Abstract
Several rodent-associated Bartonella species are human pathogens but little is known about their epidemiology. We trapped rodents and shrews around human habitations at two sites in Kenya (rural Asembo and urban Kibera) to determine the prevalence of Bartonella infection. Bartonella were detected by culture in five of seven host species. In Kibera, 60% of Rattus rattus were positive, as compared to 13% in Asembo. Bartonella were also detected in C. olivieri (7%), Lemniscomys striatus (50%), Mastomys natalensis (43%) and R. norvegicus (50%). Partial sequencing of the citrate synthase (gltA) gene of isolates showed that Kibera strains were similar to reference isolates from Rattus trapped in Asia, America, and Europe, but that most strains from Asembo were less similar. Host species and trapping location were associated with differences in infection status but there was no evidence of associations between host age or sex and infection status. Acute febrile illness occurs at high incidence in both Asembo and Kibera but the etiology of many of these illnesses is unknown. Bartonella similar to known human pathogens were detected in small mammals at both sites and investigation of the ecological determinants of host infection status and of the public health significance of Bartonella infections at these locations is warranted.
Author Summary
Bartonella are bacteria that infect many different mammal species and can cause illness in people. Several Bartonella species carried by rodents cause disease in humans but little is known about their distribution or the importance of bartonellosis as a cause of human illness. Data from Africa are particularly scarce. This study involved trapping of rodents and other small mammals at two sites in Kenya: Asembo, a rural area in Western Kenya, and Kibera, an informal urban settlement in Nairobi. Blood samples were collected from trapped animals to detect and characterize the types of Bartonella carried. At the Kibera site over half of the trapped rats were infected with Bartonella very similar to human pathogenic strains isolated from rats from other global regions. In Asembo, Bartonella were detected in four of the five animal species trapped and these Bartonella were less similar to previously identified isolates. All of the small mammals included in this study were trapped in or around human habitations. The data from this study show that Bartonella that can cause human illness are carried by the small mammals at these two sites and indicate that the public health impacts of human bartonellosis should be investigated.
Introduction
Bartonella species are Gram-negative haemotrophic bacteria that infect mammalian erythrocytes and are transmitted between hosts by blood-sucking arthropods. Over 30 species of Bartonella have been described and members of this genus infect a broad range of mammalian hosts including rodents, bats, carnivores and ruminants [1]. Arthropod vectors including fleas, sandflies, lice, ticks, bat flies and ked flies are implicated in the transmission of these pathogens [2–4]. The genus Bartonella has a global distribution. The Bartonella elizabethae complex includes several Bartonella genotypes and strains (including B. elizabethae, B. tribocorum, B. rattimassiliensis and B. queenslandensis) that have been isolated from Rattus and Bandicota species around the world [1]. Recent analyses indicate that this complex has south-east Asian origins and has been globally dispersed by Rattus species [5].
Several Bartonella species are recognized as human pathogens that cause diverse clinical presentations [6]. Among rodent-associated Bartonella species, B. elizabethae is a known cause of human endocarditis [7]. Other rodent-associated species including B. tribocorum, B. vinsonii subsp. arupensis, B. washoensis and B. alsatica have been associated with a range of symptoms in humans including fatigue, muscle and joint pain, and serious complications, such as endocarditis and neurological signs, particularly in immunocompromised patients [8,9].
Bartonella species have been identified as important causes of febrile illness in some settings. In two studies conducted in Thailand, 15% of febrile patients were diagnosed with confirmed Bartonella infection based on a four-fold rise in antibody titres, and six different Bartonella species were identified by culture from blood clots collected from febrile patients [10,11]. Non-specific clinical signs and difficulties in culturing the organism present substantial challenges to the diagnosis of bartonellosis. Consequently, Bartonella species may well be under-recognized as a cause of human disease [12]. This is particularly true for Africa, where very few data on the etiology of febrile illness are currently available [13].
In the Democratic Republic of Congo (DRC), a seroprevalence study identified IgG antibodies against Bartonella (B. henselae, B. quintana or B. clarridgeiae) in 4.5% of febrile patients [14]. Bartonella bacteraemia was detected by PCR in 10% of HIV-positive patients in South Africa [15]. Apart from these studies however, there is little information on the impact of Bartonella on human health on the African continent.
A variety of Bartonella species have been detected in animal and ectoparasite populations in Africa. Considering rodents and small mammals specifically, B. elizabethae and two other Bartonella lineages were detected in Namaqua rock mice sampled in South Africa, where 44% of the 100 individuals sampled were positive by PCR for Bartonella species [16]. B. elizabethae, B. tribocorum and a Bartonella species with intermediate species classification based on sequence data were detected in 28% of rodents and hedgehogs (n = 75) sampled in Algeria [17]. B. elizabethae, B. tribocorum and novel Bartonella species were also detected in rodents sampled in the Democratic Republic of Congo (DRC) and Tanzania [18]. Small mammals trapped in Ethiopia, had an overall Bartonella infection prevalence of 34% (n = 529) and were infected with multiple genotypes including genotypes very closely related to B. elizabethae [19]. B. elizabethae has also been detected in invasive and indigenous rodents sampled in Uganda [20] and genotypes related to B. rochalimae, B. grahamii and B. elizabethae have been detected in Mearn’s pouched mice studied in Kenya [21]. Bartonella have also been detected in fleas collected in Egypt, Morocco, DRC and Uganda [20,22–24].
