Abstract
Inhibitory interneurons with somata in strata radiatum and lacunosun-moleculare (SR/L-M) of hippocampal area CA3 receive excitatory input from pyramidal cells via the recurrent collaterals (RC), and the dentate gyrus granule cells via the mossy fibers (MFs). Here we demonstrate that Hebbian long-term potentiation (LTP) at RC synapses on SR/L-M interneurons requires the concomitant activation of calcium-impermeable AMPARs (CI- AMPARs) and NMDARs. RC LTP was prevented by voltage clamping the postsynaptic cell during high-frequency stimulation (HFS; 3 trains of 100 pulses delivered at 100 Hz every 10 s), with intracellular injections of the Ca2+ chelator BAPTA (20 mM), and with the N-methyl-D-aspartate receptor (NMDAR) antagonist D-AP5. In separate experiments, RC and MF inputs converging onto the same interneuron were sequentially activated. We found that RC LTP induction was blocked by inhibitors of the calcium/calmodulin-dependent protein kinase II (CaMKII; KN-62, 10 μM or KN-93, 10 μM) but MF LTP was CaMKII independent. Conversely, the application of the protein kinase A (PKA) activators forskolin/IBMX(50 μM/25 μM) potentiated MF EPSPs but not RC EPSPs. Together these data indicate that the aspiny dendrites of SR/L-M interneurons compartmentalize synaptic-specific Ca2+ signaling required for LTP induction at RC and MF synapses. We also show that the two signal transduction cascades converge to activate a common effector, protein kinase C (PKC). Specifically, LTP at RC and MF synapses on the same SR/LM interneuron was blocked by postsynaptic injections of chelerythrine (10 μM). These data indicate that both forms of LTP share a common mechanism involving PKC-dependent signaling modulation.
Keywords: Recurrent Commissural LTP and LTD; Calcium impermeable AMPARs, CaMKII, PKA, PKC; CA3 interneurons, feed-forward inhibition
Introduction
The majority of excitatory synapses in hippocampal area CA3 originate from the extensive recurrent collateral (RC) axons of pyramidal cells (Amaral and Witter, 1989, Ishizuka et al., 1990). The RC circuitry underlies many autoassociative network models that simulate pattern completion, i.e. the recall of activity patterns stored in area CA3 via MFs, the axons of dentate gyrus granule cells (Marr, 1971, McNaughton and Morris, 1987, O’Reilly and McClelland, 1994, Rolls, 1996). One key assumption in these computational models is that RC synapses exhibit NMDAR-mediated long-term potentiation (LTP) to recreate the original episode (O’Reilly and McClelland, 1994). Indeed, NMDAR activation in area CA3 is required for memory recall (Nakazawa et al., 2002, Fellini et al., 2009), and the induction of RC LTP in CA3 pyramidal cells (Harris and Cotman, 1986, Zalutsky and Nicoll, 1990, Magee and Johnston, 1997, Debanne et al., 1998, Bains et al., 1999). The retrieval of previously stored activity patterns also relies on the inhibitory input from local interneurons to constrain the activation of non-assembly pyramidal cells (Sahay et al., 2011). Therefore, preservation of the excitatory-to-inhibitory balance for optimal pattern separation requires that RC synapses undergo near simultaneous LTP on pyramidal cells and feed-forward interneurons (Lamsa et al., 2005). However, previous investigations of synaptic plasticity at RC synapses on CA3 interneurons have yielded varying results. Early studies reported NMDAR-independent LTD at RC synapses on stratum radiatum (SR) interneurons (Laezza et al., 1999). In contrast, NMDAR-dependent RC LTD in SR interneurons was detected during persistent bursting activity in the disinhibited slice (Stoop et al., 2003). A more recent study on the same interneuron synapse uncovered the bidirectional induction of NMDAR-dependent plasticity (LTP/ LTD), contingent on the level of postsynaptic depolarization (Laezza and Dingledine, 2004).
Hippocampal interneurons with somata in stratum radiatum and lacunosum-moleculare (SR/L-M) of area CA3 belong to a larger population of dendritic targeting GABAergic cells providing feed-forward inhibition to pyramidal cells (Lacaille and Schwartzkroin, 1988, Williams et al., 1994, Vida et al., 1998). MF synapses on SR/L-M interneurons exhibit NMDAR-independent LTP induced by cytosolic Ca2+ increase from the coactivation of L-type voltage gated calcium channels (VGCCs) and mGluR1. This form of MF LTP requires postsynaptic activation of protein kinases A (PKA) and C (PKC) (Galvan et al., 2011). Here we show that RC synapses on SR/L-M interneurons exhibit a form of Hebbian LTP that requires calcium entry via NMDARs. High-frequency stimulation (HFS) of RC and MF inputs synapsing on the same interneuron revealed that blockade of CaMKII prevented LTP induction at RC but not at MF synapses. Conversely, PKA stimulation resulted in a potentiation of MF synapses but did not affect RC synapses. We conclude that the aspiny dendrites of SR/L-M interneurons are able to compartmentalize the initial Ca2+ signaling cascades that trigger LTP at two different synaptic inputs. Nevertheless, PKC inhibition prevented the induction of both forms of LTP suggesting that PKC activation provides a point of convergence of signaling cascades originating from RC and MF synaptic activity.
Experimental procedures
Ethical approval
For the electrophysiological recordings, animal use was in accordance with the University Institutional Animal Care and Use Committee. Male Sprague-Dawley rats (35 ± 5 days old; Zivic Miller Company) were deeply anaesthetized (Nembutal, IP, 5 mg/100 gr body weight) and perfused intracardially with a modified artificial cerebrospinal fluid ACSF in which sucrose was substituted for NaCl (in mM): 210 sucrose, 2.8 KCl, 1.25 NaH2PO4, 26 NaHCOO3, 10 D-Glucose, 1 CaCl2, 4 MgCl2, at 4°C. Following 1–2 minutes of perfusion, animals were decapitated and the brains removed. Blocks of hippocampal tissue were glued to the stage of a Leica VT1000S and cut in 380 μm-thick sections. Slices were maintained for at least 120 min in an incubation solution of the following composition (in mM): 125 NaCl, 2.5 KCl, 1.2 NaH2PO4, 25 NaHCOO3, 10 D-Glucose, 0.4 ascorbic acid, 1 CaCl2 and 6 MgCl2. The solution was maintained at pH 7.3 and bubbled with O2 (95%)/CO2 (5%) mixture at room temperature. Slices were then transferred to a submersion recording chamber and superfused at constant flow (3.5 ml/min) with the following solution (in mM): 125 NaCl, 3 KCl, 1.25 Na2HPO4, 25 NaHCO3, 2 CaCl2, 1 MgCl2, 10 glucose). The temperature of the solution in the recording chamber was set at 33 ± 1°C. Interneurons were identified visually with infrared video (IR) microscopy and differential intensity contrast (DIC) optics. Patch pipettes were pulled from borosilicate glass and had resistances of 5–8 MΩ when filled with a solution containing (in mM), 120 K-methylsulfate, 10 NaCl, 10 KCl, 10 HEPES, 0.5 EGTA, 4 Mg.ATP, 0.3 Na2.GTP, 14 phosphocreatine. In some experiments, biocytin, (0.1%) was added to the pipette solution to allow subsequent morphological identification and reconstruction of the interneurons (Fig. 1A). For the AMPAR rectification experiments (Fig 1B–F), the intracellular solution was modified to contain (in mM), 120 Cesium Methanesulfonate, 10 KCl, 10 HEPES, 0.5 EGTA, 8 Phosphocreatine, 4 Mg-ATP, 0.3 Na2-GTP and 0.5 QX–314. Osmolarity was adjusted to 295 - 300 mOsm with pH 7.2 – 7.3. Access resistance was monitored throughout the length of experiments using a 5 ms, 5 mV voltage steps. Interneurons were accepted for analysis only if the seal resistance was >1GΩ and the series resistance did not change >15% of its initial value during the course of the experiment.
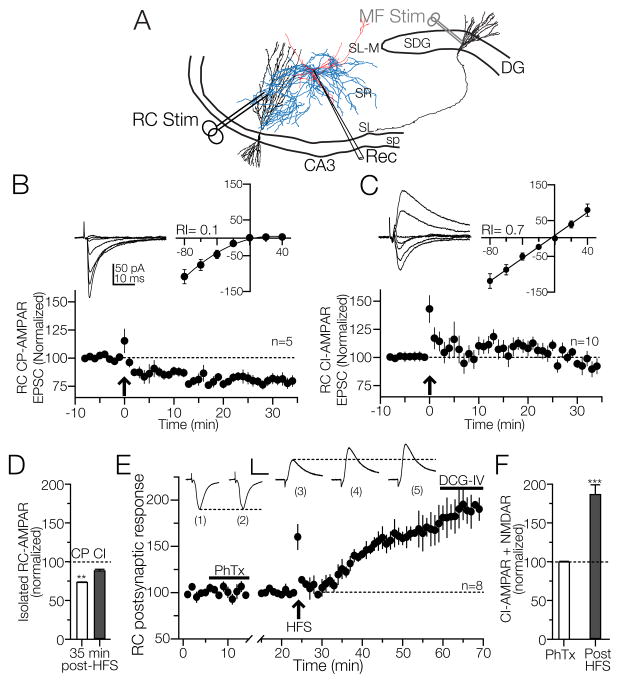
Figure 1. Composition of AMPARs at recurrent commissural (RC) synapses on CA3 interneurons.
A) Placement of stimulation electrodes and the whole cell recording pipette. Recordings were obtained from interneurons with soma in the strata radiatum (SR) and lacunosum-moleculare (SL-M). RC responses were evoked by stimulating the stratum radiatum; MF responses were evoked stimulating on the suprapyramidal blade of the dentate gyrus (SDG). B, C) Top panels. The voltage-current relationships from RC EPSCs revealed that RC synapses are comprised of CP- (19%) and CI - AMPARs (73%). Lower panels: HFS delivered to RC synapses containing CP-AMPARs induced LTD whereas HFS to RC synapses formed by CI-AMPARs results in a transient potentiation. D) Bar graph summarizing the effect of HFS on isolated RC CP-AMPARs and CI-AMPARs. E) Time-course graph averaged from 8 independent experiments in the presence of bicuculline (10 μM). The RC EPSC insensitivity to philanthotoxin (PhTx, 10 μM) was used to identify CI-AMPARs. HFS applied to this glutamatergic synapse composed of CI-AMPARs and NMDARs induces stable LTP, as summarized in the bar chart (F). **p<0.01; ***p<0.001 or higher statistical significance. Error bars indicate SEM for all the figures. Calibration bar for panel E, 50 pA / 5 ms for the voltage clamp and 2 mV / 5 ms for current clamp traces.
