Abstract
Objective
We evaluated the impact of maternal overweight/obesity and excessive weight gain on maternal serum lipids in the first and second trimester of pregnancy.
Design and Methods
Prospective data were collected for 225 women. Maternal serum lipids and fatty acids were measured at <13 weeks and between 24–28 weeks. Analyses were stratified by normal weight versus overweight/obese status and excessive vs. non-excessive weight gain.
Results
Overweight/obese women had higher baseline cholesterol (161.3±29.6 vs 149.4±26.8 mg/dL, p<0.01), LDL (80.0±19.9 vs 72.9 ±18.8 mg/dL, p<0.01) and triglycerides ( 81.7±47.2 vs 69.7±40.3 mg/dL, p=0.05) when compared to normal weight women, while HDL (43.6 ±10.4 47.6±11.5 mg/dL, p<0.01) was lower. However, cholesterol and LDL increased at a higher weekly rate in normal weight women, resulting in higher total cholesterol in normal weight women (184.1±28.1 vs. 176.0 ±32.1 mg/dL, p=0.05) at 24–28 weeks. Excessive weight gain did not affect the rate of change in lipid profiles in either group. Overweight/obese women had higher levels of arachidonic acid at both time points.
Conclusions
Overweight/obese women have significantly more atherogenic lipid profiles than normal weight women during the period of early pregnancy, delineating one physiologic pathway that could explain differences in pregnancy outcomes between normal weight and overweight/obese women.
Keywords: Pregnancy, Triglycerides, Cholesterol, Fatty Acids, Maternal Obesity, Weight gain
Introduction
Two-thirds of women in the United States are overweight or obese (body mass index (BMI) 25 kg/m2 or greater) at the time of conception.1–2 Maternal obesity is associated with an increased risk for pregnancy complications including gestational diabetes, 3 pre-eclampsia, 4 and abnormalities of fetal growth.5 In addition, offspring from obese women may be more likely to develop obesity and diabetes in their lifetime. 6 Excess maternal weight gain has also been linked to an increased risk for adverse pregnancy outcomes including gestational diabetes and fetal macrosomia.7 However, the mechanisms underlying these increased risks for pregnancy complications are incompletely understood.
Pregnancy is characterized by progressive increases in serum lipids, and this adaptation is essential to promote normal fetal growth and development.8–15 In the non-pregnant state, obesity is associated with an atherogenic phenotype including high serum concentrations of total cholesterol, low density lipoprotein (LDL) and triglycerides as well as a reduction in serum high density lipoprotein (HDL) cholesterol.16 However, less is known regarding the influence of maternal obesity or excess weight gain on serum lipid profiles during pregnancy.
There are also limited data comparing fatty acid profiles between normal weight and overweight/obese women during pregnancy. Saturated and monounsaturated long-chain fatty acids, n-6 long-chain polyunsaturated fatty acids, and trans fatty acids are important components of the Western diet. These fatty acids are linked to fetal nutrition, membrane function, synthesis of prostaglandins and other eicosanoids, and inflammation, all of which are important to the physiology of pregnancy.
We conducted this analysis to further our understanding of changes in maternal serum lipids and fatty acid profiles in overweight and obese women compared with normal weight women between the first and late second trimester of pregnancy. We also examined the impact of excess weight gain on the rate of change in maternal serum lipid profiles from the first to late second trimester. We hypothesized that changes in maternal serum lipids and fatty acid profiles between the first and second trimester in overweight/obese women may reveal pathophsiology that occurs in maternal obesity or excess weight gain, and that knowledge of these changes may improve our understanding of the increased risks for adverse pregnancy outcomes commonly seen in overweight and obese women.
Methods and Procedures
This was a secondary analysis conducted within a larger prospective cohort study designed to investigate the role of maternal nutritional influences on preterm birth at the University of Pittsburgh (Pittsburgh, PA). The parent study enrolled women from 2004–2011, and this analysis included women enrolled from 2007–2011. Women aged 14–50 years who were carrying singleton infants and planning to deliver at Magee-Womens Hospital of UPMC were eligible. Women with chronic hypertension, diabetes, renal disease, and rheumatologic disorders were excluded from the study. The Institutional Review Board of the University of Pittsburgh approved the protocol prior to study initiation and all participants provided informed consent.
Women were enrolled in the study at less than 13 weeks’ gestation, and much of the study enrollment occurred in a clinic providing care for low-income women. Baseline demographic information and medical history were collected via a structured interview, and non-fasting maternal serum was collected at less than 13 weeks’ gestation and again at 24–28 weeks’ gestation. Pregnancy outcomes including gestational age at delivery, birthweight, results of gestational diabetes screening, and diagnosis of hypertensive disorders of pregnancy (defined as new onset blood pressure >140/90 on two or more occasions 6 hours apart after 20 weeks’ gestation) were recorded from the medical record.
