Abstract
Importance
SNCA locus duplications are associated with variable clinical features and reduced penetrance but the reasons underlying this variability are unknown.
Objective
1) To report a novel family carrying a heterozygous 6.4Mb duplication of the SNCA locus with an atypical clinical presentation strongly reminiscent of frontotemporal dementia (FTD) and late-onset pallidopyramidal syndromes. 2) To study phenotype-genotype correlations in SNCA locus duplications.
Design, Setting, Participants and Data sources
We report the clinical and neuropathologic features of a family carrying a 6.4Mb duplication of the SNCA locus. To identify candidate disease modifiers, we undertake a genetic analysis in the family and conduct statistical analysis on previously published cases carrying SNCA locus duplication using regression modelling with robust standard errors to account for clustering at the family level.
Main outcome measures
To assess whether length of the SNCA locus duplication influences disease penetrance and severity, and whether extra-duplication factors have a disease-modifying role.
Results
We identified a large 6.4Mb duplication of the SNCA locus in this family. Neuropathological analysis showed extensive α-synuclein pathology with minimal phospho-tau pathology. Genetic analysis showed an increased burden of PD-related risk factors and the disease-predisposing H1/H1 MAPT haplotype. Statistical analysis of previously published cases suggested that there is a trend towards increasing disease severity and disease penetrance with increasing duplication size. The corresponding odds ratios (95% CI) from the univariate analyses were 1.17 (0.81 to 1.68) and 1.34 (0.78 to 2.31) respectively. Gender was significantly associated with both disease risk and severity; males compared to females had increased disease risk and severity and the corresponding odds ratios (95% CI) from the univariate analyses were 8.36 (1.97 to 35.42) and 5.55 (1.39 to 22.22) respectively.
Conclusions and relevance
These findings further expand the phenotypic spectrum of SNCA locus duplications. Increased dosage of genes located within the duplicated region probably cannot increase disease risk and disease severity without the contribution of additional risk factors. Identification of disease modifiers accounting for the substantial phenotypic heterogeneity of patients with SNCA locus duplications could provide insight into molecular events involved in α-synuclein aggregation.
Keywords: fronto-temporal dementia and parkinsonism, alpha-synuclein, duplication, gene
INTRODUCTION
α-Synuclein is a protein central to the pathogenesis of Parkinson’s disease (PD), a role illustrated by the identification of α-Synuclein as the principal component of Lewy Bodies (LBs) 1, the pathological hallmark of PD, and of α-Synuclein (SNCA) point mutations and multiplications as rare causes of PD 2-4. Disease severity correlates with the number of SNCA alleles in multiplication carriers. Patients with SNCA triplication present with an early-onset, aggressive form of PD, with dementia, psychiatric features and dysautonomia 5. On the other hand, SNCA duplications are not fully penetrant and are associated with variable clinical features ranging from late-onset sporadic PD to presentations indistinguishable from those in triplication carriers but the reasons underlying this variability are unknown.
Here, we report a new kindred carrying the largest non-chromosomal duplication of the SNCA locus identified to date presenting with clinical features reminiscent of frontotemporal dementia (FTD) and widespread Lewy bodies. We undertake a comprehensive assessment of candidate disease modifiers in carriers of SNCA locus duplications based on genetic analysis of the proband and on statistical analysis of previously published cases.
METHODS
Genetics
MAPT was screened through Sanger sequencing in cases III:1 and III:3 (figure 1A) because of clinical similarity to FTD 6. Because of clinical similarities to SNCA multiplication cases and widespread α-synuclein pathology, a genomic DNA sample from case III:1 was assessed for whole gene SNCA multiplications with the Multiplex-ligation dependent probe amplification (MLPA) kit P051C (MRC Holland, the Netherlands) 7. The approximate breakpoints of the duplication were determined through an Agilent custom 8×60k dense comparative genomic hybridization (CGH) array designed as part of a larger on-going study with dense spacing of probes throughout the SNCA genomic region (CP, details available on request).
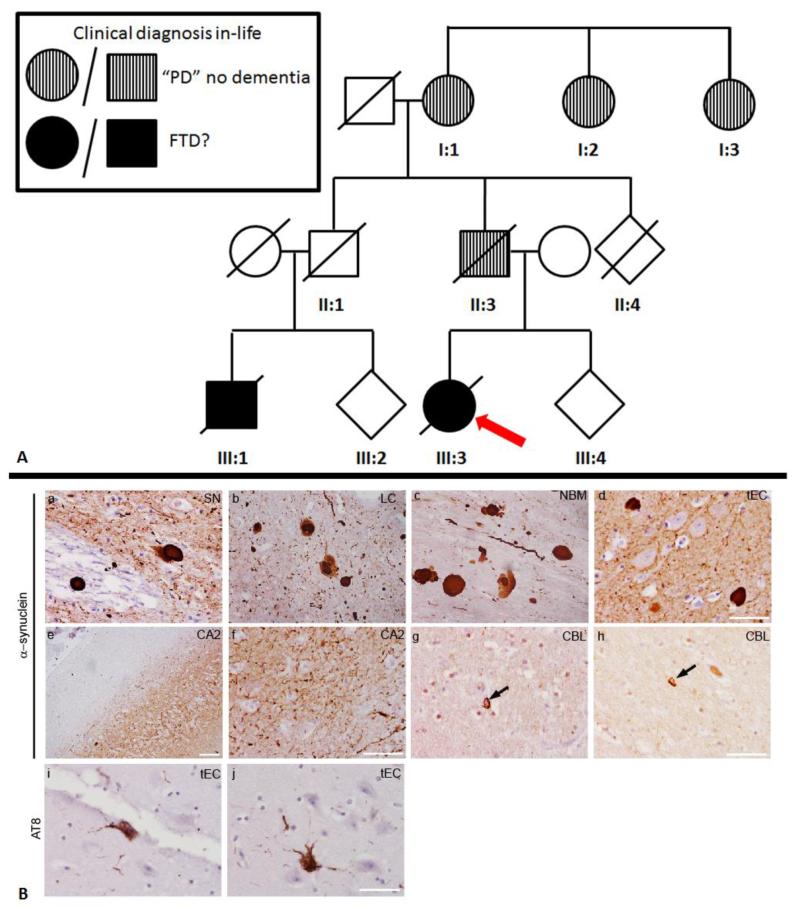
Figure 1. ‘Family tree and neuropathology findings’.
