Abstract
BACKGROUND
Young women wishing to become living kidney donors frequently ask whether nephrectomy will affect their future pregnancies.
METHODS
We conducted a retrospective cohort study of living kidney donors involving 85 women (131 pregnancies after cohort entry) who were matched in a 1:6 ratio with 510 healthy nondonors from the general population (788 pregnancies after cohort entry). Kidney donations occurred between 1992 and 2009 in Ontario, Canada, with follow-up through linked health care databases until March 2013. Donors and nondonors were matched with respect to age, year of cohort entry, residency (urban or rural), income, number of pregnancies before cohort entry, and the time to the first pregnancy after cohort entry. The primary outcome was a hospital diagnosis of gestational hypertension or preeclampsia. Secondary outcomes were each component of the primary outcome examined separately and other maternal and fetal outcomes.
RESULTS
Gestational hypertension or preeclampsia was more common among living kidney donors than among nondonors (occurring in 15 of 131 pregnancies [11%] vs. 38 of 788 pregnancies [5%]; odds ratio for donors, 2.4; 95% confidence interval, 1.2 to 5.0; P = 0.01). Each component of the primary outcome was also more common among donors (odds ratio, 2.5 for gestational hypertension and 2.4 for preeclampsia). There were no significant differences between donors and nondonors with respect to rates of preterm birth (8% and 7%, respectively) or low birth weight (6% and 4%, respectively). There were no reports of maternal death, stillbirth, or neonatal death among the donors. Most women had uncomplicated pregnancies after donation.
CONCLUSIONS
Gestational hypertension or preeclampsia was more likely to be diagnosed in kidney donors than in matched nondonors with similar indicators of baseline health. (Funded by the Canadian Institutes of Health Research and others.)
Each year, more than 27,000 persons worldwide become living kidney donors; the majority are women.1 Young female donors frequently ask whether kidney donation will affect future pregnancies.2 In late pregnancy, animals that have undergone uninephrectomy have higher levels of blood pressure and urinary protein excretion than control animals with two kidneys.3,4 In humans, the glomerular filtration rate is reduced by about 35% early after donor nephrectomy,5 and women with a similar loss of kidney function from various diseases are at increased risk for preeclampsia.6 Studies of the risk of nongestational hypertension among kidney donors, as compared with nondonors, have had conflicting results, with some studies showing an increased risk7,8 and others showing no increase in risk.9,10
A prominent 2004 international conference concluded that kidney donation poses no risk with respect to future pregnancies.11 However, two subsequent studies, one from Norway and the other from the United States, showed an increased risk of gestational hypertension and preeclampsia in pregnancies after kidney donation, as compared with pregnancies before donation.12,13 Those findings have been debated,14-17 and many transplantation programs have not incorporated this information into their informed-consent processes. We conducted this study to determine whether donors have a higher risk of gestational hypertension or preeclampsia than do nondonors with similar indicators of baseline health. We also compared other maternal and fetal outcomes.
METHODS
STUDY DESIGN
We conducted a retrospective, matched-cohort study using linked health care databases in Ontario, Canada, where citizens have universal access to hospital care and physician services. The conduct and reporting of the study followed guidelines for observational studies (see Table S1 in the Supplementary Appendix, available with the full text of this article at NEJM.org).18 The study was designed by the authors and approved by a regional ethics committee. To comply with privacy regulations for minimizing the chance of identification of a study participant, numbers of participants are suppressed in the case of 5 or fewer participants, (reported as ≤5). The data were analyzed by personnel at the Institute for Clinical Evaluative Sciences.
DATA SOURCES
We obtained information from four linked databases. The Trillium Gift of Life Network captures information on all living kidney donors in Ontario. To ensure the accuracy and completeness of the data, we manually reviewed the perioperative medical charts of all persons who underwent donor nephrectomy at five major transplantation centers in Ontario from 1992 through 2010. We retrieved data regarding demographic characteristics and vital status from Ontario’s Registered Persons Database. We retrieved data regarding pregnancies and maternal and fetal outcomes from the Discharge Abstract Database of the Canadian Institute for Health Information and the Ontario Health Insurance Plan database. These databases have been used extensively for epidemiologic and health services research, including studies of living kidney donors and maternal and fetal outcomes.19-25 Data were complete for all variables in this study except the surgical technique used for nephrectomy (open or laparoscopic), which was missing for 14% of donors and was reported only for patients with complete data.
POPULATION
Donors
We included as donors all women who donated a kidney between July 1, 1992, and April 30, 2010, in Ontario and who had at least one pregnancy with a gestation of at least 20 weeks during follow-up. (The primary outcome was assessed after 20 weeks of gestation.) Each woman’s nephrectomy date served as her cohort-entry date. To assess new events during follow-up, we excluded women in whom gestational hypertension or preeclampsia had been diagnosed before donation (in ≤5 women). We identified a matched set of nondonors for 85 of 88 study-eligible donors, as described below.