Our primary exposure of interest was maternal pre-pregnancy overweight and obesity, and this was reported as an index of weight-for-height (body mass index, BMI) and defined as the weight in kilograms (kg) divided by the square of height in meters (kg/m2). Pre-pregnancy weight was ascertained by maternal self-report at the first study visit and height was measured via a stadiometer at the baseline visit. Participants were classified as overweight or obese if their BMI was ≥25.0 kg/m2.17 Excessive early gestational weight gain was defined as gestational weight gain greater than the upper range of Institute of Medicine 2009 guidelines for each prepregnancy BMI category (underweight, normal weight, overweight, and obese).7 Specifically, the cut-off for excessive early gestational weight gain was determined by adding a first-trimester weight gain of 4.4 pounds (2.0 kg) plus second trimester weight gain per week of 1.3 pounds (0.6 kg) for underweight, 1.0 pounds (0.5 kg) for normal weight, 0.7 pounds (0.3 kg) for overweight, and 0.6 pounds (0.3 kg) for obese women.7
Study Participants
At the time of our analysis 741 women had been enrolled in the larger cohort study. A total of 225 women (30.4% of the overall cohort of women) who were enrolled in the study from 2007–2011 had serum lipids measured at less than 13 weeks’ gestation (8.3±1.9 weeks) and again at 24–28 weeks’ gestation (26.0 ± 1.1weeks) and were included in the analysis involving total cholesterol, HDL, LDL, and triglycerides. Of these patients, 53 (24%) were overweight and 82 (36% were obese). Serum fatty acid profiles were also assessed in 151 women. When compared to all women in the larger cohort, the 225 women who were included in this analysis were slightly younger (23.6 ±4.5 vs. 24.9 ±5.0 years, p<0.01), of lower parity (2.7 ±1.7 vs3.3 ±2.0 pregnancies, p<0.01), and of higher body mass index (BMI) (28.7 ±8.1 vs. 26.6 ± 6.6 kg/m2, p<0.01). The 151 women with fatty acids analyzed were similar to the 225 women in the lipid analysis with regards to age (23.6 ± 4.6 vs 23.6 ± 4.4 years, p=0.95), parity (2.5 ± 1.8 vs 2.8 ±1.6 pregnancies, p=0.21), and BMI (28.8 ±7.6 vs 28.6 ± 8.4 kg/m2, p=0.82).
Lipid Measurement
Serum total cholesterol, HDL cholesterol, LDL cholesterol, and triglyceride concentrations were determined enzymatically using specific reagents from Pointe Scientific (Canton, MI). The inter-assay variability was less than 10% for cholesterol, 10% for LDL cholesterol, and 8% for HDL cholesterol. The inter-assay variability for triglycerides was less than 5%. All samples were analyzed in duplicate and in a blinded fashion.
The following long-chain fatty acid concentrations were ascertained: total long-chain fatty acids, total monounsaturated long-chain fatty acids (MUFA), total long-chain polyunsaturated fatty acids (PUFA, C18_2n6, C18_3n6, C18_3n3, C20_2n6, C20_3n6, C20_4n6, C20_4n3, C20_5n3, C22_4n6, C22_5n3, C22_6n3, C22_5n6), total n-6 long-chain polyunsaturated fatty acids, total n-3 polyunsaturated fatty acids, arachidonic acid (C20_4n6), docohexaenoic acid (DHA, C22_6n3), and eicosapentaenoic acid (EPA, C20_5n3). Lipids were extracted using the technique of Bligh and Dryer,18 and were measured by gas column chromatography in the Heinz Laboratory at the University of Pittsburgh using a previously described protocol.19
Statistical Analysis
Statistical analyses were completed using Stata 10 software package Special Edition (StataCorp LP, College Station, TX). Distribution of variables was tested for normality using Shaprio-Wilk W-test. Maternal serum lipids were compared between the first and second trimesters using paired t-tests, and differences in maternal serum lipids and other demographic variables were compared between normal weight and overweight/obese women using chi-squared and t-tests as appropriate. Change between the first and second trimester lipid levels was calculated by subtracting the first trimester value from the second trimester value for each study subject. This was normalized to the number of weeks between the first and second trimester by dividing the calculated difference in serum lipid levels by the number of weeks that elapsed between blood draws to calculate a rate of change/week. This calculation assumed that the rate of change per week in serum lipid values was linear. Linear regression was used to identify variables independently associated with each of the serum lipid values as well as the rate of change per week for cholesterol, LDL, HDL, and triglycerides. Variables with a p value <0.10, as well as those with known or suspected biologic importance were then included in multivariable regression models to investigate the relationship among covariates. A p-value <0.05 was considered statistically significant in all analyses, and unadjusted results are shown when no relevant confounding variables were identified.
Results
Table 1 shows the clinical characteristics of normal weight compared to overweight/obese women. Although overweight and obese women had a higher pre-pregnancy BMI, they gained significantly less weight from the beginning of pregnancy to the 24–28 week time period and from the beginning of pregnancy to delivery. Overweight and obese women were also of higher parity and had higher systolic and diastolic blood pressure at the time of their first study visit. Overweight and obese women demonstrated a lower frequency of smoking, although this did not reach statistical significance. Results for the 50 gram glucose challenge test at 24–28 weeks’ gestation, gestational age at delivery, rates of preterm birth less than 37 weeks’ gestation, birthweight, and hypertensive disorders of pregnancy were similar between overweight/obese and normal weight women. There were only two women diagnosed with gestational diabetes, so we were unable to compare rates of gestational diabetes between groups.
Table 1.
