardt holds stock in Neurolutions. The other authors have no personal financial or institutional interest in any of the drugs, materials, or devices described in this article.
References
- 1.Keles GE, Lundin DA, Lamborn KR, Chang EF, Ojemann G, Berger MS. Intraoperative subcortical stimulation mapping for hemispherical perirolandic gliomas located within or adjacent to the descending motor pathways: evaluation of morbidity and assessment of functional outcome in 294 patients. J Neurosurg. 2004 Mar;100(3):369–375. doi: 10.3171/jns.2004.100.3.0369. [DOI] [PubMed] [Google Scholar]
- 2.Berger MS, Kincaid J, Ojemann GA, Lettich E. Brain mapping techniques to maximize resection, safety, and seizure control in children with brain tumors. Neurosurgery. 1989 Nov;25(5):786–792. doi: 10.1097/00006123-198911000-00015. [DOI] [PubMed] [Google Scholar]
- 3.Burchiel KJ, Clarke H, Ojemann GA, Dacey RG, Winn HR. Use of stimulation mapping and corticography in the excision of arteriovenous malformations in sensorimotor and language-related neocortex. Neurosurgery. 1989 Mar;24(3):322–327. doi: 10.1227/00006123-198903000-00002. [DOI] [PubMed] [Google Scholar]
- 4.Haglund MM, Berger MS, Shamseldin M, Lettich E, Ojemann GA. Cortical localization of temporal lobe language sites in patients with gliomas. Neurosurgery. 1994 Apr;34(4):567–576. doi: 10.1227/00006123-199404000-00001. discussion 576. [DOI] [PubMed] [Google Scholar]
- 5.Sanai N, Mirzadeh Z, Berger MS. Functional outcome after language mapping for glioma resection. N Engl J Med. 2008 Jan 3;358(1):18–27. doi: 10.1056/NEJMoa067819. [DOI] [PubMed] [Google Scholar]
- 6.Blume WT, Jones DC, Pathak P. Properties of after-discharges from cortical electrical stimulation in focal epilepsies. Clin Neurophysiol. 2004 Apr;115(4):982–989. doi: 10.1016/j.clinph.2003.11.023. [DOI] [PubMed] [Google Scholar]
- 7.Wood CC, Spencer DD, Allison T, McCarthy G, Williamson PD, Goff WR. Localization of human sensorimotor cortex during surgery by cortical surface recording of somatosensory evoked potentials. Journal of neurosurgery. 1988 Jan;68(1):99–111. doi: 10.3171/jns.1988.68.1.0099. [DOI] [PubMed] [Google Scholar]
- 8.Sloan TB. Anesthetic effects on electrophysiologic recordings. J Clin Neurophysiol. 1998 May;15(3):217–226. doi: 10.1097/00004691-199805000-00005. [DOI] [PubMed] [Google Scholar]
- 9.Boisseau N, Madany M, Staccini P, et al. Comparison of the effects of sevoflurane and propofol on cortical somatosensory evoked potentials. Br J Anaesth. 2002 Jun;88(6):785–789. doi: 10.1093/bja/88.6.785. [DOI] [PubMed] [Google Scholar]
- 10.Cedzich C, Taniguchi M, Schafer S, Schramm J. Somatosensory evoked potential phase reversal and direct motor cortex stimulation during surgery in and around the central region. Neurosurgery. 1996 May;38(5):962–970. doi: 10.1097/00006123-199605000-00023. [DOI] [PubMed] [Google Scholar]
- 11.Goldring S, Harding GW, Gregorie EM. Distinctive electrophysiological characteristics of functionally discrete brain areas: a tenable approach to functional localization. Journal of neurosurgery. 1994 Apr;80(4):701–709. doi: 10.3171/jns.1994.80.4.0701. [DOI] [PubMed] [Google Scholar]
- 12.Pfurtscheller G, Graimann B, Huggins JE, Levine SP, Schuh LA. Spatiotemporal patterns of beta desynchronization and gamma synchronization in corticographic data during self-paced movement. Clin Neurophysiol. 2003 Jul;114(7):1226–1236. doi: 10.1016/s1388-2457(03)00067-1. [DOI] [PubMed] [Google Scholar]