Nondonors
Before nephrectomy, living donors undergo rigorous health screening. We selected a similarly healthy segment of the general population, using restriction and matching.26 We randomly assigned a cohort-entry date (simulated nephrectomy date) to all women who were citizens in Ontario, according to the distribution of cohort-entry dates among donors (July 1, 1992, to April 30, 2010). We included women with an age that was within the minimum and maximum ages of donors on their cohort entry date and who had evidence of at least one pregnancy carried to 20 weeks of gestation in follow-up (731,823 women).
We identified baseline illnesses and measures of health care access from July 1, 1991 (the beginning of available database records), to the cohort-entry date. This provided a median of 11 years of baseline assessment; 99% of the women had at least 2 years of available data. We restricted the sample of eligible nondonors to women without a known medical condition before cohort entry that could preclude donation, including a diagnosis of gestational hypertension or preeclampsia. (All restrictions are listed in Table S2 in the Supplementary Appendix.) To ensure that nondonors had the same opportunity as donors to obtain health care services from physicians, we restricted the sample of eligible nondonors to women who had visited a physician at least once during the previous 2 years. (Results were not materially different when we removed this restriction in a sensitivity analysis.) These restrictions left 380,995 women (52% of the original sample) as eligible nondonors.
We then matched six eligible nondonors to each donor on the basis of baseline characteristics that might be associated with the risk of gestational hypertension or preeclampsia,27,28 including the age at the time of cohort entry, since extremes in age increase risk; the cohort-entry date (±2 years), to account for era effects; urban or rural residence (population, ≥10,000 or <10,000), since rural residence may increase risk; income (categorized into fifths of average neighborhood income), since lower income increases risk; the number of pregnancies carried to at least 20 weeks of gestation before cohort entry (0, 1, or ≥2), since previous uneventful pregnancies reduce risk; and the time to the first birth after cohort entry (live or stillbirth, matched within 2 years), since an older age during pregnancy or a greater interval from a previous pregnancy increases risk. Each nondonor could be selected only once.
STUDY OUTCOMES
Women were followed until death, emigration from the province, or the end of the observation period (March 31, 2013). The primary outcome was a hospital-based diagnostic code for either gestational hypertension or preeclampsia (from 20 weeks of gestation to 12 weeks after birth), as recorded in a health care or physician-claims database by a medical coder. Diagnostic codes are detailed in Table S3 in the Supplementary Appendix, along with information on their validation and any caveats for their use and interpretation.29-33 In a typical process, trained personnel assign standardized codes on the basis of physician-recorded diagnoses in a patient’s medical chart but do not interpret blood pressure or laboratory values. The number of eclampsia events was anticipated to be small (incidence, <0.1% of pregnancies in the general population14), and to comply with privacy regulations, such events were categorized as preeclampsia. Secondary maternal and fetal outcomes are detailed in Table S4 in the Supplementary Appendix. In multiple-birth pregnancies (only twins in our study), maternal outcomes were counted only once per pregnancy, as were fetal outcomes (e.g., any birth weight <2500 g).
STATISTICAL ANALYSIS
The primary unit of analysis was each unique pregnancy during follow-up. We used generalized linear models with generalized estimating equations for the correlation structure to compare the characteristics of donors and nondonors at the time of cohort entry. Pregnancy characteristics and outcomes were analyzed with the use of generalized linear mixed models with a random intercept and random-effects logistic-regression models, which account for the correlation structure within matched sets and in women with more than one follow-up pregnancy. We repeated the analysis of the primary outcome in three pre-specified subgroups, which were defined on the basis of the presence or absence of at least one pregnancy before cohort entry, since the risk may be higher in a first pregnancy; the time from cohort entry to pregnancy (≤2 or >2 years), since the risk may be higher in the first 2 years after nephrectomy); and the median age during pregnancy (≤32 years or >32 years), since the risk may be higher among older women. To determine whether subgroup-specific odds ratios differed, we included an interaction term in each model; these analyses were considered exploratory, since the anticipated number of events was small. All analyses were performed with the use of SAS software, version 9.3 (SAS Institute). Continuous data were summarized as medians and interquartile ranges.
RESULTS
OBSERVATION TIME
We followed 595 women (85 kidney donors and 510 nondonors) for a median of 10.9 years (11.0 years for donors and 10.9 years for nondonors), with a maximum follow-up of 20.0 years. The observation periods for 20 women (3.4%) were censored at the time of provincial emigration or death. The last donation occurred in December 2009, and the last childbirth in December 2012. There were 4361 total person-years of follow-up (646 for donors and 3715 for nondonors). Less than 2% of pregnancies were twins.
CHARACTERISTICS OF THE STUDY PARTICIPANTS
In the two study groups, the median age was 29 years (interquartile range, 26 to 32), and 29% of the women had at least one pregnancy before cohort entry (Table 1). As expected, donors had more physician visits in the year before cohort entry than nondonors because such visits are a necessary part of donor evaluation. Most donors (65%) were first-degree relatives (sibling, parent, or child) of the recipient, followed by distant relatives or genetically unrelated donors (20%) and spouses (15%). Nephrectomies were performed by means of either a laparoscopic procedure (41%) or an open procedure (59%). Before donation, the median serum creatinine level was 0.76 mg per deciliter (67 μmol per liter; interquartile range, 0.69 to 0.83 mg per deciliter [61 to 73 μmol per liter]), and the median estimated glomerular filtration rate was 114 ml per minute per 1.73 m2 of body-surface area (interquartile range, 104 to 122).
