The C-type lectin receptor CLEC-2 is expressed primarily on the surface of platelets, where it is present as a dimer, and is found at low level on a subpopulation of other haematopoietic cells, including mouse neutrophils [1-4] Clustering of CLEC-2 by the snake venom toxin rhodocytin, specific antibodies or its endogenous ligand, podoplanin, elicits powerful activation of platelets through a pathway that is similar to that used by the collagen receptor GPVI [4-6]. The cytosolic tail of CLEC-2 contains a conserved YxxL sequence preceded by three upstream acidic amino acid residues, which together form a novel motif known as a hemITAM. Ligand engagement induces tyrosine phosphorylation of the hemITAM sequence providing docking sites for the tandem-SH2 domains of the tyrosine kinase Syk across a CLEC-2 receptor dimer [3]. Tyrosine phosphorylation of Syk by Src family kinases and through autophosphorylation leads to stimulation of a downstream signalling cascade that culminates in activation of phospholipase C γ2 (PLCγ2) [4, 6].
Recently, CLEC-2 has been proposed to play a major role in supporting activation of platelets at arteriolar rates of flow [1]. Injection of a CLEC-2 antibody into mice causes a sustained depletion of the C-type lectin receptor from the platelet surface [1]. The CLEC-2-depleted platelets were unresponsive to rhodocytin but underwent normal aggregation and secretion responses following stimulation of other platelet receptors, including GPVI [1]. In contrast, there was a marked decrease in aggregate formation relative to controls when CLEC-2-depleted blood was flowed at arteriolar rates of shear over collagen (1000s−1 and 1700s−1) [1]. Furthermore, antibody-treatment significantly increased tail bleeding times and mice were unable to occlude their vessels following ferric chloride injury [1]. These data provide evidence for a critical role for CLEC-2 in supporting platelet aggregation at arteriolar rates of flow. The underlying mechanism is unclear as platelets do not express podoplanin, the only known endogenous ligand of CLEC-2.
In the present study we have investigated the role of CLEC-2 in platelet aggregation and thrombus formation using platelets from a novel mutant mouse model that lacks functional CLEC-2.
Reagents
FITC-conjugated rat α-mouse αIIbβ3, α2, GPVI, GPIbα and rat IgG antibodies were from Emfret Analytics (Würzburg, Germany). All other reagents were from previously described sources [2, 4, 7, 8].
Radiation chimeric mice
CLEC-2 mutant mice were engineered as described in the supplementary information. Due to lethality in late gestation or shortly after birth, radiation chimeric mice were made by crossing CLEC-2+/− mice and harvesting foetal livers taken between E14.5-16.5. 6 week old C57Bl/6 mice were treated with Baytril for one week followed by irradiation with two doses of 500Gy, 3hrs apart. Mice were then injected with 1.5 × 106 CLEC-2+/+ or CLEC-2−/− foetal liver cells via tail vein. The genotype of reconstituting cells was confirmed by PCR. Mice were used for experimentation 6-8 weeks post-transplantation. All procedures were undertaken with United Kingdom Home Office approval.
Platelet studies
Blood was drawn from CO2-asphyxiated mice into sodium-heparin/PPACK. Platelet-rich-plasma and washed platelets were prepared by centrifugation. Washed platelets were resuspended in modified-Tyrodes buffer [8]. Immunoprecipitation (IP), western blotting and flow cytometry studies were performed on washed platelets using α-CLEC-2 under non-reducing conditions or other antibodies according to manufacturer’s guidelines. Activation was monitored by expression of P-selectin and fibrinogen binding using flow cytometry following stimulation.
Flow adhesion and tail bleeding
Blood was drawn as previously described into sodium-heparin (5U/ml or 15U/ml) and PPACK (40μM or 120μM) to prevent thrombin generation. The two combinations of anticoagulants were used to mimic those used in previous studies, with similar results were observed with either combination [1, 9]. Glass capillary tubes (Camlab, Cambridge, UK) were coated with 100μg/ml Horm-collagen (Nycomed, Munich, Germany) for 1hr at room temperature then blocked with 5mg/ml heat-inactivated BSA for 1h at room temperature. This collagen concentration has previously been shown to be saturating for platelet adhesion [10]. Blood was perfused through the capillary for 4 min at shear rates of 1000s−1 or 1700s−1 at 37°C or room temperature followed by imaging by DIC microscopy. Samples were lysed at the end of the flow with NP-40 lysis buffer and analysed by IP and SDS-PAGE. Tail bleeding times were monitored as previously described [7].
