Abstract
Premature atherosclerotic cardiovascular disease (ASCVD) is a common and devastating complication of systemic lupus erythematosus (SLE). It is likely that immunologic derangements contribute to premature ASCVD in these patients, possibly by disrupting homeostatic mechanisms that orchestrate cholesterol balance in monocytes/macrophages in the artery wall. CD36, a macrophage scavenger receptor responsible for recognition and internalization of oxidized lipids, is a major participant in atherosclerotic foam cell formation. We hypothesized that lupus plasma would affect CD36 expression in a pro-atherogenic manner in THP-1 human monocytes and differentiated macrophages. SLE patient plasma markedly stimulated expression of CD36 message in a dose-dependent fashion in THP-1 human monocytes. A 50% volume/volume concentration of plasma derived from SLE patients increased CD36 mRNA by 71 6 8% (n = 3, P < 0.001) above 50% normal human plasma. 50% SLE patient plasma increased CD36 mRNA expression to 290 6 12% of no-plasma control (n = 3, P < 0.001), compared with only 118 6 3.7% of control in the presence of 50% normal human plasma (n = 3, not significant). 50% lupus plasma also upregulated CD36 protein expression by 482.3 6 76.2% (n = 4, P < 0.05), whereas the presence of 50% normal human plasma increased the CD36 protein level by only 239.8 6 61.9% (n = 4, P < 0.05). To our knowledge, this is the first demonstration that CD36 expression is enhanced by plasma from patients with an autoimmune disorder. Premature atherosclerosis is common in SLE patients. Upregulation of CD36 may contribute to this pathological process by increasing vulnerability to cholesterol overload. Demonstration of disrupted cholesterol homeostasis in this select group of patients provides further evidence of the involvement of the immune system in atherogenesis and may inform us of the role of CD36 in the general atherogenic process. CD36 may provide a novel therapeutic target in the treatment of ASCVD in SLE patients.
Keywords: lupus erythematosus, systemic, atherosclerosis, cholesterol, macrophage scavenger receptor, CD36
Introduction
The risk of cardiovascular disease (CVD) due to atherosclerosis is very high in systemic lupus erythematosus (SLE) and CVD is a major cause of morbidity and mortality in SLE patients (1). The pathogenesis of accelerated atherosclerosis in SLE is multifactorial and complex (2, 3). Even after controlling for traditional cardiovascular risk factors, SLE is an independent risk factor for premature atherosclerosis and death in young, premenopausal women with SLE, contributing to poor long-tem prognosis (4). Inflammatory mechanisms as well as altered cholesterol metabolism are critical in the pathogenesis of atherosclerosis and immune system dysfunction has been implicated in SLE-associated CVD (5). A pro-atherogenic lipid profile has also been observed in SLE (6, 7).
CD36, a class B scavenger receptor that is expressed on the cell surface of monocyte/macrophages, is involved in the recognition and uptake of pro-atherogenic oxidized low-density lipoprotein (LDL) (8). Endocytosis of oxidized lipids results in the deposition of excessive cholesterol and cholesteryl esters within macrophages, leading to formation of foam cells, an early and pivotal event in the atherosclerotic process. Blood-derived monocytes/macrophages within the intima of the arterial wall are the main source of foam cells. We and others have found that plasma of lupus patients exhibits atherogenic properties (9–11). In order to further explore the atherogenic nature of plasma derived from persons with SLE, we examined the effect of exposure to SLE patient plasma on CD36 expression in cultured THP-1 human monocytes.
Materials and Methods
Cell Culture
THP-1 cells (American Type Culture Collection, Rockville, MD) were cultured at 37°C in a 5% CO2 atmosphere to a density of 106 cells per ml in RPMI 1640 supplemented with 10% Fetal Bovine Serum (FBS), 50 units/ml penicillin, and 50 units/ml streptomycin (GIBCO BRL, Grand Island, NY).