- 13.Levine SP, Huggins JE, BeMent SL, et al. Identification of electrocorticogram patterns as the basis for a direct brain interface. J Clin Neurophysiol. 1999 Sep;16(5):439–447. doi: 10.1097/00004691-199909000-00005. [DOI] [PubMed] [Google Scholar]
- 14.Huggins JE, Levine SP, BeMent SL, et al. Detection of event-related potentials for development of a direct brain interface. J Clin Neurophysiol. 1999 Sep;16(5):448–455. doi: 10.1097/00004691-199909000-00006. [DOI] [PubMed] [Google Scholar]
- 15.Rohde MM, BeMent SL, Huggins JE, Levine SP, Kushwaha RK, Schuh LA. Quality estimation of subdurally recorded, event-related potentials based on signal-to-noise ratio. IEEE Trans Biomed Eng. 2002 Jan;49(1):31–40. doi: 10.1109/10.972837. [DOI] [PubMed] [Google Scholar]
- 16.Lopes da Silva FH, Pfurtscheller G, Lopes da Silva FH, Pfurtscheller G, editors. Handbook of Electroencephalography and Clinical Neurophysiology. Elsevier; Amsterdam: 1999. Event-Related Desynchronization. Elsevier. [Google Scholar]
- 17.Crone NE, Miglioretti DL, Gordon B, Lesser RP. Functional mapping of human sensorimotor cortex with electrocorticographic spectral analysis. II. Event-related synchronization in the gamma band. Brain. Dec. 1998;121(Pt 12):2301–2315. doi: 10.1093/brain/121.12.2301. [DOI] [PubMed] [Google Scholar]
- 18.Crone NE, Hao L, Hart J, Jr., et al. Electrocorticographic gamma activity during word production in spoken and sign language. Neurology. 2001 Dec 11;57(11):2045–2053. doi: 10.1212/wnl.57.11.2045. [DOI] [PubMed] [Google Scholar]
- 19.Crone NE, Sinai A, Korzeniewska A. High-frequency gamma oscillations and human brain mapping with electrocorticography. Prog Brain Res. 2006;159:275–295. doi: 10.1016/S0079-6123(06)59019-3. [DOI] [PubMed] [Google Scholar]
- 20.Breshears J, Sharma M, Anderson NR, Rashid S, Leuthardt EC. Electrocorticographic Frequency Alteration Mapping of Speech Cortex during an Awake Craniotomy: Case Report. Stereotact Funct Neurosurg. 2009;88(1):11–15. doi: 10.1159/000260074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sinai A, Bowers CW, Crainiceanu CM, et al. Electrocorticographic high gamma activity versus electrical cortical stimulation mapping of naming. Brain. 2005 Jul;128(Pt 7):1556–1570. doi: 10.1093/brain/awh491. [DOI] [PubMed] [Google Scholar]
- 22.Leuthardt EC, Miller K, Anderson NR, et al. Electrocorticographic frequency alteration mapping: a clinical technique for mapping the motor cortex. Neurosurgery. 2007 Apr;60(4 Suppl 2):260–270. doi: 10.1227/01.NEU.0000255413.70807.6E. discussion 270-261. [DOI] [PubMed] [Google Scholar]
- 23.Wu M, Wisneski K, Schalk G, et al. Electrocorticographic frequency alteration mapping for extraoperative localization of speech cortex. Neurosurgery. 2010 Feb;66(2):E407–409. doi: 10.1227/01.NEU.0000345352.13696.6F. [DOI] [PubMed] [Google Scholar]
- 24.Roland J, Brunner P, Johnston J, Schalk G, Leuthardt EC. Passive real-time identification of speech and motor cortex during an awake craniotomy. Epilepsy Behav. 2010 May 15; doi: 10.1016/j.yebeh.2010.02.017. [DOI] [PubMed] [Google Scholar]
- 25.Zhang D, Johnston JM, Fox MD, et al. Preoperative sensorimotor mapping in brain tumor patients using spontaneous fluctuations in neuronal activity imaged with fMRI: initial experience. Neurosurgery. 2009;65(6 Suppl):226. doi: 10.1227/01.NEU.0000350868.95634.CA. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Fox MD, Raichle ME. Spontaneous fluctuations in brain activity observed with functional magnetic resonance imaging. Nat Rev Neurosci. 2007 Sep;8(9):700–711. doi: 10.1038/nrn2201. [DOI] [PubMed] [Google Scholar]