A) Family tree. Red arrow depicts the proband. Information has been omitted for deidentification purposes. B) Representative images of α-synuclein immunoreactive inclusions within (a) substantia nigra (SN), (b) locus coeulus (LC), (c) nucleus basalis of Meynert (NBM) and (d) transentorhinal cortex (tEC). Severe neuritic α-synuclein deposition is shown in the CA2 region of the hippocampus at low and high magnification (e, f). α–Synuclein coiled bodies shown in the white matter of the cerebellum (g,h, CBL). Sparse AT8 (phospho-tau, Ser 202 and Thr205) immunoreactive neurofibrillary tangles were observed in the transentorhinal cortex (i,j, tEC). Scale bars represent 50 μm. PD=Parkinson’s disease; FTD=Frontotemporal dementia
In an attempt to identify risk factors potentially influencing the clinical features, a DNA sample from case III:1 was genotyped on the Immunochip, an Illumina array of custom content with a specific focus on immune-related and PD/parkinsonism genes 8-10. We focused on risk variants identified through previously published PD genome wide association studies (GWAS) and on mutations and risk factors located within PD genes (see supplementary information for methods).
Neuropathology
The brain from case III:3 was fixed by suspension in buffered 20% formalin and sampled extensively for neuropathology. Paraffin-embedded sections (8μm) from cortical, subcortical, brainstem and cerebellar regions were stained immunohistochemically for standard pathological markers including Aβ (DAKO, M0872) AT8 (Tau Ser202/Thr205, Source Bioscience 90206), TDP-43 (Abnova, H00023435-M01) and α-synuclein (Abcam ab15530). For Aβ and α-synuclein immunohistochemistry, sections required pre-treatment with formic acid for 10 minutes before antigen retrieval. Antigen retrieval was achieved by heating sections in a pressure cooker for 10 minutes in boiling citrate buffer (pH 6.0). Endogenous peroxidase activity was blocked with methanol/0.3% H2O2 followed by incubation in dried milk solution (10% in PBS, 30 minutes) to block non-specific antibody binding. Primary antibodies diluted in PBS were applied for 60 minutes at room temperature and staining was visualised by the streptavidin-biotin-peroxidase method (Vector laboratories, Peterborough, UK), using 3,3′-diaminobenzidine as the chromogen with Mayer’s haematoxylin counterstaining.
Statistical analysis of previously published cases
We reviewed previously published articles on carriers of SNCA locus duplications in order to assess whether the length of the duplication influences penetrance and disease severity and whether longer duplication sizes are related to a younger age at onset (AAO), indicating a contribution of increased dosage of other gene(s) to the development of disease. We used a duplication size threshold of 5Mb as an inclusion criterion which was chosen on the basis of the following arguments. Firstly, we hypothesised that shorter duplications are more frequently non-penetrant and that as duplication size increases, the individual exhibits a more severe phenotype ranging from unaffected, sporadic PD, PD-Dementia, to indistinguishable from triplication carriers. Secondly, we also hypothesised that, above a certain duplication length threshold, the individual will exhibit a more complex phenotype with features unusual for SNCA multiplications. Consequently, two individuals with such complex presentations, one described by Garraux et al (2012) 11 and the case reported herein carrying duplications larger than 5Mb were excluded from this analysis. Published individuals were also excluded if any of the following were true: a) there was no information on the length of the duplication, b) there was insufficient clinical information, c) clinical presentation was clearly inconsistent with parkinsonism, d) they were affected relatives of probands with SNCA duplications but their carrier status wasn’t assessed (efigure 1). Data on all eligible individuals from each family carrying the duplications were included in the study to maximise study power (eTable 1). Review of the articles’ eligibility and cataloguing of the information was undertaken by the main author (EK) and was repeated twice.
Disease severity was measured by deriving a composite score for each affected individual from the published data. Each individual was assigned a score according to a clinical presentation point system as follows: Typical PD/parkinsonism = 1, young onset (before or at 40 years) = 1, dementia = 1, hallucinations = 1, dysautonomia = 1, depression = 1, any additional features (dystonia, epilepsy, sleep disturbances, myoclonus, psychiatric features) = 1. These scores were then summed up to form a composite score, which ranged from 0 (unaffected) to 6 (severely affected).
Numerical data were summarised using mean and standard deviation or median and range depending on data distribution. Categorical data were summarised using count and percentages. We used Spearman’s rank correlation to quantify strength of monotonic association between duplication size and each of AAO and disease severity. The main outcomes of interest were disease status (binary), disease severity (ordinal) and age at onset (binary). We modelled binary outcomes using logistic regression with robust sandwich estimation of the variance to account for the clustering effect at the family level 12. We modelled ordinal outcomes using ordinal regression with the proportional odds assumption, adjusted for clusters at the family level 13. This approach has been shown to be more suitable than multilevel approaches in the presence of small number of clusters with low number of individuals 14. We investigated the individual effect of the covariates on each of the outcomes using univariate analyses. We also investigated their combined effect using multivariable analyses.
The functional form of the relationship between duplication size and each outcome was also evaluated graphically. Model fit was assessed using the Akaike Information Criterion (AIC) 15, where smaller AIC is preferred.
All statistical analyses were carried out using Stata 16. Results from modelling are presented as estimates (95 % confidence interval). Plots were generated with the statistical package R version 3.0.2 17 using the package ggplot2 18.
RESULTS
CASE REPORT
Clinical description
The proband, an English, Caucasian woman (patient III:3, figure 1A), first developed lifelong and progressive symptoms of extreme anxiety, panic disorder and hallucinations from age 8 that were managed adequately with medication up to her mid-20’s when she had to quit her job. At age 38 years, she developed right arm tremor followed by a progressive akinetic-rigid syndrome, plus a classic pill-rolling tremor, complicated by worsening obsessive behaviour, walking disturbances and falls, increased salivation, REM sleep behaviour disorder, personality changes, short-term memory impairment and poor self-care. She also had facial, head and tongue tremor accompanied by blepharospasm, dystonic neck and dysarthria. Levodopa treatment improved her walking but increased her falls. Neuropsychometry testing showed frontal and temporal deficits though neuroimaging was normal. There was a history of recurrent unexplained blackouts or seizures. EEG showed traces of alpha-rhythm, and autonomic testing indicated cardiovascular autonomic failure. On examination, she exhibited akinesia and rigidity of all four limbs, rest tremor and hypometric saccades. From the age of 42, the motor and cognitive aspects of her disorder declined further, with poor concentration, frontal release signs, wandering, repetitive speech and a profound increase in appetite particularly for sweet food, and at age 46 she was given a clinical diagnosis of possible FTDP-17 but no MAPT mutations were detected. She became bedbound and died at the age of 49 years.