Human Blood Samples
Subject Inclusion and Exclusion Criteria
Human subject studies were performed under a protocol approved by the Institutional Review Boards of both Winthrop University Hospital and NYU School of Medicine. Written informed consent was obtained from all subjects.
Levels of CD36 protein were determined in cultured THP-1 human monocytoid cells after exposure to plasma from SLE and control subjects:
Normal controls: Normal healthy volunteers, age 18–60, not on corticosteroids or any other immune-modifying medications.
SLE patients: Patients, age 18–60, fulfilled the 1982 revised criteria of the American College of Rheumatology (formerly the American Rheumatism Association) for classification of SLE (12). Patients with previous documentation of a diagnosis of a connective tissue disorder other than SLE were excluded.
Patients and controls were age- and gender-matched as closely as possible.
Experimental Conditions
When THP-1 cells had reached a density of 105–106 cells/ml, the culture media was aspirated, and the cells were rinsed twice with Dulbecco’s Phosphate Buffered Saline (DPBS) without calcium and magnesium. The cells were resuspended in fresh RPMI media without FBS and then incubated at 37°C in a 5% CO2 atmosphere for 3 hours before mRNA isolation and 6 or 12 hours before protein isolation, in six well plates, under the following conditions:
RPMI medium containing 0% human plasma.
-
RPMI medium containing 10%, 25%, and 50% human plasma from normal controls.
RPMI medium containing 10%, 25%, and 50% human plasma from SLE patients.
RNA Isolation and Message Analysis by RT-PCR
RNA was isolated using 1 ml Trizol reagent per 106 cells and dissolved in nuclease-free water. The quantity of total RNA from each condition was measured by absorption at 260 and 280 wavelengths using quartz cuvettes by ultraviolet spectrophotometry (Hitachi U2010 spectrophotometer).
RT-PCR was carried out in an Eppendorf Mastercycler Personal PCR thermocycler with reagents purchased from Applied Biosystems (Oakland, CA). Primers used in amplification reactions were generated by Sigma-Genosys (The Woodlands, TX). For each RT reaction, 1 µg of total RNA was reverse transcribed using 50 units of Murine Leukemia Virus reverse transcriptase in the presence of 20 units of RNase inhibitor in a final volume of 50 µl. The reaction mixture contained 5 mM MgCl2, 0.4 mM of each dNTP, and 2.5 µM oligo dT primers. The reaction mixtures were incubated at 42°C for 45 minutes. This was followed by heating at 95°C for 5 minutes and cooling to 5°C for five minutes.
Five µl of cDNA was taken from each RT mixture for PCR amplification using CD36 specific primers as well as glyceraldehyde-3-phosphate dehydrogenase (GAPDH) control primers. The CD36 primer pair yields a product of size 398 base pairs (13).
Nontemplate controls were included for each primer pair to check for significant levels of any contaminants. The PCR reaction was carried out using 1 unit of AmpliTaq DNA polymerase, 2 mM MgCl2, 0.4 mM of each dNTP and 0.15 µM of the upstream and downstream primers. The PCR protocol for both CD36 and GAPDH included: an initial denaturation step at 95°C for 5 minutes; 35 cycles with a denaturation step of 60 seconds at 94°C, an annealing step of 1 minute at 62°C, and an extension step of 1 minute at 72°C; and a final extension step of 7 minutes at 72°C.
In all cases, equal volumes (10 µl/lane) of amplified PCR products were mixed with 1 µl of 6× DNA loading buffer (GIBCO BRL, Carlsbad, CA) and separated by agarose gel electrophoresis on a 1.5% agarose gel. The DNA was electrophoresed at 100 volts for 30 minutes. The 1.5% agarose gel was stained with 0.5 µg/ml ethidium bromide to visualize the DNA.