- 27.Biswal B, Yetkin FZ, Haughton VM, Hyde JS. Functional connectivity in the motor cortex of resting human brain using echo-planar MRI. Magn Reson Med. 1995 Oct;34(4):537–541. doi: 10.1002/mrm.1910340409. [DOI] [PubMed] [Google Scholar]
- 28.Fox MD, Snyder AZ, Zacks JM, Raichle ME. Coherent spontaneous activity accounts for trial-to-trial variability in human evoked brain responses. Nat Neurosci. 2006 Jan;9(1):23–25. doi: 10.1038/nn1616. [DOI] [PubMed] [Google Scholar]
- 29.Cordes D, Haughton VM, Arfanakis K, et al. Mapping functionally related regions of brain with functional connectivity MR imaging. American Journal of Neuroradiology. 2000;21:1636–1644. [PMC free article] [PubMed] [Google Scholar]
- 30.Hampson M, Peterson BS, Skudlarski P, Gatenby JC, Gore JC. Detection of functional connectivity using temporal correlations in MR images. Human Brain Mapping. 2002;15:247–262. doi: 10.1002/hbm.10022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Smith SM, Fox PT, Miller KL, et al. Correspondence of the brain's functional architecture during activation and rest. Proc Natl Acad Sci U S A. 2009 Aug 4;106(31):13040–13045. doi: 10.1073/pnas.0905267106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.He B, Snyder A, Zempel J, Smyth M, Raichle M. Electrophysiological correlates of the brain's intrinsic large-scale functional architecture. Proceedings of the National Academy of Sciences. 2008;105(41):16039. doi: 10.1073/pnas.0807010105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Birbaumer N, Elbert T, Canavan AG, Rockstroh B. Slow potentials of the cerebral cortex and behavior. Physiol Rev. 1990 Jan;70(1):1–41. doi: 10.1152/physrev.1990.70.1.1. [DOI] [PubMed] [Google Scholar]
- 34.Mitzdorf U. Current source-density method and application in cat cerebral cortex: investigation of evoked potentials and EEG phenomena. Physiol Rev. 1985 Jan;65(1):37–100. doi: 10.1152/physrev.1985.65.1.37. [DOI] [PubMed] [Google Scholar]
- 35.Ko AL, Darvas F, Poliakov A, Ojemann J, Sorensen LB. Quasi-periodic fluctuations in default mode network electrophysiology. J Neurosci. 2011 Aug 10;31(32):11728–11732. doi: 10.1523/JNEUROSCI.5730-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Baria AT, Baliki MN, Parrish T, Apkarian AV. Anatomical and functional assemblies of brain BOLD oscillations. J Neurosci. 2011 May 25;31(21):7910–7919. doi: 10.1523/JNEUROSCI.1296-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kim W, Miller JW, Ojemann JG, Miller KJ. Ictal localization by invasive recording of infraslow activity with DC-coupled amplifiers. J Clin Neurophysiol. 2009 Jun;26(3):135–144. doi: 10.1097/WNP.0b013e3181a768d8. [DOI] [PubMed] [Google Scholar]
- 38.Schalk G, Miller KJ, Anderson NR, et al. Two-dimensional movement control using electrocorticographic signals in humans. J Neural Eng. 2008 Mar;5(1):75–84. doi: 10.1088/1741-2560/5/1/008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Larson-Prior LJ, Zempel JM, Nolan TS, Prior FW, Snyder AZ, Raichle ME. Cortical network functional connectivity in the descent to sleep. Proc Natl Acad Sci U S A. 2009 Mar 17;106(11):4489–4494. doi: 10.1073/pnas.0900924106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Breshears JD, Roland JL, Sharma M, et al. Stable and dynamic cortical electrophysiology of induction and emergence with propofol anesthesia. Proc Natl Acad Sci U S A. 2010 Dec 7;107(49):21170–21175. doi: 10.1073/pnas.1011949107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Vincent JL, Patel GH, Fox MD, et al. Intrinsic functional architecture in the anaesthetized monkey brain. NATURE. 2007 May 3;447(7140):83–86. doi: 10.1038/nature05758. [DOI] [PubMed] [Google Scholar]