Table 1.
Characteristics of Living Kidney Donors and Matched Nondonors at the Time of Cohort Entry.*
| Characteristic | Donors (N = 85) | Nondonors (N = 510) | P Value† |
|---|---|---|---|
| Median age (IQR) — yr | 29 (26-32) | 29 (26-32) | 1.00 |
| Period of cohort entry — no. (%) | 0.87 | ||
| 1992-1995 | 16 (19) | 92 (18) | |
| 1996-1999 | 19 (22) | 104 (20) | |
| 2000-2004 | 22 (26) | 148 (29) | |
| 2005-2009 | 28 (33) | 166 (33) | |
| Rural residence — no. (%)‡ | ≤5 (≤6) | 30 (6) | 1.00 |
| Income quintile — no. (%)§ | 1.00 | ||
| First | 12 (14) | 72 (14) | |
| Second | 15 (18) | 90 (18) | |
| Third | 13 (15) | 78 (15) | |
| Fourth | 24 (28) | 144 (28) | |
| Fifth | 16 (19) | 96 (19) | |
| One or more pregnancies before cohort entry — no. (%)¶ | 25 (29) | 150 (29) | 1.00 |
| Median time since previous pregnancy (IQR) — yr∥ | 3 (1-5) | 2 (1-5) | 0.63 |
| Median no. of physician visits in previous year (IQR)** | 4 (2-8) | 3 (1-6) | 0.002 |
For living kidney donors, the date of cohort entry was the date of nephrectomy; for nondonors, it was randomly assigned (simulated nephrectomy date) to establish the date that follow-up began. Percentages may not total 100 because of rounding. IQR denotes interquartile range.
P values were derived from generalized linear models with generalized estimating equations for the correlation structure. A normal distribution was specified when the variable was continuous, a Poisson distribution when the variable was a count, a multinomial distribution when the variable was categorical, and a binomial distribution when the variable was binary.
To comply with privacy regulations for minimizing the chance of identification of a study participant, numbers of participants are suppressed in the case of 5 or fewer participants (reported as ≤5).
Income was categorized according to fifths of average neighborhood income, with the first quintile calculated as the lowest income and the fifth quintile as the highest income. This was done only for urban residents (96% of the cohort), since it was problematic to delineate neighborhood boundaries in rural areas.
Ontario health care database records were available from July 1991. In this study, baseline records were available starting at the age of 25 years for 89% of women and starting at the age of 20 years for 61% of women.
This analysis was restricted to women with at least one previous pregnancy.
As expected, donors had more visits to primary care physicians in the year before the cohort-entry date than nondonors because such visits are a necessary part of the donor evaluation process.
PREGNANCIES
The deliveries for all 919 follow-up pregnancies (131 donor pregnancies and 788 nondonor pregnancies) were performed in hospitals (at 100 sites in Ontario). Donors and nondonors had the same median number of health care visits during pregnancy, with 10 prenatal visits and 3 ultrasono-graphic examinations (Table 2). The number of previous pregnancies and the interval between pregnancies were similar in the two groups.
Table 2.
Characteristics of Pregnancies after Cohort Entry in Living Kidney Donors and Matched Nondonors.*
| Characteristic | Pregnancies in Donors (N = 131) | Pregnancies in Nondonors (N = 788) | P Value |
|---|---|---|---|
| Median age (IQR) — yr | 32 (29-35) | 33 (30-36) | 0.94 |
| Year of pregnancy — no. of women (%) | 0.12 | ||
| 1994-1998 | 10 (8) | 61 (8) | |
| 1999-2003 | 24 (18) | 151 (19) | |
| 2004-2008 | 61 (47) | 338 (43) | |
| 2009-2012 | 36 (27) | 238 (30) | |
| Previous pregnancies — no. of women (%)† | 0.40 | ||
| 0 | 60 (46) | 360 (46) | |
| 1 | 51 (39) | 333 (42) | |
| ≥2 | 20 (15) | 95 (12) | |
| Sequence of pregnancies after cohort entry — no. of women (%) | |||
| First | 85 (65) | 510 (65) | 0.77 |
| Second | 36 (27) | 230 (29) | |
| ≥Third | 10 (8) | 48 (6) | |
| Median time since previous pregnancy (IQR) — yr‡ | 3 (2-4) | 3 (2-5) | 0.56 |
| Median time since cohort entry (IQR) — yr§ | 4 (2-7) | 4 (2-7) | 0.79 |
| Median no. of prenatal physician visits (IQR) | 10 (7-12) | 10 (8-12) | 0.06 |
| Median no. of ultrasonographic examinations (IQR) | 3 (2-4) | 3 (2-4) | 0.17 |
Percentages may not total 100 because of rounding.
Included in this category are pregnancies both before and after the date of cohort entry.
This analysis was restricted to women with at least one previous pregnancy, either before or after the date of cohort entry.