RC and MF EPSPs were evoked by extracellular stimulation via concentric bipolar electrodes (12.5 μm inner pole diameter, 125 μm outer pole diameter; FHC Inc., ME). To activate the RC input, the electrode was positioned in the stratum radiatum near the border between CA3b and CA3a. To activate the MF input, the electrode was placed in the suprapyramidal blade of the dentate gyrus (SDG; Fig. 1A). RC EPSPs exhibited shorter latency and time-to-peak than MF EPSPs (latency = 1.74 ± 0.12 ms and 3.32 ± 0.13; p<0.001; time to peak = 3.7 ± 0.22 ms and 5.61 ± 0.41; p<0.001 for RC and MF EPSPs, respectively) as previously reported (Laezza and Dingledine, 2004, Calixto et al., 2008). Pairs of stimuli were delivered at 60 ms inter-stimulus interval (ISI) to each input separately. Each pair consisted of monophasic pulses (100–400 μA; 85–100 μs duration) applied at 0.25–0.16 Hz. We applied stimulation current intensities that evoked monosynaptic RC and MF EPSP amplitudes ≤30% of the threshold amplitude required to elicit action potentials in the recorded interneurons. Cells with composite postsynaptic responses were discarded from the study. For each input paired pulse facilitation (PPF) was calculated as the ratio (PPR at 60 ms ISI) of the amplitude of the second EPSP over the first EPSP in the pair. The rectification index (RI) of the synaptic responses was obtained from the ratio of RC EPSCs at +40 and −80 mV, as previously reported (see Laezza et al., 1999). Synapses exhibiting RI > 0.6 were considered to be composed of a majority of calcium impermeable (CI) AMPARs whereas a RI < 0.3 was indicative of rectifying synapses mainly containing calcium permeable (CP) AMPARs (See Figure 1B and C). Synapses exhibiting rectification values ranging from 0.31 to 0.59 were considered to contain a mixed population of CP- and CI-AMPARs and were discarded from this study. Sequential activation of RC and MF inputs converging onto the same interneuron was delivered at 1000 ms ISI to minimize synaptic temporal summation. Control experiments were performed to confirm the long lasting duration of RC and MF LTP in the absence of the drugs used in this study. Both RC LTP (n=3) and MF LTP (n=4) exhibited duration and time-course similar to those reported in the results section. Specifically, LTP was stable for at least 100 min post-HFS (RC LTP = 204 ± 14 %; MF LTP = 164 ± 7.4 % of baseline; p<0.0001 for both inputs). Current and voltage clamp recording were obtained with an Axopatch 200B (Axon Instruments) in the presence of (−)-bicuculline methiodide (10 μM) to block GABAA- mediated responses. Signals were low-pass filtered at 5 kHz, digitized at 10 kHz, and stored for off-line analysis. Data acquisition and analysis were performed using PClamp 10 (Molecular Devices). Lack of sensitivity (<5%) of RC EPSPs to the application of the group II metabotropic glutamate receptor agonist 2S, 2′R, 3′R)-2-(2′,3′-dicarboxycyclopropyl) glycine (DCG-IV; 5 μM) was confirmed at the end of the experiments. Although DGC-IV inhibition of MF transmission in pyramidal cells is ≥90% (Kamiya et al., 1996), it is variable in interneurons (Alle et al., 2001, Lawrence and McBain, 2003, Galvan et al., 2008). Therefore, synaptic responses were considered of MF origin if the DCG-IV application resulted in ≥70% inhibition (Lawrence and McBain, 2003, Galvan et al., 2008). LTP was induced in each input by high-frequency stimulation (HFS) consisting of 3 trains of 100 pulses each at 100 Hz, repeated every 10 sec paired with a postsynaptic depolarizing current step (30 ± 0.6 pA).
Drugs
1(S),9(R)-(−)-Bicuculline methbromide; D(−)-2-Amino-5-phosphonopentanoic acid, D-AP5; (2S,2′R,3′R)-2-(2′,3′-Dicarboxycyclopro-pyl)glycine, DCG-IV; 2-Methyl-6-(phenylethynyl)pyridine hydrochloride, MPEP; (S)-(+)-α-Amino-4-carboxy-2-methylbe-nzeneacetic acid, LY 367385; KN-62, KN-93 were purchased from TOCRIS (Ellisville, MO) or Sigma Chemical (St. Louis, MO). Forskolin, IBMX and PDA were dissolved in DMSO at concentrations of 100, 10, 2, respectively, and then added to the bath solution. The concentration of DMSO in the final bath solution was 0.1%. Otherwise, drugs were dissolved in double distilled H2O.
Immunofluorescence determinations
In the first set of experiments, 6 rats were anesthetized and perfused intracardially with phosphate buffer (PB) 0.1M, pH 7.4 followed by 4% paraformaldehyde (PFA). Brains were post-fixed overnight in 4% PFA, and then transferred into 30% sucrose solution. Serial coronal sections of the brain were cut at 30 μm using a cryostat at −19°C (Leica CM1510). For immunostaining, we selected 1 every 10 slices. Sections were pretreated with an antigen retrieval citrate buffer at 80°C for 15 min and rinsed several times in 50mM Tris-buffered saline (TBS). Sections were incubated with BSA 5% in PBS 0.3% Triton X-100 for 1 h at room temperature to prevent nonspecific staining. Sections were then incubated with primary antibodies at 4°C for 24 h in BSA 5% in PBS 0.3% Triton X-100.
A second set of experiments were conducted directly on vibratome-cut slices. The first group consisted of naïve slices incubated for 20 min with ACSF. The second group comprised slices in which the HFS protocol was applied on the stratum radiatum of CA3c, as described above. Slices were next fixed in PFA (4%) 5 and 30 min after delivering the tetanic stimulation. Both groups were post-fixed during 24 h and next they were transferred to 30% sucrose solution. Slices were then resectioned into 30 μm sections for the immunohistochemical experiments. Following antibodies were used: mouse anti- CAMKII (1:500, Abcam), rabbit anti-CAMKII (1:1000, Abcam), mouse anti-Cam α-phospho (1:500 Abcam), rabbit anti-GAD67 (1:100, Santa Cruz), mouse anti-GAD67 (1:1000, Millipore) and rabbit anti-Calbindin D-28 K (1:300, Millipore). As negative controls, some slices of the same tissue cut into 30 μm thickness were simultaneously processed in the absence the primary antibody. After rinsing 3 times, slices were incubated with secondary antibodies for 2 h at room temperature as follows: goat anti-rabbit Alexa 488 (1:500, Molecular probes), goat anti-mouse Cy5 (1:500, Molecular probes), goat anti-mouse TRITC (1:100, Jackson Immunoresearch) and goat anti-rabbit TRITC (1:100, Jackson Immunoresearch). Finally, slices were rinsed three times in PBS and mounted in slides using Vectashield with DAPI mounting medium (Vector). All slices were examined with an epifluorescent microscope (Axio Scope, Carl Zeiss).
Morphological reconstructions
Following recordings, slices were fixed in cold 4% paraformaldehyde for 72 hrs., transferred into an anti-freeze solution (a one-to-one mixture of glycerol and ethylene glycol in 0.1M phosphate buffer), and stored at −80°C. Slices were then cut into 60 μm sections on a vibratome, reacted with 1% H202, and placed in blocking serum with 0.5% Triton X-100 for 2 hrs at room temperature. Biocytin-labeled neurons were incubated with ABC-peroxidase and developed using the Ni-enhanced DAB chromogen. Interneurons were reconstructed using the Neurolucida tracing system (MicroBrightField, Inc., Williston, VT) on a Axioplan 2 Zeiss microscope equipped with DIC, a 100x (NA =1.4) planapochromatic lens and additional Optovar magnification of 1.6x (final optical magnification, 1,600x; screen magnification, 7,200x). For the reconstructions, all sections containing the cell were used.
Statistics
Group measures are expressed as means ± S.E.M. Normality of the populations were tested with Kolmogorov-Smirnov test (P < 0.05), followed by one way ANOVA and a Student-Newman-Keuls all pairwise comparisons (P < 0.05). In all cases differences were considered significant if P was less than alpha = 0.05. In the figures, statistical significance is denoted as follows: * P < 0.05, ** P < 0.01 and *** P < 0.001 (or higher).
Results
Anatomical and electrophysiological properties of SR/L-M CA3 interneurons
Whole cell recordings were obtained from 90 SR interneurons and 37 L-M localized in area CA3b. Interneuron somata were typically positioned 120 ± 10 μm to 300 ± 10 μm from the boundary between stratum pyramidale and stratum lucidum and 150–250 μm from the medial extend of the superior blade of the dentate gyrus and 50 to 150 μm below the slice surface (Fig. 1A). The predominant morphology of the recorded cells was bipolar with dendritic arborizations extended horizontally, and had similar passive properties (Calixto et al., 2008, Ascoli et al., 2009). Both types of interneurons exhibited adapting (accommodating) firing patterns with spike adaptation ratio of the first to last inter-spike interval >3.0. These interneurons provide feedforward and lateral inhibition to pyramidal cells (Chitwood et al., 1999, Ascoli et al., 2009).
Characterization of AMPARs targeted by the RC input to SR/L-M CA3 interneurons
It is well known that glutamatergic synaptic transmission in hippocampal area CA3 is mediated by GluR2-lacking calcium permeable CP-AMPARs, and GluA2-containing calcium impermeable CI AMPARs. In addition, different forms of synaptic plasticity of these responses have been characterized for MF synapses on CA3 interneurons (Toth and McBain, 1998, Toth et al., 2000) and RC synapses (Laezza et al., 1999).
In the presence of bicuculline and D-AP5, RC-evoked voltage-current relationships obtained from −80 mV to +40 mV in steps of 20 mV revealed that the RC input to SR/L-M CA3 interneurons forms at least three types of AMPAR synapses. The first group was characterized by a strong inward rectification curve (5 out of 26 recorded cells; rectification
index: 0.1 ± 0.17; Fig. 1B; upper panel). HFS of these synapses induced a stable RC-LTD (RC EPSC, 74.2 ± 0.8% of baseline at 35 min post HFS; p<0.001 RM-ANOVA; Fig 1B, lower panel; N = 5). A second group (19 out of 26) expressed a linear V-I relationship (rectification index: 0.7 ± 0.13; Fig 1C, upper panel). In 10 of these interneurons, HFS induced a transitory potentiation that lasted up to 20 min before returning to baseline values followed by a mild synaptic depression (RC EPSC, 94.6 ± 2.2% of baseline at 35 min post HFS; p<0.001 RM-ANOVA; Fig 1C, lower panel; N = 10). The remaining 9 cells of this group showed a small PTP but no potentiation (RC EPSC, 104.6 ± 4% of baseline at 35 min post HFS). Two additional cells displayed an irregular V-I response. Similar responses were previously described for mixed AMPAR-containing synapses (Toth and McBain, 1998, Toth et al., 2000); these cells were discarded from the present study. These results support the notion that in CA3 interneurons the isolated RC CP AMPARs express LTD whereas MF CI AMPARs express NMDAR independent LTP (Galvan et al., 2008). In contrast, RC synapses composed of isolated CI AMPARs are unable to undergo LTP.