The first objective of this study was to determine the presence and prevalence of Bartonella infections in small mammals trapped at rural and urban locations in Kenya. We also aimed to characterize the Bartonella isolates obtained using partial sequences of the citrate synthase (gltA) gene and to compare the Bartonella genotypes detected in these distinct Kenyan populations with each other and with Bartonella detected in small mammals in other parts of the world.
Materials and Methods
Study sites
Cross-sectional rodent trapping surveys were conducted within two locations: Asembo, a rural area on the northern shore of Lake Victoria in Nyanza Province western Kenya (Latitude-0.1443, longitude 34.3468) and Kibera, an urban informal settlement in Nairobi City (Latitude-1.3156, longitude 36.7820, Fig. 1). These locations are the study sites for ongoing population-based human health surveillance [25]. In Asembo, subsistence farming is the primary occupation for 65% of household heads, 13% work in the informal economy and 5% are salaried [25]. Households are clustered into compounds of closely related family units. Livestock ownership is common: 44% of Asembo households own cattle and 43% own at least one sheep or goat. In contrast, in urban Kibera, 53% of heads of household are salaried and 43% work in the informal sector [25]. Ownership of large livestock species in Kibera is very rare and prohibited by City Council law.
Fig 1. Map of Kenya indicating the location of study sites.
Small mammal trapping and sample collection
In Asembo, trapping was conducted over the period of July—August 2009. Traps were placed at 50 compounds that were a randomly selected subset of livestock-owning compounds enrolled in a larger study of zoonoses epidemiology [26]. Within each selected compound, five or six medium-sized foldable Sherman traps (H.B. Sherman Traps Inc., Tallahassee, FL) were placed for three or four nights. Traps were placed in three categories of habitat: within occupied dwellings; within outbuildings, which included unoccupied dwellings, stores, latrines or kitchens separate from the main dwelling; and outside, in areas within the compound yard. In Kibera, trapping was conducted over the period of September—November 2008. The overall study site was divided for this study into five trapping zones of similar area and within each zone a 50m x 50m trapping area was defined (Fig. 2). Within each of the five trapping zones, medium-sized foldable Sherman traps were placed for a minimum of two consecutive nights and a maximum of six nights with the aim of trapping approximately 50 rodents per zone. In Kibera, all traps were placed indoors at 270 occupied dwellings.
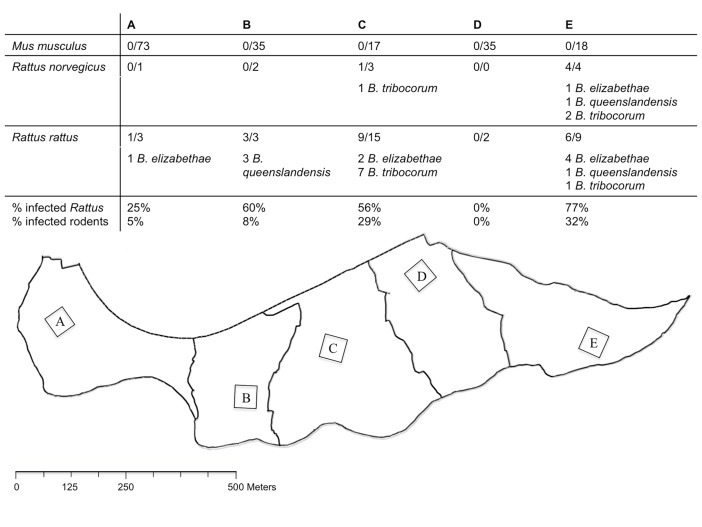
Fig 2. Map and summary of rodent trapping sites in Kibera.
Summary of the rodents trapped and Bartonella isolates obtained within different trapping zones (A to E) at the Kibera study site.
All trapped animals from both locations were euthanized by overdose of the inhalant anesthetic halothane and whole blood was collected by cardiocentesis using aseptic technique. Blood samples were processed to remove serum and the remaining blood clots frozen at -80°C prior to testing. Blood clots were shipped on dry ice to the Bartonella laboratory at the Centers for Disease Control and Prevention, Fort Collins, Colorado for laboratory testing. Morphometric data were collected from each trapped animal for species identification at the National Museums of Kenya. The Asembo small mammals were submitted for archiving under accession numbers NMK 171860—NMK 171922. The Kibera rodent population included in this study is as described previously [27].
Bartonella culture
Culture was performed using previously described techniques [28]. Briefly, blood clots were re-suspended 1:4 in brain heart infusion broth supplemented with 5% amphotericin B, then plated onto agar supplemented with 5% sterile rabbit blood and incubated at 35°C in an aerobic atmosphere of 5% carbon dioxide for up to 30 days. Bacterial colonies were presumptively identified as Bartonella based on their morphology. Subcultures of Bartonella colonies from the original agar plate were streaked onto secondary agar plates and incubated at the same conditions until sufficient growth was observed, usually between 5 and 7 days. Pure cultures were harvested and stored in 10% ethanol.