Tyrosine phosphorylation of CLEC-2
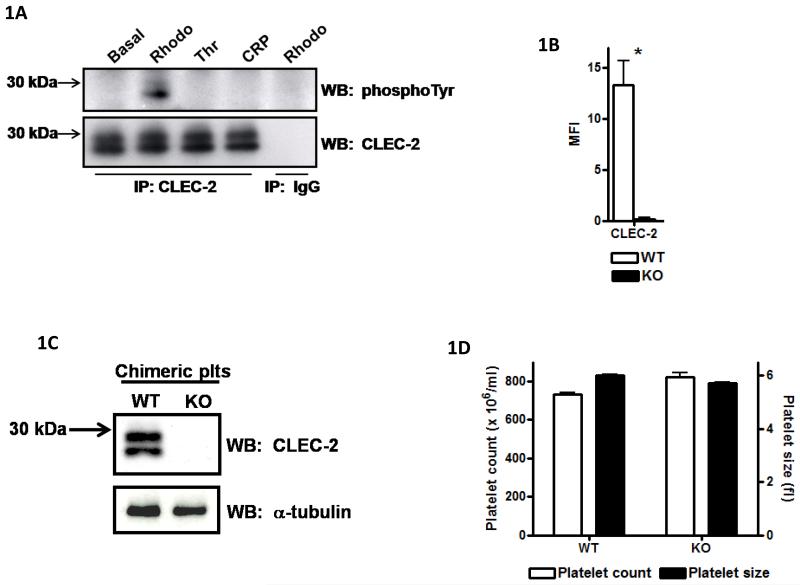
The proposal that CLEC-2 plays a critical role in supporting platelet aggregation under shear [1] suggests the presence of an endogenous ligand that is either released or expressed on the surface following platelet activation. However, we have not observed a significant increase in tyrosine phosphorylation of CLEC-2 in mouse platelets under aggregating conditions in the absence of shear or at arteriolar shear on a collagen matrix as illustrated in Figure-1A and Supplementary-A or in longer exposures (not shown). The former studies were performed in washed platelets and in platelet-rich-plasma and the latter in whole blood. Similar results were also observed for human platelets (not shown). As previously shown, CLEC-2 runs as a dimer due to glycosylation and both forms are phosphorylated [4]. These results provide evidence against the presence of an activating ligand for CLEC-2 in platelets or in plasma but do not rule out the presence of a non-stimulatory ligand for CLEC-2 which may support aggregation through an adhesive-based mechanism.
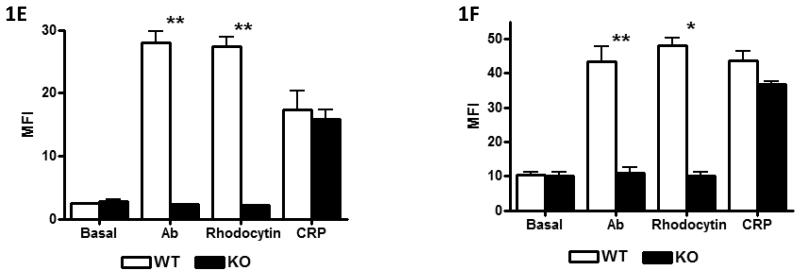
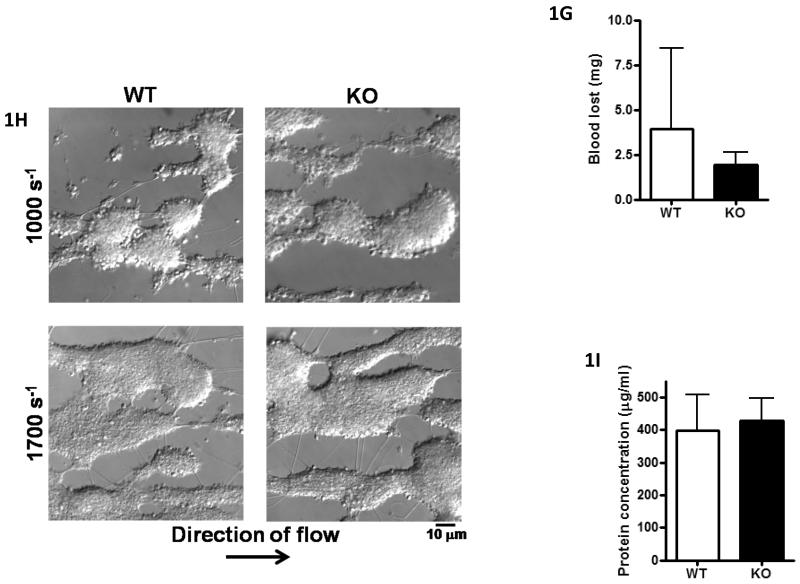
Figure-1. CLEC-2−/− platelets do not respond to CLEC-2 agonists but have normal tail bleeding and aggregation on collagen under flow.