It has been previously shown that early on postnatal development (P12-P20) HFS stimulation applied to RC synapses on CA3 interneurons containing CI-AMPARs/NMDARs do not exhibit LTP (Laezza and Dingledine, 2004). To test if this is the case for mature hippocampi, experiments were performed in slices from P30-P40 animals. Following a stable 8 min baseline of RC EPSCs (recorded at −70 mV) in the presence of bicuculline, philanthotoxin (10 μM) was added to the perfusion medium. In 8 interneurons, RC EPSCs were minimally sensitive to PhTx (3.1 ± 2% of sensitivity; p>0.1, one-way ANOVA; Fig. 1E). After the washout of PhTx, the recordings were switched to current clamp mode, and a 5-min baseline was recorded followed by HFS of the RC input. The RC EPSPs exhibited PTP (149.05 ± 8.28 % of baseline; p<0.001) followed LTP that lasted up to one hour. RC EPSPs were insensitive to DCG-IV (5 μM; RC LTP = 183.9 ± 10 % of baseline at 40 min post HFS; p<0.001; RM ANOVA; RC LTP in the presence of DCG-IV = 191.2 ± 7 % of baseline at 60 min post HFS; p<0.001; RM-ANOVA). No statistical difference in RC LTP magnitude was found in the presence of DCG-IV (p > 0.15; one-way ANOVA). In two additional cells, RC EPSCs were highly sensitive to PhTx (78.13 ± 9% of sensitivity) indicating that these synapses were mainly composed of CP-AMPAR. These cells were discarded. In summary, the majority of RC synapses on SR/L-M interneurons were mainly comprised of CI-AMPARs (75%). The remaining synapses contained either CP-AMPARs (19.4%) or a mixed population of CP- and CI-AMPARs (5.5%). These data suggest that RC LTP may require the co-activation of CI-AMPARs and NMDARs.
Requirements for the induction of RC LTP in CA3 interneurons
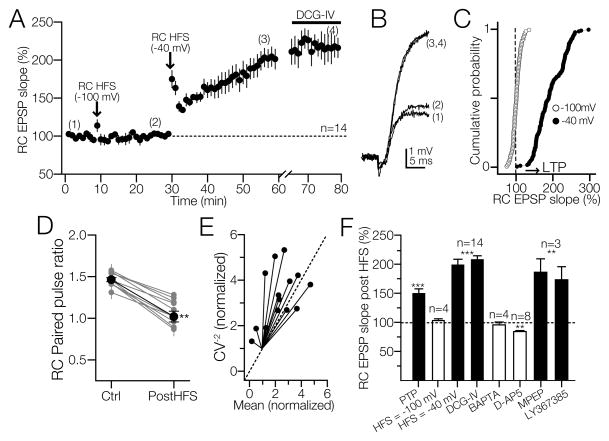
One distinct anatomical feature of area CA3 is the dense local connectivity via the RC axons of CA3 pyramidal cells making synapses on neighboring CA3 pyramidal cells (Ishizuka et al., 1990, Sik et al., 1993, Li et al., 1994). In contrast to MF LTP, RC LTP in CA3 pyramidal cells requires NMDAR activation for the postsynaptic Ca2+influx (Harris and Cotman, 1986, Zalutsky and Nicoll, 1990). In a separate group of interneurons, HFS was applied while the membrane potential was voltage clamped at −100 mV to prevent action potential firing. In these conditions, no significant changes in the RC EPSP slope was observed (102.3 ± 1 % at 5 min pre-HFS; 102.5 ± 2 % at 15 min post-HFS; p > 0.45, RM-ANOVA; N = 6; Fig. 2A – D). A second HFS train paired with a depolarizing pulse (50 pA) to evoke burst of action potentials, was delivered to the RC input while the cell was held in current clamp mode at −60 mV. This paired stimulus protocol resulted in a robust increase in RC EPSPs slope that lasted up to 45 min and was insensitive to DCG-IV (PTP = 152 ± 7% of baseline; RC LTP = 195 ± 8 % of baseline at 30 min post-HFS; p<0.001; RM ANOVA; RC LTP in the presence of DCG-IV = 218.9 ± 16 % of baseline at 50 min post HFS; p<0.001; RM ANOVA; N = 6; Fig. 2A – D). The requirement for concomitant pre- and postsynaptic activation for RC LTP induction in SR/L-M interneurons indicates that this form of synaptic plasticity is Hebbian. To further investigate the locus of expression of RC LTP, the paired pulse facilitation (PPF, at ISI 60 ms) was monitored in all the experiments. Forty min after the induction of RC LTP, RC PPF exhibited a systematic reduction compared to control (RC PPR control= 1.46 ± 0.02; RC PPR at 40 min post HFS = 1.02 ± 0.06; p < 0.0001, one-way ANOVA; Fig. 2D). We also plotted the coefficient of variation (CV−2) against the mean of the RC EPSP values at 40 min following the application of HFS. The distribution of the data values (Fig. 2E) were close located to the identity line (dashed line). The decrease in the PPF and the changes in the CV−2 following the induction of RC LTP, strongly suggest that RC LTP has a presynaptic component of expression (Malinow and Tsien, 1990, Alle et al., 2001).
Figure 2. The requirements of LTP induction at RC synapses on CA3 SR/SL-M interneurons.
All experiments were performed in the presence of bicuculline (10 μM). A) Average time-course of RC EPSPs from 6 separate experiments. HFS applied while cells were held in voltage clamp at −100 mV did not express RC LTP (2) but subsequent HFS paired with a postsynaptic depolarization step (−40 mV) elicited RC LTP that lasted up to one hour (3), and was insensitive to DCG-IV (4). B) Average of RC EPSP slopes from 10 consecutive sweeps at the times indicated in 2A. C) Cumulative probability of responses when HFS was applied at −100 mV (empty circles) or HFS at −40 mV (filled circles). Dash line represents the RC EPSP baseline. Arrowhead indicates an increase in the EPSP slope above 25% of baseline value. D) The paired pulse ratio of RC EPSPs at 60 ms ISI showed a persistent decrease at 40 min after the application of the HFS. E) Coefficient of variation (CV−2) plotted against mean of the synaptic responses at 40 min post HFS; the values were normalized to their respective controls. The distribution of the data points with respect to the identity line (dashed) is indicative of a presynaptic locus of expression for RC LTP. F) Bar graph summarizing the magnitude of RC PTP and LTP with respect to baseline and the lack of effect of DCG-IV (8 additional experiments were performed without PhTx; the LTP magnitude was similar to that shown in 2A). In cells loaded with BAPTA (20 mM) HFS failed to induce LTP. In the presence of D-AP5 (50 μM), HFS triggered a significant depression of RC EPSPs. RC LTP was insensitive to the pharmacological blockade of group I mGluRs with MPEP (50 μM) or LY367385 (100 μM). **p<0.01; ***p<0.001 or higher statistical significance.
It has been reported that RC LTP induction in CA3 pyramidal cells is prevented with postsynaptic Ca2+ chelation (Zalutsky and Nicoll, 1990). Thus, we investigated whether RC synapses on CA3 interneurons also require postsynaptic Ca2+ influx to induce LTP. Cells were loaded with BAPTA (20 mM) for at least 15 min before the experiments. BAPTA did not affect PTP (142 ± 9 % of baseline; p<0.001) but prevented changes in RC EPSPs slopes at 15 min (100 ± 4.1 % of baseline; p>0.05; one-way ANOVA) and 35 min post-HFS (94.8 ± 5 % of baseline; p>0.05; one-way ANOVA, N = 4; Fig. 2C).
CA3 interneurons also express group I mGluRs (Baude et al., 1993, Lujan et al., 1996), which contributes to several forms of synaptic plasticity in hippocampal interneurons. For example, at MF synapses on SR/L-M interneurons, mGluR1 is a metaplastic switch controlling the polarity of long-term synaptic plasticity (Galvan et al., 2008). At CA1 stratum oriens interneuron synapses, mGluR1 is required for the induction of Hebbian LTP (Perez et al., 2001, Lapointe et al., 2004, Topolnik et al., 2006). In the next series of experiments, we investigated whether the group I mGluRs is involved in RC LTP induction in SR/L-M interneurons. The mGluR5 antagonist MPEP (50 μM) did not block the induction of RC LTP (PTP = 162.7 ± 29 %; LTP at 30 min post HFS = 185 ± 23 % of baseline; p<0.001; one-way ANOVA; N = 3; Fig. 2C). Similar results were found from experiments in which the mGluR1 was blocked with bath perfused LY 367385 (100 μM) for at least 10 min before the experiment. RC HFS was delivered after EPSP baseline was collected for 8 min. In 3 cells, HFS applied to the RC input induced PTP followed by LTP with a magnitude similar to those obtained in the experiments described in Fig. 2A (PTP = 142 ± 11 % of baseline; LTP at 30 min post HFS = 172.2 ± 22.4 % of baseline; p<0.001; RM-ANOVA; N = 3; Fig. 2C). Collectively these data show that the induction of RC LTP in SR/L-M CA3 does not require activation of the group I mGluRs.
Induction of RC LTP in CA3 interneurons requires CAMKII activity
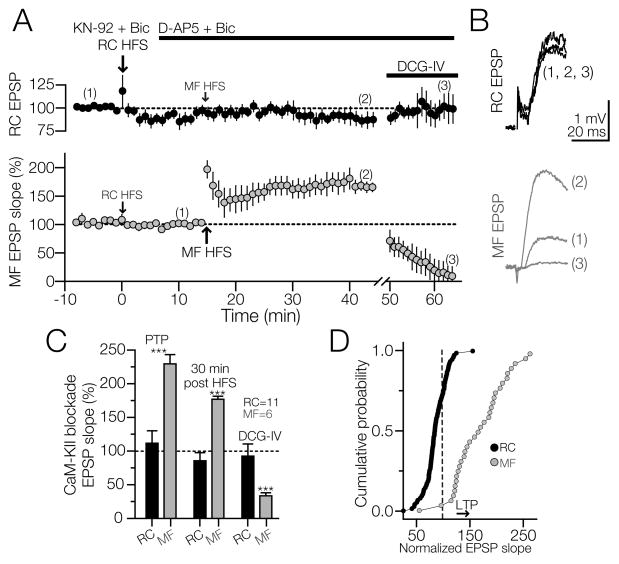
Ca2+/calmodulin-dependent kinase II (CaMKII) plays a key role in the induction of NMDAR-dependent LTP of CA1 pyramidal cells of hippocampus (Malinow et al., 1989, Hvalby et al., 1994, Lledo et al., 1995, Wang and Kelly, 1995, 1996). In addition, CaMKII up-regulates the glutamatergic transmission of CA1 fast spiking non-pyramidal cells (Wang and Kelly, 2001), and is required for the induction of NMDAR-dependent LTP in interneurons located in CA1 stratum radiatum (Lamsa et al., 2007). Furthermore, the dependence on CaMKII activation for the induction of CA3-CA3 LTP has been documented in organotypic slices (Pavlidis et al., 2000, Lu and Hawkins, 2006). Given the dependency of NMDAR-mediated LTP on CaMKII in CA1 interneurons (Lamsa et al., 2007), we postulated that RC LTP in CA3 SR/L-M interneurons also requires CaMKII autophosphorylation. To test this hypothesis, we sought to determine whether CaMKII inhibition prevented induction of RC LTP. Hippocampal slices were incubated in the presence of the cell-permeable inhibitor of CaMKII, KN-62 (10 μM) or the more selective and potent CaMKII blocker KN-93 (10 μM) for 50–60 min prior to the experiment. In these experiments, RC and MF inputs converging onto the same interneuron were consecutively stimulated (see Fig. 1A for stimulation electrodes position) at 1000 ms ISI (see Experimental procedures). Following the incubation with KN-62 or KN-93, stable EPSP slopes were recorded for 8 min prior to the delivery of HFS to the RC input. As predicted, the slope of the RC EPSP was unchanged following the incubation with KN-62 (91.7 ± 3.76 % at 5 min post-HFS; and 89.9 ± 3.3 % at 15 min of baseline post-HFS; p>0.5 RM-ANOVA; N = 5) or KN-93 (91 ± 5 % at 5 min post-HFS; and 85 ± 12 % at 15 min post-HFS; p>0.5 RM-ANOVA; N = 6; Fig. 3A, top panel). In the same experiment, D-AP5 (50 μM) was subsequently added to the perfusion bath to isolate the AMPAR component of the MF-mediated transmission. A second HFS applied to the MF input induced a robust PTP followed by a sustained increase in MF EPSP slope that lasted 30 min and was sensitive to DCG-IV (5 μM) (PTP = 228.6 ± 13.6 of baseline; p<0.001; LTP = 176.7 ± 5 % at 30 min post HFS; p<0.001; DCG-IV depression of the MF response = 32.9 ± 4 % of baseline; p<0.001; RM-ANOVA; N = 6; Fig 3A, bottom panel). In contrast, RC EPSPs were insensitive to DCG-IV (94.8 ± 2.75% of baseline 1 hour post-FS; p>0.15; one-way ANOVA; Fig. 3A, top panel; Fig. 3A – 3C).
