Abstract
Objective
To examine whether a caregiver's attachment style is associated with patient cognitive trajectory after traumatic brain injury (TBI).
Setting
National Institute of Neurological Disorders and Stroke, National Institutes of Health, Bethesda, Maryland.
Participants
Forty Vietnam War veterans with TBI and their caregivers.
Main
Outcome Measure
Cognitive performance, measured by the Armed Forces Qualification Test percentile score, completed at 2 time points: preinjury and 40 years postinjury.
Design
On the basis of caregivers’ attachment style (secure, fearful, preoccupied, dismissing), participants with TBI were grouped into a high or low group. To examine the association between cognitive trajectory of participants with TBI and caregivers’ attachment style, we ran four 2 × 2 analysis of covariance on cognitive performances.
Results
After controlling for other factors, cognitive decline was more pronounced in participants with TBI with a high fearful caregiver than among those with a low fearful caregiver. Other attachment styles were not associated with decline.
Conclusion and Implication
Caregiver fearful attachment style is associated with a significant decline in cognitive status after TBI. We interpret this result in the context of the neural plasticity and cognitive reserve literatures. Finally, we discuss its impact on patient demand for healthcare services and potential interventions.
Keywords: attachment style (AS), caregiver, cognitive reserve, fearful, neural plasticity, traumatic brain injury (TBI)
Traumatic brain injury (TBI) is a worldwide health and socioeconomic concern. Approximately 3.2 to 5.3 million Americans are currently living with a TBI-related disorder. Fourteen percent of combat veterans sustained a brain injury during the Vietnam War,1 and 294,172 active-duty individuals sus tained a TBI during OEF (Operation Enduring Freedom)/OIF (Operation Iraqi Freedom)/OND (Operation New Dawn) conflicts (2000-2013).2 In the Vietnam War, there were more gunshot and fragment wounds (35% of the injuries vs 19% in OEF/OIF), whereas blast-related injuries were more prevalent during OIF/OEF combat (81% vs 65% during the Vietnam War).3 As a consequence, there were more penetrating TBIs during the Vietnam War, whereas OIF/OEF injuries were mainly mild or moderate closed-head injuries with only 1.4% being penetrating TBIs.4,5 Individuals with a TBI are often faced with physical, cognitive, and social limitations that may persist for their lifetime. These limitations are challenging not only for the individual but also for family members and friends.6,7 However, there are limited data concerning the impact of caregivers on cognitive evolution of individuals with TBI.
A study by Taylor et al,8 on TBI in children noted that the family's response to the new situation may affect the recovery of the child. On the other end of the developmental spectrum, some research has examined individuals with dementia and their caregivers,9–12 showing that a stimulating environment predicts a slower cognitive and functional decline in this population.9 Individuals with dementia are able to delay nursing home placement when the caregiver is the spouse, is in good health, provides positive interactions, and spends less time providing care.10,11 Also, the caregiver's attachment style (AS) plays a role in the behavior of the individual with dementia: the more avoidant the caregiver's AS, the more aggression and agitation are exhibited by the patient.12
Attachment style affects the way individuals cope with emotional events and interact with others, including those to whom they are attracted.13–15 The roots of AS lie in John Bowlby's16 work on what he called the attachment behavioral system. Bowlby16 believed that attachment behavior has a biological function such as protection and was innate in most mammals. Also, he highlighted that an AS was established during early childhood according to the primary attachment figure (mostly the mother) but then it remains mainly stable throughout life, as confirmed by recent studies.17–19 Individual differences in AS were predicted by Bowlby and then systematically studied in mother-infant relationships by Mary Ainsworth.20 Inspired by this developmental literature, it was shown that adults can be classified into 4 attachment categories (secure, preoccupied, fearful, and dismissing).21 Preoccupied AS is defined by a negative self-esteem and positive sociability, whereas dismissing AS individuals present a positive self-esteem but are socially avoidant. Secure AS consists of positive self-esteem and sociability, whereas fearful AS individuals have a low self-esteem and are socially anxious and avoidant.22,23 The association between caregiver AS and behavioral outcomes in individuals with TBI has not been explored yet.
Animal models show compelling evidence that enriched environments induce neurogenesis, synaptogenesis, and dendritic growth, which potentially propel recovery after a brain injury.24,25 Living in an enriched environment enhances damage-induced neurogenesis in the adult brain26 and has been shown to slow functional deterioration in neurodegenerative disorders, such as in a rat model of Huntington disease.27 Interestingly, a recent study of adult rats showed that an enriched environment has a protective effect on glutamate excitotoxicity, reducing oxidative damage.28 In contrast, an impoverished environment has a negative impact on neural plasticity. For instance, rats that live in a traditional laboratory cage develop hippocampal atrophy.29 There are few such studies on nonhuman primates, but in adult marmosets, exposure to a complex environment for 1 month enhanced the length and complexity of dendrites and increased dendritic spines and synaptophysin in the hippocampus and frontal cortex.30
Regions preferentially involved in anxiety, for example, the hippocampus, prefrontal cortex (PFC), and amygdala, are particularly plastic and capable of transformation as a result of activity and experience.29,31,32 Adaptive plasticity observed in the hippocampus and medial PFC (mPFC) is reversed or inhibited by chronic stress, which also causes dendritic atrophy in the mPFC and hippocampus, and hypertrophy in the amygdala.32 The PFC and the amygdala are also some of the most common brain areas affected in TBI.33
In humans, cognitive reserve, the apparent result of lifetime intellectual activity, influences the timing of cognitive decline in aging and delays clinical progression in neurodenerative disorders.34,35 Intelligence, education, and occupation are associated with increased synaptic density, neurogenesis, and synaptic plasticity.34,35
We hypothesize that the caregiver plays a key role in determining the richness of the individual's environment, thereby affecting the trajectory of long-term cognitive change after TBI. Anxious individuals experience increased distress, as has been shown among melanoma survivors.23 This suggests that a caregiver with a fearful AS might not only limit social interactions of individuals with TBI but also increase stress in the environment with a resultant negative effect on plastic or protective processes or capacities important for recovery or preservation of cognitive capacities in individual with TBI across the life span. We hypothesize that cognitive decline in an individual with TBI, which is greater than in controls,1 could be exacerbated if his or her caregiver has a high fearful AS than if his or her caregiver has a low fearful AS. While the current observational study could not be used to determine a causal relationship between AS and cognitive decline, we sought to establish whether an association could be detected.