The GAPDH controls (10 µl/lane) were loaded at two concentrations, 1 and 0.20 µg/µl of starting total RNA amount. The DNA samples electrophoresed in agarose gel were visualized and photographed under ultraviolet light (320 nm) using a Kodak trans-illuminator. The gel images were photo-documented, and net intensities were measured with Kodak Digital Science 1D, version 2.0.3, after imaging with Kodak Digital Science Electrophoresis Documentation and Analysis System 120. All experimental results were normalized to the mean density of GAPDH.
Protein Isolation and Western Blot
Immediately after the incubation period, cells were collected directly from the culture dishes. Total cell lysate was isolated for Western immunoblotting using RIPA lysis buffer (98% PBS, 1% Igepal CA-630, 0.5% sodium deoxycholate, 0.1% sodium dodecyl sulfate). Protein concentrations were measured using the BCA Protein Assay Reagent (Pierce, Rockford, IL).
Protein samples (20 µg/lane) were boiled for 5 minutes, loaded onto a 10% polyacrylamide gel, electrophoresed for 1.5 hours at 100 volts, and then transferred to nitrocellulose membranes in a semi-dry transblot apparatus for 1 hour at 100 volts. Membranes were stained with Ponceau red to verify uniformity of protein loading in each lane. The nitrocellulose membranes were blocked for 4 hours at 4°C in blocking solution (3% nonfat dry milk dissolved in 1 × Tween 20 TBS [TTBS]) and then immersed in a 1:200 dilution of primary anti-CD36 antibody or 1:2000 dilution of anti-beta-actin antibody in blocking solution overnight at 4°C. The primary antibody was a mouse monoclonal IgM anti-peptide antibody raised against human CD36 protein (Product SC-7309, Santa Cruz Biotechnology, Santa Cruz, CA). The following day, the membrane was washed 5 times in TTBS for 5 minutes per wash and then incubated at room temperature in a 1:1000 dilution of an alkaline phosphatase-conjugated goat anti-mouse IgM secondary antibody in blocking solution. The 5 washes in TTBS were repeated, and then the immunoreactive protein was visualized using the BCIP/NBT alkaline phosphatase substrate system (GIBCO BRL) according to the manufacturer’s directions.
Band intensities for Western blot protein samples were quantified using Kodak Digital Science 1D, version 2.0.3, after imaging with Kodak Digital Science Electrophoresis Documentation and Analysis System 120.
Statistical Analysis of Experimental Data
Statistical analysis was performed using SigmaStat v2.03 (SPSS Inc., Chicago, IL). Pairwise comparison was made between each treatment condition and control using Student’s t test. Data are presented as the mean ± SEM. Each experimental condition from each individual patient was performed in triplicate so that a given n, such as n = 4, indicates that three repetitions of the same experimental conditions were performed using plasma from 4 individual patients for a total of 12 wells.
Results
SLE Patient Plasma, but Not Normal Human Plasma, Increases the Level of mRNA for CD36
Table 1 highlights the major demographic features and characteristics of patients enrolled in the study. Control subject information is summarized in Table 2.
Table 1.
Characteristics of Lupus Plasma Study Population
| SLE patients (n = 13) Mean (C.I.) |
|
|---|---|
| Age (yr) | 37.7 (5.5) |
| Ethnicity (%) | |
| African American | 23 |
| Asian | 8 |
| Caucasian | 46 |
| Hispanic | 23 |
| Gender: % women | 92.3 |
| Disease duration (years) | 9.5 (7.2) |
| Serum cholesterol (mg/dl) | 152.7 (17.5) |
| LDL cholesterol (mg/dl) | 77.8 (17.3) |
| HDL cholesterol (mg/dl) | 58.0 (11.2) |
| Triglyceride (mg/dl) | 90.9 (28.0) |
| CRP (mg/L) | 18.9 (51.0)a |
| ESR (mm/hr) | 21.8 (14.3) |
| % patients on prednisone | 75 |
| % patients on statins | 0 |
Based on 5 out of 13 patients.
Table 2.