A) Washed platelets (5×108/ml) under basal, rhodocytin (300nM), thrombin (1U/ml) or CRP-aggregated (3μg/ml) conditions were lysed with 2xNP40 lysis buffer. Lysates were immunoprecipitated with α-CLEC-2 or non-specific IgG antibodies and protein G sepharose. Precipitated proteins were separated by non-reducing SDS-PAGE and western blotted for phosphotyrosine and CLEC-2. Results are representative of 3 experiments. B) Washed platelets (2×107/ml) were stained with 1μg α-CLEC-2 or non-specific IgG for 20min followed by staining with FITC-conjugated α-rat IgG for 20min in the dark. Platelets were analysed by flow cytometry and non-specific IgG readings subtracted. Results are from 8 experiments. *p<0.05. C) Washed platelets (2×108/ml) were lysed with an equal volume of 2x non-reducing SDS sample buffer, separated by SDS-PAGE and western blotted for CLEC-2 and α-tubulin as a loading control. Results are representative of 3 experiments. D) Platelet number (×106/ml) and platelet size (fl) were analysed using a Blood Function Analyser. Results are from 8 experiments. E+F) Washed platelets (2×107/ml) were stimulated with rhodocytin (100nM), α-CLEC-2 (10μg) or CRP (10μg/ml) for 2 min followed by staining with 5μl FITC-conjugated α-P-selectin antibody (E) or FITC-fibrinogen (F), for 30 min in the dark. Platelets were then analysed by flow cytometry. Results are from 3-4 experiments. **p<0.001, *p<0.05. G) A 0.2cm portion of tail was removed from anaesthetised mice. The assay was halted if blood loss had stopped for more than 5min. The amount of blood lost was determined by weight (shown ± standard deviation). Results are from 14 mice per group. H, I) Anticoagulated (to prevent thrombin generation) whole blood was passed through collagen-coated glass capillaries at a shear rate of 1000s−1 or 1700s−1 for 4 minutes. (H) Platelets were imaged by DIC microscopy then (I) lysed with NP40 lysis buffer and protein concentration determined (result shown for 1000s−1 only). Results are representative of 3-4 experiments.
CLEC-2 mutant mice
Genetic targeting of CLEC-2 to remove exons 3 and 4 and introduction of an early STOP codon was performed by Taconic Artemis (Cologne, Germany) (Supplementary-B). Mice were bred as heterozygotes on a mixed C57Bl/6 × 129Sv background. The majority of the knockouts die either before or just after birth, with only three mice surviving to 4 weeks out of more than 150 mice that were genotyped at this time, and both were extremely sick and had to be sacrificed. The phenotype of the mutant embryos is similar to that recently reported by Bertozzi et al [11] with abundant blood filled lymphatics and severe oedema, and will be described in detail in a future publication. Foetal livers were taken from E14.5-E16.5 mice and used as donor cells for lethally irradiated mice to generate CLEC-2-deficient haematopoietic cells and wild-type controls.