METHODS
Participants
A subgroup of male participants was selected from the Vietnam Head Injury Study (VHIS), a prospective and wide-ranging study of veterans who sustained brain damage from penetrating head injuries (TBI) during the Vietnam War. The VHIS consists of 4 phases that stretched over more than 40 years. Phase 1 occurred during the Vietnam War (1967-1970) and included medical records of 1221 veterans who survived the first week post-TBI. Phase 2 occurred between 1981 and 1984 at the Walter Reed Army Medical Center and was a follow-up of 520 individuals with TBI among the initial 1221 participants collected in phase 1. Phase 3 occurred from 2003 to 2006 at the National Naval Medical Center (Bethesda, Maryland), with an extensive neuropsychological follow-up of 199 individuals with TBI. In phase 4 (2008-2012), participants and their caregivers came to the National Institute of Neurological Disorders and Stroke of the National Institutes of Health (NINDS/NIH), Bethesda, Maryland, for a 5-day study. The caregivers completed a series of questionnaires and an interview.
From the 134 phase 4 participants with penetrating injuries, we selected individuals if (a) they had a documented induction Armed Forces Qualification Test (AFQT) score (preinjury), (b) they were accompanied by a caregiver during their phase 4 evaluation, (c) the care-giver had known the individual since preinjury or within 5 years postinjury (mean years since caregiver and participant with TBI knew each other: 42.10 ± 6.57 years). The final sample consisted of 40 couples (see Table 1). All veterans had completed the AFQT-7A upon military induction and during VHIS phase 4.
Table 1.
Demographic characteristics of the entire sample (40 participants with TBI and 40 caregivers)
| Mean (SD) | |
|---|---|
| Participants with TBI | |
| Age, y | 63.28 (3.404) |
| Education, y | 13.97 (1.915) |
| Handedness (right:left) | 37:3 |
| Gender (male:female) | 40:0 |
| Loss of consciousness | |
| None | n = 13 |
| Momentary | n = 5 |
| 1-15 min | n = 8 |
| 15 min to 1 d | n = 5 |
| >1 d | n = 6 |
| Unknown | n = 3 |
| Posttraumatic amnesia | |
| None | n = 20 |
| <1 h | n = 2 |
| 1 h to <1 d | n = 0 |
| 1 d to <1 wk | n = 7 |
| 1 wk to < 1 mo | n = 5 |
| ≥1 mo | n = 4 |
| Unknown | n = 2 |
| Caregivers | |
| Age, y | 61.52 (3.948) |
| Education, y | 13.75 (2.072) |
| Gender (male:female) | 3:37 |
| Relation to participant with TBI (spouse:sibling:friend) | 36:3:1 |
Abbreviations: SD, standard deviation; TBI, traumatic brain injury.
All participants gave written informed consent, and the study was approved by the institutional review board of the NINDS/NIH, Bethesda, Maryland.
Clinical assessment
Participants underwent assessments of their global functioning (Functional Status Questionnaire36), personality (NEO-Five Factor Inventory37), posttraumatic stress disorder (Mississippi PTSD [posttraumatic stress disorder]38), depression (Beck Depression Inventory39), memory (Wechsler Memory Scale40), language (Boston Naming Test41 and Token Test42), executive functions (from the Delis-Kaplan Executive Function System43: Verbal Fluency, Sorting Test, Trail Making Test), and visual perception (Visual Object and Space Perception Battery44). Also, caregivers’ depression (Center for Epidemiological Studies Depression Scale)45 and burden (Zarit Burden Inventory)46 were evaluated.
Measures
Armed Forces Qualification Test
The AFQT-7A contains 100 multiple-choice questions on word knowledge, arithmetic word problems, object function matching, as well as mental imagery. Fifty minutes is allowed for completion. The difference in the AFQT percentile score from preinjury to phase 4 was used as the measure of cognitive trajectory. AFQT correlates strongly with the Wechsler Adult Intelligence Test47 and has a good validity and reliability (0.7 and 0.73, respectively).48
Relationship Questionnaire and Relationship Scale Questionnaire
The Relationship Questionnaire (RQ) is a measure of adult attachment based on a 4-category model: secure, preoccupied, dismissing, and fearful.21 In this model, secure attachment consists of a positive view of self and others, preoccupied attachment is a negative view of self but a positive view of others, dismissing attachment is a positive view of self and negative view of others, and fearful attachment is a negative view of self and other.49,50 The RQ consists of 4 paragraphs; participants rate how much each of the 4 paragraphs represent them on a 7-point Likert scale. The Relationship Scale Questionnaire (RSQ) is a similar measure that contains 30 statements in which participants respond on a 5-point Likert scale to the extent that each statement is consistent with their feelings about close relationships.13 The participants with TBI and their caregiver completed both questionnaires. A recent study confirmed good test-retest short-time reliability and internal consistency, as well as a solid factor analysis.51
A combination of the RQ and RSQ scores for each person, on each of the 4 ASs, was used to establish a continuous rating.13 Continuous indexes were obtained by first converting the raw scores for each AS on the RQ and the RSQ to standardized z-scores.