In her family history, her father (II:3), paternal grandmother (I:1) and two paternal great-aunts (I:2, I:3) suffered from PD with no documented dementia. Her paternal cousin (III:1) was diagnosed as having possible FTDP-17 in his early 50’s when he presented with memory problems, hallucinations and falls, complicated by alcoholism. This progressed with increasing cognitive decline and disorientation and rigidity at which point his progressive condition was managed by local services.
Pathology
Neuropathological examination showed neuronal loss which was most severe in the substantia nigra, moderate in the locus coeruleus and dorsal motor nucleus of the vagus and mild in the nucleus basalis of Meynert and cerebellar Purkinje cells. Neuronal loss was not observed in other regions including the hippocampus. Widespread Lewy bodies and Lewy neurites affecting brainstem, limbic and neocortical regions corresponding to neocortical Lewy body pathology, Braak stage 619,20 (figure 1B, a-d) were detected. The hippocampus showed mild accumulation of α–synuclein inclusions with severe neuritic pathology in the CA2 region (figure 1B, e, f). Occasional oligodenrdrocytes contained α–synuclein immunoreactive inclusions with coiled body morphology. No inclusions resembling glial cytoplasmic inclusions were observed (figure 1B, g, h). Minimal phospho-tau pathology was present in the transentorhinal cortex corresponding to Braak and Braak stage I (figure 1B, I, j) and there were sparse diffuse cortical amyloid β deposits. TDP-43 pathology was not present.
Genetics
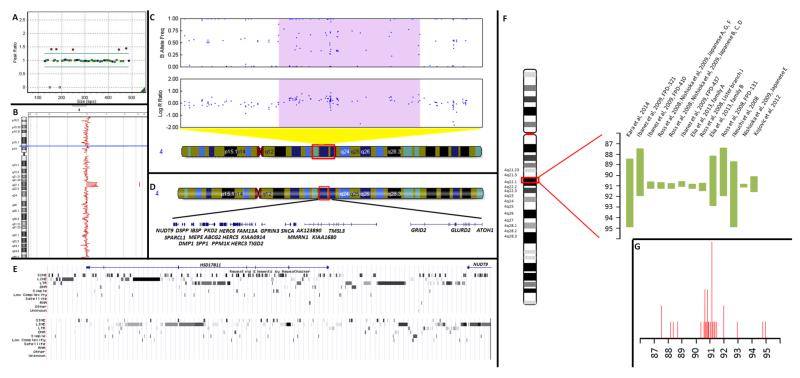
Patient III:1 was negative for MAPT point mutations but was found to carry a heterozygous SNCA duplication on MLPA (figure 2A). CGH arrays showed that the duplication extended over approximately 6.4Mb (chr4:88349207-94751141, corresponding to the first and the last duplicated probes respectively, human build 37) and contained 37 genes including SNCA (figure 2B,C)(table 1). The first and last non-duplicated probes are A_14_P119434 (chr4:88295927-88295986) and A_14_P124103 (chr4:94859877-94859936) respectively (figure 2D). The breakpoints are located centromeric to intron 1 of the NUDT9 gene (NM_198038.2), and telomeric to the ATOH1 gene (figure 2E).
Figure 2. ‘Genetic findings’.
A) MLPA results depicting the duplication. B) Minimum duplicated region as identified by the CGH array (chr4:88,349,207-94,751,141, HB37). C) Minimum duplicated region as identified by the Immunochip (chr4: 88,231,429-94,816,144 HB37) with an increased logR ratio and probes forming 4 distinct genotype clusters 46. Region depicted in figure is chr4: 83,666,791-97,713,411 (HB37). D) Genes included within the minimum duplicated region (chr4:88,349,207-94,751,141, HB37) as identified by the CGH array. E) Breakpoint regions and repetitive sequences identified through UCSC repeat masker. Upper panel centromeric (chr4:88,249,207-88,349,207), lower panel telomeric (chr4: 94,751,141-94,851,141). F) Comparison of the length of the duplicated/triplicated region between our case and published cases (band sizes are approximate). Sizes represent minimum length based on Human Build 36. G) Location of breakpoints. Height of peak corresponds to the number of cases with a breakpoint in the particular region. Breakpoint location could correspond to regions of recombination hotspots 21,47. The case reported by Garraux et al (2012) 11 was excluded from both images.
Table 1.
List of genes included in the duplicated region in case III:1. Generated with NCBI Map Viewer (http://www.ncbi.nlm.nih.gov/projects/mapview/).