DNA extraction, PCR and sequencing
The identity of presumptive Bartonella isolates was confirmed by PCR amplification and sequencing of a specific fragment of the Bartonella citrate synthase (gltA) gene. Crude DNA extracts were obtained from bacterial cultures by heating a heavy suspension of the microorganisms. Two oligonucleotides (BhCS.781.p and BhCS.1137.n) were used as PCR primers to generate a 379-bp amplicon of the Bartonella gltA gene [29]. PCR products were separated by 1.5% agarose gel electrophoresis and visualized by ethidium bromide staining. Sequencing reactions were carried out in a PTC 200 Peltier Thermal cycler (Applied Biosystems; Foster City, California) using the same primers as the initial PCR assay.
Phylogenetic analysis
Sequences were analysed using Lasergene 12 Core Suite (DNASTAR, Madison, WI) to determine sequence consensus for the gltA amplicons. Unique gltA sequences generated through this study were submitted to GenBank (accession numbers KM233484—KM233492). The Clustal V program within the MegAlign module of Lasergene was used to compare homologous Bartonella gltA sequences generated in this study with others available from the GenBank database. Phylogenetic trees were constructed using the neighbor-joining method with the Kimura’s 2-parameter distance model and bootstrap calculations were carried out with 1000 replicates. B. tamiae was used as the outgroup. A criterion of >96% homology was used to define similarity of study sequences to known Bartonella species [30].
Ecological analysis
Generalized linear models were used to examine associations between individual Bartonella infection status (culture positive or negative for Bartonella) and host and environmental variables in R (Version 3.0.3) [31]. Binomial family models with a logit link function were used and p values ≤ 0.05 were considered statistically significant. Variables examined included host species, sex, mass and trapping location. Data from the Asembo (S1 Table) and Kibera (S2 Table) sites were analysed separately.
Ethics statement
Written informed consent for trapping was obtained from representatives of the study households. The protocols and consent forms were reviewed and approved by the Animal Care and Use and Ethical Review Boards of the Kenya Medical Research Institute (#1191). The study protocols were also approved by the Institutional Animal Care and Use Committee and Institutional Review Board of the U.S. Centers for Disease Control and Prevention (#5410) and complied with the Public Health Service Policy on Humane Care and Use of Laboratory Animals.
Results
Small mammal trapping
A total of 49 small mammals trapped at 29 compounds in Asembo and 220 rodents trapped at 143 households in Kibera were included in this study. The small mammals trapped in Asembo included Crocidura olivieri (n = 16, African giant shrew), and rodents of the species Lemniscomys striatus (n = 2, striped grass mouse), Mastomys natalensis (n = 14, Natal multimammate mouse), Mus minutoides (n = 1, pygmy mouse), and Rattus rattus (n = 16, black rat). All of the rodents trapped in Kibera were Mus musculus (n = 178, house mouse), Rattus norvegicus (n = 10, brown rat) or Rattus rattus (n = 32) (Table 1).
Table 1. Summary of the species and number of small mammals trapped at different locations in the two study sites that were tested for Bartonella.
| Site | Species | Trap Location | |||
|---|---|---|---|---|---|
| Indoor | Outbuildings | Outside | Location Not Recorded | ||
| Asembo | Crocidura olivieri | 5 | 1 | 10 | 0 |
| Lemniscomys striatus | 0 | 0 | 2 | 0 | |
| Mastomys natalensis | 1 | 1 | 10 | 2 | |
| Mus minutoides | 0 | 0 | 1 | 0 | |
| Rattus rattus | 6 | 8 | 1 | 1 | |
| Total | 12 | 10 | 24 | 3 | |
| Kibera | Mus musculus | 178 | - | - | - |
| Rattus norvegicus | 10 | - | - | - | |
| Rattus rattus | 32 | - | - | - | |
| Total | 220 | - | - | - | |
Bartonella culture
Ten of the 49 (21%) animals trapped in Asembo were culture-positive for Bartonella, including: Crocidura olivieri (n = 1, 7%); Lemniscomys striatus (n = 1, 50%); Mastomys natalensis (n = 6, 43%); and Rattus rattus (n = 2, 13%). Overall, 24 of the 220 (11%) animals trapped in Kibera were culture positive: including Rattus norvegicus (n = 5, 50%) and R. rattus (n = 19, 60%). None of the 178 samples collected from Mus musculus in Kibera were positive (Table 2). Culture-positive Rattus species were trapped in four of the five trapping grids established at the Kibera site (Fig. 2).
Table 2. Summary of the number and species of the Bartonella isolates obtained and the prevalence of Bartonella infection in each population.