Maternal characteristics and pregnancy outcomes by lean vs. overweight/obese status
| Demographics | Normal (n=90) | Overweight/Obese (n=135) | P |
|---|---|---|---|
|
| |||
| Age (years) | 23.0 (±3.7) | 24.0 (±4.9) | 0.11 |
|
| |||
| Parity | |||
| 1 | 27 (30) | 27 (20) | 0.03 |
| 2 | 30 (33) | 35 (26) | |
| ≥3 | 33 (37) | 73 (54) | |
|
| |||
| Race | |||
| White | 34 (37.8) | 43 (32.2) | |
| Black | 38 (42.2) | 72 (54.1) | 0.18 |
| Other | 18 (20.0) | 18 (13.5) | |
|
| |||
| Education (years) | 12.2 (±1.7) | 12.5 (±2.0 | 0.15 |
|
| |||
| Yearly Income | |||
| <25,000 | 65 (77.4) | 103 (77.4) | |
| 25,000–75,000 | 17 (20.2) | 30 (22.6) | 0.19 |
| >75,000 | 2 (2.4) | 0 | |
|
| |||
| BMI (mean) | 21.6 (±2.3) | 33.4 (±7.1) | <0.01 |
|
| |||
| Total weight gain (lbs) | 38.9 (±16.8) | 24.8 (±17.0) | <0.01 |
|
| |||
| Weight gain 24–28 weeks (lbs) | 21.9 (±12.3) | 13.0 (±14.2) | <0.01 |
|
| |||
| Weight gain >IOM Recommendations at 24–28 weeks | 50 (55.6) | 66 (49.0) | 0.36 |
|
| |||
| Smoking | 44 (49.4) | 51 (38.1) | 0.09 |
|
| |||
| Any alcohol use | 6 (6.7) | 7 (5.2) | 0.65 |
|
| |||
| SBP | 108.1 (±10.7) | 115.8 (±7.5) | <0.01 |
|
| |||
| DBP | 65.2 (±8.1) | 70.7 (±6.0) | <0.01 |
|
| |||
| GA at enrollment | 8.3 (±2.0) | 8.3 (±1.8) | 0.98 |
|
| |||
| GA age at 24–28 week visit | 26.1 (±1.2) | 25.9 (±1.1) | 0.17 |
|
| |||
| 50 g GCT results (mg/dL) | 92.4 (±21.3) | 96.7 (±22.1) | 0.17 |
|
| |||
| GA at delivery (weeks) | 39.2 (±1.7) | 38.9 (±2.3) | 0.30 |
|
| |||
| Preterm birth (<37 weeks) | 5 (5.6%) | 12 (8.9%) | 0.35 |
|
| |||
| Birthweight (grams) | 3215 (±556) | 3227 (±594) | 0.86 |
|
| |||
| Hypertensive disorders of pregnancy | 8 (8.9%) | 10 (7.4%) | 0.67 |
All variables presented as mean (± standard deviation) or n (percent). BMI (Body Mass Index), lbs (pounds), IOM (Institute of Medicine), SBP (Systolic Blood Pressure), DBP (Diastolic Blood Pressure), GA (gestational age), GCT (glucose challenge test)
As shown in table 1, total cholesterol, LDL, HDL, and triglycerides increased from <13 weeks to 24–28 weeks’ gestation in both normal weight and overweight/obese women. Analysis stratified by normal weight versus overweight/obese status demonstrated that there were marked differences in serum lipids between the two groups. At less than 13 weeks’ gestation overweight/obese women had higher total cholesterol, LDL cholesterol, and triglycerides. In contrast, HDL was lower in overweight/obese women when compared to normal weight women (Table 2). The magnitude and direction of these differences were unchanged when adjusted for smoking, parity, gestational age at blood draw, and maternal race (data not shown).
Table 2.
Maternal Serum Lipid Values in the 1st and 2nd Trimester
| 1st Trimester | 2nd trimester | |||||||
|---|---|---|---|---|---|---|---|---|
| Normal Weight | Overweight/Obese | P | Normal Weight | Overweight/ Obese | P | P^ | P* | |
| Total Cholesterol (mg/dL) | 149.4 (±26.8) | 161.3 (±29.6) | <0.01 | 184.1 (±28.1) | 176.0 (±32.1) | 0.05 | <0.01 | <0.01 |
| LDL Cholesterol (mg/dL) | 72.9 (±18.8) | 80.0 (±19.9) | <0.01 | 115.5 (±23.5) | 111.0 (±24.2) | 0.17 | <0.01 | <0.01 |
| HDL Cholesterol (mg/dL) | 47.6 (±11.5) | 43.6 (±10.4) | <0.01 | 56.9 (±16.0) | 53.0 (16.9) | 0.08 | <0.01 | <0.01 |
| Triglycerides (mg/dL) | 69.7 (±40.3) | 81.7 (±47.2) | 0.05 | 134.0 (±55.6) | 138.3 (±59.8) | 0.59 | <0.01 | <0.01 |
All variables presented as mean (± standard deviation). LDL (Low Density Lipoprotein), HDL (High Density Lipoprotein). P^ is the p-value comparing first and second trimester lipid results in normal weight women, while P* represents the same comparison in overweight/obese women
By the second trimester, total cholesterol was slightly higher in normal weight women when compared to overweight/obese women. There was also a trend towards higher HDL cholesterol in normal weight women and no difference in either LDL cholesterol or triglycerides between normal weight and overweight/obese women (Table 2). Adjustment of these results for parity, weight gain from pre-pregnancy to the time of the 24–28 week blood draw, race, and gestational age at the time of blood draw did not affect the magnitude and direction of these changes (data not shown).