Characteristics of Control Study Population
| Controls (n = 8) Mean (C.I.) |
|
|---|---|
| Age (yr) | 36.1 (5.5)a |
| Ethnicity (%) | |
| African American | 12.5 |
| Asian | 12.5 |
| Caucasian | 75 |
| Hispanic | 0 |
| Gender: % women | 87.5a |
No significant difference from lupus study patients.
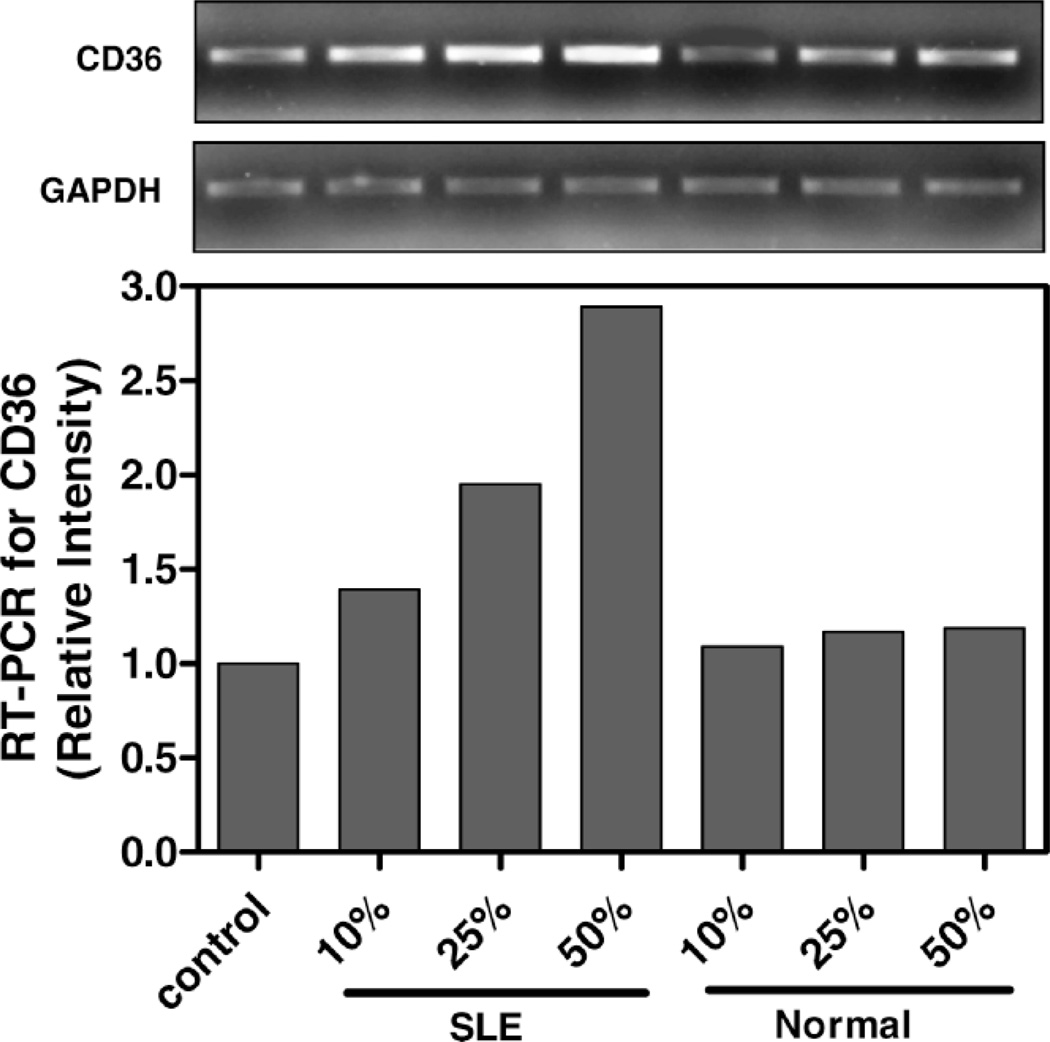
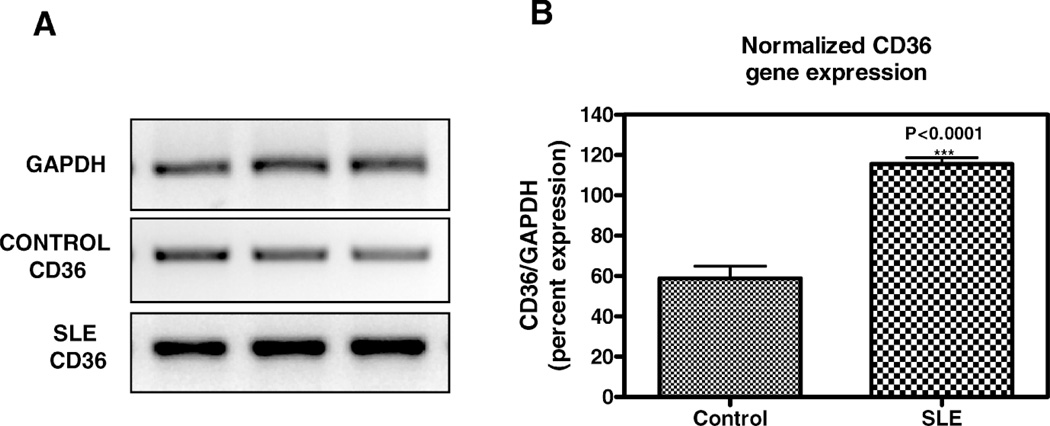
SLE patient plasma markedly stimulated expression of CD36 message in a dose-dependent fashion in THP-1 human monocytes (Fig. 1). After a 3-hour incubation, a 50% volume/volume concentration of plasma derived from SLE patients increased CD36 mRNA by 71 ± 8% (n = 3, P < 0.001) above 50% normal human plasma. 50% SLE patient plasma increased CD36 mRNA expression to 290 ± 12% of no plasma control (n = 3, P < 0.001), compared with only 118 ± 3.7% of control in the presence of 50% normal human plasma (n = 3, not significant, Holm-Sidak). The increase in CD36 message level in SLE plasma-treated THP-1 human monocytes compared to normal human plasma-treated THP-1 human monocytes persisted up to 24 hours of incubation (data not shown). Figure 2 provides a further demonstration of augmented CD36 mRNA expression in the presence of a single concentration (50% volume/volume) of SLE plasma versus control normal human plasma. Figure 2A shows RT-PCR results for a representative patient and control (age- and sex-matched) performed in triplicate. Figure 2B graphs the normalized difference in CD36 message level in 3 patients versus 3 controls.
Figure 1.
CD36 message level in THP-1 monocytes increase with SLE plasma exposure. THP-1 monocytes were incubated in increasing concentrations of SLE patient or normal control plasma for 3 hours as indicated. Total RNA isolated from cells exposed to each condition was reverse transcribed and amplified by PCR with GAPDH message as an internal standard. Representative experiment from a total of three SLE patients studied.
Figure 2.
Effect of SLE plasma versus normal human plasma on CD36 mRNA in THP-1 monocytes. THP-1 monocytes were incubated in 50% volume/volume SLE patient or normal control plasma for 3 hours as indicated. Following incubation, total RNA isolated from cells exposed to each condition was reverse transcribed and amplified by PCR with GAPDH message as an internal standard. A) Representative experiment from a total of three SLE patients and three controls studied. Photograph of ethidium bromide-stained PCR-amplified bands corresponding to message for CD36 and GAPDH as indicated. B) Gene expression levels were graphed as relative mRNA expression. The data represent the mean and SEM of three independent experiments.