Blood was drawn from chimeric mice 7–8 weeks post-transplantation. Antibody staining by flow cytometry confirmed that platelets had no detectable CLEC-2 on their surface (Figure-1B), whereas levels of GPVI, αIIbβ3, GPIbα and α2 were similar in the two populations (Supplementary-C). The lack of expression of CLEC-2 protein in platelets was confirmed by western blotting (Figure-1C). Platelet size and counts were similar between both sets of mice (Figure-1D). Activation of αIIbβ3 and α-granule secretion as measured using FITC-fibrinogen and P-selectin staining were abrogated in response to rhodocytin or an α-CLEC-2 antibody in mutant but not control platelets, whereas the response to agonists for other receptors was unaffected, as shown for collagen-related-peptide (CRP) which is selective to GPVI (Figure-1E+F). These data confirm loss of functional CLEC-2 and demonstrate that the C-type lectin receptor is not required for platelet activation by other agonists in a low shear environment, in agreement with the results of May et al [1]. These data also confirm CLEC-2 as the major platelet receptor for the snake toxin rhodocytin.
Tail bleeding and platelet aggregation under flow are not altered in the absence of CLEC-2
Tail bleeding times were measured in the CLEC-2-deficient mice following removal of 0.2cm of tail as previously described [7]. There was no significant difference in the bleeding times of CLEC-2-deficient and irradiated wild-type mice (Figure-1G). These observations contrast with the increase in tail bleeding observed in antibody-depleted mice [1] and in our studies in mice-deficient in the major signalling receptor for collagen, the GPVI-FcRγ-chain complex [7, 12]. There was also no apparent difference in platelet aggregation on collagen when blood was flowed at 1000s−1 or 1700s−1 for 4 min (Figure 1H) using the same combination of anticoagulants as used by May et al [1]. Similar results were also obtained at room temperature (not shown). Measurement of protein and surface area confirmed the lack of a significant difference in aggregate formation at 1000s−1 or 1700s−1 (Figure 1I and not shown).
These results provide evidence against a role for CLEC-2 in supporting platelet aggregation at arteriolar shear in vitro and in haemostasis as monitored by tail bleeding time in contrast to the observations of May et al [1] using a specific antibody to deplete CLEC-2 from the platelet surface. This conclusion is consistent with the observation of the absence of tyrosine phosphorylation of CLEC-2 in platelets under aggregating conditions, which argues against the presence of an activating CLEC-2 ligand. The differing observations using the two approaches suggest that antibody depletion of CLEC-2 has effects that are additional to loss of CLEC-2 that impair platelet activation.
Following submission of this study, a manuscript describing the generation of a new CLEC-2 knockout mouse was published [13]. In agreement with our observations but in contrast to those of May et al [1], Suzuki-Inoue et al [13] reported that tail bleeding was not altered in mice deficient in CLEC-2. On the other hand, they observed a partial decrease in platelet aggregation on collagen at a shear rate of 2000s−1, which is similar to the observation of May et al [1]. We do not know the explanation for the difference to the observations found in the present study as the anticoagulant was the same and the shear was only slightly higher than the maximal rate that we used of 1700s−1. We note however that this experiment was performed twice and that the platelet count was measured one week before the experiment rather than on the day itself. Given the bleeding that is seen in the intestine of the chimeric mice there could have been a decrease in platelet count on the day of the experiment. Suzuki-Inoue et al [13] reported that CLEC-2 undergoes a homophilic interaction, although the results of our study would suggest that such an interaction does not give rise to tyrosine phosphorylation of CLEC-2. We have been unable to analyse thrombus formation in the mesentery of the CLEC-2 chimeric mice due to the extensive bleeding in this region that occurs upon dissection, which is also seen in mice deficient in Syk, which plays a critical role in platelet activation by CLEC-2 [14].
Supplementary Material
Supplementary A) Platelet-rich-plasma under basal, rhodocytin (300nM), thrombin (1U/ml) and GPRP (2.5mM) or CRP-aggregated (3μg/ml) conditions were pelleted and rapidly lysed with NP40 lysis buffer. Whole blood was passed through collagen-coated glass capillaries at a shear rate of 1000s−1. Platelets were then lysed with NP40 lysis buffer. Lysates were immunoprecipitated with α-CLEC-2 or non-specific IgG antibodies and protein G sepharose. Precipitated proteins were separated by non-reducing SDS-PAGE and western blotted for phosphotyrosine and CLEC-2. Results are representative of 3 experiments.