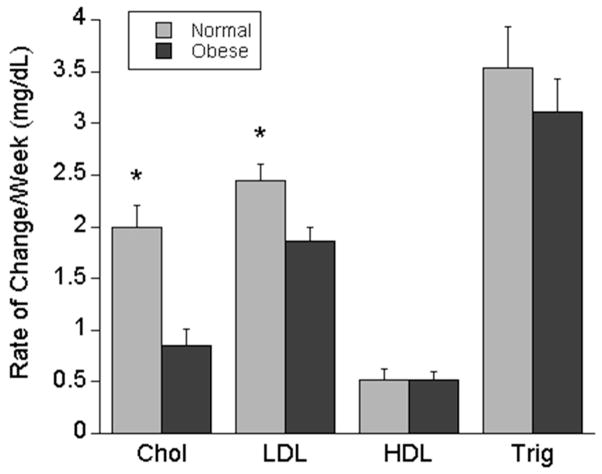
The differences in maternal total cholesterol, LDL, HDL, and triglycerides between normal weight and overweight/obese women in the first and late second trimester suggested that these lipid values have markedly different trajectories between the two groups. Because of this, we sought to examine factors that were associated with the average weekly rate of change for each of these serum lipid values. Univariate analysis of overweight/obese status, smoking, parity, weight gain from pre-pregnancy until the 24–28 week blood draw, and maternal race demonstrated that overweight/obese status was the only variable significantly associated with both the average weekly rate of change in cholesterol (−1.1, 95% CI −1.3 – −0.6, p<0.01) and LDL (−0.6, 95% CI −1.0 – −0.2, p<0.01). Maternal race was associated with the average weekly rate of change in triglycerides (−0.9, 95% CI −1.6 – −.2, p=0.02), but had no significant effect on the relationship between the rate of change in triglycerides and maternal overweight/obese status. Because the relationship between maternal overweight/obese status and the rate of change in lipids were unchanged by any covariates, the results of the unadjusted analysis are shown demonstrating that overweight/obese women had a rate of increase in their total cholesterol and LDL that was significantly lower that the rate of increase in normal weight women (Figure 1). There were no differences in the rate of change in HDL cholesterol or triglycerides between normal weight and overweight/obese women (Figure 1). Furthermore, there was no difference in the weekly rate of change in maternal serum total cholesterol, LDL cholesterol, HDL cholesterol or triglycerides between women with nonexcessive or excessive weight gain in either normal weight or overweight/obese women (Table 3).
Figure 1.
Average change in maternal serum lipids/week in normal weight compared to overweight/obese women. Legend: *p<0.01 normal weight compared to overweight/obese women. Chol (cholesterol), LDL (Low Density Lipoprotein), HDL (High Density Lipoprotein), Trig (triglycerides) 190×159mm (72 x 72 DPI)
Table 3.
Weekly Increase in Maternal Serum Lipid Values by Appropriate or Excessive Weight Gain
| Normal Weight | Overweight/Obese | |||||
|---|---|---|---|---|---|---|
| Nonexcessive Weight Gain (n=40) | Excessive Weight Gain (n=50) | P | Nonexcessive Weight Gain (n=68) | Excessive Weight Gain(n=66) | P | |
| Cholesterol (mg/dL) | 2.1 (±1.6) | 1.9 (±2.0) | 0.62 | 0.7 (±2.1) | 1.0 (±1.7) | 0.33 |
| LDL Cholesterol (mg/dL) | 2.4 (±1.5) | 2.5 (±1.7) | 0.85 | 1.9 (±1.7) | 1.8 (±1.5) | 0.79 |
| HDL Cholesterol (mg/dL) | 0.5 (±0.9) | 0.5 (±0.9) | 0.97 | 0.5 (±0.8) | 0.5 (±0.9) | 0.77 |
| Triglycerides (mg/dL) | 3.9 (±3.5) | 3.2 (±4.1) | 0.44 | 3.2 (±3.8) | 3.1 (±3.3) | 0.88 |
All variables presented as mean (± standard deviation). LDL (Low Density Lipoprotein), HDL (High Density Lipoprotein)
Finally, we examined differences in the maternal serum fatty acid profile between normal and overweight/obese women between the first and late second trimester of pregnancy. Total fatty acids increased from <13 weeks to 24–28 weeks in both normal weight and overweight/obese women. Concentrations of MUFA, PUFA, saturated fatty acids, omega-3 fatty acids, omega-6 fatty acids, arachidonic acid and DHA also increased between the first and late second trimesters in both normal weight and overweight/obese women, while EPA was unchanged. Maternal serum arachadonic acid levels were higher in overweight/obese women in both the first and late second trimester, but there were no significant differences in the concentrations of total fatty acids or any of the other subfractions of fatty acids between normal weight and overweight/obese women (table 4). In addition, there was no difference in the weekly rate of change for each of the fatty acids between normal weight and overweight/obese women (data not shown).
Table 4.