| List of genes included within duplication region in case III:1 | ||||
|---|---|---|---|---|
| Start1 | Stop1 | Gene Symbol | Description | Known role in disease |
| 88343728 | 88380606 | NUDT9 | nudix (nucleoside diphosphate linked moiety X)-type motif 9 | NA |
| 88394487 | 88450655 | SPARCL1 | SPARC-like 1 (hevin) | NA |
| 88529681 | 88538025 | DSPP | dentin sialophosphoprotein | dentinogenesis imperfecta-148,49 |
| 88571454 | 88585513 | DMP1 | dentin matrix acidic phosphoprotein 1 | Autosomal recessive hypophosphatemia50 |
| 88707075 | 88707514 | CHCHD2P7 | coiled-coil-helix-coiled-coil-helix domain containing 2 pseudogene 7 | NA |
| 88720702 | 88733601 | IBSP | integrin-binding sialoprotein | NA |
| 88742563 | 88767969 | MEPE | matrix extracellular phosphoglycoprotein | NA |
| 88812995 | 88815167 | HSP90AB3P | heat shock protein 90kDa alpha (cytosolic), class B member 3, pseudogene | NA |
| 88896802 | 88904563 | SPP1 | secreted phosphoprotein 1 | NA |
| 88928799 | 88998931 | PKD2 | polycystic kidney disease 2 (autosomal dominant) | Autosomal dominant polycystic kidney disease (ADPKD) 51 |
| 89011416 | 89152474 | ABCG2 | ATP-binding cassette, sub-family G (WHITE), member 2 | NA |
| 89084691 | 89085162 | RPL31P24 | ribosomal protein L31 pseudogene 24 | NA |
| 89178761 | 89205983 | PPM1K | protein phosphatase, Mg2+/Mn2+ dependent, 1K | NA |
| 89299891 | 89364249 | HERC6 | HECT and RLD domain containing E3 ubiquitin protein ligase family member 6 | NA |
| 89378268 | 89427321 | HERC5 | HECT and RLD domain containing E3 ubiquitin protein ligase 5 | NA |
| 89428154 | 89431638 | LOC728333 | nuclear receptor coactivator 4 pseudogene | NA |
| 89442129 | 89444952 | PIGY | phosphatidylinositol glycan anchor biosynthesis, class Y | NA |
| 89442129 | 89444952 | PYURF | PIGY upstream reading frame | NA |
| 89448713 | 89449318 | LOC100129137 | CD53 molecule pseudogene | NA |
| 89513647 | 89629686 | HERC3 | HECT and RLD domain containing E3 ubiquitin protein ligase 3 | NA |
| 89605999 | 89606105 | RNU6-33P | RNA, U6 small nuclear 33, pseudogene | NA |
| 89617066 | 89619023 | NAP1L5 | nucleosome assembly protein 1-like 5 | NA |
| 89630940 | 89651254 | FAM13A-AS1 | FAM13A antisense RNA 1 | NA |
| 89647105 | 89978346 | FAM13A | family with sequence similarity 13, member A | NA |
| 90031864 | 90033424 | LOC731282 | uncharacterized LOC731282 | NA |
| 90033968 | 90036052 | TIGD2 | tigger transposable element derived 2 | NA |
| 90165429 | 90229161 | GPRIN3 | GPRIN family member 3 | NA |
| 90645250 | 90759447 | SNCA | synuclein, alpha | PD 2 |
| 90757552 | 90763142 | LOC644248 | uncharacterized LOC644248 | NA |
| 90816052 | 90875780 | MMRN1 | multimerin 1 | NA |
| 91048684 | 92523370 | CCSER1 | coiled-coil serine-rich protein 1 | NA |
| 91759636 | 91760266 | TMSB4XP8 | thymosin beta 4, X-linked pseudogene 8 | NA |
| 93103426 | 93105206 | LOC133083 | peptidase (mitochondrial processing) alpha pseudogene | NA |
| 93225550 | 94693649 | GRID2 | glutamate receptor, ionotropic, delta 2 | Cerebellar ataxia 52 |
| 93623496 | 93623786 | MTND1P19 | MT-ND1 pseudogene 19 | NA |
| 93743117 | 93744164 | LOC100422562 | guanine nucleotide binding protein (G protein), beta polypeptide 2-like 1 pseudogene | NA |
| 94750078 | 94751142 | ATOH1 | atonal homolog 1 (Drosophila) | NA |
Base pairs, HB37
The patient was homozygous for the wild type allele for all rare SNPs or probes encoding mutations on the Immunochip apart from 1 heterozygous parkin variant that is probably non-pathogenic (rs1801334) (MAF 0.04 in European populations as catalogued in ensembl) (eTable 2) and had a MAPT haplotype of H1/H1. The patient carried a total of 18/46 risk alleles for 23 GWAS SNPs (eTable 3) placing him in the 3rd risk quintile shown to confer approximately a 77% increase in PD risk21-23.
Statistical analysis
Table 2 describes the demographic and clinical characteristics of individuals included in the statistical analysis. Overall, 73% (27/37) of individuals were affected, with a higher proportion of females compared to males in the unaffected group (90% versus 10 %). The median (range) age at onset of disease was 47 (31-71) years. The median (range) composite score was 2 (0-6). The median (range) duplication size for affected and unaffected individuals was 0.63 (0.2-5) and 0.6 (0.4-3.47) Mb respectively. There was a weak positive monotonic association between duplication size and composite score (rho=0.17, p=0.314) and between duplication size and age at onset (rho=0.12, p=0.560).
Table 2. Demographic and clinical characteristics of study sample by disease status.
Demographic and clinical characteristics of individuals included in the statistical analysis.
| Disease status | Overall | ||
|---|---|---|---|
| Variable | Unaffected | Affected | |
| Individuals [n (%)] | 10 (27%) | 27 (73%) | 37 (100%) |
| Gender | |||
| Male [n (%)] | 1 (7.14%) | 13 (92.86%) | 14 (37.84%) |
| Female [n (%)] | 9 (39.13%) | 14 (60.87%) | 23 (62.16%) |
| Members per family [median (range)] | NA | NA | 2 (1-7) |
| Age at Onset [median (range)] | NA | 47 (31-71) | 47 (31-71) |
| Duplication size [median (range)]2 | 0.6 (0.4-3.47) | 0.63 (0.2-5) | 0.6 (0.2-5) |
| Composite score [median (range)] | 0 (0-0) | 2 (0-6) | 2 (0-6) |
Mean (standard deviation-SD): 1.17 (1.22) (unaffected), 2.09 (2.12) (affected), 1.84 (1.95) (overall)
Table 3 provides estimates of the effect of duplication size and gender on disease status, age at onset and disease severity. Overall, only the effect of gender on disease status and disease severity reached statistical significance for both univariate and multivariate models (p<0.05). The odds of being affected were more than 8-fold for males compared to females. The corresponding OR (95% CI) was 8.36 (1.97 to 35.42). The odds of being affected increased by approximately 34% for each unit increase in duplication size. The corresponding OR (95% CI) was 1.34 (0.78 to 2.31). Males had a 77% greater chance of developing an early onset form of disease in comparison to females with an OR of 0.23 (95% CI: 0.04 to 1.28), whereas increase in duplication size by one unit resulted in a 6% decrease in the chance of developing late-onset disease (OR, 95% CI: 0.94, 0.71 to 1.24). Males had over 5-fold increase in the chance of developing a more severe disease, and disease severity increased by 17% for each unit increase in duplication size. The corresponding OR (95% CI) were 5.55 (1.39, 22.22) and 1.17 (0.81, 1.68) respectively. (All ORs quoted in-text refer to univariate models).
Table 3.