| Site | Species | N tested | N (%) Bartonella positive | Bartonella species identified | |||
|---|---|---|---|---|---|---|---|
| birtlesii-like | elizabethae | tribocorum | queenslandensis | ||||
| Asembo | Crocidura olivieri | 16 | 1 (7%) | 1 | - | - | - |
| Lemniscomys striatus | 2 | 1 (50%) | - | - | 1 | - | |
| Mastomys natalensis | 14 | 6 (43%) | - | 1 | 5 | - | |
| Mus minutoides | 1 | 0 (0%) | - | - | - | - | |
| Rattus rattus | 16 | 2 (13%) | - | 2 | - | - | |
| Kibera | Mus musculus | 178 | 0 (0%) | - | - | - | |
| Rattus norvegicus | 10 | 5 (50%) | - | 1 | 3 | 1 | |
| Rattus rattus | 32 | 19 (60%) | - | 7 | 8 | 4 | |
Phylogenetic analysis
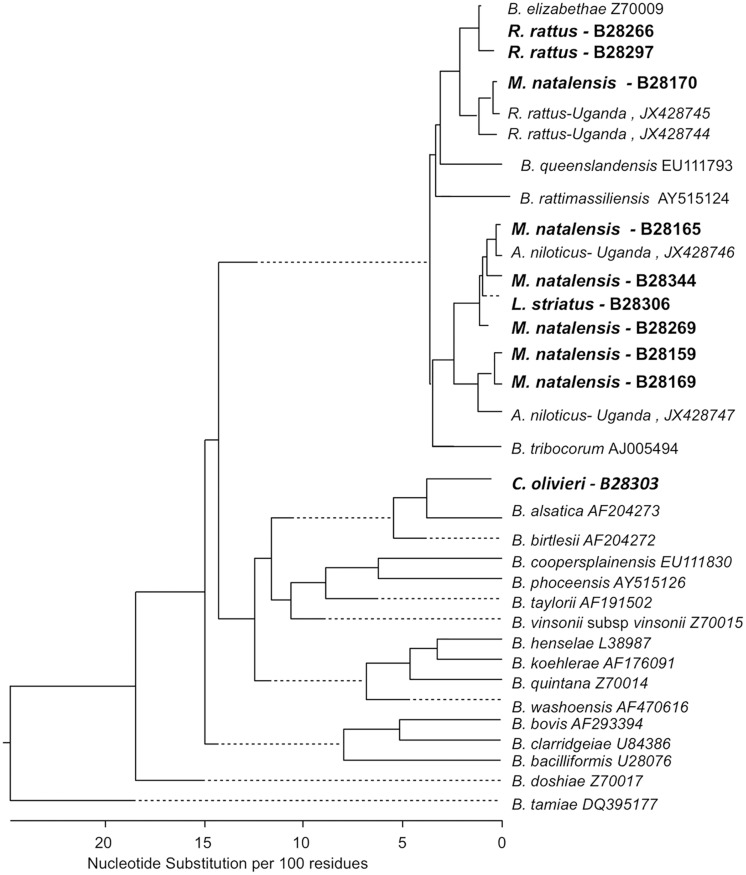
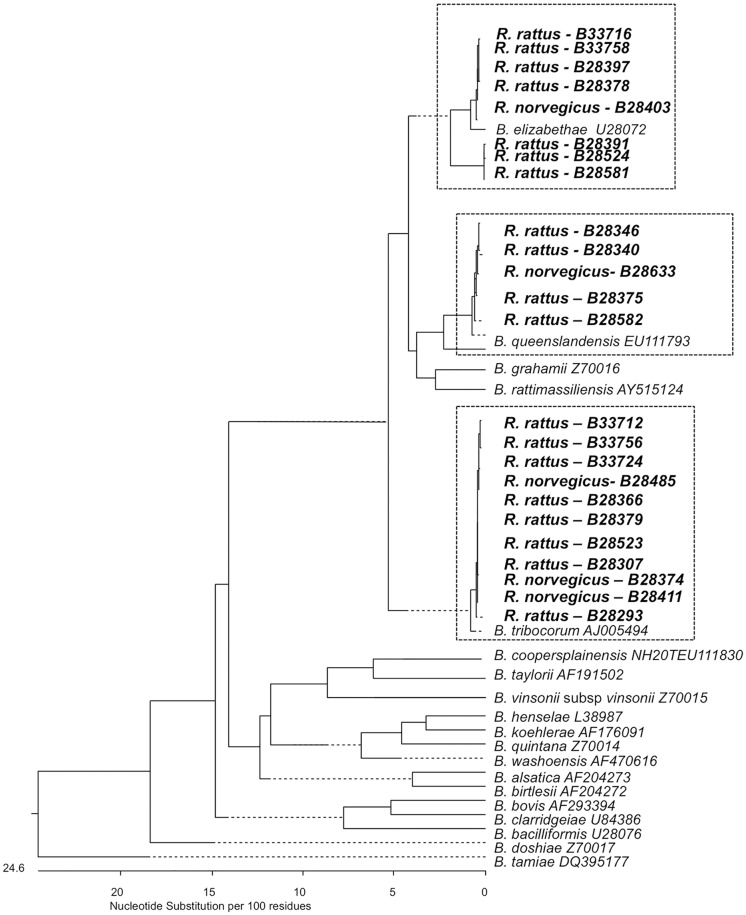
Information on gltA sequences was obtained from all 34 culture-positive animals. The phylogenetic relationships between the isolates obtained in this study and previously described Bartonella species are shown in Figs 3 and 4. Bartonella detected in one M. natalensis and two R. rattus trapped in Asembo belong to the B. elizabethae species complex based on the similarity of the gltA sequences (Fig. 3 & Table 2). Five additional sequences detected in M. natalensis and one detected in L. striatus were most closely related to B. tribocorum. None of the strains obtained from M. natalensis or L. striatus were identical (≥ 96% sequence identity) to reference strains of the Bartonella species described previously in Rattus species trapped elsewhere. The strain of Bartonella cultured from a C. olivieri was not very similar to any previously described Bartonella reference species but has 93.8% similarity B. birtlesii. Three pathogenic Bartonella species (B. elizabethae, B. tribocorum and B. queenslandensis) were detected in the two Rattus species sampled at the Kibera site. The gltA sequences for all Bartonella strains from Kibera rodents were identical (≥ 96% sequence identity) to reference isolates that are typical of Bartonella detected in Rattus populations globally (Table 2 & Fig. 4).
