Abstract
Acetyl-CoA carboxylase (ACCase, EC 6.4.1.2) catalyzes the synthesis of malonyl-CoA, the first intermediate in fatty acid synthesis. We studied the localization of two forms, the prokaryote and the eukaryote forms, of ACCase in pea leaves by comparing the biotin polypeptides of the two ACCases in protein extract from leaves and plastids. We found that the two forms of ACCase were in different cell compartments of pea leaves; the prokaryote form was in the plastids, and the eukaryote form was elsewhere, probably in the cytosol. This result suggested the existence of two sites of malonyl-CoA synthesis. The Gramineae, such as rice and wheat, which lack the accD gene encoding one of the subunits of the prokaryote form of ACCase in their chloroplast genomes, did not have the prokaryote form of the enzyme but had the eukaryote form. The selective grass herbicides of the diphenoxypropionic acid type and the cyclohexanedione type, in vitro, inhibited plastidic ACCase of the eukaryote form from wheat but did not inhibit that of the prokaryote form from pea, suggesting that the origin of the tolerance of intact pea plant toward these herbicides is partly in the insensitivity of the prokaryote form of the enzyme. The origin of the susceptibility of the Gramineae plants toward these herbicides seems to lie in the presence of the herbicide-sensitive eukaryote form and the absence of the insensitive prokaryote form due to the lack of the accD gene in plastid.
Full text
PDF

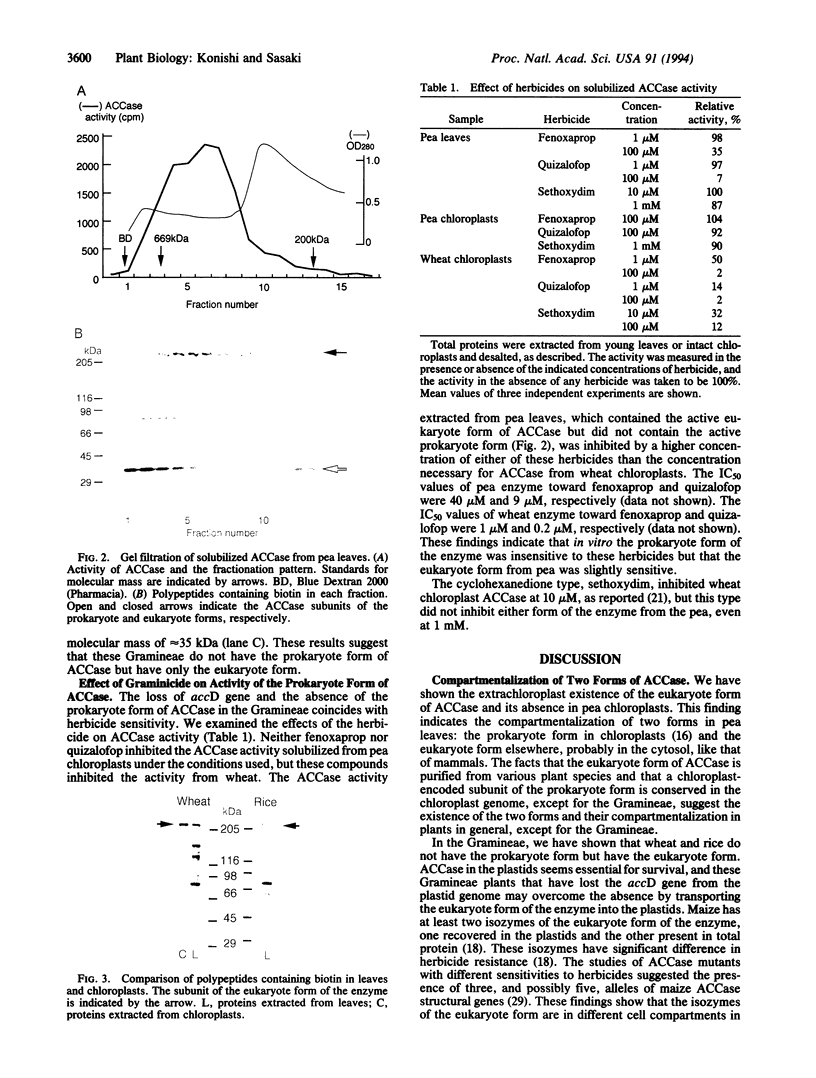

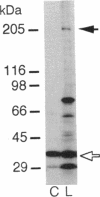
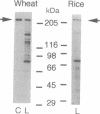
Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Baldet P., Alban C., Axiotis S., Douce R. Localization of free and bound biotin in cells from green pea leaves. Arch Biochem Biophys. 1993 May 15;303(1):67–73. doi: 10.1006/abbi.1993.1256. [DOI] [PubMed] [Google Scholar]
- Bolton P., Harwood J. L. Fatty acid biosynthesis by a particulate preparation from germinating pea. Biochem J. 1977 Nov 15;168(2):261–269. doi: 10.1042/bj1680261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burton J. D., Gronwald J. W., Somers D. A., Connelly J. A., Gengenbach B. G., Wyse D. L. Inhibition of plant acetyl-coenzyme A carboxylase by the herbicides sethoxydim and haloxyfop. Biochem Biophys Res Commun. 1987 Nov 13;148(3):1039–1044. doi: 10.1016/s0006-291x(87)80236-x. [DOI] [PubMed] [Google Scholar]