For living kidney donors, the date of cohort entry was the date of nephrectomy, and for nondonors, it was randomly assigned (simulated nephrectomy date) to establish the date that follow-up began.
STUDY OUTCOMES
Gestational hypertension or preeclampsia (the primary outcome) was diagnosed in 53 women (15 donors and 38 nondonors) at 28 hospitals (Table 3). The risk of this outcome was higher among donors than among nondonors (11% vs. 5%; odds ratio for donors, 2.4; 95% confidence interval [CI], 1.2 to 5.0; P = 0.01). Each component of the primary outcome was also more common among donors (odds ratio, 2.5 for gestational hypertension and 2.4 for preeclampsia). The two groups did not differ significantly with respect to other secondary maternal or fetal outcomes. There were no maternal deaths, stillbirths, or neonatal deaths in either group.
Table 3.
Maternal and Fetal Outcomes of Pregnancies after Cohort Entry in Living Kidney Donors and Matched Nondonors.
| Outcome | Pregnancies in Donors (N =131) | Pregnancies in Nondonors (N=788) | Odds Ratio (95% CI) | P Value* |
|---|---|---|---|---|
| no. of events (%) | ||||
| Primary outcome: gestational hypertension or preeclampsia | 15 (11) | 38 (5) | 2.4 (1.2-5.0) | 0.01 |
| Secondary outcomes | ||||
| Gestational hypertension† | 7 (5) | 17 (2) | 2.5 (0.9-6.5) | 0.06 |
| Preeclampsia | 8 (6) | 21 (3) | 2.4 (1.0-5.6) | 0.05 |
| Cesarean section | 41 (31) | 224 (28) | 1.2 (0.7-2.1) | 0.44 |
| Postpartum hemorrhage | ≤5 (≤4)‡ | 24 (3) | 0.9 (0.3-2.9) | 0.91 |
| Preterm birth with gestation of <37 wk | 10 (8) | 52 (7) | 1.2 (0.5-2.5) | 0.70 |
| Low birth weight of <2500 g | 8 (6) | 31 (4) | 1.7 (0.7-4.0) | 0.21 |
P values were derived from random-effects logistic-regression models for binary outcome data, accounting for the correlation structure within matched sets and in women with multiple pregnancies.
When diagnostic codes for both gestational hypertension and preeclampsia were present in a given pregnancy, the outcome was counted as a diagnosis of preeclampsia.
To comply with privacy regulations for minimizing the chance of identification of a study participant, numbers of participants are suppressed in the case of 5 or fewer participants (reported as ≤5).
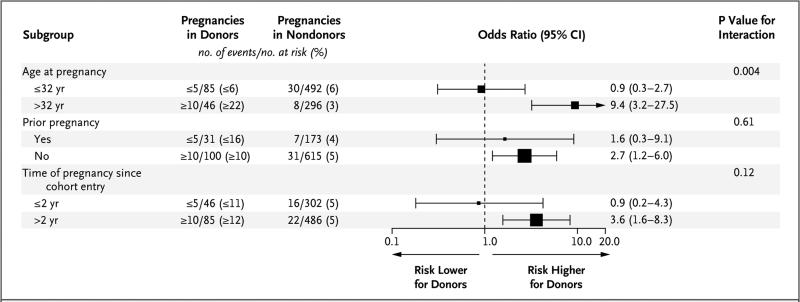
SUBGROUP AND ADDITIONAL ANALYSES
In subgroup analyses, the odds ratio for the primary outcome in donors as compared with nondonors was significantly higher among women who were older than 32 years of age than among those who were 32 years of age or younger (P = 0.004 for interaction) (Fig. 1). In additional analyses, among both donors and nondonors, gestational hypertension or preeclampsia was associated with an increased likelihood of cesarean section or low birth weight (Table S5 in the Supplementary Appendix). In an analysis that eliminated most of the health restrictions in the non-donor group before cohort entry but continued to match eligible nondonors with donors on the basis of baseline characteristics, the increased risk of the primary outcome among donors persisted (11% vs. 4%; odds ratio, 3.2; 95% CI, 1.5 to 6.8; P = 0.002). (See Table S6 in the Supplementary Appendix for nondonor restrictions, characteristics, and outcomes.)
Figure 1. Subgroup Analyses of the Association between Kidney Donation and the Risk of Gestational Hypertension or Preeclampsia.
The size of the squares that represent the odds ratios is proportional to the precision of the estimate, so that the size is larger when the 95% confidence interval is narrower. The horizontal lines indicate 95% confidence intervals. To comply with privacy regulations for minimizing the chance of identification of a study participant, the exact numbers of events in donors are not reported.
DISCUSSION
In this Canadian cohort, gestational hypertension or preeclampsia was more likely to be diagnosed in living kidney donors than in matched nondonors with similar indicators of baseline health (incidence, 11% vs. 5%). Other important maternal and fetal outcomes did not differ significantly between the two groups, and there were no maternal or perinatal deaths. Most women had uncomplicated pregnancies after kidney donation.