Fig 3. Phylogenetic tree of Asembo Bartonella isolates.
Phylogenetic tree of Bartonella isolates obtained from Asembo small mammals (shown in bold) and previously described reference strains from sylvatic rodents trapped in Africa based on sequence analysis of the citrate synthase (gltA) gene. The phylogenetic tree was constructing using the neighbor-joining method. Dotted lines indicate negative branch lengths.
Fig 4. Phylogenetic tree of Kibera Bartonella isolates.
Phylogenetic tree of Bartonella isolates obtained from Kibera rodents (shown in bold) and previously described reference strains for globally dispersed Bartonella species based on sequence analysis of the citrate synthase (gltA) gene. The phylogenetic tree was constructing using the neighbor-joining method. Dotted lines indicate negative branch lengths.
Ecological analysis
At the Asembo location, there was a weak association between individual infection status and host species (likelihood ratio test p = 0.053) where infection probability was higher in M. natalensis individuals than in the reference species C. olivieri (OR = 11.25, 95% CI = 1.15–110.47, p = 0.038). Approximately half (24/49) of the small mammals trapped in Asembo were trapped outside (Table 1). None of the Bartonella positive animals trapped in Asembo were trapped within occupied dwellings. Two positive R. rattus were trapped in outbuildings but all other positive animals (one C. olivieri, one Lemniscomys striatus and six M. natalensis) were trapped outside. There were no statistically significant associations between the probability of Bartonella infection and small mammal sex or mass within the Asembo population. At the Kibera location, there was a clear influence of genus upon infection probability. None of the 178 Mus trapped were Bartonella positive but 24/42 Rattus were positive indicating much higher infection probability in Rattus (OR = Infinite). Considering the data for Rattus individuals only, there were no statistically significant associations between the probability of Bartonella infection and rodent species, sex, or mass. The proportion of infected Rattus and proportion of infected rodents overall varied by trapping zone in Kibera (Fig. 2). There was no statistically significant difference in the probability of Bartonella infection in Rattus from different trapping zones. However the sample size for this analysis was small and the existence of zones where no positive individuals were trapped complicate this analyses and its interpretation. Descriptively, the trapping data from Kibera fall into two groups. In zones A, B and D few Rattus were trapped (Fig. 2), the rodent populations in these zones were dominated by Mus musculus and only four Bartonella isolates were identified in the combined rodent populations from these three zones (Fig. 2). In contrast, in trapping zones C and E, Rattus made up larger proportions of the total trapped population (51% in zone C and 40% in zone E) and more Bartonella isolates of several species were identified in these populations.
Discussion
This study reports isolation of Bartonella strains from rodent and shrew species in Asembo and Kibera, Kenya. Bartonella strains were found in several small mammal species with variation observed in the infection prevalence and in the strains of Bartonella detected between host species and study sites. The majority of Bartonella isolates obtained from these Kenyan mammals are genetically similar to reference strains of known human pathogens.
Several recent studies indicate that the prevalence of Bartonella infection in Rattus in Africa may be low in contrast to the frequently high prevalences observed in Asian Rattus populations [18–20,32]. It has been argued that this pattern of lower prevalence in African Rattus populations could be attributed to host escape during colonization [19,20], a phenomenon where relatively small founding populations of invading species can leave their parasites behind when colonizing new areas [33]. Consistent with this, a relatively low prevalence was seen in R. rattus from Asembo (13%). However, the high infection prevalence observed in Rattus trapped at the Kibera site (e.g. 50% R. norvegicus and 60% R. rattus) is more similar to prevalence values observed in studies of Asian Rattus populations than to other African populations [19]. There are multiple possible explanations for the difference in the prevalence observed in Rattus at these two sites. Phylogenetic analyses indicate that B. elizabethae complex strains originated in Southeast Asia and have been disseminated throughout Asia, Europe, Africa, Australia and the Americas through multiple dispersal events of commensal Rattus species [5]. The Kibera study site is near the centre of Nairobi, the Kenyan capital, and is likely to have greater international connectivity (in terms of international rodent movement through trade etc.) than the Asembo site, which is more rural. The higher prevalence observed at the Kibera site could therefore be explained by repeated introduction of Rattus and their associated Bartonella species to this site [19,33]. Further analyses would however be needed to elucidate the colonization history of Rattus and their associated Bartonella at these sites specifically. The number of species trapped in Kibera was smaller than the number trapped in Asembo, indicating a simpler species composition at this site and these data could also suggest a possible dilution effect of the increased community complexity in Asembo on Bartonella prevalence [34]. Finally, temporal dynamics in host and ectoparasite population structure are known to affect Bartonella infection prevalence [21,35]. This study involved cross-sectional trapping surveys conducted at different times of year in the two study locations. There are few data on the seasonal variation in the abundance or diversity of the rodent populations at these sites but it is likely that there are seasonal influences upon rodent abundance and diversity with differences between the urban Kibera site and the more rural Asembo site in the seasonal population dynamics observed [36]. It is therefore possible that differences in the sampling time may have contributed to the differences in infection prevalence seen between these two Rattus populations.