SLE Plasma Increases CD36 Protein Expression
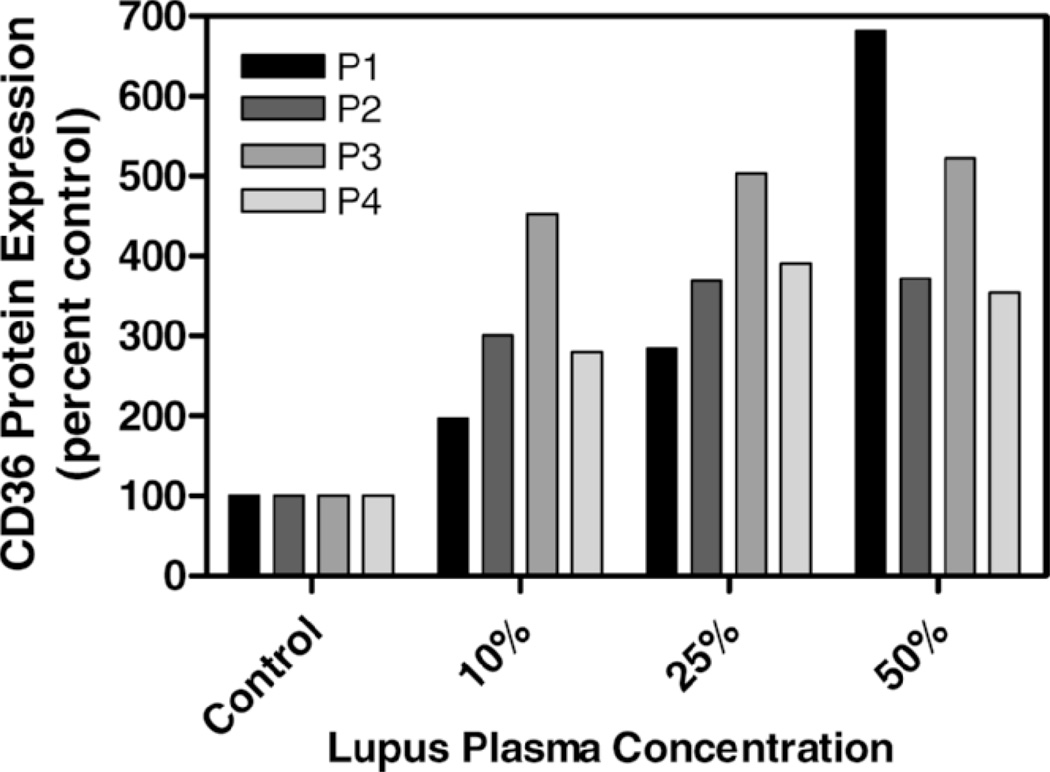
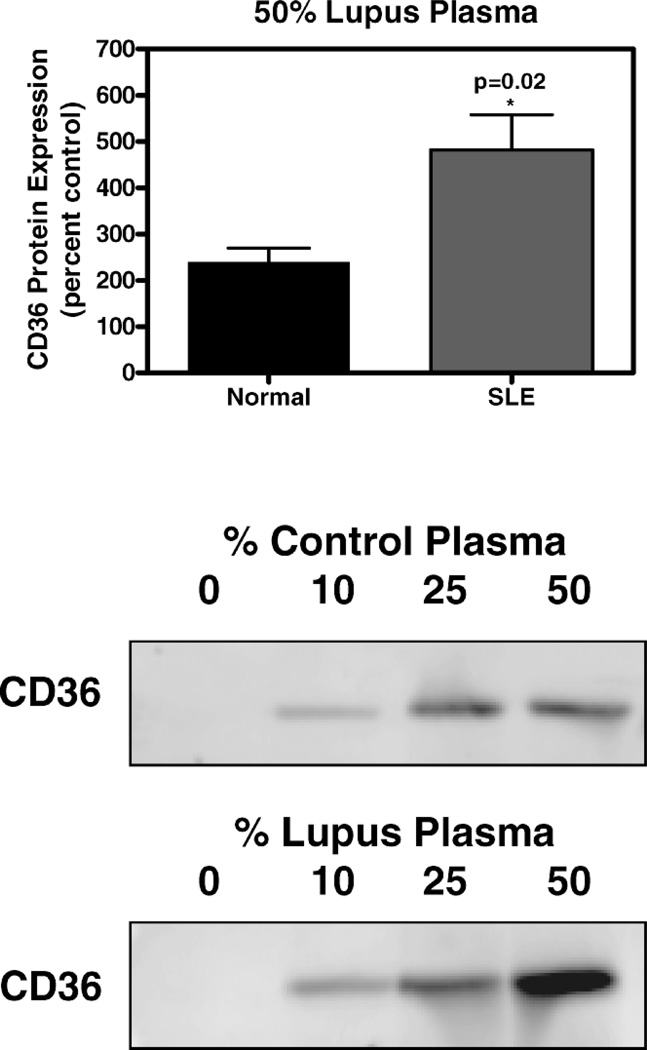
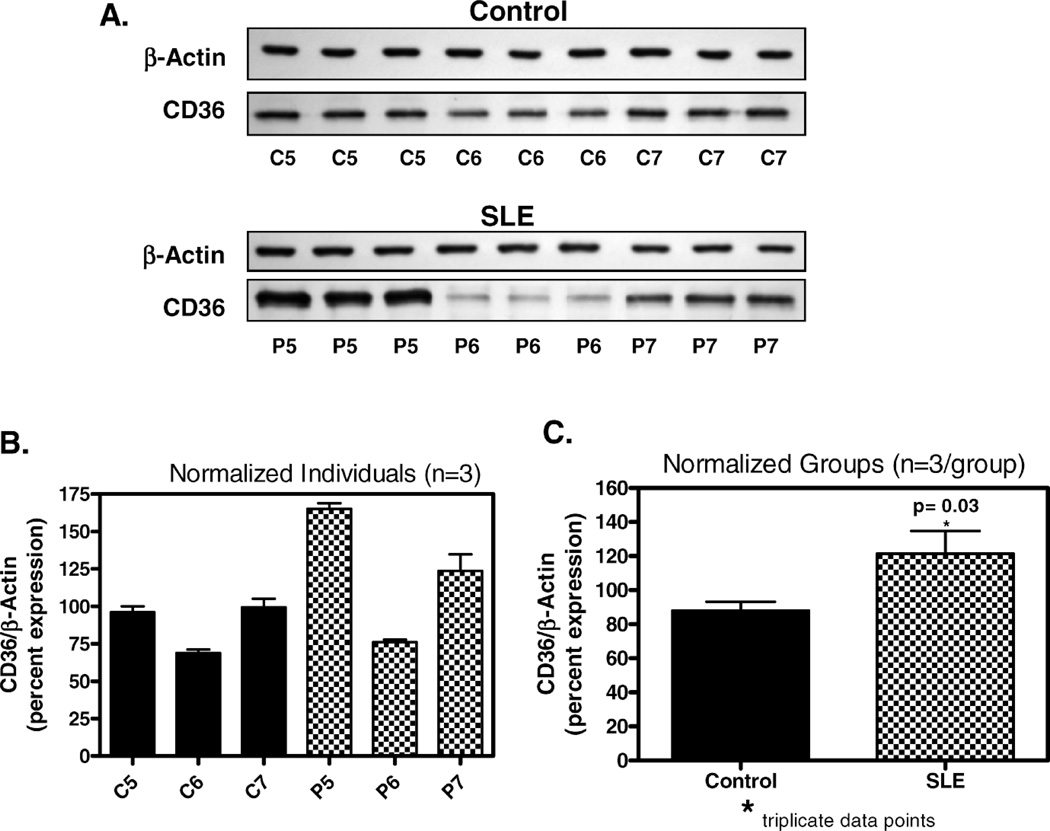
CD36 protein expression in THP-1 cells exposed to lupus plasma was markedly increased versus control plasma after 6 hours of incubation and the difference between lupus and control remained significant at 12 hours. In an evaluation of plasma samples from four individual SLE patients, the presence of 50% lupus plasma upregulated CD36 protein expression 4.8-fold (n = 4, P < 0.05), whereas the presence of 50% normal human plasma increased the CD36 protein level by only 2.4-fold (n = 4, P < 0.05) after 6 hours (Fig. 3). The concentration-dependent increase in THP-1 CD36 protein with SLE plasma exposure is shown in Figure 4. Figure 5 shows experimental data at 12 hours from 3 SLE patients and 3 controls with corresponding beta-actin levels. Figure 5A demonstrates enhanced CD36 expression in THP-1 monocytes exposed to 50% SLE plasma as compared to 50% control plasma in two of the three SLE patients. Figure 5B is a graphic representation of this data after normalization to beta-actin and Figure 5C pools the data from the 3 SLE patients and the 3 controls used in parts 5A and 5B.