Maternal Serum Fatty Acid Values in the 1st and 2nd Trimester
| 1st Trimester | 2nd Trimester | |||||||
|---|---|---|---|---|---|---|---|---|
| Normal (n=62) | Overweight/ Obese (n=89) | P | Normal (n=62) | Overweight/ Obese (n=89) | P | P^ | P* | |
| Total fatty acids (mg/dL) | 230.2 (±47.4) | 234.2 (±56.7) | 0.65 | 367.1 (±86.4) | 356.1 (±80.5) | 0.43 | <0.01 | <0.01 |
| MUFA (mg/dL) | 43.9 (±13.1) | 44.1 (±15.2) | 0.91 | 73.0 (±24.4) | 70.4 (±21.9) | 0.49 | <0.01 | <0.01 |
| PUFA (mg/dL) | 110.2 (±22.3) | 111.0 (±23.6) | 0.83 | 162.7 (±37.8) | 158.5 (±33.5) | 0.48 | <0.01 | <0.01 |
| Saturated fatty acids (mg/dL) | 74.9 (±16.0) | 78.0 (±20.2) | 0.32 | 129.4 (±31.2) | 125.7 (±31.3) | 0.48 | <0.01 | <0.01 |
| Omega 3 fatty acids (mg/dL) | 8.0 (±3.1) | 8.2 (±2.3) | 0.59 | 12.6 (±3.7) | 12.2 (±3.4) | 0.50 | <0.01 | <0.01 |
| Omega 6 fatty acids (mg/dL) | 102.2 (±20.6) | 102.8 (±21.8) | 0.87 | 150.1 (±35.3) | 146.3 (±31.1) | 0.49 | <0.01 | <0.01 |
| Arachidonic Acid (204:n6) (mg/dL) | 22.2 (±5.0) | 24.3 (±5.5) | 0.02 | 28.3 (±5.9) | 30.6 (±7.0) | 0.04 | <0.01 | <0.01 |
| DHA (226:n3) (mg/dL) | 4.5 (±1.9) | 4.8 (±1.4) | 0.26 | 7.4 (±2.3) | 7.8 (±2.0) | 0.24 | <0.01 | <0.01 |
| EPA (205:n3) (mg/dL) | 0.79 (±0.75) | 0.77 (±0.40) | 0.83 | 0.81 (±0.47) | 0.73 (±0.46) | 0.29 | 0.79 | 0.50 |
All data presented as mean (±standard deviation). MUFA (monounsaturated fatty acid), PUFA (polyunsaturated fatty acid), DHA (Docosahexaenoic acid), EPA (Eicosapentaenoic acid). P^ is the p-value comparing first and second trimester fatty acid results in normal weight women, while P* represents the same comparison in overweight/obese women
Discussion
Overweight and obese pregnant women demonstrate a more atherogenic lipid profile in early pregnancy when compared to normal weight women. Maternal serum total cholesterol, LDL cholesterol, HDL cholesterol, and triglycerides increase in all women from the first to the late second trimester. However, overweight/obese women demonstrate a significantly blunted increase in maternal serum total cholesterol and LDL cholesterol between the first and late second trimesters. This results in slightly higher levels of total cholesterol in normal weight women by the late second trimester when compared to overweight and obese women with no difference between the two groups in LDL cholesterol. These results remained constant even after adjustment for factors such as maternal weight gain. Of note, the rate of change in maternal serum lipids between the first and late second trimester was not different in women with excessive compared to non-excessive weight gain. The incidence of pregnancy complications were similar between the normal weight and overweight/obese women in our study, indicating that the differences seen in overweight and obese women may represent a physiologic response to pregnancy.
Our results complement those recently published by Vahratian, et al, who examined longitudinal changes in maternal serum lipids in normal weight and overweight/obese women 20. They also demonstrated that overweight/obese women display marked alterations in the trajectory of maternal serum lipids, including a lower rate of change in LDL cholesterol and total cholesterol in overweight and obese women when compared to normal weight women. However, this cohort was homogenous with regards to race and socioeconomic status, and the authors did not assess the impact of maternal weight gain. We have demonstrated that this lower rate of increase in total and LDL cholesterol in overweight/obese women between the first and late second trimester is not explained by differences in maternal weight gain. Our results also provide unique insight into the similar composition of fatty acid profiles between normal weight and overweight/obese women during pregnancy with the exception of higher arachidonic acid concentrations in overweight/obese women.
Dyslipidemia in early pregnancy is significant because more atherogenic lipid profiles are associated with a variety of pregnancy complications, including spontaneous preterm birth,21 gestational diabetes,22 and pre-eclampsia.12, 23–24 However, most of the available data is cross sectional, and it is unknown what gestational age or duration of dyslipidemia is necessary to contribute to adverse pregnancy outcomes. The mechanisms linking early pregnancy dyslipidemia to pregnancy complications are incompletely understood. Shallow placentation has been associated with approximately one-third of spontaneous preterm births,25–26 and pre-eclampsia is also associated with abnormal implantation.27 Factors such as maternal obesity14 and hypercholesterolemia28–29 may predispose to LDL oxidation, and previous work has demonstrated that oxidized LDL inhibited invasion of extravillous cytotrophoblasts in a concentration-dependent manner.30 These data highlight one possible pathway whereby maternal hyperlipidemia in early pregnancy may influence placentation.
The physiology leading to different trajectories of total cholesterol and LDL cholesterol between normal weight and overweight/obese women are not fully elucidated. Triglycerides and cholesterol increase across normal pregnancy, but they do not differ significantly from non-pregnant controls until approximately 9 weeks’ gestation.31 Our first trimester samples were measured at a mean of 8.3 weeks’ gestation, indicating that these values likely represent pre-pregnancy physiology. In normal pregnancy, lipoprotein alterations appear to be related to changes in sex hormones. Increased plasma prolactin and estrogen are correlated with plasma and VLDL triglyceride concentrations,32 and progesterone is correlated with low-density lipoprotein cholesterol concentrations.33 Maternal serum lipoprotein lipids and insulin resistance both increase in parallel to maternal plasma hormone levels in normal pregnancy,32–33 and maternal insulin resistance contributes to plasma lipid perturbations due to an increases in adipose tissue lipolysis. Increased lipolysis provides a surplus of plasma free fatty acid substrate for hepatic triglyceride synthesis and secretion. Additionally, maternal lipoprotein lipase activity declines in liver and adipose tissue, and this leads to decreased peripheral catabolism of VLDL triglycerides.34 These physiologic adaptations may differ significantly between normal weight and overweight/obese individuals, and we speculate that altered first trimester physiology could lead to downstream alterations in placental function and physiologic adaptation in overweight and obese women. Maternal serum lipids changed at the same rate between women with and without excessive weight gain, suggesting that corrections to maternal dyslipidemia may need to occur prior to conception if they could improve outcomes. The majority of overweight and obese women in our study had normal pregnancy outcomes, and more work is needed to understand what are pathologic versus physiologic adaptations in serum lipids among overweight and obese women.