Computed tomographic acquisition and analysis
Computed tomographic (CT) scans were acquired on a GE Medical Systems Light Speed Plus CT scanner in helical mode. Images were reconstructed with 1-mm overlapping slice thickness and a 1-mm interval. Lesion volume was determined from CT scans by manual tracing using the Analysis of Brain Lesion (ABLe) software52,53 implemented in MEDx (Medical Numerics Inc, Sterling, Virginia) with enhancements to support the Automated Anatomical Labeling atlas. The tracing was performed by a trained neuropsychiatrist and then reviewed by J.G., an experienced observer, who was blind to the results of the clinical evaluations. A consensus judgment determined the final outline of the lesion. On the basis of the lesion volume, we determined the percentage of volume loss [lesion volume (cm3) × 100/total brain volume (cm3)].
Statistical analysis
We used IBM SPSS (version 16 for Mac, www.spss.com) and applied a level of significance of P < .05 (2-tailed) to all analyses and a Bonferroni correction to analyses of covariance (ANCOVAs) (a level of significance for P < .013). We report the effect size when the analyses reached the level of significance.
On the basis of our hypothesis, we split the patient-caregiver pairs by the median score of the caregiver fearful AS and placed them into a “high fearful” (HF) or “low fearful” (LF) group. To compare the LF and HF groups on demographic, clinical variables, percentage of brain volume loss, and AS (i.e., secure, fearful, dismissing and preoccupied) of the caregiver and the participants with TBI, we used independent-samples t tests.
To examine the association between cognitive trajectory of participants with TBI and caregivers’ fearful AS, we ran a 2 × 2 ANCOVA on cognitive performances (mean AFQT percentile scores) with trajectory (preinjury, postinjury) as a within-subjects factor and AS (LF, HF) as a between-subjects factor. Caregiver secure and dismissing ASs, as well as caregiver depression and NEO Conscientiousness variable of participants with TBI were integrated as covariates in the model (the only measures, with caregiver fearful AS, that were significantly different between LF and HF).
Also, we controlled for the other AS by repeating the aforementioned analysis 3 more times, each time using a different AS as the between-subjects factor and the remaining behavioral variables that differed significantly between LF and HF as covariates.
RESULTS
Fearful AS
On the basis of a median split on caregivers’ fearful AS z-scores, 20 patient-caregiver pairs were assigned to the LF and 20 pairs to the HF group. There were no significant differences between the groups on demographic, clinical, or total percent volume loss (see Table 2).
TABLE 2.
Descriptive and inferential statistics (mean and standard deviation) of demographic, neuropsychological, and psychiatric data of the LF and HF groups
| LF (n = 20), mean (SD) | HF (n = 20), mean (SD) | Statistics, P | |
|---|---|---|---|
| Participants with TBI | |||
| Age, y | 63.85 (4.38) | 62.70 (1.98) | .291 |
| Education, y | 14.15 (2.11) | 13.80 (1.74) | .570 |
| Handedness (right:left) | 16:4 | 17:3 | .667 |
| Secure AS (z score) | 0.099 (0.974) | –1.134 (0.727) | .398 |
| Fearful AS (z score) | –0.243 (0.580) | 0.215 (0.903) | .065 |
| Preoccupied AS (z score) | –0.243 (0.750) | 0.155 (0.811) | .116 |
| Dismissing AS (z score) | –0.003 (0.894) | 0.068 (0.989) | .813 |
| NEO Neurotic | 44.300 (9.370) | 49.25 (15.172) | .222 |
| NEO Extrovert | 50.60 (9.779) | 45.40 (12.592) | .153 |
| NEO Openness | 44.60 (9.422) | 45.15 (8.425) | .847 |
| NEO Agreeable | 50.40 (10.065) | 49.60 (12.424) | .824 |
| NEO Conscientiousness | 54.95 (10.650) | 46.65 (13.747) | .039a |
| Functional Status Questionnaire | 96.50 (18.763) | 92.30 (20.846) | .507 |
| Mississippi PTSD (total raw) | 73.35 (21.3555) | 84.30 (26.821) | .161 |
| Beck Depression Inventory | 5.35 (7.527) | 10.65 (10.358) | .073 |
| Wechsler Memory Scale (total Memory scaled score) | 95.10 (19.523) | 95.84 (15.446) | .993 |
| Boston Naming Test (total raw) | 53.00 (6.936) | 53.47 (5.531) | .816 |
| Token Test (total correct) | 97.05 (5.424) | 97.37 (2.543) | .817 |
| Verbal Fluency (letter, raw) | 28.05 (12.931) | 28.25 (9.358) | .931 |
| Sorting Test (combined description composite scaled score) | 10.20 (3.04) | 9.68 (2.89) | .590 |
| Trail Making Test (number letter set loss error) | 0.40 (0.821) | 0.21 (0.419) | .374 |
| Visual Object and Space Perception Battery | 19.20 (1.056) | 19.74 (0.562) | .057 |
| Total percent volume loss, cm3 | 3.9 (4.69) | 2.51 (2.30) | .243 |
| Caregivers | |||
| Age, y | 62.15 (4.660) | 60.9 (3.076) | .323 |
| Education, y | 13.65 (1.872) | 13.85 (2.300) | .765 |
| Gender (male:female) | 2:18 | 1:19 | .545 |
| Relation to participant with TBI (spouse:sibling:friend) | 17:2:1 | 19:1:0 | .486 |
| Center for Epidemiological Studies | 7.20 (8.40) | 13.10 (7.45) | .024a |
| Depression Scale | |||
| Zarit Burden Inventory | 15.45 (13.617) | 21.35 (13.743) | .181 |
| Secure AS (z score) | 0.411 (0.855) | –0.372 (0.818) | .005a |
| Fearful AS (z score) | –0.725 (0.327) | 0.646 (0.786) | <.001a |
| Preoccupied AS (z score) | –0.083 (0.941) | 0.014 (0.810) | .731 |
| Dismissing AS (z score) | –0.452 (0.740) | 0.408 (0.901) | .002a |
Abbreviations: AS, attachment style; HF, high fearful; LF, low fearful; PTSD, posttraumatic stress disorder; SD, standard deviation.