Figure 3. RC LTP but not MF LTP requires CaMKII activity.
The slices were incubated with the CaMKII inhibitor KN-62 (10 μM, n=5) or KN-93 (10 μM, n=6) for 50–60 min before experiments and simultaneous recordings of RC and MF EPSPs in the same interneuron were performed in the presence of bicuculline. A) Averaged time-course graph of RC (upper panel) and MF EPSP slopes (lower panel). RC HFS failed to induce LTP. Ten min after HFS, D-AP5 was added to the bath and a second HFS train was applied to the MF input. MF HFS induced a PTP followed by a robust MF LTP sensitive to DCG-IV. HFS on RC did not affect MF EPSPs nor did the HFS on MF affected RC EPSPs indicating that the two inputs converging onto the same cell were functionally independent. B) RC and MF EPSPs slopes averaged from 10 continuous sweeps obtained at the times indicated in the experiment of 3A. C) Summary of the changes for RC and MF EPSPs in the presence of CaMKII blockade. Because similar effects were obtained with KN-62 and KN-93, the data were pooled. Error bars indicate SEM. D) Cumulative probability chart shows induction of MF LTP and blockade of RC LTP in the presence of KN-93. Dash line represents the EPSP baseline, for both MF and RC EPSP slopes. Arrowhead indicates a change in the slope above (right arrowhead) or below (left arrowhead) 25% of baseline value. In some experiments, RC HFS induced a depression of the synaptic response in the presence of CaMKII antagonists. ***p<0.001 or higher statistical significance.
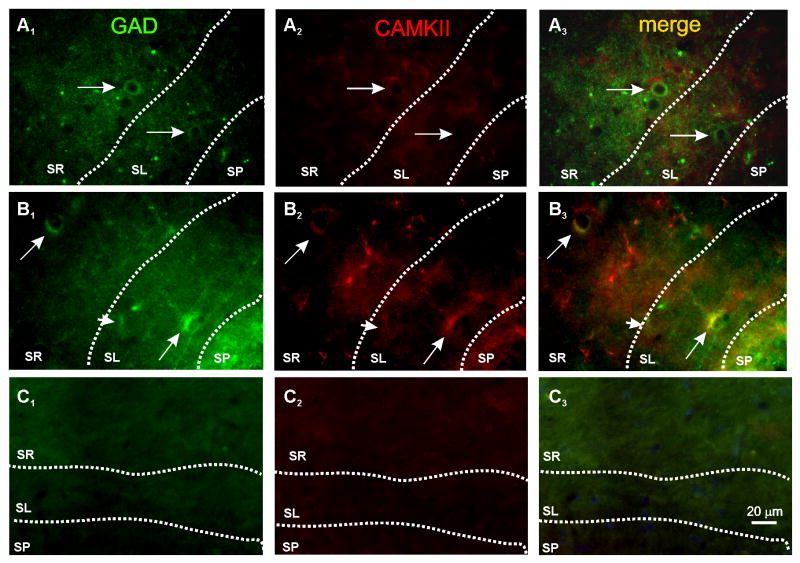
The results described above indicate that CaMKII activity is required for LTP in CA3 SR/L-M interneurons. However, CaMKII has not been directly observed in CA1 interneurons (Liu and Jones, 1996, Sik et al., 1998) but see (Lamsa et al., 2007). Therefore, to determine whether CaMKII is detected in these interneurons, we performed double-immunofluorescence staining on hippocampal sections for the CaMKII isoforms (see the experimental procedures for details) and glutamate decarboxylase enzyme (GAD-67), the limiting enzyme for GABA synthesis present in interneurons. In slices prepared from rats that were transcardially perfused with PFA, the coexpression of GAD and CaMKII in interneurons of the stratum lucidum was virtually inexistent (3 interneurons in 150 slices analyzed). We therefore conducted immunohistochemical experiments in slices prepared for in vitro recordings before and 5 min after HFS. We found that 32 out of 89 (36%) interneurons co-expressed the phosphorylated α subunit of CaMKII and GAD+ whereas in non-stimulated slices, only 4 out of 90 were immunopositive. As shown in Fig. 4, the merging of the confocal images revealed that GAD-67 immunopositive populations of interneurons located in strata radiatum/lacunosum moleculare of area CA3 also were immunopositive for CaMKII. Together, these results suggest that CaMKII is postsynaptically expressed in CA3 interneurons in an activity-dependent manner.
Figure 4. Co-localization of phospho-CaMKII and GAD67 in CA3 interneurons.
Photomicrographs depicting the co-expression of phospho-CaMKII and GAD67. A1-3) Immunofluorescent images of CA3 hippocampal area of a non-stimulated slice. B) Co-expression of GAD67 (B1; green) and phospho-CaMKII (B2; red) was observed in the somatic region of 2 interneurons, one located in stratum radiatum (SR; arrow), and a second one in stratum lucidum (SL; arrow) of CA3b (merge in yellow; B3) of a stimulated slice. The arrowhead signals a GAD67- positive interneuron devoid of CAMKII immunoreactivity. These results were obtained from a slice that was fixated 5 min after HFS. C) Control experiment in which the IHC was conducted in the absence of the first antibody. The calibration bar at the bottom of the right hand panel corresponds to 20 μm and applies to all photomicrographs.
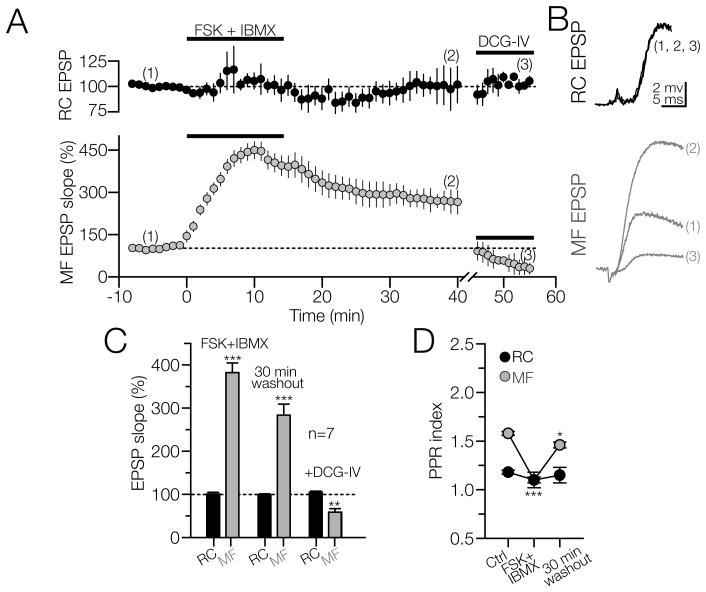
Application of forskolin/IBMX does not potentiate RC EPSPs in CA3 interneurons
Among the multiple kinases required for LTP induction, the cAMP-dependent protein kinase (PKA) plays an essential role at the Schaffer to CA1 pyramidal cell synapse (Frey et al., 1993, Huang et al., 1994, Blitzer et al., 1995, Duffy and Nguyen, 2003) and at the MF to CA3 pyramidal cell synapse (Weisskopf et al., 1994, Villacres et al., 1998, Calixto et al., 2003). PKA activity is also required for the induction of MF LTP in dentate gyrus basket cells (Alle et al., 2001), and CA3 interneurons in SL-M (Galvan et al., 2010). However, Adenylyl cyclase (AC) stimulation has been reported to have mild effects on RC EPSPs in CA3 pyramidal cells and interneurons (Weisskopf et al., 1994, Galvan et al., 2010). We tested whether the signal transduction through the cAMP-PKA cascade plays a role in RC LTP induction in CA3 interneurons. In the presence of bicuculline, a stable baseline of RC and MF EPSPs were concurrently evoked in the same interneuron for 8 min. The co-application of the AC stimulator forskolin (FSK, 50 μM) with the non-specific inhibitor of cAMP phosphodiesterase IBMX (25 μM) had contrasting effects on the EPSPs evoked from RC and MF. RC EPSPs were insensitive to AC stimulation during or after washout of the drugs (105.3 ± 8% of baseline at 10 min after the onset of FSK+IBMX; p>0.05, RM-ANOVA. 97 ± 3% of baseline at 30 min after washout; p>0.15; N = 7; Fig. 5A, top panel; Figs. 5B and 5C). In contrast, the FSK+IBMX treatment induced a fast and sustained potentiation of MF EPSPs for at least 30 min after the washout of drugs (440 ± 29.6 % of baseline 10 min after the onset of FSK+IBMX; p<0.001; 265 ± 42 % of baseline after 30 min washout; p<0.0001, RM-ANOVA, N = 7; Fig. 5A, bottom panel; Fig. 5B and C). DCG-IV (5 μM) depressed the MF EPSPs but had no effect on RC EPSPs (RC EPSP in the presence of DCG-IV, 105 ± 2 % of baseline; p>0.05; MF EPSP sensitivity to DCG-IV = 58.7 ± 8 % of baseline; p<0.001, RM-ANOVA). In addition, the PPF ratio of the EPSPs was monitored during these experiments, as illustrated in Fig. 5D. The RC EPSPs remained unchanged in the presence or after 30 min washout of FSK+IBMX (RC-PPF control = 1.18 ± 0.02; during FSK+IBMX = 1.1 ± 0.8; 30 min after washout = 1.15 ± 0.08, p>0.6; One-way ANOVA). In agreement with our previous results (Galvan et al., 2010), the FSK/IBMX-induced potentiation of the MF EPSP was associated with a decrease in the PPF ratio during the drug application but exhibited a slight recovery after 30 min washout (MF-PPF control = 1.57 ± 0.02; during FSK+IBMX = 1.1 ± 0.3; p<0.001; 30 min after washout = 1.46 ± 0.03; p<0.05. One-way ANOVA). Although presynaptic PKA activation is sufficient to produce a robust but transient potentiation of transmission at MF synapses on CA3 interneurons, the increased PKA activation in the postsynaptic cell is required for the maintenance of FSK/IBMX-induced MF potentiation (Galvan et al., 2010). The lack of effects of PKA on RC synapses suggests that in CA3 interneurons PKA is exposed to compartmentalized pools of cAMP locally generated by adenylate cyclases and phosphodiesterases (Michel and Scott, 2002).
Figure 5. Forskolin and IBMX potentiate MF EPSPs but not RC EPSPs in CA3 interneurons.