Total fatty acids and levels of MUFA, PUFA, saturated fatty acids, omega-3 fatty acids, omega-6 fatty acids, arachidonic acid, and DHA increased between the first and late second trimester of pregnancy consistent with increasing fetal requirements to ensure normal fetal growth and development.8 Fatty acids have important roles as an energy source, membrane building blocks, and regulators of gene expression. They are also the precursors of eicosanoids such as prostaglandins, prostacyclins, thromboxanes, and leukotrienes, and these signaling molecules regulate diverse processes including gene expression, cell differentiation, immunity, and inflammation. Higher levels of arachidonic acid in the first and late second trimester of pregnancy in overweight and obese women were the only significant differences between groups. Arachidonic acid is metabolized to both proinflammatory and anti-inflammatory molecules,35–36 and while arachadonic acid supplementation does not appear to have proinflammatory effects in healthy individuals, it may counter the anti-inflammatory effects of omega-3 fatty acid supplementation.37 Our results demonstrate that levels of the majority of fatty acids are constant across a wide range of maternal weights, and the differences in arachadonic acid deserves further attention given the clinical importance of arachidonic acid in inflammatory pathways and fetal neurodevelopment.
One limitation of our study is the relatively small sample size, which hindered our ability to separately analyze women who were overweight and obese. In addition, there were a limited number of women with pregnancy complications such as pre-eclampsia or gestational diabetes. There was a trend towards higher 50 gram glucose challenge test results and higher rates of preterm delivery in overweight/obese women, and it is possible that these differences would have been significant with a larger sample size. We are unable to comment on the differences in lipid trajectories between normal weight and obese women with pregnancy complications, but this is an important area of future investigation. In addition, we do not have lipid data for the third trimester so we are unable to comment on lipid trajectories over the entire pregnancy. Finally, the use of non-fasting lipids may introduce some variability into our results. For example, non-fasting triglycerides may vary by up to 20%, and LDL by 10%.38 However, serum lipids are associated with disease risk on both the fasting and nonfasting state indicating that non-fasting lipids have clinical relevance.39–40
One strength is that our sample was derived from an economically and racially diverse population, which increases the generalizability of our results. We are also unaware of any previous studies that have compared the maternal serum fatty acid profile between normal weight and overweight/obese women, providing novel insight into the physiologic changes in fatty acids seen in women of different weight categories.
We chose to focus on the first and late second trimester of pregnancy because understanding the physiologic differences that occur in early and mid-pregnancy is crucial for developing tools to identify women at increased risk as well as designing interventions prior to the onset of clinical disease. Our results help define the physiologic changes that occur in overweight and obese women when compared to normal weight women in the first and late second trimester of pregnancy. These findings may have important implications for the design of interventions, particularly with regards to possible adverse affects of early pregnancy lipids on placental implantation and development. These data are relevant in understanding the causes, contributors, prevention, and treatment of pre-eclampsia, disorders of fetal growth, and gestational diabetes mellitus. Future studies are required to determine whether screening for and intervening on early pregnancy dyslipidemia can improve pregnancy or postnatal outcomes.
What is already known about this subject?
Maternal serum lipids increase throughout gestation to support normal fetal growth and development
Obesity in pregnancy is associated with an increased risk for pregnancy complications, but the mechanisms underlying this risk are not completely understood
What this study adds
To the best of our knowledge this is the first study to examine the impact of excessive maternal weight gain on maternal serum lipids
There are limited data assessing the impact of maternal obesity on maternal serum lipid profiles
These data contribute to our understanding of the physiologic adaptations to pregnancy in overweight and obese women
Acknowledgments
Funding Source: This project was supported by NIH RO1 HD052732 (to H.S.)
All authors participated in the study design. C.S. and J.C. carried out the data analysis and interpretation, and all authors were involved in writing the paper and had final approval of the submitted and published versions.