Measures that survived statistical significance.
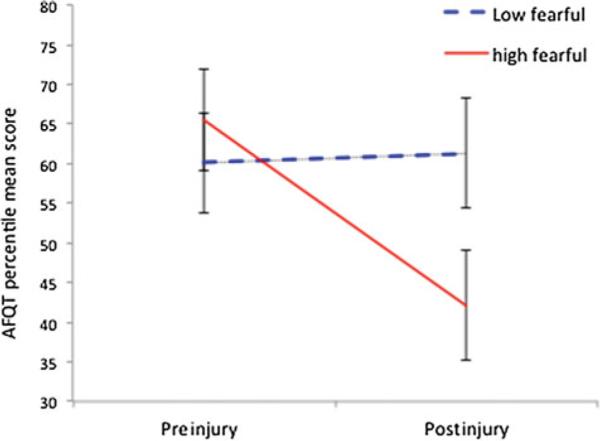
The ANCOVA evaluating the association between cognitive trajectory of participants with TBI and care-givers’ fearful AS showed a significant interaction effect for trajectory × AS (F1,34 = 9.328; P = .004) (effect size: η2 = 0.215) but no main effect for trajectory (F1,34 = 4.252; P = .047) or for AS (F1,34 = 0.508; P = .481). The covariates, dismissing AS (F1,34 = 0.597; P = .445), secure AS (F1,34 = 1.962; P = .170), caregiver depression (F1,34 = 1.167; P = .288), ness and NEO Conscientious-(F1,34 = 1.237; P = .274), were not significantly related to trajectory. Post hoc analysis showed that participants with TBI with HF caregivers performed significantly worse 40 years postinjury than at preinjury (t19 = 4.360; P < .001), whereas participants with TBI with (see LF caregivers were stable (t19 = 0.545; P = .592) Figures 1 and 2).
Figure 1.
Cognitive performances measured with AFQT over time. Mean values and standard error of the mean for the AFQT percentile scores are shown for the participants with TBI of the low and high fearful caregiver groups at preinjury and 40 years postinjury. AFQT indicates Armed Forces Qualification Test; TBI, traumatic brain injury.
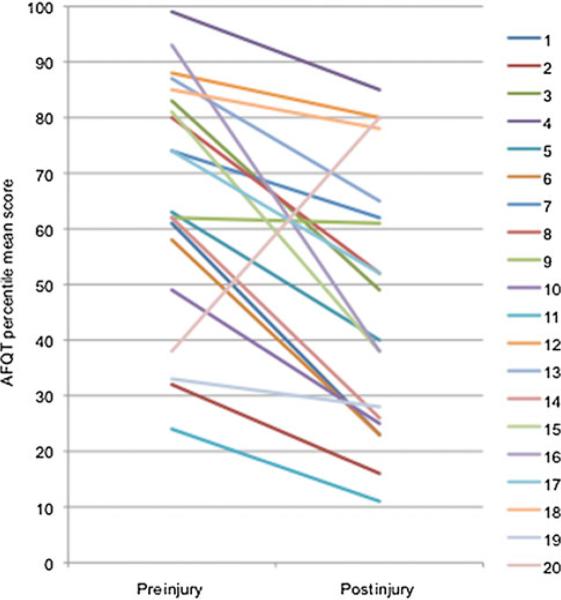
Figure 2.
Individual cognitive decline slopes of the 20 participants with TBI of the high fearful group. AFQT indicates Armed Forces Qualification Test; TBI, traumatic brain injury. Participant with TBI 20 is an outlier but it is not unheard of for someone to show improvement over a lifetime of experience. In his case, he completed his GED after leaving the military (which means after his pre-injury AFQT). He also reported experiencing some difficulties with english and reading while in school. In the post-injury AFQT, his vocabulary subscore is about the same than his arithmetic one. Although we don't have the detail of his pre-injury AFQT, we can speculate that he might have improved greatly his semantic knowledge in the meanwhile.
Secure, preoccupied, and dismissing ASs
Using secure caregiver AS as a between-subjects factor, we found a main effect for trajectory (F1,34 = 8.554; P = .006; η2 = 0.201) but no main effect for AS (F1,34 = 0.037; P = .849), nor a significant interaction effect for trajectory × AS (F1,34 = 3.455; P = .072).