Simultaneous recordings of RC and MF EPSPs in the same interneuron in the presence of bicuculline. A) Graph averaged from 6 independent experiments showing the time course of RC and MF EPSP and the effect of the adenylyl cyclase stimulator forskolin (FSK; 50 μM) and the nonspecific phosphodiesterase inhibitor IBMX (25 μM). RC EPSPs were unaffected by the FSK/IBMX or by DCG-IV treatment. In contrast, MF EPSPs dramatically increased during the FSK/IBMX treatment (2, filled circles) and thirty minutes after washout the MF EPSP remained potentiated. MF EPSPs were reduced by DCG-IV (3, filled circles). B) Representative RC and MF EPSP slopes averaged from 10 continuous sweeps at the times indicated by the corresponding numbers in 5A. C) Summary of the effects of FSK/IBMX application on MF and RC EPSP slopes. D) The paired pulse ratio (PPR) for the MF EPSP was decreased during the FSK/IBMX stimulation, and partially recovered 30 min after the washout of the drugs. RC PPR did not change during the experiment. *p<0.05; **p<0.01; ***p<0.001 or higher statistical significance.
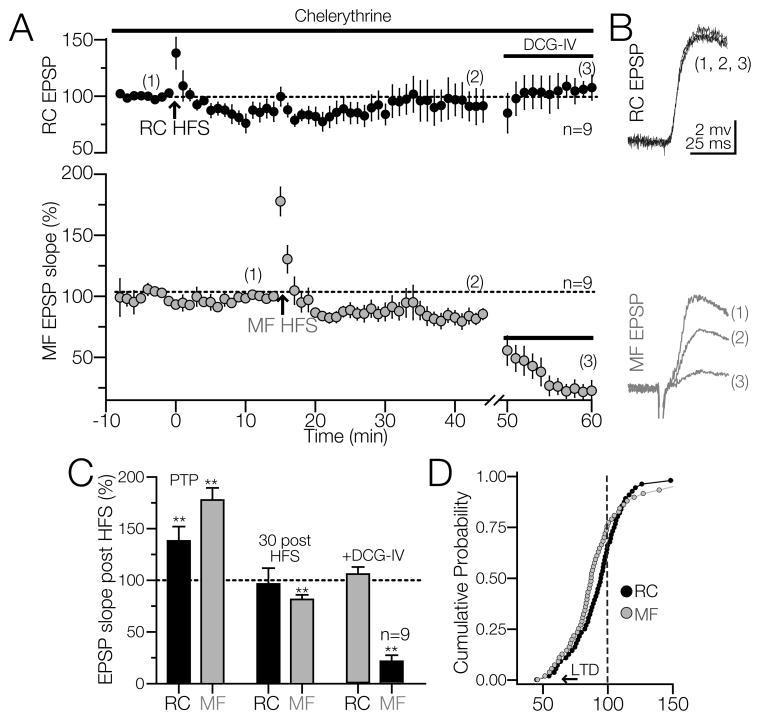
Induction of RC and MF LTP in CA3 interneurons rely on postsynaptic PKC activation
Previous studies have shown that PKC is essential for LTP induction at the Schaffer/collateral to CA1 pyramidal cell synapse (Malinow et al., 1989, Hvalby et al., 1994, Wang and Kelly, 1995, Hussain and Carpenter, 2005) and at the MF to CA3 pyramidal cell synapse (Son et al., 1996, Hussain and Carpenter, 2005, Kwon and Castillo, 2008). To assess whether postsynaptic PKC is required for the induction of RC LTP we loaded interneurons with PKC blocker chelerythrine (10 μM); (Kwon and Castillo, 2008, Galvan et al., 2010). In these experiments, a baseline for RC and MF EPSPs was recorded in the same interneuron in the presence of bicuculline. Chelerythrine had little effect on PTP of RC and MF EPSPs but prevented LTP induction at both inputs (RC PTP = 133.2 ± 5.7% of baseline; p<0.001; RC at 30 min post-HFS = 91.5 ± 4% of baseline; p>0.05, one-way ANOVA; MF PTP = 188.2 ± 10% of baseline; p<0.001; MF at 30 min post-HFS, 85.5 ± 4.4 of baseline; p<0.01; one-way ANOVA; N = 9, for both inputs; Fig 6A – 6D). DCG-IV decreased the MF responses without affecting the RC EPSP slopes of CA3 interneurons (RC EPSP in the presence of DCG-IV = 105.4 ± 5% of baseline; p>0.05, one-way ANOVA; MF EPSP in the presence of DCG-IV = 62.6 ± 5% of baseline; p<0.001, one-way ANOVA. The blockade of PKC with chelerythrine demonstrates that postsynaptic PKC signaling is required for the induction of RC and MF LTP in SR/L-M CA3 interneurons (See model in Fig. 7).
Figure 6. Postsynaptic PKC activity is required for LTP at RC and MF synapses on CA3 interneurons.
Cells were loaded with the PKC inhibitor, chelerythrine (10 μM) for at least 15 min before the experiment. A) HFS failed to induce RC LTP and triggered a significant MF LTD. B) Representative RC and MF EPSP slopes obtained from 10 consecutive traces at the indicated time of 6A. C) Summary of the effects of HFS; RC and MF PTP were unaffected but induction of LTP was blocked. D) Cumulative probability of responses for RC and MF following the HFS. Dash line represents the EPSPs baseline. Arrowhead indicates a decrease in the slope below 25% of baseline value. **p<0.01 or higher statistical significance.
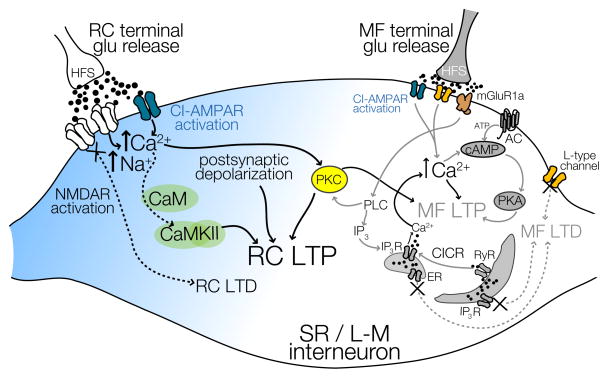
Figure 7. Schematic representation of the compartmentalized signaling cascades for LTP induction at RC and MF synapses on the spiny dendrite of CA3 SR/L-M interneurons.
LTP at both synapses mostly containing CI-AMPARs has a strong dependence on presynaptic activation and rise in postsynaptic calcium levels. MF LTP induction requires two sources of calcium: (1) from the extracellular space via L-type VGCCs and (2) via mGluR1 dependent phospholipase C (PLC) pathway to promote IP3-mediated Ca2+ mobilization and the AC-cAMP-PKA signaling cascade (see Galván et al., 2008 and 2010 for details). In contrast, RC LTP only requires calcium from the activation of NMDARs, and is dependent on CaMKII activation. PKC-dependent signaling modulation is the point of convergence for signaling molecules originating from RC and MF synaptic inputs.
Discussion
The contribution of NMDARs to the induction of long-term plasticity in hippocampal interneurons may be different at synapses expressing CI- and CP-AMPARs (Lei and McBain, 2002, Laezza and Dingledine, 2011). For example, previous investigations on CA3 stratum radiatum interneurons reported a form of RC NMDAR-independent LTD that required the coactivation of postsynaptic CP-AMPARs and presynaptic mGluR7 (Laezza et al., 1999). A subsequent study of the same interneuron synapse revealed a form of LTP mediated by CP-AMPARs and NMDARs (Laezza and Dingledine, 2004). In the same study, RC LTD was induced by calcium influx either through CP-AMPARs or NMDARs, depending on the postsynaptic membrane potential. However, a comparison between those data and our present results may be problematic because of age differences in the rats used in the two studies (P9-P12 vs. P30-P40, respectively). Here we show that in the absence of functional NMDARs, RC synapse mostly containing CI-AMPARs exhibit a comparatively small but significant LTD that relies on calcium entry, possibly via L-type VGCCs (Galvan et al., 2008).
We also demonstrate that RC LTP exclusively depends on CaMKII activity, in agreement with the findings that GAD-67 positive SR/L-M interneurons are immunoreactive to CaMKII isoforms. However, by conducting immunohistofluorescence experiments to detect CAMKII and phospho-CAMII, we found phospho-CAMII in 36 % of interneurons of SL and SR only if the recorded slices were fixed 5 min after the HFS. If the slices were fixed after more than 30 min post-HFS, the labeling of CaMKII and phospho-CaMKII was not detected. This may suggest that HFS transiently elicits phosphorylation of CaMKII or de novo expression of phospho-CaMKII. Earlier work on CA1 interneurons with somata in stratum pyramidale revealed that CaMKII activity up-regulates AMPAR mediated transmission by inducing the conversion of inactive-to-active synapses (Wang and Kelly, 2001). While all four CaMKII isoforms (α, β, γ, and δ) are present in the brain (Takaishi et al., 1992), CaMKIIα and CaMKIIβ are predominantly found in neurons. CaMKIIα expression is localized to excitatory neuronal populations (Jones et al., 1994) but it has not been found in GABAergic neurons (Benson et al., 1992, Ochiishi et al., 1994, Sik et al., 1998). Autophosphorylation of CaMKIIα is essential for NMDAR-dependent LTP in the hippocampus (Lisman et al., 2002) and in the neocortex (Hardingham et al., 2003). In the CaMKIIα T286A-mutant mice, NMDAR-dependent LTP expression at the Schaffer commissural-CA1 pyramidal cell synapse is absent (Giese et al., 1998, Cooke et al., 2006). However, in the same strain of mutant mice, LTP is inducible at the medial perforant path input to dentate gyrus granule cells (Cooke et al., 2006), and in CA1 inhibitory interneurons (Lamsa et al., 2007). Therefore, the induction of some forms of NMDAR-dependent LTP do not_rely on the auto phosphorylation of threonine 286 in the CaMKIIα isoform (Lamsa et al., 2007). Because there are no isoform-selective inhibitors of CaMKII, we were unable to determine whether the specific activation of CaMKIIα plays a key role in RC LTP. In agreement with previous reports that CaMKII auto phosphorylation is not involved in MF LTP in CA3 pyramidal cells (Salin et al., 1996, Kakegawa et al., 2004). CaMKII inhibition did not prevent the subsequent induction of MF LTP in the same interneuron. Taken together, our data suggest that the initial steps required for the induction of RC LTP in SR/L-M interneurons are similar to those reported to underlie NMDAR dependent LTP at synapses containing CI-AMPAR located on the spiny dendrites of pyramidal cells.
The sustained activation of the AC-cAMP-PKA effector system by forskolin elicited robust MF potentiation but did not affect RC synapses in the same interneuron. The contrasting effects of forskolin on RC and MF synapses have been previously documented in CA3 pyramidal cells (Weisskopf et al., 1994). Interestingly, the signaling cascades for LTP induction differ across different interneuron subtypes, likely reflecting a diversity in dendritic Ca2+ signaling in these cells (Goldberg and Yuste, 2005, Camire and Topolnik, 2012). For example, MF synapses on dentate gyrus basket cells and SR/L-M interneurons also undergo long lasting synaptic enhancement during AC stimulation with forskolin (Alle et al., 2001, Galvan et al., 2010). In contrast, naïve MF synapses in stratum lucidum interneurons are insensitive to forskolin stimulation (Maccaferri et al., 1998, Lawrence and McBain, 2003) indicating lack of PKA-mediated signaling.