Notably, no bartonellae were detected in Mus musculus trapped in Kibera despite a large number of tested animals and high infection prevalence observed in Rattus trapped in the same locations. Low-level Bartonella infection has been reported from Mus trapped in Ethiopia but the absence of Bartonella in Mus was also reported in a small-scale study from Nigeria [19,37].
All of the Kibera rodents were trapped within residents’ homes. In contrast, although nearly half of the animals trapped in Asembo were trapped indoors or in outbuildings, none of the positive animals at this site were trapped indoors, and only two positive animals were trapped in outbuildings. Approximately one third of the animals trapped outside in Asembo however were culture positive for Bartonella. The two culture positive animals trapped in outbuildings were R. rattus and they were carrying Bartonella similar to the B. elizabethae reference strain (Fig. 2). This species was most commonly found indoors or in outbuildings (14/15 records) and therefore may pose a risk due to closer human contact, even though only 2/16 were positive.
Many of the Bartonella detected at in this study (except the birtlesii-like isolates from Crocidura) belong to the Bartonella elizabethae complex and many of the strains identified in invasive Rattus hosts particularly are closely related to known human pathogens. All of the Bartonella strains isolated from Kibera rodents have ≥ 96% sequence identity with strains that are common in Rattus species sampled in Asia and on several other continents [5]. In contrast, several strains isolated from Asembo rodents and shrews were less similar to the international reference strains from Rattus and were more similar to isolates gathered previously from Ugandan rodents, suggesting a longer history of circulation of these strains within these species at the Asembo site. The identification of similar B. tribocorum sequences in Mastomys and Lemniscomys individuals trapped in Asembo suggests an absence of strong host-pathogen associations in these populations.
There is a high incidence of acute febrile illness in people in both Asembo and Kibera [25]. A variety of pathogens are known to account for a proportion of febrile illness in Asembo and Kibera but considerable proportions remain unexplained [38–41]. Bartonella species have been identified as important causes of human febrile illness in several global settings but there has been little investigation of the impact of bartonellosis upon human health in Africa particularly and it is conceivable that Bartonella may be an important cause of febrile illness in these study populations.
The data presented from the Asembo and Kibera sites indicate clear differences in: the prevalence of Bartonella infection in the same host (Rattus species) at the two sites; the prevalence of infection in different hosts trapped at the same sites; the abundance of different infected hosts between the two locations and also between trapping zones in Kibera; the strains of Bartonella detected and finally in the locations within communities where rodents overall and Bartonella infected rodents were trapped. The impact of this variation in rodent host community composition, infection prevalence, ectoparasite vector preferences, and other ecological factors need to be understood to evaluate human Bartonella infection risks at these sites. The data presented here suggest that investigations of the multi-host infection dynamics of Bartonella and the public health significance of Bartonella infections at these Kenyan locations and others where there are close associations between people and small mammals are warranted.
Supporting Information
(XLS)
(XLSX)
Acknowledgments
We thank the field staff at the National Museums of Kenya and team at the KEMRI/CDC Public Health and Research Collaboration as well as Stella Kiambi, Samuel Chege, John Mugo and Gilbert Ogango in Kibera, and Samuel Asembo, Michael Otieno, James Oyigo, and Pauline Otieno in Asembo for assistance with the field components of this study. We would also like to thank the Director of Veterinary Services, Nairobi and residents of Kibera and Asembo for their support throughout the research project.
Data Availability
All relevant data are within the paper and its Supporting Information files. The unique sequences generated through this study have been submitted to GenBank (accession numbers KM233484 - KM233492).