Two previous studies have examined pregnancy outcomes after living kidney donation: a national study conducted in Norway12 and a single-center study conducted in Minnesota.13
The incidences of gestational hypertension, preeclampsia, and other maternal and fetal outcomes after donation in these studies were similar to the estimates in our study (Table 4). In the two previous studies, the analyses compared outcomes in a group of women who were pregnant before donation with outcomes in a group of women who were pregnant after donation. The Minnesota study surveyed donors by asking them to recall outcomes many years after pregnancy, and more than 24% of women were lost to follow-up.13 The Norwegian study provided an additional comparison between donor pregnancies and nondonor pregnancies among women in the same birth registry. However, on average, the maternal age was 5 years older among donors than among nondonors, and that comparison did not account for between-group differences in prognostic factors.12
Table 4.
Study Characteristics, Donor Characteristics, and Maternal and Fetal Outcomes in Retrospective Studies from Norway and Minnesota, as Compared with the Ontario Study.*
| Characteristic or Outcome | Norway Study | Minnesota Study | Ontario Study |
|---|---|---|---|
| Study characteristics | |||
| No. of transplantation centers | 1 | 1 | 5 |
| Health care system | Public universal health care | Private insurance | Public universal health care |
| Data source | National birth registry | Study data | Provincial health care data |
| Outcomes recorded at time of pregnancy | Yes; mandatory reporting to birth registry | No; self-reported patient surveys completed an average of 4 yr after postdonation pregnancies and 12 yr after first pregnancies | Yes; mandatory hospital reporting during pregnancy and fee-for-service physician claims |
| Eligible pregnancies | Gestation of>16 wk | All pregnancies | Gestation of >20 wk |
| Loss to follow-up after donation | <4%† | 24-39% | <4% |
| Primary groups being compared | Pregnancies before and after donation | Pregnancies before and after donation | Follow-up pregnancies in matched donors and nondonors |
| Type of nondonor comparison | Sample from same data source, but not selected fordonor similarity and no statistical adjustment for between-group differences at baseline | General population estimates from published literature; other than race, not selected fordonor similarity | Sample from the same data source, selected for donor similarity on the basis of demographic and other prognostic factors |
| Reporting of blood-pressure or kidney function values during pregnancy | No | No | No |
| Donor characteristics | |||
| No. of women | 69 | 239 | 85 |
| Period of donation | 1967-2002 | 1963-2007 | 1992-2009 |
| Family history of kidney failure — % | NR | 96 | 65 |
| Mean glomerular filtration rate before donation — ml/min/1.73 m2‡ | NR | 91 | 114 |
| White race — % | 98 | 97 | 70 |
| No. of pregnancies after donation | 106 | 490 | 131 |
| Mean age at time of donation — yr | 27 | 26 | 29 |
| Mean age at time of pregnancy — yr | 32 | 29 | 32 |
| One or more pregnancies before donation — no. (%) | NR | 98 (41) | 25 (29) |
| Mean or median interval between donation and subsequent pregnancy — yr | 5 | 5 | 4 |
| Outcomes after donation | |||
| Maternal — no./total no. (% of pregnancies) | |||
| Gestational hypertension or preeclampsia§ | 9/106 (8) | 55/490 (11) | 15/131 (11) |
| Gestational hypertension | 3/106 (3) | 28/490 (6) | 7/131 (5) |
| Preeclampsia | 6/106 (6) | 27/490 (6) | 8/131 (6) |
| Death | NR | NR | 0 |
| Fetal — no./total no. (% of pregnancies) | |||
| Preterm birth with gestation of<37 wk | 10/106 (9) | 35/490 (7) | 10/131 (8) |
| Birth weight <2500 g | 9/106 (8) | NR | 8/131 (6) |
| Stillbirth¶ | 3/106 (3) | 2/490 (<1) | 0 |
| Neonatal death <28 days after birth | 0 | NR | 0 |
Data are from Reisaeter et al.12 (for the Norway study) and Ibrahim et al.13 (for the Minnesota study). The Ontario study is the Donor Nephrectomy Outcomes Research (DONOR) Network study reported here. Details regarding other aspects of the studies are provided in Table S7 in the Supplementary Appendix. NR denotes not reported.
The loss to follow-up was not reported in the Norway study. However, since Norway has a national birth registry and national health care, the only loss to follow-up would probably be from national emigration.
The glomerular filtration rate was estimated with the use of the Modification of Diet in Renal Disease (MDRD) equation in the Minnesota study and the Chronic Kidney Disease Epidemiology Collaboration formula in the Ontario study.
In the Norway study, gestational hypertension was defined as a blood pressure of 140/90 mm Hg or more or an increase in diastolic blood pressure of at least 15 mm Hg or systolic blood pressure of at least 30 mm Hg from the woman's average blood pressure before 20 weeks of gestation, without proteinuria (which was defined as urinary excretion of ≥0.3 g of protein per day, usually equivalent to 1+ or more on a standard urine test strip); preeclampsia was defined as gestational hypertension with proteinuria. In the Minnesota study, gestational hypertension was defined as the receipt of hypertension treatment during pregnancy, but not before or after; preeclampsia was typically defined as hypertension associated with new-onset proteinuria or edema, according to the woman's recall of the diagnosis by the primary care provider.