- Dahlin C., Cline K. Developmental Regulation of the Plastid Protein Import Apparatus. Plant Cell. 1991 Oct;3(10):1131–1140. doi: 10.1105/tpc.3.10.1131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ebel J., Hahlbrock K. Enzymes of flavone and flavonol-glycoside biosynthesis. Coordinated and selective induction in cell-suspension cultures of Petroselinum hortense. Eur J Biochem. 1977 May 2;75(1):201–209. doi: 10.1111/j.1432-1033.1977.tb11518.x. [DOI] [PubMed] [Google Scholar]
- Egin-Bühler B., Ebel J. Improved purification and further characterization of acetyl-CoA carboxylase from cultured cells of parsley (Petroselinum hortense). Eur J Biochem. 1983 Jun 15;133(2):335–339. doi: 10.1111/j.1432-1033.1983.tb07467.x. [DOI] [PubMed] [Google Scholar]
- Egli M. A., Gengenbach B. G., Gronwald J. W., Somers D. A., Wyse D. L. Characterization of Maize Acetyl-Coenzyme A Carboxylase. Plant Physiol. 1993 Feb;101(2):499–506. doi: 10.1104/pp.101.2.499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gornicki P., Haselkorn R. Wheat acetyl-CoA carboxylase. Plant Mol Biol. 1993 Jun;22(3):547–552. doi: 10.1007/BF00015984. [DOI] [PubMed] [Google Scholar]
- Guchhait R. B., Polakis S. E., Dimroth P., Stoll E., Moss J., Lane M. D. Acetyl coenzyme A carboxylase system of Escherichia coli. Purification and properties of the biotin carboxylase, carboxyltransferase, and carboxyl carrier protein components. J Biol Chem. 1974 Oct 25;249(20):6633–6645. [PubMed] [Google Scholar]
- Hiratsuka J., Shimada H., Whittier R., Ishibashi T., Sakamoto M., Mori M., Kondo C., Honji Y., Sun C. R., Meng B. Y. The complete sequence of the rice (Oryza sativa) chloroplast genome: intermolecular recombination between distinct tRNA genes accounts for a major plastid DNA inversion during the evolution of the cereals. Mol Gen Genet. 1989 Jun;217(2-3):185–194. doi: 10.1007/BF02464880. [DOI] [PubMed] [Google Scholar]
- Kannangara C. G., Stumpf P. K. Fat metabolism in higher plants. LIV. A procaryotic type acetyl CoA carboxylase in spinach chloroplasts. Arch Biochem Biophys. 1972 Sep;152(1):83–91. doi: 10.1016/0003-9861(72)90196-8. [DOI] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Mishina M., Kamiryo T., Tanaka A., Fukui S., Numa S. Acetyl-coenzyme-A carboxylase of Candida Lipolytica. 1. Purification and properties of the enzyme. Eur J Biochem. 1976 Dec;71(1):295–300. doi: 10.1111/j.1432-1033.1976.tb11115.x. [DOI] [PubMed] [Google Scholar]
- Nikolau B. J., Wurtele E. S., Stumpf P. K. Use of streptavidin to detect biotin-containing proteins in plants. Anal Biochem. 1985 Sep;149(2):448–453. doi: 10.1016/0003-2697(85)90596-2. [DOI] [PubMed] [Google Scholar]