Irrespective of the main source of postsynaptic Ca2+ influx that triggers RC and MF LTP, both forms of Hebbian plasticity involve PKC activation. Furthermore, postsynaptic application of chelerythrine prevented the induction of both forms of LTP, thus confirming the participation of PKC activation in NMDAR-dependent LTP (Ling et al., 2002) and NMDAR-independent LTP at MF synapses (Kwon and Castillo, 2008, Galvan et al., 2010).
SR/L-M interneurons lack dendritic spines, which provide the necessary biochemical compartment for input-specific plasticity in pyramidal cells (Yuste and Denk, 1995, Goldberg et al., 2003, Bourne and Harris, 2008). However, the dendritic shafts of CA1 interneurons possess specialized asymmetric synaptic junctions that use glutamate as neurotransmitter (Harris and Landis, 1986), and experience dendritic remodeling driven by synaptic activity (Chen et al., 2011, Guirado et al., 2013). Another example of complex signaling in aspiny dendrites is present in fast-spiking interneurons of the neocortex. These interneurons possess highly localized Ca2+ signaling due to the presence of microdomains associated with CP-AMPARs, potentially allowing synapse-specific biochemical compartmentalization in the absence of dendritic spines (Goldberg et al., 2003, Goldberg and Yuste, 2005). In part, dendritic compartmentalization in the aspiny dendrite may be due to specific barriers to calcium diffusion, and the movement of second messenger molecule (Soler-Llavina and Sabatini, 2006). We hypothesize that at RC and MF synapses, CI-AMPARs also have spatially restricted Ca2+ micro domains associated with NMDARs and L-type VGCCs/mGluR1, respectively. The contrasting induction requirements for RC and MF LTP also suggest that scaffolding and anchoring proteins adjacent to RC and MF synapses are different. While little information is available regarding the anchoring proteins expressed on hippocampal interneurons (Sik et al., 2000), our data suggest that different groups of scaffolding proteins may be coupled to excitatory synapses on interneurons (Wong and Scott, 2004, Sanderson and Dell’Acqua, 2011). It is possible that compartmentalization of signaling cascades also could be due to the spatial segregation of MF and RC synapses onto different dendritic branches (Cosgrove et al., 2010).
At the Schaffer-CA1 pyramidal cell synapse, LTP expression requires incorporation of new AMPARs following HFS. The delivery of GluR1-containing AMPAR requires CaMKII activity in a PDZ protein dependent fashion (Hayashi et al., 2000, Poncer et al., 2002, Malinow, 2003) but see (Adesnik and Nicoll, 2007). Similarly, in CA3 pyramidal cells RC LTP but not MF LTP is expressed by the replacement of AMPARs with newly incorporated CP AMPARs. Although we have no direct evidence for the incorporation of newly synthesized CP-AMPARs in SR/L-M interneurons, RC LTP occurs at synapses mainly comprised of CI-AMPARs and requires NMDAR and CaMKII activation. A parsimonious hypothesis is that RC LTP expression in these interneurons results from the incorporation of newly synthesized CP-AMPARs. The trafficking of CP-AMPARs is triggered by postsynaptic CaMKII activity, a mechanism that is absent at the MF synapse (Kakegawa et al., 2004). This is in agreement with our findings showing that MF LTP in SR/L-M interneurons is unaffected by CaMKII blockade.
Computational and behavioral studies (McNaughton and Morris, 1987, Treves and Rolls, 1992, O’Reilly and McClelland, 1994, Lisman, 1999, Leutgeb et al., 2007) have proposed that during pattern separation, the dentate gyrus has the ability to generate sparse memory representations conveyed to the CA3 network via the MF pathway. These studies also suggest that the RC connectivity between CA3 pyramidal cells operates as an autoassociative network capable of reestablishing previously stored representations based on noisy or degraded cues via pattern completion. Pattern separation and pattern completion involve the obligatory contribution of the parallel activation of feed-forward inhibitory interneurons to maintain the temporal window for synaptic integration and restrict the spurious activation of non-assembly pyramidal cells (Pouille and Scanziani, 2001, Perez-Orive et al., 2002, Sahay et al., 2011). The preservation of the balance between monosynaptic excitation and disynaptic inhibition requires near simultaneous LTP induction at excitatory synapses on pyramidal cells and interneurons (Lamsa et al., 2005, Carvalho and Buonomano, 2009, Rolls, 2013). Our results indicate that SR/L-M feed-forward inhibitory interneurons in area CA3 have the ability to express two mechanistically distinct forms of Hebbian LTP at CI-AMPAR synapses. Functionally, synapse-specific compartmentalization of MF and RC LTP signaling in the aspiny dendrite enables SR/L-M interneurons to participate in the dual mnemonic processes of pattern separation and pattern completion.
CONCLUSION
The aspiny dendrites of CA3 SR/L-M interneurons compartmentalize the initial steps in the signaling transduction cascades implicated in the induction of Hebbian LTP at RC and MF synapses predominantly containing CI-AMPARs. Both forms of synaptic plasticity were prevented by postsynaptic injections of the calcium chelator BAPTA. However, RC LTP depends on Ca2+ influx via the NMDARs whereas MF LTP requires cytosolic Ca2+ increase from the coactivation of L-type VGCCs and mGluR1 (Galvan et al., 2008). Despite the absence of dendritic spines, SR/L-M interneurons have the capability to spatially restrict the signaling calcium cascades that lead to two mechanistically distinct forms of Hebbian LTP.
Acknowledgments
Financial support
EJG is supported by Conacyt México CB-2011-01-166241 and INFR-2012-01-187757. RG is supported by Conacyt México, I020/193/10 FON.INST.-29-10. GB is supported by NIH grant R01 GM066018.
Experiments were performed at the University of Pittsburgh, USA and Cinvestav-Sur, México City. Conception and early experiments: TPR and EJG who also designed, performed and analyzed the electrophysiological data. EJG and GB wrote the manuscript. RG, GGL and EL conducted the IHC experiments. All the authors read and agreed the interpretation of the results.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Adesnik H, Nicoll RA. Conservation of glutamate receptor 2-containing AMPA receptors during long-term potentiation. J Neurosci. 2007;27:4598–4602. doi: 10.1523/JNEUROSCI.0325-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alle H, Jonas P, Geiger JR. PTP and LTP at a hippocampal mossy fiber-interneuron synapse. Proc Natl Acad Sci U S A. 2001;98:14708–14713. doi: 10.1073/pnas.251610898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amaral DG, Witter MP. The three-dimensional organization of the hippocampal formation: a review of anatomical data. Neuroscience. 1989;31:571–591. doi: 10.1016/0306-4522(89)90424-7. [DOI] [PubMed] [Google Scholar]
- Ascoli GA, Brown KM, Calixto E, Card JP, Galvan EJ, Perez-Rosello T, Barrionuevo G. Quantitative morphometry of electrophysiologically identified CA3b interneurons reveals robust local geometry and distinct cell classes. J Comp Neurol. 2009;515:677–695. doi: 10.1002/cne.22082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bains JS, Longacher JM, Staley KJ. Reciprocal interactions between CA3 network activity and strength of recurrent collateral synapses. Nat Neurosci. 1999;2:720–726. doi: 10.1038/11184. [DOI] [PubMed] [Google Scholar]
- Baude A, Nusser Z, Roberts JD, Mulvihill E, McIlhinney RA, Somogyi P. The metabotropic glutamate receptor (mGluR1 alpha) is concentrated at perisynaptic membrane of neuronal subpopulations as detected by immunogold reaction. Neuron. 1993;11:771–787. doi: 10.1016/0896-6273(93)90086-7. [DOI] [PubMed] [Google Scholar]
- Benson DL, Isackson PJ, Gall CM, Jones EG. Contrasting patterns in the localization of glutamic acid decarboxylase and Ca2+/calmodulin protein kinase gene expression in the rat central nervous system. Neuroscience. 1992;46:825–849. doi: 10.1016/0306-4522(92)90188-8. [DOI] [PubMed] [Google Scholar]
- Blitzer RD, Wong T, Nouranifar R, Iyengar R, Landau EM. Postsynaptic cAMP pathway gates early LTP in hippocampal CA1 region. Neuron. 1995;15:1403–1414. doi: 10.1016/0896-6273(95)90018-7. [DOI] [PubMed] [Google Scholar]
- Bourne JN, Harris KM. Balancing structure and function at hippocampal dendritic spines. Annu Rev Neurosci. 2008;31:47–67. doi: 10.1146/annurev.neuro.31.060407.125646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calixto E, Galvan EJ, Card JP, Barrionuevo G. Coincidence detection of convergent perforant path and mossy fibre inputs by CA3 interneurons. J Physiol. 2008 doi: 10.1113/jphysiol.2008.152751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calixto E, Thiels E, Klann E, Barrionuevo G. Early maintenance of hippocampal mossy fiber--long-term potentiation depends on protein and RNA synthesis and presynaptic granule cell integrity. J Neurosci. 2003;23:4842–4849. doi: 10.1523/JNEUROSCI.23-12-04842.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Camire O, Topolnik L. Functional compartmentalisation and regulation of postsynaptic Ca2+ transients in inhibitory interneurons. Cell Calcium. 2012;52:339–346. doi: 10.1016/j.ceca.2012.05.001. [DOI] [PubMed] [Google Scholar]