Footnotes
Competing Interests: The authors report no competing interests
References
- 1.Vahratian A. Prevalence of overweight and obesity among women of childbearing age: results from the 2002 National Survey of Family Growth. Matern Child Health J. 2009;13:268–73. doi: 10.1007/s10995-008-0340-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Chu SY, Callaghan WM, Kim SY, et al. Maternal obesity and risk of gestational diabetes mellitus. Diabetes Care. 2007;30:2070–6. doi: 10.2337/dc06-2559a. [DOI] [PubMed] [Google Scholar]
- 3.Solomon CG, Willett WC, Carey VJ, et al. A prospective study of pregravid determinants of gestational diabetes mellitus. JAMA. 1997;278:1078–83. [PubMed] [Google Scholar]
- 4.Sibai BM, Gordon T, Thom E, et al. Risk factors for preeclampsia in healthy nulliparous women: a prospective multicenter study. The National Institute of Child Health and Human Development Network of Maternal-Fetal Medicine Units. Am J Obstet Gynecol. 1995;172:642–8. doi: 10.1016/0002-9378(95)90586-3. [DOI] [PubMed] [Google Scholar]
- 5.Hyperglycaemia and Adverse Pregnancy Outcome (HAPO) Study: associations with maternal body mass index. BJOG. 2010;117:575–84. doi: 10.1111/j.1471-0528.2009.02486.x. [DOI] [PubMed] [Google Scholar]
- 6.Dabelea D, Crume T. Maternal environment and the transgenerational cycle of obesity and diabetes. Diabetes. 2011;60:1849–55. doi: 10.2337/db11-0400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Rasmussen KM, Catalano PM, Yaktine AL. New guidelines for weight gain during pregnancy: what obstetrician/gynecologists should know. Curr Opin Obstet Gynecol. 2009;21:521–6. doi: 10.1097/GCO.0b013e328332d24e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Herrera E, Amusquivar E, Lopez-Soldado I, Ortega H. Maternal lipid metabolism and placental lipid transfer. Horm Res. 2006;65 (Suppl 3):59–64. doi: 10.1159/000091507. [DOI] [PubMed] [Google Scholar]
- 9.Lippi G, Albiero A, Montagnana M, et al. Lipid and lipoprotein profile in physiological pregnancy. Clin Lab. 2007;53:173–7. [PubMed] [Google Scholar]
- 10.Ustun Y, Engin-Ustun Y, Dokmeci F, Soylemez F. Serum concentrations of lipids and apolipoproteins in normal and hyperemetic pregnancies. J Matern Fetal Neonatal Med. 2004;15:287–90. doi: 10.1080/14767050410001680028. [DOI] [PubMed] [Google Scholar]
- 11.Belo L, Caslake M, Santos-Silva A, et al. LDL size, total antioxidant status and oxidised LDL in normal human pregnancy: a longitudinal study. Atherosclerosis. 2004;177:391–9. doi: 10.1016/j.atherosclerosis.2004.07.023. [DOI] [PubMed] [Google Scholar]
- 12.Enquobahrie DA, Williams MA, Butler CL, Frederick IO, Miller RS, Luthy DA. Maternal plasma lipid concentrations in early pregnancy and risk of preeclampsia. Am J Hypertens. 2004;17:574–81. doi: 10.1016/j.amjhyper.2004.03.666. [DOI] [PubMed] [Google Scholar]
- 13.Couch SC, Philipson EH, Bendel RB, Pujda LM, Milvae RA, Lammi-Keefe CJ. Elevated lipoprotein lipids and gestational hormones in women with diet-treated gestational diabetes mellitus compared to healthy pregnant controls. J Diabetes Complications. 1998;12:1–9. doi: 10.1016/s1056-8727(97)00007-x. [DOI] [PubMed] [Google Scholar]
- 14.Sanchez-Vera I, Bonet B, Viana M, et al. Changes in plasma lipids and increased low-density lipoprotein susceptibility to oxidation in pregnancies complicated by gestational diabetes: consequences of obesity. Metabolism. 2007;56:1527–33. doi: 10.1016/j.metabol.2007.06.020. [DOI] [PubMed] [Google Scholar]
- 15.Montelongo A, Lasuncion MA, Pallardo LF, Herrera E. Longitudinal study of plasma lipoproteins and hormones during pregnancy in normal and diabetic women. Diabetes. 1992;41:1651–9. doi: 10.2337/diab.41.12.1651. [DOI] [PubMed] [Google Scholar]
- 16.Howard BV, Ruotolo G, Robbins DC. Obesity and dyslipidemia. Endocrinol Metab Clin North Am. 2003;32:855–67. doi: 10.1016/s0889-8529(03)00073-2. [DOI] [PubMed] [Google Scholar]
- 17.Obesity: preventing and managing the global epidemic. Report of a WHO consultation. World Health Organ Tech Rep Ser. 2000;894:i–xii. 1–253. [PubMed] [Google Scholar]
- 18.Bligh EG, Dyer WJ. A rapid method of total lipid extraction and purification. Can J Biochem Physiol. 1959;37:911–7. doi: 10.1139/o59-099. [DOI] [PubMed] [Google Scholar]
- 19.Kramer MS, Kahn SR, Platt RW, et al. Antioxidant vitamins, long-chain fatty acids, and spontaneous preterm birth. Epidemiology. 2009;20:707–13. doi: 10.1097/EDE.0b013e3181a818c5. [DOI] [PubMed] [Google Scholar]
- 20.Vahratian A, Misra VK, Trudeau S, Misra DP. Prepregnancy body mass index and gestational age-dependent changes in lipid levels during pregnancy. Obstet Gynecol. 2010;116:107–13. doi: 10.1097/AOG.0b013e3181e45d23. [DOI] [PubMed] [Google Scholar]