B) Exons 3 and 4 of the CLEC-2 gene locus were flanked with LoxP sites and the puromycin selection cassette was flanked with FRT (Flippase recognition target) sites. Cross-breeding of targeted mice with Flp-expressing mice generates floxed mice missing the puromycin cassette. Further cross-breeding of the floxed mice with Cre-expressing mice generates a mutated CLEC-2 locus missing exons 3 and 4 and containing a premature STOP codon preventing expression of exons 5 and 6.
C) Washed platelets (2×107/ml) were stained with 3μl FITC-conjugated α-GPVI, α-αIIbβ3, α-α2, α-GPIbα or non-specific IgG antibodies for 30min in the dark. Platelets were then analysed by flow cytometry and non-specific IgG readings subtracted. Results are from 3-4 experiments.
Acknowledgements
We would like to thank Dr Caetano Reis e Sousa for the CLEC-2 antibody and for valuable discussion, Dr Johannes Eble for providing rhodocytin, Dr Beata Grygielska for genotyping and Ian Ricketts in the Biomedical Service Unit for generating the radiation chimeric mice. This work was supported by the Wellcome Trust (073107 and 088410) and the British Heart Foundation (PG/07/116). L.N-N holds a postdoctoral position funded by Fundación Séneca. S.P.W. holds a BHF Chair (CH/03/003).
References
- 1.May F, Hagedorn I, Pleines I, Bender M, Vogtle T, Eble J, Elvers M, Nieswandt B. CLEC-2 is an essential platelet-activating receptor in hemostasis and thrombosis. Blood. 2009;114:3464–72. doi: 10.1182/blood-2009-05-222273. [DOI] [PubMed] [Google Scholar]
- 2.Kerrigan AM, Dennehy KM, Mourao-Sa D, Faro-Trindade I, Willment JA, Taylor PR, Eble JA, Reis e Sousa C, Brown GD. CLEC-2 is a phagocytic activation receptor expressed on murine peripheral blood neutrophils. J Immunol. 2009;182:4150–7. doi: 10.4049/jimmunol.0802808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hughes CE, Pollitt AY, Mori J, Eble JA, Tomlinson MG, Hartwig JH, O’Callaghan CA, Futterer K, Watson SP. CLEC-2 activates Syk through dimerization. Blood. 2010;115:2947–55. doi: 10.1182/blood-2009-08-237834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Suzuki-Inoue K, Fuller GL, Garcia A, Eble JA, Pohlmann S, Inoue O, Gartner TK, Hughan SC, Pearce AC, Laing GD, Theakston RD, Schweighoffer E, Zitzmann N, Morita T, Tybulewicz VL, Ozaki Y, Watson SP. A novel Syk-dependent mechanism of platelet activation by the C-type lectin receptor CLEC-2. Blood. 2006;107:542–9. doi: 10.1182/blood-2005-05-1994. [DOI] [PubMed] [Google Scholar]
- 5.Suzuki-Inoue K, Kato Y, Inoue O, Kaneko MK, Mishima K, Yatomi Y, Yamazaki Y, Narimatsu H, Ozaki Y. Involvement of the snake toxin receptor CLEC-2, in podoplanin-mediated platelet activation, by cancer cells. J Biol Chem. 2007;282:25993–6001. doi: 10.1074/jbc.M702327200. [DOI] [PubMed] [Google Scholar]
- 6.Fuller GL, Williams JA, Tomlinson MG, Eble JA, Hanna SL, Pohlmann S, Suzuki-Inoue K, Ozaki Y, Watson SP, Pearce AC. The C-type lectin receptors CLEC-2 and Dectin-1, but not DC-SIGN, signal via a novel YXXL-dependent signaling cascade. J Biol Chem. 2007;282:12397–409. doi: 10.1074/jbc.M609558200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kalia N, Auger JM, Atkinson B, Watson SP. Critical Role of FcR gamma-Chain, LAT, PLCgamma2 and Thrombin in Arteriolar Thrombus Formation upon Mild, Laser-Induced Endothelial Injury In Vivo. Microcirculation. 2008;15:325–35. doi: 10.1080/10739680701728822. [DOI] [PubMed] [Google Scholar]