Using dismissing caregiver AS as a between-subjects factor, we found no main effects for trajectory (F1,34 = 5.555; P = .024) or AS (F1,34 = 0.056; P = .815), nor a significant interaction effect for trajectory × AS (F1,34 = 0.355; P = .555).
Finally, using preoccupied caregiver AS as a between-subjects factor, we found a main effect for trajectory (F1,34 = 8.554; P = .006; η2 = 0.201) but no main effect for AS (F1,34 = 0.037; P = .849), nor a significant interaction effect for trajectory × AS (F1,34 = 3.455; P = .072).
DISCUSSION
In a 40-year follow-up study, we investigated the association between caregiver AS and the cognitive decline of a homogeneous population of participants with TBI: US males of similar age and education, who sustained their TBI during combat in Vietnam and who knew their caregivers for 42 years on average. Our results indicate that participants with TBI whose caregiver scored high on fearful AS (as measured at follow-up) had a significantly larger cognitive decline from preinjury to the present. Since we used an observational study design and the AS and the final cognitive outcome were measured concurrently, it is not possible to draw firm conclusions regarding causal relationships. The relationship observed could be due to a third unmeasured variable, and it is also conceivable that cognitive decline led to a more fearful AS. However, we can consider several alternative hypotheses.
On the basis of animal and human research on neural plasticity and cognitive reserve, we predicted that a fearful AS would be associated with cognitive decline in individuals with TBI, possibly by depriving persons with TBI of the protective effect of more positive styles. In line with our prediction, participants with TBI whose caregiver scored high on fearful AS showed a significantly greater degree of cognitive decline than those whose caregiver scored low on fearful AS.
By being generally more anxious and avoiding social situations, it is likely that HF caregivers’ increased stress in the environment of participants with TBI and reduced the richness of social activities of participants with TBI. In contrast, LF caregivers may allow participants with TBI to be challenged more and increase the richness of their environments.
The consequences of environmental complexity on brain recovery and cognitive decline have been thoroughly studied in animals24,25,28,30,54,55 and show the protective effect of complex housing by modulating the damage-induced neurogenesis and dendritic growth.24–26,30,54 These findings in rodents are supported by research on nonhuman primates.30 Cognitive enrichment not only is a protective factor for cognitive decline but also potentially improves cognition in aging populations via cognitive stimulation.56 In addition, some authors argued that if participants engaged in cognitively stimulating activities such as completing puzzles, reading, or learning new games or activities as they are aging or postinjury, this would increase their cognitive reserve.57,58 Interestingly, the large effect size found for the association between caregivers’ AS and cognitive decline of individuals with TBI is equal to or even greater than the medium to large effect size found in cognitive reserve studies.59,60
Factors other than the caregiver certainly influence cognitive decline and might have confounded the group differences we report. However, we found no signifi-cant between-group differences for AS participants on language (naming, comprehension), executive functions (mental flexibility, verbal fluency, abstract reasoning), memory or visual perception, posttraumatic stress disorder, functional status, or brain volume loss at their phase 4 evaluation.
It is also possible that participants predisposed to particular cognitive trajectories might have become paired with caregivers with particular ASs. Although individuals, regardless of their own AS, are overall more attracted by secure individuals, some studies found other patterns of attraction.15,61 Those with an insecure AS are more likely to be attracted by insecure partners than secure individuals. In the current study, we didn't find any significant difference between LF and HF participants’ AS and personality traits.
There have been very few studies that have examined interventions to modify AS; this limitation may be due to AS being considered a trait. A recent study by Kinley and Reyno62 found increased secure attachment and decreased fearful attachment after 6 weeks of intensive group psychotherapy. Another intervention that could be explored is cognitive-behavioral therapy, as it has shown short-term and long-term efficacy for anxiety.63 Since fearful AS is associated with anxiety disorder,64 it might be valuable to treat anxiety in caregivers in the hope of protecting or enhancing cognition in participants.
The study has some limitations. While the VHIS sample has many advantages, such as homogeneity and baseline data, it may limit the generalizability of the results to populations more diverse in terms of sex, age, race, and socioeconomic status. Moreover, the study design did not allow for conclusions regarding the direction of the association between cognitive decline and AS, nor does it allow inference of causal relationship. Although we showed a large effect size of the association between caregivers’ AS and cognitive trajectory of participants with TBI, we are unsure of the mediator factor(s) of this association. An interesting research direction would be to analyze caregiver's behavioral data, measuring, for example, how much the caregiver controls the environment of participants with TBI or how much the caregiver protects the participant with TBI. Finally, the association between caregivers’ AS and specific cognitive functions could not be determined because of the limitations of the AFQT; we were not able to address this issue. Our participants with TBI were older than 60 years at the time of this evaluation. On the basis of our extensive 1-week inpatient evaluation, we did not detect symptoms of dementia in this cohort but we were able to document exacerbated cognitive decline that was dependent on a number of factors such as lesion characteristics, prein-jury cognitive status, presence or absence of epilepsy, and so on.
CONCLUSION
Animal and human literature on neural plasticity supports the fact that a stimulating environment enhances neurogenesis, synaptogenesis, and dendrite growth. Similarly, a deprived environment has negative effects on neural plasticity and can reduce cognitive reserve. Care-givers exercise important effects on the environment of individuals with TBI beyond providing physical, social, and emotional support. In the current study, we found an association between caregivers’ AS and cognitive trajectory of individuals with TBI. While caregiver burden and coping have been studied extensively, we urge future research to also take into account the possible effects of the caregiver on recovery and maintenance of functional abilities. Also, it might be prudent to evaluate caregivers along with individuals with TBI after the injury in order to develop cost-effective caregiver interventions targeting AS, thereby potentially reducing long-term cognitive decline in the patient.