- Carvalho TP, Buonomano DV. Differential effects of excitatory and inhibitory plasticity on synaptically driven neuronal input-output functions. Neuron. 2009;61:774–785. doi: 10.1016/j.neuron.2009.01.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cooke SF, Wu J, Plattner F, Errington M, Rowan M, Peters M, Hirano A, Bradshaw KD, Anwyl R, Bliss TV, Giese KP. Autophosphorylation of alphaCaMKII is not a general requirement for NMDA receptor-dependent LTP in the adult mouse. J Physiol. 2006;574:805–818. doi: 10.1113/jphysiol.2006.111559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cosgrove KE, Galvan EJ, Meriney SD, Barrionuevo G. Area CA3 interneurons receive two spatially segregated mossy fiber inputs. Hippocampus. 2010;20:1003–1009. doi: 10.1002/hipo.20713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen JL, Flanders GH, Lee WC, Lin WC, Nedivi E. Inhibitory dendrite dynamics as a general feature of the adult cortical microcircuit. J Neurosci. 2011;31:12437–12443. doi: 10.1523/JNEUROSCI.0420-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chitwood RA, Hubbard A, Jaffe DB. Passive electrotonic properties of rat hippocampal CA3 interneurones. J Physiol. 1999;515(Pt 3):743–756. doi: 10.1111/j.1469-7793.1999.743ab.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Debanne D, Gahwiler BH, Thompson SM. Long-term synaptic plasticity between pairs of individual CA3 pyramidal cells in rat hippocampal slice cultures. J Physiol. 1998;507(Pt 1):237–247. doi: 10.1111/j.1469-7793.1998.237bu.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duffy SN, Nguyen PV. Postsynaptic application of a peptide inhibitor of cAMP-dependent protein kinase blocks expression of long-lasting synaptic potentiation in hippocampal neurons. J Neurosci. 2003;23:1142–1150. doi: 10.1523/JNEUROSCI.23-04-01142.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fellini L, Florian C, Courtey J, Roullet P. Pharmacological intervention of hippocampal CA3 NMDA receptors impairs acquisition and long-term memory retrieval of spatial pattern completion task. Learn Mem. 2009;16:387–394. doi: 10.1101/lm.1433209. [DOI] [PubMed] [Google Scholar]
- Frey U, Huang YY, Kandel ER. Effects of cAMP simulate a late stage of LTP in hippocampal CA1 neurons. Science. 1993;260:1661–1664. doi: 10.1126/science.8389057. [DOI] [PubMed] [Google Scholar]
- Galvan EJ, Calixto E, Barrionuevo G. Bidirectional Hebbian plasticity at hippocampal mossy fiber synapses on CA3 interneurons. J Neurosci. 2008;28:14042–14055. doi: 10.1523/JNEUROSCI.4848-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galvan EJ, Cosgrove KE, Barrionuevo G. Multiple forms of long-term synaptic plasticity at hippocampal mossy fiber synapses on interneurons. Neuropharmacology. 2011;60:740–747. doi: 10.1016/j.neuropharm.2010.11.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galvan EJ, Cosgrove KE, Mauna JC, Card JP, Thiels E, Meriney SD, Barrionuevo G. Critical involvement of postsynaptic protein kinase activation in long-term potentiation at hippocampal mossy fiber synapses on CA3 interneurons. J Neurosci. 2010;30:2844–2855. doi: 10.1523/JNEUROSCI.5269-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giese KP, Fedorov NB, Filipkowski RK, Silva AJ. Autophosphorylation at Thr286 of the alpha calcium-calmodulin kinase II in LTP and learning. Science. 1998;279:870–873. doi: 10.1126/science.279.5352.870. [DOI] [PubMed] [Google Scholar]
- Goldberg JH, Tamas G, Aronov D, Yuste R. Calcium microdomains in aspiny dendrites. Neuron. 2003;40:807–821. doi: 10.1016/s0896-6273(03)00714-1. [DOI] [PubMed] [Google Scholar]
- Goldberg JH, Yuste R. Space matters: local and global dendritic Ca2+ compartmentalization in cortical interneurons. Trends Neurosci. 2005;28:158–167. doi: 10.1016/j.tins.2005.01.005. [DOI] [PubMed] [Google Scholar]
- Guirado R, Perez-Rando M, Sanchez-Matarredona D, Castillo-Gomez E, Liberia T, Rovira-Esteban L, Varea E, Crespo C, Blasco-Ibanez JM, Nacher J. The Dendritic Spines of Interneurons Are Dynamic Structures Influenced by PSA-NCAM Expression. Cereb Cortex. 2013 doi: 10.1093/cercor/bht156. [DOI] [PubMed] [Google Scholar]
- Hardingham N, Glazewski S, Pakhotin P, Mizuno K, Chapman PF, Giese KP, Fox K. Neocortical long-term potentiation and experience-dependent synaptic plasticity require alpha-calcium/calmodulin-dependent protein kinase II autophosphorylation. J Neurosci. 2003;23:4428–4436. doi: 10.1523/JNEUROSCI.23-11-04428.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harris EW, Cotman CW. Long-term potentiation of guinea pig mossy fiber responses is not blocked by N-methyl D-aspartate antagonists. Neurosci Lett. 1986;70:132–137. doi: 10.1016/0304-3940(86)90451-9. [DOI] [PubMed] [Google Scholar]
- Harris KM, Landis DM. Membrane structure at synaptic junctions in area CA1 of the rat hippocampus. Neuroscience. 1986;19:857–872. doi: 10.1016/0306-4522(86)90304-0. [DOI] [PubMed] [Google Scholar]
- Hayashi Y, Shi SH, Esteban JA, Piccini A, Poncer JC, Malinow R. Driving AMPA receptors into synapses by LTP and CaMKII: requirement for GluR1 and PDZ domain interaction. Science. 2000;287:2262–2267. doi: 10.1126/science.287.5461.2262. [DOI] [PubMed] [Google Scholar]
- Huang YY, Li XC, Kandel ER. cAMP contributes to mossy fiber LTP by initiating both a covalently mediated early phase and macromolecular synthesis-dependent late phase. Cell. 1994;79:69–79. doi: 10.1016/0092-8674(94)90401-4. [DOI] [PubMed] [Google Scholar]
- Hussain RJ, Carpenter DO. A comparison of the roles of protein kinase C in long-term potentiation in rat hippocampal areas CA1 and CA3. Cell Mol Neurobiol. 2005;25:649–661. doi: 10.1007/s10571-005-4045-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hvalby O, Hemmings HC, Jr, Paulsen O, Czernik AJ, Nairn AC, Godfraind JM, Jensen V, Raastad M, Storm JF, Andersen P, et al. Specificity of protein kinase inhibitor peptides and induction of long-term potentiation. Proc Natl Acad Sci U S A. 1994;91:4761–4765. doi: 10.1073/pnas.91.11.4761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ishizuka N, Weber J, Amaral DG. Organization of intrahippocampal projections originating from CA3 pyramidal cells in the rat. J Comp Neurol. 1990;295:580–623. doi: 10.1002/cne.902950407. [DOI] [PubMed] [Google Scholar]
- Jones EG, Huntley GW, Benson DL. Alpha calcium/calmodulin-dependent protein kinase II selectively expressed in a subpopulation of excitatory neurons in monkey sensory-motor cortex: comparison with GAD-67 expression. J Neurosci. 1994;14:611–629. doi: 10.1523/JNEUROSCI.14-02-00611.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kakegawa W, Tsuzuki K, Yoshida Y, Kameyama K, Ozawa S. Input- and subunit-specific AMPA receptor trafficking underlying long-term potentiation at hippocampal CA3 synapses. Eur J Neurosci. 2004;20:101–110. doi: 10.1111/j.1460-9568.2004.03461.x. [DOI] [PubMed] [Google Scholar]
- Kamiya H, Shinozaki H, Yamamoto C. Activation of metabotropic glutamate receptor type 2/3 suppresses transmission at rat hippocampal mossy fibre synapses. J Physiol. 1996;493(Pt 2):447–455. doi: 10.1113/jphysiol.1996.sp021395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kwon HB, Castillo PE. Long-term potentiation selectively expressed by NMDA receptors at hippocampal mossy fiber synapses. Neuron. 2008;57:108–120. doi: 10.1016/j.neuron.2007.11.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lacaille JC, Schwartzkroin PA. Stratum lacunosum-moleculare interneurons of hippocampal CA1 region. I. Intracellular response characteristics, synaptic responses, and morphology. J Neurosci. 1988;8:1400–1410. doi: 10.1523/JNEUROSCI.08-04-01400.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laezza F, Dingledine R. Voltage-controlled plasticity at GluR2-deficient synapses onto hippocampal interneurons. J Neurophysiol. 2004;92:3575–3581. doi: 10.1152/jn.00425.2004. [DOI] [PubMed] [Google Scholar]
- Laezza F, Dingledine R. Induction and expression rules of synaptic plasticity in hippocampal interneurons. Neuropharmacology. 2011;60:720–729. doi: 10.1016/j.neuropharm.2010.12.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laezza F, Doherty JJ, Dingledine R. Long-Term Depression in Hippocampal Interneurons: Joint Requirement for Pre- and Postsynaptic Events. Science. 1999;285:1411–1414. doi: 10.1126/science.285.5432.1411. [DOI] [PubMed] [Google Scholar]
- Lamsa K, Heeroma JH, Kullmann DM. Hebbian LTP in feed-forward inhibitory interneurons and the temporal fidelity of input discrimination. Nat Neurosci. 2005;8:916–924. doi: 10.1038/nn1486. [DOI] [PubMed] [Google Scholar]
- Lamsa K, Irvine EE, Giese KP, Kullmann DM. NMDA receptor-dependent long-term potentiation in mouse hippocampal interneurons shows a unique dependence on Ca(2+)/calmodulin-dependent kinases. J Physiol. 2007;584:885–894. doi: 10.1113/jphysiol.2007.137380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lapointe V, Morin F, Ratte S, Croce A, Conquet F, Lacaille JC. Synapse-specific mGluR1-dependent long-term potentiation in interneurones regulates mouse hippocampal inhibition. J Physiol. 2004;555:125–135. doi: 10.1113/jphysiol.2003.053603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lawrence JJ, McBain CJ. Interneuron diversity series: containing the detonation--feedforward inhibition in the CA3 hippocampus. Trends Neurosci. 2003;26:631–640. doi: 10.1016/j.tins.2003.09.007. [DOI] [PubMed] [Google Scholar]
- Lei S, McBain CJ. Distinct NMDA Receptors Provide Differential Modes of Transmission at Mossy Fiber-Interneuron Synapses. Neuron. 2002;33:921–933. doi: 10.1016/s0896-6273(02)00608-6. [DOI] [PubMed] [Google Scholar]
- Leutgeb JK, Leutgeb S, Moser MB, Moser EI. Pattern separation in the dentate gyrus and CA3 of the hippocampus. Science. 2007;315:961–966. doi: 10.1126/science.1135801. [DOI] [PubMed] [Google Scholar]
- Li XG, Somogyi P, Ylinen A, Buzsaki G. The hippocampal CA3 network: an in vivo intracellular labeling study. J Comp Neurol. 1994;339:181–208. doi: 10.1002/cne.903390204. [DOI] [PubMed] [Google Scholar]
- Ling DS, Benardo LS, Serrano PA, Blace N, Kelly MT, Crary JF, Sacktor TC. Protein kinase Mzeta is necessary and sufficient for LTP maintenance. Nat Neurosci. 2002;5:295–296. doi: 10.1038/nn829. [DOI] [PubMed] [Google Scholar]
- Lisman J, Schulman H, Cline H. The molecular basis of CaMKII function in synaptic and behavioural memory. Nat Rev Neurosci. 2002;3:175–190. doi: 10.1038/nrn753. [DOI] [PubMed] [Google Scholar]
- Lisman JE. Relating hippocampal circuitry to function: recall of memory sequences by reciprocal dentate-CA3 interactions. Neuron. 1999;22:233–242. doi: 10.1016/s0896-6273(00)81085-5. [DOI] [PubMed] [Google Scholar]
- Liu XB, Jones EG. Localization of alpha type II calcium calmodulin-dependent protein kinase at glutamatergic but not gamma-aminobutyric acid (GABAergic) synapses in thalamus and cerebral cortex. Proc Natl Acad Sci U S A. 1996;93:7332–7336. doi: 10.1073/pnas.93.14.7332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu FM, Hawkins RD. Presynaptic and postsynaptic Ca(2+) and CamKII contribute to long-term potentiation at synapses between individual CA3 neurons. Proc Natl Acad Sci U S A. 2006;103:4264–4269. doi: 10.1073/pnas.0508162103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lujan R, Nusser Z, Roberts JD, Shigemoto R, Somogyi P. Perisynaptic location of metabotropic glutamate receptors mGluR1 and mGluR5 on dendrites and dendritic spines in the rat hippocampus. Eur J Neurosci. 1996;8:1488–1500. doi: 10.1111/j.1460-9568.1996.tb01611.x. [DOI] [PubMed] [Google Scholar]