Estimates for univariate (A) and multivariate models (B). OR represents the odds of affected disease status or late age at onset (> 40 years) for models 1 and 2 respectively (logistic regression models); OR >1 represents the odds of being affected or having a late AAO. OR represents the odds of severe disease outcome compared to mild and moderate for model 3 (ordinal logistic regression), where OR > 1 indicates worse outcome. OR=odds ratio, 95% CI=95% confidence intervals, AIC= Akaike Information Criterion.
| A. Estimates for Univariate models | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| Model 1 | Model 2 | Model 3 | |||||||
| Outcome |
Disease status (Logistic regression with robust standard errors) |
Age at Onset (Logistic regression with robust standard errors) |
Disease severity (Ordinal logistic regression with robust standard errors) |
||||||
| Independent variable | OR (95% CI) | p-value | AIC | OR (95% CI) | p-value | AIC | OR (95% CI) | p-value | AIC |
| Duplication size | 1.34 (0.78, 2.31) | 0.285 | 45.31 | 0.94 (0.71, 1.24) | 0.662 | 39.49 | 1.17 (0.81, 1.68) | 0.395 | 83.78 |
| Gender | |||||||||
| female | base | base | base | ||||||
| male | 8.36 (1.97, 35.42) | 0.004** | 41.99 | 0.23 (0.04, 1.28) | 0.094 | 36.49 | 5.55 (1.39, 22.22) | 0.016* | 78.09 |
| B. Estimates for Multivariate models | |||||||||
| Duplication size | 1.35 (0.77, 2.37) | 0.297 | 42.40 | 0.95 (0.69, 1.31) | 0.766 | 38.43 | 1.12 (0.78, 1.62) | 0.532 | 79.61 |
| Gender | |||||||||
| female | base | base | base | ||||||
| male | 8.26 (1.81, 37.75) | 0.006** | 0.24 (0.04, 1.29) | 0.095 | 5.27 (1.29, 21.50) | 0.021* | |||
DISCUSSION AND CONCLUSION
A total of 29 kindreds carrying SNCA locus duplications have been reported in the literature 24, with pathology information on 4 duplication patients (3 belonging to the same family) 25-29 (eTable 4). The size of the duplicated region has been studied in 16 kindreds/sporadic cases (eTable 4) and has been found to vary greatly, from 0.2Mb 30to 41.2Mb 11 containing from 2 to 150 genes (figure 2E,F, eTable 5). The case described here carries the largest ‘submicroscopic’ duplication of the SNCA locus reported to date.
Even though we were not able to determine the exact breakpoints of the duplication, in accordance with Ibanez et al (2009) 31, we showed that these lay within regions rich in repetitive elements and especially transposable elements (long interspersed elements-LINEs, short interspersed elements-SINEs, and long terminal repeat-LTR retrotransposons) facilitating the insertion of additional copies of flanking sequences in the genome 32, allowing non-homologous recombination events leading to genomic structural rearrangements. The 4q22 region is known to be inherently prone to disruption as a fragile site (FRA4F), which interestingly was originally described as a 7 Mb region corresponding very closely to the duplicated region in this report 33. Subsequent work has extended the fragile site from 88200000-99100000Mb 34 but the centromeric boundary still corresponds to the centromeric breakpoint in our report. Other reported copy number variants (CNVs) are mostly within the confines of the fragile site, although a breakpoint centromeric to the proposed FRA4F boundary has been documented in some cases (figure 2F, G).
This report further emphasises the clinical variability and expands the phenotypic spectrum associated with SNCA duplications and represents the first case with such a complex presentation and substantial overlap with other dementing and psychiatric disorders. SNCA duplications are characterised by incomplete penetrance and variable clinical presentation 27,35 occasionally being indistinguishable from sporadic PD 31 or having the core clinical features of young-onset PD-dementia (YOPD-dementia) with dysautonomia, depression and hallucinations, resembling the phenotype of SNCA triplications 5. Our case exhibits some of the main features associated with SNCA multiplications (YOPD-dementia, hallucinations, dysautonomia) which are, however, part of a more complicated phenotype reminiscent of FTD. Obsessive compulsive disorder (OCD) and anxiety have not been previously reported in any patient with an SNCA duplication, nor was it present in any other member of our family. Thus although it is possible these were causally related in our case III:3, it is more likely a coincidental occurrence. However, this does make it difficult to establish with certainty the exact age at onset of the patient (possibly age 8 or probably age 38 years). Intrafamilial variability is marked as two members had an FTD-like phenotype and 4 family members from older generations apparently had typical PD. CaseII:1, an obligate carrier, was an alcoholic, as was his son III:1, and died at age 70 without documented parkinsonism or dementia.
Neuropathological examination showed features corresponding to idiopathic PD and SNCA duplication cases, with widespread Lewy pathology extending into neocortical regions (Braak stage 6), and no TDP-43 pathology 25,27-29. There was only minimal Alzheimer pathology with tau pathology limited to Braak and Braak stage I and sparse diffuse amyloid β deposition in the cortex. Although previous studies have suggested ‘cross-seeding’ between α-synuclein and tau 36, the relatively low accumulation of tau compared to α-synuclein containing inclusions suggests that this tau accumulation was a normal consequence of aging. Unlike other reported cases of SNCA duplication, neuronal loss was restricted and did not affect the hippocampus 25,28. In further contrast, oligodendroglial inclusions with coiled body morphology were observed, but there were no structures resembling glial cytoplasmic inclusions25. However, as has been reported, hippocampal pathology, particularly neuritic α–synuclein deposition was severe, especially in the CA2 region.
Given that SNCA duplications are frequently non-penetrant 24,27 it is possible that mutations in another gene are responsible for the unusual clinical presentation of the proband. However, there are several lines of evidence supporting the pathogenicity of the duplication: the widespread Lewy body pathology and other pathological features are consistent with SNCA duplications, the pattern of inheritance of the disease in the family is autosomal dominant with possible reduced penetrance, and other affected relatives have phenotypes that have been previously associated with SNCA duplication. Thus, even though we were unable to confirm complete segregation due to the lack of DNA samples, the SNCA duplication is most likely to be responsible for the disease in this family.