- Ogihara Y., Terachi T., Sasakuma T. Intramolecular recombination of chloroplast genome mediated by short direct-repeat sequences in wheat species. Proc Natl Acad Sci U S A. 1988 Nov;85(22):8573–8577. doi: 10.1073/pnas.85.22.8573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohlrogge J. B., Kuhn D. N., Stumpf P. K. Subcellular localization of acyl carrier protein in leaf protoplasts of Spinacia oleracea. Proc Natl Acad Sci U S A. 1979 Mar;76(3):1194–1198. doi: 10.1073/pnas.76.3.1194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohlrogge J. B., Kuhn D. N., Stumpf P. K. Subcellular localization of acyl carrier protein in leaf protoplasts of Spinacia oleracea. Proc Natl Acad Sci U S A. 1979 Mar;76(3):1194–1198. doi: 10.1073/pnas.76.3.1194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roessler P. G., Ohlrogge J. B. Cloning and characterization of the gene that encodes acetyl-coenzyme A carboxylase in the alga Cyclotella cryptica. J Biol Chem. 1993 Sep 15;268(26):19254–19259. [PubMed] [Google Scholar]
- Sasaki Y., Hakamada K., Suama Y., Nagano Y., Furusawa I., Matsuno R. Chloroplast-encoded protein as a subunit of acetyl-CoA carboxylase in pea plant. J Biol Chem. 1993 Nov 25;268(33):25118–25123. [PubMed] [Google Scholar]
- Shinozaki K., Ohme M., Tanaka M., Wakasugi T., Hayashida N., Matsubayashi T., Zaita N., Chunwongse J., Obokata J., Yamaguchi-Shinozaki K. The complete nucleotide sequence of the tobacco chloroplast genome: its gene organization and expression. EMBO J. 1986 Sep;5(9):2043–2049. doi: 10.1002/j.1460-2075.1986.tb04464.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takai T., Yokoyama C., Wada K., Tanabe T. Primary structure of chicken liver acetyl-CoA carboxylase deduced from cDNA sequence. J Biol Chem. 1988 Feb 25;263(6):2651–2657. [PubMed] [Google Scholar]
- Tanabe T., Wada K., Okazaki T., Numa S. Acetyl-coenzyme-A carboxylase from rat liver. Subunit structure and proteolytic modification. Eur J Biochem. 1975 Sep 1;57(1):15–24. doi: 10.1111/j.1432-1033.1975.tb02272.x. [DOI] [PubMed] [Google Scholar]
- Wakil S. J., Stoops J. K., Joshi V. C. Fatty acid synthesis and its regulation. Annu Rev Biochem. 1983;52:537–579. doi: 10.1146/annurev.bi.52.070183.002541. [DOI] [PubMed] [Google Scholar]
- Walker K. A., Harwood J. L. Localization of chloroplastic fatty acid synthesis de novo in the stroma. Biochem J. 1985 Mar 1;226(2):551–556. doi: 10.1042/bj2260551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Silva J., Loader N. M., Jarman C., Windust J. H., Hughes S. G., Safford R. The isolation and sequence analysis of two seed-expressed acyl carrier protein genes from Brassica napus. Plant Mol Biol. 1990 Apr;14(4):537–548. doi: 10.1007/BF00027499. [DOI] [PubMed] [Google Scholar]