In the Norway study, a fetus was recorded as stillborn if it died before or during labor. In the Minnesota study, stillbirth was recorded from reports of fetal deaths.
The strengths of our study include a manual review of all perioperative donor charts, careful selection of similar donors and nondonors, and minimal loss to follow-up (<4%). Our study population had access to a system of universal health care benefits, in which all health care encounters were recorded, and the pregnancies of donors and nondonors had similarly high levels of health surveillance (with medians of 10 prenatal visits and 3 ultrasonographic examinations).
Our study has certain limitations. First, data with respect to blood pressure, renal function, body-mass index, and medication use during pregnancy were not available in our data sources. Second, accurate racial information was not available,34 although 71% of Ontario citizens are white, as are approximately 70% of donors. Hyper-tension after kidney donation is more common among black donors than among white donors,8,10,35 and whether the same is true of hypertension during pregnancy requires future study (<3% of Ontario citizens are black). Third, confidence intervals for risk estimates were wide. Fourth, physicians use clinical judgment when applying accepted diagnostic criteria for gestational hypertension and preeclampsia, and not all diagnoses have the same medical significance. It remains possible that gestational hypertension and preeclampsia were more likely to be diagnosed and recorded among donors than among nondonors despite similar clinical presentations in the two groups. Urine protein may rise after nephrectomy,36 which could also increase the chance of a diagnosis of preeclampsia among donors.
In addition, some donors may have had a genetic predisposition to kidney disease, which could have increased the risk of our primary study outcome among those in whom this condition developed. Sixty-five percent of the donors had a first-degree relative with kidney failure, and we assume that few nondonors had a similar family history, although such information was not available for nondonors. There were too few events to reliably assess the effect of family history on outcomes. Nevertheless, three details warrant consideration. First, the donors had to have excellent health to qualify for nephrectomy, and women who had signs of kidney disease during donor evaluation were excluded from donation. Second, the average time between donation and a subsequent pregnancy was only 4 years, which was a short interval for new kidney disease to develop. Third, given our study inclusion criteria, the 29% of donors who had been pregnant before donation had pregnancies that were uncomplicated by gestational hypertension or preeclampsia despite any genetic predisposition. Thus, it seems unlikely that a genetic predisposition to kidney disease in isolation would explain the study findings. However, a genetic predisposition in combination with a reduced glomerular filtration rate from donor nephrectomy could amplify the risk of gestational hypertension or preeclampsia.
Living kidney donation is an important treatment option for kidney failure that clearly benefits many families and society. The ethical practice of living kidney donation requires that professionals in the transplantation field provide donors with up-to-date, accurate information about risks (including pregnancy risks2) and acknowledge the limitations of what is known.
In theory, randomized trials could generate estimates of donor risk that are less prone to bias; however, randomized trials of donation are not ethically feasible. An alternative approach would be to perform a large, multicenter, prospective cohort study in which carefully selected donors and nondonors are enrolled over a period of several years and then followed for a decade, with adjudicated pregnancy outcomes, but this approach would also face many logistical challenges. An increased risk of gestational hyper-tension and preeclampsia among kidney donors is biologically plausible3,4,6,7 and has been identified in two previous studies in Norway12 and the United States13 and now in Canada. Although there is some uncertainty regarding the true magnitude of risk, having reviewed all the evidence and associated limitations, we believe it is conscionable to act. Information on this potential risk should be included in clinical practice guidelines, shared in the informed-consent processes for potential donors and their recipients when a woman has reproductive potential, and used to guide the care of pregnant donors.
Our study and others show that probabilities of the most serious maternal and fetal outcomes remain low and are not significantly increased after donation.12,13 It is unknown whether the same holds true in countries in which women lack access to a similar quality of health care. For this reason, there may be a role for government programs to cover the costs of recommended pregnancy care for donors who lack health insurance, including any costs related to the treatment of hypertension.37,38
Supplementary Material
Acknowledgments
Supported by a grant from the Canadian Institute of Health Research (RN117985-251529); the Academic Medical Organization of Southwestern Ontario; the Kidney Foundation of Canada; the Lawson Health Research Institute; the Division of Nephrology, Department of Medicine, Clinical Investigator Program, and the Schulich School of Medicine and Dentistry at Western University; the Dr. Adam Linton Chair in Kidney Health Analytics; the Ontario Ministry of Health and Long-term Care; the Institute for Clinical Evaluative Sciences; the Ontario Kidney, Dialysis, and Transplantation Research Program; and a grant from the National Institutes of Health (R01-DK096008).
Footnotes
Disclosure forms provided by the authors are available with the full text of this article at NEJM.org.