The reasons underlying this clinical variability associated with SNCA duplication are currently unknown, although several preliminary hypotheses emerge from our analyses that require validation in future studies on larger cohorts of SNCA duplication carriers. We found trends for association between duplication size and disease status, age at onset and disease severity that didn’t reach statistical significance though association with gender did. Even though our study was underpowered to detect association (n=37) 37,38, it is possible that larger duplication size may not independently produce disease without the contribution of additional risk factors. Gender-specific risk factors are an intriguing possibility as regression model fitting was improved after adjusting for gender (table 3), and males with SNCA duplications had an 8-fold increase in risk for developing PD. A predilection for male involvement is well known for sporadic PD 39,40. Alternatively, an increased burden of other risk factors could have a disease-modifying role; the patient we report carried one single heterozygous parkin rare variant, had an H1H1 MAPT haplotype and an accumulation of risk alleles in loci identified through GWAS. Similarly, Itokawa et al have reported a patient with a SNCA duplication carrying a PD-risk factor (p.G2385R) within LRRK2 35. Other unexplored possibilities include increased dosage of common or rare variants with a regulatory effect on expression or other properties of α-synuclein 41,42, mosaicism 43, environmental factors 44, or stochasticity 45.
In conclusion, patients in whom complex presentations of onset in early adulthood with dementia and parkinsonism complicated by multiple cognitive, psychiatric and motor features should be considered for genetic testing for SNCA multiplication. Understanding of the genetic modifiers influencing the phenotype of SNCA duplications may be important in elucidating the mechanisms underlying α-synuclein accumulation and the formation of Lewy bodies.
Supplementary Material
AKNOWLEDGMENTS
The authors would like to thank the patients and their families for their help and sample donation. This study was supported by the Parkinson’s disease foundation (HH, EK), and the Wellcome Trust/MRC Joint Call in Neurodegeneration award (WT089698) to the UK Parkinson’s Disease Consortium whose members are from the UCL Institute of Neurology, the University of Sheffield, and the MRC Protein Phosphorylation Unit at the University of Dundee. JHolton is supported by the Reta Lila Weston Institute for Neurological Studies, the Multiple System Atrophy Trust, Alzheimer’s Research UK and Parkinson’s UK. AK is supported by the Multiple System Atrophy Trust. This work was supported in part by the Intramural Research Program of the National Institute on Aging, National Institutes of Health, Department of Health and Human Services; project ZO1 .AG000949-08. The authors are grateful to the Queen Square Brain Bank and the South West Dementia Brain Bank (SWDBB) for provision of human tissue. This study was supported by the National Institute for Health Research University College London Hospitals Biomedical Research Centre. EK and HH had full access to all the data in the study and take responsibility for the integrity of the data and the accuracy of the data analysis. Kailash P Bhatia received funding for travel from GlaxoSmithKline, Orion Corporation, Ipsen, and Merz Pharmaceuticals, LLC; serves on the editorial boards of Movement Disorders and Therapeutic Advances in Neurological Disorders; receives royalties from the publication of Oxford Specialist Handbook of Parkinson’s Disease and Other Movement Disorders (Oxford University Press, 2008); received speaker honoraria from GlaxoSmithKline, Ipsen, Merz Pharmaceuticals, LLC, and Sun Pharmaceutical Industries Ltd.; personal compensation for scientific advisory board for GSK and Boehringer Ingelheim; received research support from Ipsen and from the Halley Stewart Trust through Dystonia Society UK, and the Wellcome Trust MRC strategic neurodegenerative disease initiative award (Ref. number WT089698), a grant from the Dystonia Coalition and a grant from Parkinson’s UK (Ref. number G-1009). The funding organisations or sponsors weren’t involved in the design and conduct of the study; collection, management, analysis, and interpretation of the data; and preparation, review, or approval of the manuscript; and decision to submit the manuscript for publication.
Footnotes
Conflict of interest: Kailash P Bhatia received funding for travel from GlaxoSmithKline, Orion Corporation, Ipsen, and Merz Pharmaceuticals, LLC; serves on the editorial boards of Movement Disorders and Therapeutic Advances in Neurological Disorders; receives royalties from the publication of Oxford Specialist Handbook of Parkinson’s Disease and Other Movement Disorders (Oxford University Press, 2008); received speaker honoraria from GlaxoSmithKline, Ipsen, Merz Pharmaceuticals, LLC, and Sun Pharmaceutical Industries Ltd.; personal compensation for scientific advisory board for GSK and Boehringer Ingelheim; received research support from Ipsen and from the Halley Stewart Trust through Dystonia Society UK, and the Wellcome Trust MRC strategic neurodegenerative disease initiative award (Ref. number WT089698), a grant from the Dystonia Coalition and a grant from Parkinson’s UK (Ref. number G-1009).
URLs
UCSC genome browser http://genome.ucsc.edu/
R package ggplot2 http://cran.r-project.org/web/packages/ggplot2/index.html
STATA package gologit2 http://www3.nd.edu/~rwilliam/gologit2/
REFERENCES
- 1.Spillantini MG, Schmidt ML, Lee VM, Trojanowski JQ, Jakes R, Goedert M. Alpha-synuclein in Lewy bodies. Nature. 1997 Aug 28;388(6645):839–840. doi: 10.1038/42166. [DOI] [PubMed] [Google Scholar]
- 2.Polymeropoulos MH, Lavedan C, Leroy E, et al. Mutation in the alpha-synuclein gene identified in families with Parkinson’s disease. Science. 1997 Jun 27;276(5321):2045–2047. doi: 10.1126/science.276.5321.2045. [DOI] [PubMed] [Google Scholar]