- 42.Schalk G, McFarland DJ, Hinterberger T, Birbaumer N, Wolpaw JR. BCI2000: a general-purpose brain-computer interface (BCI) system. IEEE Trans Biomed Eng. 2004 Jun;51(6):1034–1043. doi: 10.1109/TBME.2004.827072. [DOI] [PubMed] [Google Scholar]
- 43.Nunez PL, Srinivasan R, Westdorp AF, et al. EEG coherency. I: Statistics, reference electrode, volume conduction, Laplacians, cortical imaging, and interpretation at multiple scales. Electroencephalogr Clin Neurophysiol. 1997 Nov;103(5):499–515. doi: 10.1016/s0013-4694(97)00066-7. [DOI] [PubMed] [Google Scholar]
- 44.White BR, Bauer AQ, Snyder AZ, Schlaggar BL, Lee JM, Culver JP. Imaging of functional connectivity in the mouse brain. PLoS One. 2011;6(1):e16322. doi: 10.1371/journal.pone.0016322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Fox MD, Zhang D, Snyder AZ, Raichle ME. The global signal and observed anticorrelated resting state brain networks. Journal of Neurophysiology. 2009;101(6):3270. doi: 10.1152/jn.90777.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Håberg A, Kvistad KA, Unsgård G, Haraldseth O. Preoperative blood oxygen level-dependent functional magnetic resonance imaging in patients with primary brain tumors: clinical application and outcome. Neurosurgery. 2004;54(4):902. doi: 10.1227/01.neu.0000114510.05922.f8. [DOI] [PubMed] [Google Scholar]
- 47.Mueller WM, Yetkin FZ, Hammeke TA, et al. Functional magnetic resonance imaging mapping of the motor cortex in patients with cerebral tumors. Neurosurgery. 1996;39(3):515. doi: 10.1097/00006123-199609000-00015. [DOI] [PubMed] [Google Scholar]
- 48.Schiffbauer H, Berger MS, Ferrari P, Freudenstein D, Rowley HA, Roberts TPL. Preoperative magnetic source imaging for brain tumor surgery: a quantitative comparison with intraoperative sensory and motor mapping. Journal of neurosurgery. 2002;97(6):1333–1342. doi: 10.3171/jns.2002.97.6.1333. [DOI] [PubMed] [Google Scholar]
- 49.Shimony JS, Zhang D, Johnston JM, Fox MD, Roy A, Leuthardt EC. Resting-state spontaneous fluctuations in brain activity: a new paradigm for presurgical planning using fMRI. Acad Radiol. 2009 May;16(5):578–583. doi: 10.1016/j.acra.2009.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Boveroux P, Vanhaudenhuyse A, Bruno MA, et al. Breakdown of within-and between-network resting state functional magnetic resonance imaging connectivity during propofol-induced loss of consciousness. Anesthesiology. 2010;113(5):1038. doi: 10.1097/ALN.0b013e3181f697f5. [DOI] [PubMed] [Google Scholar]
- 51.Martuzzi R, Ramani R, Qiu M, Rajeevan N, Constable RT. Functional connectivity and alterations in baseline brain state in humans. NEUROIMAGE. 2010;49(1):823–834. doi: 10.1016/j.neuroimage.2009.07.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Spencer SS. Neural networks in human epilepsy: evidence of and implications for treatment. Epilepsia. 2002 Mar;43(3):219–227. doi: 10.1046/j.1528-1157.2002.26901.x. [DOI] [PubMed] [Google Scholar]
- 53.Zhang X, Tokoglu F, Negishi M, et al. Social network theory applied to resting-state fMRI connectivity data in the identification of epilepsy networks with iterative feature selection. J Neurosci Methods. 2011 Jul 15;199(1):129–139. doi: 10.1016/j.jneumeth.2011.04.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental Video 1. Video illustrates the anatomy-based approach to systematic seed selection and resting-state SCP network identification. It also demonstrates qualitative correspondence between stimulation mapping and resting-state SCP mapping results.