Footnotes
Drs Krueger and Grafman have joint senior authorship.
The authors declare no conflicts of interest.
REFERENCES
- 1.Raymont V, Greathouse A, Reding K, Lipsky R, Salazar A, Grafman J. Demographic, structural and genetic predictors of late cognitive decline after penetrating head injury. Brain. 2008;131(pt 2):543–558. doi: 10.1093/brain/awm300. [DOI] [PubMed] [Google Scholar]
- 2.Department of Defense [March 5, 2014];DoD worldwide total numbers for TBI, 2000-2013. http://www.dvbic.org/dod-worldwide-numbers-tbi.
- 3.Owens BD, Kragh JF, Jr, Wenke JC, Macaitis J, Wade CE, Holcomb JB. Combat wounds in Operation Iraqi Freedom and Operation Enduring Freedom. J Trauma. 2008;64(2):295–299. doi: 10.1097/TA.0b013e318163b875. [DOI] [PubMed] [Google Scholar]
- 4.Terrio H, Brenner LA, Ivins BJ, et al. Traumatic brain injury screening: preliminary findings in a US Army Brigade Combat Team. J Head Trauma Rehabil. 2009;24(1):14–23. doi: 10.1097/HTR.0b013e31819581d8. [DOI] [PubMed] [Google Scholar]
- 5.French LM. Military traumatic brain injury: an examination of important differences. Ann N Y Acad Sci. 2010;1208:38–45. doi: 10.1111/j.1749-6632.2010.05696.x. [DOI] [PubMed] [Google Scholar]
- 6.Schmidt AT, Orsten KD, Hanten GR, Li X, Levin HS. Family environment influences emotion recognition following paediatric traumatic brain injury. Brain Inj. 2010;24(13/14):1550–1560. doi: 10.3109/02699052.2010.523047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Yeates KO, Swift E, Taylor HG, et al. Short- and long-term social outcomes following pediatric traumatic brain injury. J Int Neuropsychol Soc. 2004;10(3):412–426. doi: 10.1017/S1355617704103093. [DOI] [PubMed] [Google Scholar]
- 8.Taylor HG, Yeates KO, Wade SL, Drotar D, Stancin T, Burant C. Bidirectional child-family influences on outcomes of traumatic brain injury in children. J Int Neuropsychol Soc. 2001;7(6):755–767. doi: 10.1017/s1355617701766118. [DOI] [PubMed] [Google Scholar]
- 9.Tschanz JT, Piercy K, Corcoran CD, et al. Caregiver coping strategies predict cognitive and functional decline in dementia: the cache county dementia progression study. Am J Geriatr Psychiatry. 2013;21(1):57–66. doi: 10.1016/j.jagp.2012.10.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wright LK. AD spousal caregivers. Longitudinal changes in health, depression, and coping. J Gerontol Nurs. 1994;20(10):33–48. doi: 10.3928/0098-9134-19941001-08. [DOI] [PubMed] [Google Scholar]
- 11.Wright LK. Alzheimer's disease afflicted spouses who remain at home: can human dialectics explain the findings? Soc Sci Med. 1994;38(8):1037–1046. doi: 10.1016/0277-9536(94)90220-8. [DOI] [PubMed] [Google Scholar]
- 12.Perren S, Schmid R, Herrmann S, Wettstein A. The impact of attachment on dementia-related problem behavior and spousal caregivers’ well-being. Attach Hum Dev. 2007;9(2):163–178. doi: 10.1080/14616730701349630. [DOI] [PubMed] [Google Scholar]
- 13.Ognibene TC, Collins NL. Adult attachment styles, perceived social support and coping strategies. J Soc Pers Relat. 1998;15(3):323–345. [Google Scholar]
- 14.Vrticka P, Vuilleumier P. Neuroscience of human social interactions and adult attachment style. Front Hum Neurosci. 2012;6:212. doi: 10.3389/fnhum.2012.00212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Mikulincer M, Shaver P. Attachment in Adulthood. Structure, Dynamics, and Change. Guilford Press; New York, NY: 2007. [Google Scholar]
- 16.Bowlby J. The making and breaking of affectional bonds, part I: aetiology and psychopathology in the light of attachment theory. An expanded version of the Fiftieth Maudsley Lecture, delivered before the Royal College of Psychiatrists, 19 November 1976. Br J Psychiatry. 1977;130:201–210. doi: 10.1192/bjp.130.3.201. [DOI] [PubMed] [Google Scholar]
- 17.Kirkpatrick LA, Davis KE. Attachment style, gender, and relationship stability: a longitudinal analysis. J Pers Soc Psychol. 1994;66(3):502–512. doi: 10.1037//0022-3514.66.3.502. [DOI] [PubMed] [Google Scholar]
- 18.Klohnen EC, Bera S. Behavioral and experiential patterns of avoidantly and securely attached women across adulthood: a 31-year longitudinal perspective. J Pers Soc Psychol. 1998;74(1):211–223. doi: 10.1037//0022-3514.74.1.211. [DOI] [PubMed] [Google Scholar]
- 19.Main M, Kaplan N, Cassidy J. Security in infancy, childhood, and adulthood: a move to the level of representation. Monogr Soc Res Child. 1985;50(1):66–104. [Google Scholar]
- 20.Ainsworth MD. Infant-mother attachment. Am Psychol. 1979;34(10):932–937. doi: 10.1037//0003-066x.34.10.932. [DOI] [PubMed] [Google Scholar]