- Lledo PM, Hjelmstad GO, Mukherji S, Soderling TR, Malenka RC, Nicoll RA. Calcium/calmodulin-dependent kinase II and long-term potentiation enhance synaptic transmission by the same mechanism. Proc Natl Acad Sci U S A. 1995;92:11175–11179. doi: 10.1073/pnas.92.24.11175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maccaferri G, Tóth K, McBain CJ. Target-Specific Expression of Presynaptic Mossy Fiber Plasticity. Science. 1998;279:1368–1371. doi: 10.1126/science.279.5355.1368. [DOI] [PubMed] [Google Scholar]
- Magee JC, Johnston D. A synaptically controlled, associative signal for Hebbian plasticity in hippocampal neurons. Science. 1997;275:209–213. doi: 10.1126/science.275.5297.209. [DOI] [PubMed] [Google Scholar]
- Malinow R. AMPA receptor trafficking and long-term potentiation. Philos Trans R Soc Lond B Biol Sci. 2003;358:707–714. doi: 10.1098/rstb.2002.1233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malinow R, Schulman H, Tsien RW. Inhibition of postsynaptic PKC or CaMKII blocks induction but not expression of LTP. Science. 1989;245:862–866. doi: 10.1126/science.2549638. [DOI] [PubMed] [Google Scholar]
- Malinow R, Tsien RW. Presynaptic enhancement shown by whole-cell recordings of long-term potentiation in hippocampal slices. Nature. 1990;346:177–180. doi: 10.1038/346177a0. [DOI] [PubMed] [Google Scholar]
- Marr D. Simple memory: a theory for archicortex. Philos Trans R Soc Lond B Biol Sci. 1971;262:23–81. doi: 10.1098/rstb.1971.0078. [DOI] [PubMed] [Google Scholar]
- McNaughton N, Morris RG. Chlordiazepoxide, an anxiolytic benzodiazepine, impairs place navigation in rats. Behav Brain Res. 1987;24:39–46. doi: 10.1016/0166-4328(87)90034-9. [DOI] [PubMed] [Google Scholar]
- Michel JJ, Scott JD. AKAP mediated signal transduction. Annu Rev Pharmacol Toxicol. 2002;42:235–257. doi: 10.1146/annurev.pharmtox.42.083101.135801. [DOI] [PubMed] [Google Scholar]
- Nakazawa K, Quirk MC, Chitwood RA, Watanabe M, Yeckel MF, Sun LD, Kato A, Carr CA, Johnston D, Wilson MA, Tonegawa S. Requirement for hippocampal CA3 NMDA receptors in associative memory recall. Science. 2002;297:211–218. doi: 10.1126/science.1071795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Reilly RC, McClelland JL. Hippocampal conjunctive encoding, storage, and recall: avoiding a trade-off. Hippocampus. 1994;4:661–682. doi: 10.1002/hipo.450040605. [DOI] [PubMed] [Google Scholar]
- Ochiishi T, Terashima T, Yamauchi T. Specific distribution of Ca2+/calmodulin-dependent protein kinase II alpha and beta isoforms in some structures of the rat forebrain. Brain Res. 1994;659:179–193. doi: 10.1016/0006-8993(94)90877-x. [DOI] [PubMed] [Google Scholar]
- Pavlidis P, Montgomery J, Madison DV. Presynaptic protein kinase activity supports long-term potentiation at synapses between individual hippocampal neurons. J Neurosci. 2000;20:4497–4505. doi: 10.1523/JNEUROSCI.20-12-04497.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perez-Orive J, Mazor O, Turner GC, Cassenaer S, Wilson RI, Laurent G. Oscillations and sparsening of odor representations in the mushroom body. Science. 2002;297:359–365. doi: 10.1126/science.1070502. [DOI] [PubMed] [Google Scholar]
- Perez Y, Morin F, Lacaille JC. A hebbian form of long-term potentiation dependent on mGluR1a in hippocampal inhibitory interneurons. Proc Natl Acad Sci U S A. 2001;98:9401–9406. doi: 10.1073/pnas.161493498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poncer JC, Esteban JA, Malinow R. Multiple mechanisms for the potentiation of AMPA receptor-mediated transmission by alpha-Ca2+/calmodulin-dependent protein kinase II. J Neurosci. 2002;22:4406–4411. doi: 10.1523/JNEUROSCI.22-11-04406.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pouille F, Scanziani M. Enforcement of temporal fidelity in pyramidal cells by somatic feed-forward inhibition. Science. 2001;293:1159–1163. doi: 10.1126/science.1060342. [DOI] [PubMed] [Google Scholar]
- Rolls ET. A theory of hippocampal function in memory. Hippocampus. 1996;6:601–620. doi: 10.1002/(SICI)1098-1063(1996)6:6<601::AID-HIPO5>3.0.CO;2-J. [DOI] [PubMed] [Google Scholar]
- Rolls ET. A quantitative theory of the functions of the hippocampal CA3 network in memory. Front Cell Neurosci. 2013;7:98. doi: 10.3389/fncel.2013.00098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sahay A, Wilson DA, Hen R. Pattern separation: a common function for new neurons in hippocampus and olfactory bulb. Neuron. 2011;70:582–588. doi: 10.1016/j.neuron.2011.05.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salin Paul A, Scanziani M, Malenka Robert C, Nicoll Roger A. Distinct short-term plasticity at two excitatory synapses in the hippocampus. PNAS. 1996;93:13304–13309. doi: 10.1073/pnas.93.23.13304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanderson JL, Dell’Acqua ML. AKAP signaling complexes in regulation of excitatory synaptic plasticity. Neuroscientist. 2011;17:321–336. doi: 10.1177/1073858410384740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sik A, Gulacsi A, Lai Y, Doyle WK, Pacia S, Mody I, Freund TF. Localization of the A kinase anchoring protein AKAP79 in the human hippocampus. Eur J Neurosci. 2000;12:1155–1164. doi: 10.1046/j.1460-9568.2000.00002.x. [DOI] [PubMed] [Google Scholar]
- Sik A, Hajos N, Gulacsi A, Mody I, Freund TF. The absence of a major Ca2+ signaling pathway in GABAergic neurons of the hippocampus. Proc Natl Acad Sci U S A. 1998;95:3245–3250. doi: 10.1073/pnas.95.6.3245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sik A, Tamamaki N, Freund TF. Complete axon arborization of a single CA3 pyramidal cell in the rat hippocampus, and its relationship with postsynaptic parvalbumin-containing interneurons. Eur J Neurosci. 1993;5:1719–1728. doi: 10.1111/j.1460-9568.1993.tb00239.x. [DOI] [PubMed] [Google Scholar]
- Soler-Llavina GJ, Sabatini BL. Synapse-specific plasticity and compartmentalized signaling in cerebellar stellate cells. Nat Neurosci. 2006;9:798–806. doi: 10.1038/nn1698. [DOI] [PubMed] [Google Scholar]
- Son H, Madelian V, Carpenter DO. The translocation and involvement of protein kinase C in mossy fiber-CA3 long-term potentiation in hippocampus of the rat brain. Brain Res. 1996;739:282–292. doi: 10.1016/s0006-8993(96)00836-0. [DOI] [PubMed] [Google Scholar]
- Stoop R, Conquet F, Zuber B, Voronin LL, Pralong E. Activation of metabotropic glutamate 5 and NMDA receptors underlies the induction of persistent bursting and associated long-lasting changes in CA3 recurrent connections. J Neurosci. 2003;23:5634–5644. doi: 10.1523/JNEUROSCI.23-13-05634.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takaishi T, Saito N, Tanaka C. Evidence for distinct neuronal localization of gamma and delta subunits of Ca2+/calmodulin-dependent protein kinase II in the rat brain. J Neurochem. 1992;58:1971–1974. doi: 10.1111/j.1471-4159.1992.tb10079.x. [DOI] [PubMed] [Google Scholar]
- Topolnik L, Azzi M, Morin F, Kougioumoutzakis A, Lacaille JC. mGluR1/5 subtype-specific calcium signalling and induction of long-term potentiation in rat hippocampal oriens/alveus interneurones. J Physiol. 2006;575:115–131. doi: 10.1113/jphysiol.2006.112896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toth K, McBain CJ. Afferent-specific innervation of two distinct AMPA receptor subtypes on single hippocampal interneurons. Nat Neurosci. 1998;1:572–578. doi: 10.1038/2807. [DOI] [PubMed] [Google Scholar]
- Toth K, Suares G, Lawrence JJ, Philips-Tansey E, McBain CJ. Differential mechanisms of transmission at three types of mossy fiber synapse. J Neurosci. 2000;20:8279–8289. doi: 10.1523/JNEUROSCI.20-22-08279.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Treves A, Rolls ET. Computational constraints suggest the need for two distinct input systems to the hippocampal CA3 network. Hippocampus. 1992;2:189–199. doi: 10.1002/hipo.450020209. [DOI] [PubMed] [Google Scholar]
- Vida I, Halasy K, Szinyei C, Somogyi P, Buhl EH. Unitary IPSPs evoked by interneurons at the stratum radiatum-stratum lacunosum-moleculare border in the CA1 area of the rat hippocampus in vitro. J Physiol. 1998;506(Pt 3):755–773. doi: 10.1111/j.1469-7793.1998.755bv.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Villacres EC, Wong ST, Chavkin C, Storm DR. Type I adenylyl cyclase mutant mice have impaired mossy fiber long-term potentiation. J Neurosci. 1998;18:3186–3194. doi: 10.1523/JNEUROSCI.18-09-03186.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang JH, Kelly P. Calcium-calmodulin signalling pathway up-regulates glutamatergic synaptic function in non-pyramidal, fast spiking rat hippocampal CA1 neurons. J Physiol. 2001;533:407–422. doi: 10.1111/j.1469-7793.2001.0407a.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang JH, Kelly PT. Postsynaptic injection of CA2+/CaM induces synaptic potentiation requiring CaMKII and PKC activity. Neuron. 1995;15:443–452. doi: 10.1016/0896-6273(95)90048-9. [DOI] [PubMed] [Google Scholar]
- Wang JH, Kelly PT. Regulation of synaptic facilitation by postsynaptic Ca2+/CaM pathways in hippocampal CA1 neurons. J Neurophysiol. 1996;76:276–286. doi: 10.1152/jn.1996.76.1.276. [DOI] [PubMed] [Google Scholar]
- Weisskopf MG, Castillo PE, Zalutsky RA, Nicoll RA. Mediation of hippocampal mossy fiber long-term potentiation by cyclic AMP. Science. 1994;265:1878–1882. doi: 10.1126/science.7916482. [DOI] [PubMed] [Google Scholar]
- Williams S, Samulack DD, Beaulieu C, LaCaille JC. Membrane properties and synaptic responses of interneurons located near the stratum lacunosum-moleculare/radiatum border of area CA1 in whole-cell recordings from rat hippocampal slices. J Neurophysiol. 1994;71:2217–2235. doi: 10.1152/jn.1994.71.6.2217. [DOI] [PubMed] [Google Scholar]
- Wong W, Scott JD. AKAP signalling complexes: focal points in space and time. Nat Rev Mol Cell Biol. 2004;5:959–970. doi: 10.1038/nrm1527. [DOI] [PubMed] [Google Scholar]
- Yuste R, Denk W. Dendritic spines as basic functional units of neuronal integration. Nature. 1995;375:682–684. doi: 10.1038/375682a0. [DOI] [PubMed] [Google Scholar]
- Zalutsky RA, Nicoll RA. Comparison of two forms of long-term potentiation in single hippocampal neurons. Science. 1990;248:1619–1624. doi: 10.1126/science.2114039. [DOI] [PubMed] [Google Scholar]