- 8.Suzuki-Inoue K, Inoue O, Frampton J, Watson SP. Murine GPVI stimulates weak integrin activation in PLCgamma2−/− platelets: involvement of PLCgamma1 and PI3-kinase. Blood. 2003;102:1367–73. doi: 10.1182/blood-2003-01-0029. [DOI] [PubMed] [Google Scholar]
- 9.Auger JM, Kuijpers MJ, Senis YA, Watson SP, Heemskerk JW. Adhesion of human and mouse platelets to collagen under shear: a unifying model. Faseb J. 2005;19:825–7. doi: 10.1096/fj.04-1940fje. [DOI] [PubMed] [Google Scholar]
- 10.Thornber K, McCarty OJ, Watson SP, Pears CJ. Distinct but critical roles for integrin alphaIIbbeta3 in platelet lamellipodia formation on fibrinogen, collagen-related peptide and thrombin. Febs J. 2006;273:5032–43. doi: 10.1111/j.1742-4658.2006.05500.x. [DOI] [PubMed] [Google Scholar]
- 11.Bertozzi CC, Schmaier AA, Mericko P, Hess PR, Zou Z, Chen M, Chen CY, Xu B, Lu MM, Zhou D, Sebzda E, Santore MT, Merianos DJ, Stadtfeld M, Flake AW, Graf T, Skoda R, Maltzman JS, Koretzky GA, Kahn ML. Platelets regulate lymphatic vascular development through CLEC-2-SLP-76 signaling. Blood. 2010 doi: 10.1182/blood-2010-02-270876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Best D, Senis YA, Jarvis GE, Eagleton HJ, Roberts DJ, Saito T, Jung SM, Moroi M, Harrison P, Green FR, Watson SP. GPVI levels in platelets: relationship to platelet function at high shear. Blood. 2003;102:2811–8. doi: 10.1182/blood-2003-01-0231. [DOI] [PubMed] [Google Scholar]
- 13.Suzuki-Inoue K, Inoue O, Ding G, Nishimura S, Hokamura K, Eto K, Kashiwagi H, Tomiyama Y, Yatomi Y, Umemura K, Shin Y, Hirashima M, Ozaki Y. Essential in vivo roles of the c-type lectin receptor CLEC-2: Embryonic/neonatal lethality of CLEC-2-deficient mice by blood/lymphatic misconnections and impaired thrombus formation of CLEC-2-deficient platelets. J Biol Chem. 2010 doi: 10.1074/jbc.M110.130575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Turner M, Mee PJ, Costello PS, Williams O, Price AA, Duddy LP, Furlong MT, Geahlen RL, Tybulewicz VL. Perinatal lethality and blocked B-cell development in mice lacking the tyrosine kinase Syk. Nature. 1995;378:298–302. doi: 10.1038/378298a0. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary A) Platelet-rich-plasma under basal, rhodocytin (300nM), thrombin (1U/ml) and GPRP (2.5mM) or CRP-aggregated (3μg/ml) conditions were pelleted and rapidly lysed with NP40 lysis buffer. Whole blood was passed through collagen-coated glass capillaries at a shear rate of 1000s−1. Platelets were then lysed with NP40 lysis buffer. Lysates were immunoprecipitated with α-CLEC-2 or non-specific IgG antibodies and protein G sepharose. Precipitated proteins were separated by non-reducing SDS-PAGE and western blotted for phosphotyrosine and CLEC-2. Results are representative of 3 experiments.
B) Exons 3 and 4 of the CLEC-2 gene locus were flanked with LoxP sites and the puromycin selection cassette was flanked with FRT (Flippase recognition target) sites. Cross-breeding of targeted mice with Flp-expressing mice generates floxed mice missing the puromycin cassette. Further cross-breeding of the floxed mice with Cre-expressing mice generates a mutated CLEC-2 locus missing exons 3 and 4 and containing a premature STOP codon preventing expression of exons 5 and 6.
C) Washed platelets (2×107/ml) were stained with 3μl FITC-conjugated α-GPVI, α-αIIbβ3, α-α2, α-GPIbα or non-specific IgG antibodies for 30min in the dark. Platelets were then analysed by flow cytometry and non-specific IgG readings subtracted. Results are from 3-4 experiments.