References
- 1.Horvat LD, Shariff SZ, Garg AX. Global trends in the rates of living kidney donation. Kidney Int. 2009;75:1088–98. doi: 10.1038/ki.2009.20. [DOI] [PubMed] [Google Scholar]
- 2.Tong A, Chapman JR, Wong G, Kanellis J, McCarthy G, Craig JC. The motivations and experiences of living kidney donors: a thematic synthesis. Am J Kidney Dis. 2012;60:15–26. doi: 10.1053/j.ajkd.2011.11.043. [DOI] [PubMed] [Google Scholar]
- 3.Packham DK, Hewitson TD, Whit-worth JA, Kincaid-Smith PS. Glomerulo-sclerosis and hyalinosis in rabbits. Pathology. 1992;24:164–9. doi: 10.3109/00313029209063165. [DOI] [PubMed] [Google Scholar]
- 4.Gibson KJ, Thomson CL, Boyce AC, Karime BM, Lumbers ER. Effects of a reduction in maternal renal mass on pregnancy and cardiovascular and renal function of the pregnant ewe. Am J Physiol Renal Physiol. 2006;290:F1153–F1162. doi: 10.1152/ajprenal.00241.2005. [DOI] [PubMed] [Google Scholar]
- 5.Poggio ED, Braun WE, Davis C. The science of stewardship: due diligence for kidney donors and kidney function in living kidney donation — evaluation, determinants, and implications for outcomes. Clin J Am Soc Nephrol. 2009;4:1677–84. doi: 10.2215/CJN.02740409. [DOI] [PubMed] [Google Scholar]
- 6.Nevis IF, Reitsma A, Dominic A, et al. Pregnancy outcomes in women with chronic kidney disease: a systematic review. Clin J Am Soc Nephrol. 2011;6:2587–98. doi: 10.2215/CJN.10841210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Boudville N, Prasad GV, Knoll G, et al. Meta-analysis: risk for hypertension in living kidney donors. Ann Intern Med. 2006;145:185–96. doi: 10.7326/0003-4819-145-3-200608010-00006. [DOI] [PubMed] [Google Scholar]
- 8.Doshi MD, Goggins MO, Li L, Garg AX. Medical outcomes in African American live kidney donors: a matched cohort study. Am J Transplant. 2013;13:111–8. doi: 10.1111/j.1600-6143.2012.04303.x. [DOI] [PubMed] [Google Scholar]
- 9.Ibrahim HN, Foley R, Tan L, et al. Long-term consequences of kidney donation. N Engl J Med. 2009;360:459–69. doi: 10.1056/NEJMoa0804883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lentine KL, Schnitzler MA, Xiao H, et al. Racial variation in medical outcomes among living kidney donors. N Engl J Med. 2010;363:724–32. doi: 10.1056/NEJMoa1000950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Delmonico F. A Report of the Amsterdam Forum on the care of the live kidney donor: data and medical guidelines. Transplantation. 2005;79(Suppl):S53–S66. [PubMed] [Google Scholar]
- 12.Reisaeter AV, Røislien J, Henriksen T, Irgens LM, Hartmann A. Pregnancy and birth after kidney donation: the Norwegian experience. Am J Transplant. 2009;9:820–4. doi: 10.1111/j.1600-6143.2008.02427.x. [DOI] [PubMed] [Google Scholar]
- 13.Ibrahim HN, Akkina SK, Leister E, et al. Pregnancy outcomes after kidney donation. Am J Transplant. 2009;9:825–34. doi: 10.1111/j.1600-6143.2009.02548.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Nevis IF, Garg AX. Maternal and fetal outcomes after living kidney donation. Am J Transplant. 2009;9:661–8. doi: 10.1111/j.1600-6143.2009.02623.x. [DOI] [PubMed] [Google Scholar]
- 15.Nayak-Rao S. Pregnancy after kidney donation — placing things in perspective. Saudi J Kidney Dis Transpl. 2011;22:552–3. [PubMed] [Google Scholar]
- 16.Josephson MA. Transplantation: pregnancy after kidney donation: more questions than answers. Nat Rev Nephrol. 2009;5:495–7. doi: 10.1038/nrneph.2009.129. [DOI] [PubMed] [Google Scholar]
- 17.Ahmadi AR, Lafranca JA, Claessens LA, et al. Shifting paradigms in eligibility criteria for live kidney donation: a systematic review. Kidney Int. 2014 Apr 30; doi: 10.1038/ki.2014.118. (Epub ahead of print) [DOI] [PubMed] [Google Scholar]
- 18.von Elm E, Altman DG, Egger M, Pocock SJ, Gøtzsche PC, Vandenbroucke JP. The Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) statement: guidelines for reporting observational studies. Ann Intern Med. 2007;147:573–7. doi: 10.7326/0003-4819-147-8-200710160-00010. [Erratum, Ann Intern Med 2008;148:168.]