Funding Statement
This research was supported by the Wellcome Trust, UK (http://www.wellcome.ac.uk; Grant number 081828/B/06/Z). JEBH and SC receive funding support from the BBSRC, UK (http://www.bbsrc.ac.uk; grant BB/J010367/1). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1. Kosoy M, Hayman DT, Chan KS (2012) Bartonella bacteria in nature: where does population variability end and a species start? Infect Genet Evol 12(5): 894–904. 10.1016/j.meegid.2012.03.005 [DOI] [PubMed] [Google Scholar]
- 2. Billeter SA, Levy MG, Chomel BB, Breitschwerdt EB (2008) Vector transmission of Bartonella species with emphasis on the potential for tick transmission. Med Vet Entomol 22(1): 1–15. 10.1111/j.1365-2915.2008.00713.x [DOI] [PubMed] [Google Scholar]
- 3. Billeter SA, Hayman DT, Peel AJ, Baker K, Wood JL, et al. (2012) Bartonella species in bat flies (Diptera: Nycteribiidae) from western Africa. Parasitology 139(3): 324–329. 10.1017/S0031182011002113 [DOI] [PubMed] [Google Scholar]
- 4. Morse SF, Olival KJ, Kosoy M, Billeter S, Patterson BD, et al. (2012) Global distribution and genetic diversity of Bartonella in bat flies (Hippoboscoidea, Streblidae, Nycteribiidae). Infect Genet Evol 12(8): 1717–1723. 10.1016/j.meegid.2012.06.009 [DOI] [PubMed] [Google Scholar]
- 5. Hayman DT, McDonald KD, Kosoy MY (2013) Evolutionary history of rat-borne Bartonella: the importance of commensal rats in the dissemination of bacterial infections globally. Ecol Evol 3(10): 3195–3203. 10.1002/ece3.702 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Breitschwerdt EB, Kordick DL (2000) Bartonella infection in animals: Carriership, reservoir potential, pathogenicity, and zoonotic potential for human infection. Clin Microbiol Rev 13(3): 428–438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Ellis BA, Regnery RL, Beati L, Bacellar F, Rood M, et al. (1999) Rats of the genus Rattus are reservoir hosts for pathogenic Bartonella species: An Old World origin for a New World disease? J Infect Dis 180(1): 220–224. [DOI] [PubMed] [Google Scholar]
- 8. Anderson BE, Neuman MA (1997) Bartonella spp. as emerging human pathogens. Clin Microbiol Rev 10(2): 203–219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Breitschwerdt EB, Maggi RG, Duncan AW, Nicholson WL, Hegarty BC, et al. (2007) Bartonella species in blood of immunocompetent persons with animal and arthropod contact. Emerg Infect Dis 13(6): 938–941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Bhengsri S, Baggett HC, Peruski LF Jr., Morway C, Bai Y, et al. (2010) Bartonella spp. infections, Thailand. Emerg Infect Dis 16(4): 743–745. 10.3201/eid1604.090699 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Kosoy M, Bai Y, Sheff K, Morway C, Baggett H, et al. (2010) Identification of Bartonella infections in febrile human patients from Thailand and their potential animal reservoirs. Am J Trop Med Hyg 82(6): 1140–1145. 10.4269/ajtmh.2010.09-0778 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Rattanavong S, Fournier PE, Chu V, Frichitthavong K, Kesone P, et al. (2014) Bartonella henselae Endocarditis in Laos—'The Unsought Will Go Undetected'. PLoS Negl Trop Dis 8(12): e3385 10.1371/journal.pntd.0003385 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Crump JA, Morrissey AB, Nicholson WL, Massung RF, Stoddard RA, et al. (2013) Etiology of Severe Non-malaria Febrile Illness in Northern Tanzania: A Prospective Cohort Study. PLoS Negl Trop Dis 7(7): e2324 10.1371/journal.pntd.0002324 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Laudisoit A, Iverson J, Neerinckx S, Shako JC, Nsabimana JM, et al. (2011) Human seroreactivity against Bartonella species in the Democratic Republic of Congo. Asian Pac J Trop Med 4(4): 320–322. 10.1016/S1995-7645(11)60094-1 [DOI] [PubMed] [Google Scholar]
- 15. Frean J, Arndt S, Spencer D (2002) High rate of Bartonella henselae infection in HIV-positive outpatients in Johannesburg, South Africa. Trans R Soc Trop Med Hyg 96(5): 549–550. [DOI] [PubMed] [Google Scholar]
- 16. Brettschneider H, Bennett NC, Chimimba CT, Bastos AD (2012) Bartonellae of the Namaqua rock mouse, Micaelamys namaquensis (Rodentia: Muridae) from South Africa. Vet Microbiol 157(1–2): 132–136. [DOI] [PubMed] [Google Scholar]
- 17. Bitam I, Rolain JM, Kernif T, Baziz B, Parola P, et al. (2009) Bartonella species detected in rodents and hedgehogs from Algeria. Clin Microbiol Infect 15(Suppl 2): 102–103. 10.1111/j.1469-0691.2008.02180.x [DOI] [PubMed] [Google Scholar]
- 18. Gundi VA, Kosoy MY, Makundi RH, Laudisoit A (2012) Identification of diverse Bartonella genotypes among small mammals from Democratic Republic of Congo and Tanzania. Am J Trop Med Hyg 87(2): 319–326. 10.4269/ajtmh.2012.11-0555 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Meheretu Y, Leirs H, Welegerima K, Breno M, Tomas Z, et al. (2013) Bartonella prevalence and genetic diversity in small mammals from Ethiopia. Vector Borne Zoonotic Diseases 13(3): 164–175. 10.1089/vbz.2012.1004 [DOI] [PubMed] [Google Scholar]
- 20. Billeter SA, Borchert JN, Atiku LA, Mpanga JT, Gage KL, et al. (2014) Bartonella species in invasive rats and indigenous rodents from Uganda. Vector Borne Zoonotic Diseases 14(3): 182–188. 10.1089/vbz.2013.1375 [DOI] [PubMed] [Google Scholar]