- 21.Catov JM, Bodnar LM, Kip KE, et al. Early pregnancy lipid concentrations and spontaneous preterm birth. Am J Obstet Gynecol. 2007;197:610, e1–7. doi: 10.1016/j.ajog.2007.04.024. [DOI] [PubMed] [Google Scholar]
- 22.Enquobahrie DA, Williams MA, Qiu C, Luthy DA. Early pregnancy lipid concentrations and the risk of gestational diabetes mellitus. Diabetes Res Clin Pract. 2005;70:134–42. doi: 10.1016/j.diabres.2005.03.022. [DOI] [PubMed] [Google Scholar]
- 23.Bayhan G, Kocyigit Y, Atamer A, Atamer Y, Akkus Z. Potential atherogenic roles of lipids, lipoprotein(a) and lipid peroxidation in preeclampsia. Gynecol Endocrinol. 2005;21:1–6. doi: 10.1080/09513590500097382. [DOI] [PubMed] [Google Scholar]
- 24.Aksoy H, Kumtepe Y, Akcay F, Yildirim AK. Correlation of P-selectin and lipoprotein(a), and other lipid parameters in preeclampsia. Clin Exp Med. 2002;2:39–43. doi: 10.1007/s102380200005. [DOI] [PubMed] [Google Scholar]
- 25.Germain AM, Carvajal J, Sanchez M, Valenzuela GJ, Tsunekawa H, Chuaqui B. Preterm labor: placental pathology and clinical correlation. Obstet Gynecol. 1999;94:284–9. doi: 10.1016/s0029-7844(99)00324-5. [DOI] [PubMed] [Google Scholar]
- 26.Arias F, Rodriquez L, Rayne SC, Kraus FT. Maternal placental vasculopathy and infection: two distinct subgroups among patients with preterm labor and preterm ruptured membranes. Am J Obstet Gynecol. 1993;168:585–91. doi: 10.1016/0002-9378(93)90499-9. [DOI] [PubMed] [Google Scholar]
- 27.Ray JG, Diamond P, Singh G, Bell CM. Brief overview of maternal triglycerides as a risk factor for pre-eclampsia. BJOG. 2006;113:379–86. doi: 10.1111/j.1471-0528.2006.00889.x. [DOI] [PubMed] [Google Scholar]
- 28.Lavy A, Brook GJ, Dankner G, Ben Amotz A, Aviram M. Enhanced in vitro oxidation of plasma lipoproteins derived from hypercholesterolemic patients. Metabolism. 1991;40:794–9. doi: 10.1016/0026-0495(91)90005-h. [DOI] [PubMed] [Google Scholar]
- 29.Cominacini L, Garbin U, Pastorino AM, et al. Predisposition to LDL oxidation in patients with and without angiographically established coronary artery disease. Atherosclerosis. 1993;99:63–70. doi: 10.1016/0021-9150(93)90051-u. [DOI] [PubMed] [Google Scholar]
- 30.Pavan L, Hermouet A, Tsatsaris V, et al. Lipids from oxidized low-density lipoprotein modulate human trophoblast invasion: involvement of nuclear liver X receptors. Endocrinology. 2004;145:4583–91. doi: 10.1210/en.2003-1747. [DOI] [PubMed] [Google Scholar]
- 31.Potter JM, Nestel PJ. The hyperlipidemia of pregnancy in normal and complicated pregnancies. Am J Obstet Gynecol. 1979;133:165–70. doi: 10.1016/0002-9378(79)90469-1. [DOI] [PubMed] [Google Scholar]
- 32.Glueck CJ, Fallat RW, Scheel D. Effects of estrogenic compounds on triglyceride kinetics. Metabolism. 1975;24:537–45. doi: 10.1016/0026-0495(75)90078-5. [DOI] [PubMed] [Google Scholar]
- 33.Miller VT. Dyslipoproteinemia in women. Special considerations. Endocrinol Metab Clin North Am. 1990;19:381–98. [PubMed] [Google Scholar]
- 34.Herrera E, Lasuncion MA, Gomez-Coronado D, Aranda P, Lopez-Luna P, Maier I. Role of lipoprotein lipase activity on lipoprotein metabolism and the fate of circulating triglycerides in pregnancy. Am J Obstet Gynecol. 1988;158:1575–83. doi: 10.1016/0002-9378(88)90193-7. [DOI] [PubMed] [Google Scholar]
- 35.Harris WS, Mozaffarian D, Rimm E, et al. Omega-6 fatty acids and risk for cardiovascular disease: a science advisory from the American Heart Association Nutrition Subcommittee of the Council on Nutrition, Physical Activity, and Metabolism; Council on Cardiovascular Nursing; and Council on Epidemiology and Prevention. Circulation. 2009;119:902–7. doi: 10.1161/CIRCULATIONAHA.108.191627. [DOI] [PubMed] [Google Scholar]
- 36.Serhan CN, Chiang N, Van Dyke TE. Resolving inflammation: dual anti-inflammatory and pro-resolution lipid mediators. Nat Rev Immunol. 2008;8:349–61. doi: 10.1038/nri2294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Li B, Birdwell C, Whelan J. Antithetic relationship of dietary arachidonic acid and eicosapentaenoic acid on eicosanoid production in vivo. J Lipid Res. 1994;35:1869–77. [PubMed] [Google Scholar]
- 38.Sidhu D, Naugler C. Fasting time and lipid levels in a community-based population: a crosssectional study. Arch Intern Med. 2012;172:1707–10. doi: 10.1001/archinternmed.2012.3708. [DOI] [PubMed] [Google Scholar]
- 39.Langsted A, Freiberg JJ, Nordestgaard BG. Fasting and nonfasting lipid levels: influence of normal food intake on lipids, lipoproteins, apolipoproteins, and cardiovascular risk prediction. Circulation. 2008;118:2047–56. doi: 10.1161/CIRCULATIONAHA.108.804146. [DOI] [PubMed] [Google Scholar]
- 40.Bansal S, Buring JE, Rifai N, Mora S, Sacks FM, Ridker PM. Fasting compared with nonfasting triglycerides and risk of cardiovascular events in women. JAMA. 2007;298:309–16. doi: 10.1001/jama.298.3.309. [DOI] [PubMed] [Google Scholar]