- 19.Ray JG, Schull MJ, Urquia ML, You JJ, Guttmann A, Vermeulen MJ. Major radio-diagnostic imaging in pregnancy and the risk of childhood malignancy: a population-based cohort study in Ontario. PLoS Med. 2010;7(9):e1000337. doi: 10.1371/journal.pmed.1000337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lam N, Huang A, Feldman LS, et al. Acute dialysis risk in living kidney donors. Nephrol Dial Transplant. 2012;27:3291–5. doi: 10.1093/ndt/gfr802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Garg AX, Pouget J, Young A, et al. Fracture risk in living kidney donors: a matched cohort study. Am J Kidney Dis. 2012;59:770–6. doi: 10.1053/j.ajkd.2012.01.013. [DOI] [PubMed] [Google Scholar]
- 22.Garg AX, Meirambayeva A, Huang A, et al. Cardiovascular disease in kidney donors: matched cohort study. BMJ. 2012;344:e1203. doi: 10.1136/bmj.e1203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Thomas SM, Lam NN, Welk BK, et al. Risk of kidney stones with surgical intervention in living kidney donors. Am J Transplant. 2013;13:2935–44. doi: 10.1111/ajt.12446. [DOI] [PubMed] [Google Scholar]
- 24.Thomas SM, Lam NN, Huang A, et al. Risk of serious gastrointestinal bleeding in living kidney donors. Clin Transplant. 2014;28:530–9. doi: 10.1111/ctr.12344. [DOI] [PubMed] [Google Scholar]
- 25.Garg AX, Prasad GV, Thiessen-Phil-brook HR, et al. Cardiovascular disease and hypertension risk in living kidney donors: an analysis of health administrative data in Ontario, Canada. Transplantation. 2008;86:399–406. doi: 10.1097/TP.0b013e31817ba9e3. [DOI] [PubMed] [Google Scholar]
- 26.Lin J, Kramer H, Chandraker AK. Mortality among living kidney donors and comparison populations. N Engl J Med. 2010;363:797–8. doi: 10.1056/NEJMc1002100. [DOI] [PubMed] [Google Scholar]
- 27.Steegers EA, von Dadelszen P, Duvekot JJ, Pijnenborg R. Pre-eclampsia. Lancet. 2010;376:631–44. doi: 10.1016/S0140-6736(10)60279-6. [DOI] [PubMed] [Google Scholar]
- 28.Sibai B, Dekker G, Kupferminc M. Pre-eclampsia. Lancet. 2005;365:785–99. doi: 10.1016/S0140-6736(05)17987-2. [DOI] [PubMed] [Google Scholar]
- 29.Hadfield RM, Lain SJ, Cameron CA, Bell JC, Morris JM, Roberts CL. The prevalence of maternal medical conditions during pregnancy and a validation of their reporting in hospital discharge data. Aust N Z J Obstet Gynaecol. 2008;48:78–82. doi: 10.1111/j.1479-828X.2007.00818.x. [DOI] [PubMed] [Google Scholar]
- 30.Korst LM, Gregory KD, Gornbein JA. Elective primary caesarean delivery: accuracy of administrative data. Paediatr Perinat Epidemiol. 2004;18:112–9. doi: 10.1111/j.1365-3016.2003.00540.x. [DOI] [PubMed] [Google Scholar]
- 31.Yasmeen S, Romano PS, Schembri ME, Keyzer JM, Gilbert WM. Accuracy of obstetric diagnoses and procedures in hospital discharge data. Am J Obstet Gynecol. 2006;194:992–1001. doi: 10.1016/j.ajog.2005.08.058. [DOI] [PubMed] [Google Scholar]
- 32.Klemmensen AK, Olsen SF, Osterdal ML, Tabor A. Validity of preeclampsia-related diagnoses recorded in a national hospital registry and in a postpartum interview of the women. Am J Epidemiol. 2007;166:117–24. doi: 10.1093/aje/kwm139. [DOI] [PubMed] [Google Scholar]
- 33.Geller SE, Ahmed S, Brown ML, Cox SM, Rosenberg D, Kilpatrick SJ. International Classification of Diseases-9th revision coding for preeclampsia: how accurate is it? Am J Obstet Gynecol. 2004;190:1629–33. doi: 10.1016/j.ajog.2004.03.061. [DOI] [PubMed] [Google Scholar]
- 34.Reese PP, Huverserian A, Bloom RD. Pregnancy outcomes among live kidney donors. Am J Transplant. 2009;9:1967. doi: 10.1111/j.1600-6143.2009.02719.x. [DOI] [PubMed] [Google Scholar]
- 35.Lentine KL, Schnitzler MA, Xiao H, et al. Consistency of racial variation in medical outcomes among publicly and privately insured living kidney donors. Transplantation. 2014;97:316–24. doi: 10.1097/01.TP.0000436731.23554.5e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Garg AX, Muirhead N, Knoll G, et al. Proteinuria and reduced kidney function in living kidney donors: a systematic review, meta-analysis, and meta-regression. Kidney Int. 2006;70:1801–10. doi: 10.1038/sj.ki.5001819. [DOI] [PubMed] [Google Scholar]
- 37.Sickand M, Cuerden MS, Klarenbach SW, et al. Reimbursing live organ donors for incurred non-medical expenses: a global perspective on policies and programs. Am J Transplant. 2009;9:2825–36. doi: 10.1111/j.1600-6143.2009.02829.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Yang RC, Thiessen-Philbrook H, Klarenbach S, Vlaicu S, Garg AX. Insurability of living organ donors: a systematic review. Am J Transplant. 2007;7:1542–51. doi: 10.1111/j.1600-6143.2007.01793.x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.