- 3.Singleton AB, Farrer M, Johnson J, et al. alpha-Synuclein locus triplication causes Parkinson’s disease. Science. 2003 Oct 31;302(5646):841. doi: 10.1126/science.1090278. [DOI] [PubMed] [Google Scholar]
- 4.Ibanez P, Bonnet AM, Debarges B, et al. Causal relation between alpha-synuclein gene duplication and familial Parkinson’s disease. Lancet. 2004 Sep 25 1;Oct 25 1;364(9440):1169–1171. doi: 10.1016/S0140-6736(04)17104-3. [DOI] [PubMed] [Google Scholar]
- 5.Gwinn K, Devine MJ, Jin LW, et al. Clinical features, with video documentation, of the original familial lewy body parkinsonism caused by alpha-synuclein triplication (Iowa kindred) Mov Disord. 2011 Sep;26(11):2134–2136. doi: 10.1002/mds.23776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kara E, Ling H, Pittman AM, et al. The MAPT p.A152T variant is a risk factor associated with tauopathies with atypical clinical and neuropathological features. Neurobiol Aging. 2012 Sep;33(9):2231e2237–2231 e2214. doi: 10.1016/j.neurobiolaging.2012.04.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Schouten JP, McElgunn CJ, Waaijer R, Zwijnenburg D, Diepvens F, Pals G. Relative quantification of 40 nucleic acid sequences by multiplex ligation-dependent probe amplification. Nucleic Acids Res. 2002 Jun 15;30(12):e57. doi: 10.1093/nar/gnf056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Plagnol V, Nalls MA, Bras JM, et al. A two-stage meta-analysis identifies several new loci for Parkinson’s disease. PLoS Genet. 2011 Jun;7(6):e1002142. doi: 10.1371/journal.pgen.1002142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Jostins L, Ripke S, Weersma RK, et al. Host-microbe interactions have shaped the genetic architecture of inflammatory bowel disease. Nature. 2012 Nov 1;491(7422):119–124. doi: 10.1038/nature11582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Faraco J, Lin L, Kornum BR, et al. ImmunoChip study implicates antigen presentation to T cells in narcolepsy. PLoS Genet. 2013;9(2):e1003270. doi: 10.1371/journal.pgen.1003270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Garraux G, Caberg JH, Vanbellinghen JF, et al. Partial trisomy 4q associated with young-onset dopa-responsive parkinsonism. Arch Neurol. 2012 Mar;69(3):398–400. doi: 10.1001/archneurol.2011.802. [DOI] [PubMed] [Google Scholar]
- 12.Williams RL. A note on robust variance estimation for cluster-correlated data. Biometrics. 2000 Jun;56(2):645–646. doi: 10.1111/j.0006-341x.2000.00645.x. [DOI] [PubMed] [Google Scholar]
- 13.Harrell FE, Jr., Margolis PA, Gove S, et al. WHO/ARI Young Infant Multicentre Study Group Development of a clinical prediction model for an ordinal outcome: the World Health Organization Multicentre Study of Clinical Signs and Etiological agents of Pneumonia, Sepsis and Meningitis in Young Infants. Stat Med. 1998 Apr 30;17(8):909–944. doi: 10.1002/(sici)1097-0258(19980430)17:8<909::aid-sim753>3.0.co;2-o. [DOI] [PubMed] [Google Scholar]
- 14.Austin PC. Estimating multilevel logistic regression models when the number of clusters is low: a comparison of different statistical software procedures. Int J Biostat. 2010;6(1) doi: 10.2202/1557-4679.1195. Article 16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Chirwa ED, Griffiths PL, Maleta K, Norris SA, Cameron N. Multi-level modelling of longitudinal child growth data from the Birth-to-Twenty Cohort: a comparison of growth models. Ann Hum Biol. 2013 Oct 11; doi: 10.3109/03014460.2013.839742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.StataCorp. Stata Statistical Software: Release 12. StataCorp LP; College Station, TX: 2011. [Google Scholar]
- 17.Team RC. R: A language and environment for statistical computing. R Foundation for Statistical Computing. Vienna, Austria: 2013. URL http://www.R-project.org/ [Google Scholar]
- 18.Wickham H. ggplot2: elegant graphics for data analysis. Springer; New York: 2009. [Google Scholar]
- 19.Braak H, Alafuzoff I, Arzberger T, Kretzschmar H, Del Tredici K. Staging of Alzheimer disease-associated neurofibrillary pathology using paraffin sections and immunocytochemistry. Acta Neuropathol. 2006 Oct;112(4):389–404. doi: 10.1007/s00401-006-0127-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.McKeith IG, Dickson DW, Lowe J, et al. Diagnosis and management of dementia with Lewy bodies: third report of the DLB Consortium. Neurology. 2005 Dec 27;65(12):1863–1872. doi: 10.1212/01.wnl.0000187889.17253.b1. [DOI] [PubMed] [Google Scholar]
- 21.Nalls MA, Plagnol V, Hernandez DG, et al. Imputation of sequence variants for identification of genetic risks for Parkinson’s disease: a meta-analysis of genome-wide association studies. Lancet. 2011 Feb 19;377(9766):641–649. doi: 10.1016/S0140-6736(10)62345-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hernandez DG, Nalls MA, Ylikotila P, et al. Genome wide assessment of young onset Parkinson’s disease from Finland. PLoS One. 2012;7(7):e41859. doi: 10.1371/journal.pone.0041859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kara E, Xiromerisiou G, Spanaki C, et al. Assessment of Parkinson’s disease risk loci in Greece. Neurobiol Aging. 2013 Sep 27; doi: 10.1016/j.neurobiolaging.2013.07.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Elia A, Petrucci S, Fasano A, et al. Alpha-synuclein gene duplication: Marked intrafamilial variability in two novel pedigrees. Mov Disord. 2013 doi: 10.1002/mds.25518. [DOI] [PubMed] [Google Scholar]
- 25.Ikeuchi T, Kakita A, Shiga A, et al. Patients homozygous and heterozygous for SNCA duplication in a family with parkinsonism and dementia. Arch Neurol. 2008 Apr;65(4):514–519. doi: 10.1001/archneur.65.4.514. [DOI] [PubMed] [Google Scholar]
- 26.Wakabayashi K, Hayashi S, Ishikawa A, et al. Autosomal dominant diffuse Lewy body disease. Acta Neuropathol. 1998 Aug;96(2):207–210. doi: 10.1007/s004010050883. [DOI] [PubMed] [Google Scholar]
- 27.Nishioka K, Hayashi S, Farrer MJ, et al. Clinical heterogeneity of alpha-synuclein gene duplication in Parkinson’s disease. Ann Neurol. 2006 Feb;59(2):298–309. doi: 10.1002/ana.20753. [DOI] [PubMed] [Google Scholar]