- 21.Bartholomew K, Horowitz LM. Attachment styles among young adults: a test of a four-category model. J Pers Soc Psychol. 1991;61(2):226–244. doi: 10.1037//0022-3514.61.2.226. [DOI] [PubMed] [Google Scholar]
- 22.Lopez FG, Gormley B. Stability and change in adult attachment style over the first-year college transition: relations to self-confidence, coping, and distress patterns. J Couns Psychol. 2002;49(3):355–364. [Google Scholar]
- 23.Hamam-Raz Y, Solomon Z. Psychological adjustment of melanoma survivors. J Individ Diff. 2006;27(3):172–182. [Google Scholar]
- 24.Saucier DM, Yager JY, Armstrong EA. Housing environment and sex affect behavioral recovery from ischemic brain damage. Behav Brain Res. 2010;214(1):48–54. doi: 10.1016/j.bbr.2010.04.039. [DOI] [PubMed] [Google Scholar]
- 25.Briones TL, Rogozinska M, Woods J. Environmental experience modulates ischemia-induced amyloidogenesis and enhances functional recovery. J Neurotrauma. 2009;26(4):613–625. doi: 10.1089/neu.2008.0707. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 26.Kozorovitskiy Y, Gould E. Adult neurogenesis: a mechanism for brain repair? J Clin Exp Neuropsychol. 2003;25(5):721–732. doi: 10.1076/jcen.25.5.721.14580. [DOI] [PubMed] [Google Scholar]
- 27.Hockly E, Cordery PM, Woodman B, et al. Environmental enrichment slows disease progression in R6/2 Huntington's disease mice. Ann Neurol. 2002;51(2):235–242. doi: 10.1002/ana.10094. [DOI] [PubMed] [Google Scholar]
- 28.Briones TL, Rogozinska M, Woods J. Modulation of ischemia-induced NMDAR1 activation by environmental enrichment decreases oxidative damage. J Neurotrauma. 2011;28(12):2485–2492. doi: 10.1089/neu.2011.1842. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 29.Leuner B, Gould E. Structural plasticity and hippocampal function. Annu Rev Psychol. 2010;61:111–140. C111–C113. doi: 10.1146/annurev.psych.093008.100359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kozorovitskiy Y, Gross CG, Kopil C, et al. Experience induces structural and biochemical changes in the adult primate brain. Proc Natl Acad Sci U S A. 2005;102(48):17478–17482. doi: 10.1073/pnas.0508817102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Holtmaat A, Svoboda K. Experience-dependent structural synaptic plasticity in the mammalian brain. Nat Rev Neurosci. 2009;10(9):647–658. doi: 10.1038/nrn2699. [DOI] [PubMed] [Google Scholar]
- 32.Leuner B, Shors TJ. Stress, anxiety, and dendritic spines: what are the connections? Neuroscience. 2013;251:108–119. doi: 10.1016/j.neuroscience.2012.04.021. [DOI] [PubMed] [Google Scholar]
- 33.Arciniegas DB, Zasler ND, Vanderploeg RD, Jaffee MS. Management of Adults With Traumatic Brain Injury. American Psychiatric Association; Arlington, VA: 2013. pp. 10–11. [Google Scholar]
- 34.Moretti L, Cristofori I, Weaver SM, Chau A, Portelli JN, Grafman J. Cognitive decline in older adults with a history of traumatic brain injury. Lancet Neurol. 2012;11(12):1103–1112. doi: 10.1016/S1474-4422(12)70226-0. [DOI] [PubMed] [Google Scholar]
- 35.Nithianantharajah J, Hannan AJ. The neurobiology of brain and cognitive reserve: mental and physical activity as modulators of brain disorders. Prog Neurobiol. 2009;89(4):369–382. doi: 10.1016/j.pneurobio.2009.10.001. [DOI] [PubMed] [Google Scholar]
- 36.Jette AM, Davies AR, Cleary PD, et al. The Functional Status Questionnaire: reliability and validity when used in primary care. J Gen Intern Med. 1986;1(3):143–149. doi: 10.1007/BF02602324. [DOI] [PubMed] [Google Scholar]
- 37.Costa PT, McCrae RR. The NEO Personality Inventory Manual. Psychological Assessment Resources; Odessa, FL: 1985. [Google Scholar]
- 38.Keane TM, Caddell JM, Taylor KL. Mississippi Scale for Combat-Related Posttraumatic Stress Disorder: three studies in reliability and validity. J Consult Clin Pyschol. 1988;56(1):85–90. doi: 10.1037//0022-006x.56.1.85. [DOI] [PubMed] [Google Scholar]
- 39.Beck AT, Rush AJ, Shaw BF, Emery D. Cognitive Therapy of Depression. Guilford Press; New York, NY: 1979. [Google Scholar]
- 40.Wechsler D. Wechsler Adult Intelligence Scale. 3rd ed. The Psychological Corporation; San Antonio, TX: 1997. [Google Scholar]
- 41.Kaplan E, Goodglass H, Weintraub S. Boston Naming Test. 2nd ed. Lippincott Williams & Wilkins; Philadelphia, PA: 2001. [Google Scholar]
- 42.Boller F, Vignolo LA. Latent sensory aphasia in hemisphere-damaged patients: an experimental study with the Token Test. Brain. 1966;89(4):815–830. doi: 10.1093/brain/89.4.815. [DOI] [PubMed] [Google Scholar]
- 43.Delis DC, Kaplan E, Kramer JH. Delis-Kaplan Executive Function System. The Psychological Corporation; San Antonio, TX: 2001. [Google Scholar]