- 21. Young HS, Dirzo R, Helgen KM, McCauley DJ, Billeter SA, et al. (2014) Declines in large wildlife increase landscape-level prevalence of rodent-borne disease in Africa. Proc Natl Acad Sci U S A 111(19): 7036–7041. 10.1073/pnas.1404958111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Boudebouch N, Sarih M, Beaucournu JC, Amarouch H, Hassar M, et al. (2011) Bartonella clarridgeiae, B. henselae and Rickettsia felis in fleas from Morocco. Ann Trop Med Parasitol 105(7): 493–498. 10.1179/1364859411Y.0000000038 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Sackal C, Laudisoit A, Kosoy M, Massung R, Eremeeva ME, et al. (2008) Bartonella spp. and Rickettsia felis in fleas, Democratic Republic of Congo. Emerg Infect Dis 14: 1972–1974. 10.3201/eid1412.080610 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Loftis AD, Reeves WK, Szumlas DE, Abbassy MM, Helmy IM, et al. (2006) Surveillance of Egyptian fleas for agents of public health significance: Anaplasma, Bartonella, Coxiella, Ehrlichia, Rickettsia, and Yersinia pestis . Am J Trop Med Hyg 75: 41–48. [PubMed] [Google Scholar]
- 25. Feikin DR, Olack B, Bigogo GM, Audi A, Cosmas L, et al. (2011) The burden of common infectious disease syndromes at the clinic and household level from population-based surveillance in rural and urban Kenya. PLoS One 6: e16085 10.1371/journal.pone.0016085 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Knobel DL, Maina AN, Cutler SJ, Ogola E, Feikin DR, et al. (2013) Coxiella burnetii in humans, domestic ruminants, and ticks in rural western Kenya. Am J Trop Med Hyg 88(3): 513–518. 10.4269/ajtmh.12-0169 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Halliday JE, Knobel DL, Allan KJ, Bronsvoort BMdC, Handel I, et al. (2013) Urban leptospirosis in Africa: a cross-sectional survey of Leptospira infection in rodents in the Kibera urban settlement, Nairobi, Kenya. Am J Trop Med Hyg 89(6): 1095–1102. 10.4269/ajtmh.13-0415 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Kosoy MY, Regnery RL, Tzianabos T, Marston EL, Jones DC, et al. (1997) Distribution, diversity, and host specificity of Bartonella in rodents from the Southeastern United States. Am J Trop Med Hyg 57(5): 578–588. [DOI] [PubMed] [Google Scholar]
- 29. Norman AF, Regnery R, Jameson P, Greene C, Krause DC (1995) Differentiation of Bartonella-like isolates at the species level by PCR-restriction fragment length polymorphism in the citrate synthase gene. J Clin Microbiol 33(7): 1797–1803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. La Scola B, Zeaiter Z, Khamis A, Raoult D (2003) Gene-sequence-based criteria for species definition in bacteriology: the Bartonella paradigm. Trends Microbiol 11(7): 318–321. [DOI] [PubMed] [Google Scholar]
- 31. R Core Team (2014) R: A Language and Environment for Statistical Computing. Vienna, Austria. [Google Scholar]
- 32. Pretorius AM, Beati L, Birtles RJ (2004) Diversity of bartonellae associated with small mammals inhabiting Free State province, South Africa. Int J Syst Evol Microbiol 54: 1959–1967. [DOI] [PubMed] [Google Scholar]
- 33. Torchin ME, Lafferty KD, Dobson AP, McKenzie VJ, Kuris AM (2003) Introduced species and their missing parasites. Nature 421(6923): 628–630. [DOI] [PubMed] [Google Scholar]
- 34. Keesing F, Holt RD, Ostfeld RS (2006) Effects of species diversity on disease risk. Ecol Lett 9: 485–498. [DOI] [PubMed] [Google Scholar]
- 35. Telfer S, Begon M, Bennett M, Bown KJ, Burthe S, et al. (2007) Contrasting dynamics of Bartonella spp. in cyclic field vole populations: the impact of vector and host dynamics. Parasitology 134(Pt 3): 413–425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Holt J, Davis S, Leirs H (2006) A model of Leptospirosis infection in an African rodent to determine risk to humans: Seasonal fluctuations and the impact of rodent control. Acta Trop 99(2–3): 218–225. [DOI] [PubMed] [Google Scholar]
- 37. Kamani J, Morick D, Mumcuoglu KY, Harrus S (2013) Prevalence and diversity of Bartonella species in commensal rodents and ectoparasites from Nigeria, West Africa. PLoS Negl Trop Dis 7(5): e2246 10.1371/journal.pntd.0002246 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Katz MA, Lebo E, Emukule G, Njuguna HN, Aura B, et al. (2012) Epidemiology, seasonality, and burden of influenza and influenza-like illness in urban and rural Kenya, 2007–2010. J Infect Dis 206(Suppl 1): S53–60. 10.1093/infdis/jis530 [DOI] [PubMed] [Google Scholar]
- 39. Breiman RF, Cosmas L, Njuguna H, Audi A, Olack B, et al. (2012) Population-based incidence of typhoid fever in an urban informal settlement and a rural area in kenya: implications for typhoid vaccine use in Africa. PLoS One 7(1): e29119 10.1371/journal.pone.0029119 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Feikin DR, Njenga MK, Bigogo G, Aura B, Aol G, et al. (2012) Etiology and incidence of viral and bacterial acute respiratory illness among older children and adults in rural western Kenya, 2007–2010. PLoS One 7(8): e43656 10.1371/journal.pone.0043656 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Tabu C, Breiman RF, Ochieng B, Aura B, Cosmas L, et al. (2012) Differing burden and epidemiology of non-Typhi Salmonella bacteremia in rural and urban Kenya, 2006–2009. PloS one 7(2): e31237 10.1371/journal.pone.0031237 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(XLS)
(XLSX)
Data Availability Statement
All relevant data are within the paper and its Supporting Information files. The unique sequences generated through this study have been submitted to GenBank (accession numbers KM233484 - KM233492).