- 28.Obi T, Nishioka K, Ross OA, et al. Clinicopathologic study of a SNCA gene duplication patient with Parkinson disease and dementia. Neurology. 2008 Jan 15;70(3):238–241. doi: 10.1212/01.wnl.0000299387.59159.db. [DOI] [PubMed] [Google Scholar]
- 29.Ross OA, Braithwaite AT, Skipper LM, et al. Genomic investigation of alpha-synuclein multiplication and parkinsonism. Ann Neurol. 2008 Jun;63(6):743–750. doi: 10.1002/ana.21380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Nishioka K, Ross OA, Ishii K, et al. Expanding the clinical phenotype of SNCA duplication carriers. Mov Disord. 2009 Sep 15;24(12):1811–1819. doi: 10.1002/mds.22682. [DOI] [PubMed] [Google Scholar]
- 31.Ibanez P, Lesage S, Janin S, et al. Alpha-synuclein gene rearrangements in dominantly inherited parkinsonism: frequency, phenotype, and mechanisms. Arch Neurol. 2009 Jan;66(1):102–108. doi: 10.1001/archneurol.2008.555. [DOI] [PubMed] [Google Scholar]
- 32.Ivics Z, Izsvak Z. Repetitive elements and genome instability. Semin Cancer Biol. 2010 Aug;20(4):197–199. doi: 10.1016/j.semcancer.2010.08.002. [DOI] [PubMed] [Google Scholar]
- 33.Rozier L, El-Achkar E, Apiou F, Debatisse M. Characterization of a conserved aphidicolin-sensitive common fragile site at human 4q22 and mouse 6C1: possible association with an inherited disease and cancer. Oncogene. 2004 Sep 9;23(41):6872–6880. doi: 10.1038/sj.onc.1207809. [DOI] [PubMed] [Google Scholar]
- 34.Fungtammasan A, Walsh E, Chiaromonte F, Eckert KA, Makova KD. A genome-wide analysis of common fragile sites: what features determine chromosomal instability in the human genome? Genome Res. 2012 Jun;22(6):993–1005. doi: 10.1101/gr.134395.111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Itokawa K, Sekine T, Funayama M, et al. A case of alpha-synuclein gene duplication presenting with head-shaking movements. Mov Disord. 2013 Mar;28(3):384–387. doi: 10.1002/mds.25243. [DOI] [PubMed] [Google Scholar]
- 36.Guo JL, Covell DJ, Daniels JP, et al. Distinct alpha-Synuclein Strains Differentially Promote Tau Inclusions in Neurons. Cell. 2013 Jul 3;154(1):103–117. doi: 10.1016/j.cell.2013.05.057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Bonett DG, Right TAW. SAMPLE SIZE REQUIREMENTS FOR ESTIMATING PEARSON, KENDALL AND SPEARMAN CORRELATIONS. PSYCHOMETRIKA. 2000;65(1):23–28. [Google Scholar]
- 38.Hsieh FY. Sample size tables for logistic regression. Stat Med. 1989 Jul;8(7):795–802. doi: 10.1002/sim.4780080704. [DOI] [PubMed] [Google Scholar]
- 39.Wooten GF, Currie LJ, Bovbjerg VE, Lee JK, Patrie J. Are men at greater risk for Parkinson’s disease than women? J Neurol Neurosurg Psychiatry. 2004 Apr;75(4):637–639. doi: 10.1136/jnnp.2003.020982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Taylor KS, Cook JA, Counsell CE. Heterogeneity in male to female risk for Parkinson’s disease. J Neurol Neurosurg Psychiatry. 2007 Aug;78(8):905–906. doi: 10.1136/jnnp.2006.104695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Spencer CC, Plagnol V, Strange A, et al. Dissection of the genetics of Parkinson’s disease identifies an additional association 5′ of SNCA and multiple associated haplotypes at 17q21. Hum Mol Genet. 2011 Jan 15;20(2):345–353. doi: 10.1093/hmg/ddq469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Rhinn H, Qiang L, Yamashita T, et al. Alternative alpha-synuclein transcript usage as a convergent mechanism in Parkinson’s disease pathology. Nat Commun. 2012;3:1084. doi: 10.1038/ncomms2032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Proukakis C, Houlden H, Schapira AH. Somatic alpha-synuclein mutations in Parkinson’s disease: Hypothesis and preliminary data. Mov Disord. 2013 May 14; doi: 10.1002/mds.25502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Baranzini SE, Mudge J, van Velkinburgh JC, et al. Genome, epigenome and RNA sequences of monozygotic twins discordant for multiple sclerosis. Nature. 2010 Apr 29;464(7293):1351–1356. doi: 10.1038/nature08990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Xiromerisiou G, Houlden H, Sailer A, Silveira-Moriyama L, Hardy J, Lees AJ. Identical twins with Leucine rich repeat kinase type 2 mutations discordant for Parkinson’s disease. Mov Disord. 2012 Sep 1;27(10):1323. doi: 10.1002/mds.24924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Simon-Sanchez J, Scholz S, Fung HC, et al. Genome-wide SNP assay reveals structural genomic variation, extended homozygosity and cell-line induced alterations in normal individuals. Hum Mol Genet. 2007 Jan 1;16(1):1–14. doi: 10.1093/hmg/ddl436. [DOI] [PubMed] [Google Scholar]
- 47.Simon-Sanchez J, Schulte C, Bras JM, et al. Genome-wide association study reveals genetic risk underlying Parkinson’s disease. Nat Genet. 2009 Dec;41(12):1308–1312. doi: 10.1038/ng.487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Zhang X, Zhao J, Li C, et al. DSPP mutation in dentinogenesis imperfecta Shields type II. Nat Genet. 2001 Feb;27(2):151–152. doi: 10.1038/84765. [DOI] [PubMed] [Google Scholar]
- 49.Xiao S, Yu C, Chou X, et al. Dentinogenesis imperfecta 1 with or without progressive hearing loss is associated with distinct mutations in DSPP. Nat Genet. 2001 Feb;27(2):201–204. doi: 10.1038/84848. [DOI] [PubMed] [Google Scholar]
- 50.Lorenz-Depiereux B, Bastepe M, Benet-Pages A, et al. DMP1 mutations in autosomal recessive hypophosphatemia implicate a bone matrix protein in the regulation of phosphate homeostasis. Nat Genet. 2006 Nov;38(11):1248–1250. doi: 10.1038/ng1868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Mochizuki T, Wu G, Hayashi T, et al. PKD2, a gene for polycystic kidney disease that encodes an integral membrane protein. Science. 1996 May 31;272(5266):1339–1342. doi: 10.1126/science.272.5266.1339. [DOI] [PubMed] [Google Scholar]
- 52.Hills LB, Masri A, Konno K, et al. Deletions in GRID2 lead to a recessive syndrome of cerebellar ataxia and tonic upgaze in humans. Neurology. 2013 Sep 27; doi: 10.1212/WNL.0b013e3182a841a3. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.