- 44.Warrington EK, James M. Visual Object and Space Perception Battery (VOSP) Thames Valley test Company; Suffolk, English: 1991. [Google Scholar]
- 45.Radloff LS. The CES-D scale: a self-report depression scale for research in the general population. Appl Psychol Meas. 1977;1:385–401. [Google Scholar]
- 46.Zarit SH, Todd PA, Zarit JM. Subjective burden of husbands and wives as caregivers: a longitudinal study. Gerontologist. 1986;26(3):260–266. doi: 10.1093/geront/26.3.260. [DOI] [PubMed] [Google Scholar]
- 47.Grafman J, Jonas BS, Martin A, et al. Intellectual function following penetrating head injury in Vietnam veterans. Brain. 1988;111(pt 1):169–184. doi: 10.1093/brain/111.1.169. [DOI] [PubMed] [Google Scholar]
- 48.US Department of Defense . A Test Manual for the Armed Services Vocational Aptitude Battery. US Military Entrance Processing Command; Chicago, IL: 1984. [Google Scholar]
- 49.Griffin DW, Bartholomew K. The metaphysics of measurement: the case of adult attachment. Adv Pers Relationships. 1994;5:17–52. [Google Scholar]
- 50.Bartholomew K, Shaver PR. Methods of assessing adult attachment: do they converge? In: Simpson JA, Rholes WS, editors. Attachment Theory and Close Relationships. Guilford Press; New York, NY: 1998. pp. 25–45. [Google Scholar]
- 51.Guedeney N, Fermanian J, Bifulco A. [Construct validation study of the Relationship Scales Questionnaire (RSQ) on an adult sample]. Encephale. 2010;36(1):69–76. doi: 10.1016/j.encep.2008.12.006. [DOI] [PubMed] [Google Scholar]
- 52.Makale M, Solomon J, Patronas NJ, Danek A, Butman JA, Grafman J. Quantification of brain lesions using interactive automated software. Behav Res Methods Instrum Comput. 2002;34(1):6–18. doi: 10.3758/bf03195419. [DOI] [PubMed] [Google Scholar]
- 53.Solomon J, Raymont V, Braun A, Butman JA, Grafman J. User-friendly software for the analysis of brain lesions (ABLe). Comput Methods Programs Biomed. 2007;86(3):245–254. doi: 10.1016/j.cmpb.2007.02.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Briones TL, Klintsova AY, Greenough WT. Stability of synaptic plasticity in the adult rat visual cortex induced by complex environment exposure. Brain Res. 2004;1018(1):130–135. doi: 10.1016/j.brainres.2004.06.001. [DOI] [PubMed] [Google Scholar]
- 55.Leuner B, Kozorovitskiy Y, Gross CG, Gould E. Diminished adult neurogenesis in the marmoset brain precedes old age. Proc Natl Acad Sci U S A. 2007;104(43):17169–17173. doi: 10.1073/pnas.0708228104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Milgram NW, Siwak-Tapp CT, Araujo J, Head E. Neuroprotective effects of cognitive enrichment. Ageing Res Rev. 2006;5(3):354–369. doi: 10.1016/j.arr.2006.04.004. [DOI] [PubMed] [Google Scholar]
- 57.Wilson RS, Mendes De Leon CF, Barnes LL, et al. Participation in cognitively stimulating activities and risk of incident Alzheimer disease. JAMA. 2002;287(6):742–748. doi: 10.1001/jama.287.6.742. [DOI] [PubMed] [Google Scholar]
- 58.Baldivia B, Andrade MA, Amodeo Bueno OF. Contribution of education, occupation and cognitively stimulating activities to the formation of cognitive reserve. Dement Neuropsychol. 2008;2(3):173–182. doi: 10.1590/S1980-57642009DN20300003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Valenzuela M, Sachdev P. Can cognitive exercise prevent the onset of dementia? Systematic review of randomized clinical trials with longitudinal follow-up. Am J Geriatr Psychiatry. 2009;17(3):179–187. doi: 10.1097/JGP.0b013e3181953b57. [DOI] [PubMed] [Google Scholar]
- 60.Honer WG, Barr AM, Sawada K, et al. Cognitive reserve, presynaptic proteins and dementia in the elderly. Transl Psychiatry. 2012;2:e114. doi: 10.1038/tp.2012.38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Carnelley KB, Pietromonaco PR, Jaffe K. Depression, working models of others, and relationship functioning. J Pers Soc Psychol. 1994;66(1):127–140. doi: 10.1037//0022-3514.66.1.127. [DOI] [PubMed] [Google Scholar]
- 62.Kinley JL, Reyno SM. Attachment style changes following intensive short-term group psychotherapy. Int J Group Psychother. 2013;63(1):53–75. doi: 10.1521/ijgp.2013.63.1.53. [DOI] [PubMed] [Google Scholar]
- 63.DiMauro J, Domingues J, Fernandez G, Tolin DF. Long-term effectiveness of CBT for anxiety disorders in an adult outpatient clinic sample: a follow-up study. Behav Res Ther. 2013;51(2):82–86. doi: 10.1016/j.brat.2012.10.003. [DOI] [PubMed] [Google Scholar]
- 64.Marganska A, Gallagher M, Miranda R. Adult attachment, emotion dysregulation, and symptoms of depression and generalized anxiety disorder. Am J Orthopsychiatry. 2013;83(1):131–141. doi: 10.1111/ajop.12001. [DOI] [PubMed] [Google Scholar]