Figure 3.
Effect of plasma from four individual SLE patients on CD36 protein in THP-1 monocytes. Cultured THP-1 cells were untreated (control, 0% plasma) or exposed for 6 hours to increasing concentrations of plasma from 4 distinct SLE patients. Total cellular protein was isolated and run on an SDS-polyacrylamide gel and immunoblotted with mouse monoclonal IgM anti-peptide antibody raised against human CD36 protein.
Figure 4.
CD36 protein expression in THP-1 monocytes exposed to SLE plasma and healthy control plasma. Cultured THP-1 monocytes were exposed for 6 hours to SLE or control plasma as indicated. Total cellular protein was isolated and run on an SDS-polyacrylamide gel and immunoblotted with mouse monoclonal IgM anti-peptide antibody raised against human CD36 protein. Upper portion of figure is a graphic representation of the difference in CD36 protein level in cells incubated for 12 hours in SLE patient plasma versus control plasma (n = 4 for each). Lower portion of figure shows marked increase in CD36 band intensity with increasing percentage of plasma from a single representative SLE patient versus a single control normal human plasma patient.
Figure 5.
Effect of SLE plasma on CD36 protein in THP-1 monocytes. Cultured THP-1 monocytes were exposed for 12 hours to SLE or control plasma as indicated. Total cellular protein was isolated and run on an SDS-polyacrylamide gel and immunoblotted with mouse monoclonal IgM anti-peptide antibody raised against human CD36 protein. A) CD36 expression in 3 control subjects with corresponding beta-actins (C5, C6, C7) compared to 3 SLE patients (P5, P6, P7) shows elevated CD36 expression in 2 of the 3 SLE patients. B) Graphic representation of immunoblot results from section A with normalization to beta-actin. C) Combined data from Figure B representing aggregate of controls versus patients for this experiment, normalized to beta-actin with statistically significant difference in CD36 expression.
Discussion
We report here the first demonstration that CD36 expression in human monocytes is stimulated by exposure to plasma from SLE patients. The THP-1 human monocytic leukemia cell line was chosen because it is highly differentiated, shares many properties with normal human monocytes, including expression of scavenger receptors and cholesterol transport proteins, and is a well-accepted model for atherosclerosis used in our laboratory and numerous others (9, 14, 15). The cells were used unstimulated in monocyte form. The plasma samples were added directly to the media without adjusting for differences in protein content. Total protein in plasma may vary greatly depending on factors such as an increase in immunoglobulins or a decrease in albumin in disease states such as that under study (16–19). Thus, normalization of test plasma to protein concentration may bias the ultimate determination. We therefore chose to use undiluted plasma in order to keep intact the proportion of bioactive factor(s) that could affect the expression of CD36 and other proteins related to the transport of cholesterol.
CD36 is a transmembrane glycoprotein of the class B scavenger receptor family that has pro-atherogenic properties (20, 21). Human and animal studies as well as cell culture experiments have provided strong evidence that CD36 influences propensity to foam cell formation and development of atherosclerosis (22–26). In murine models of atherosclerosis, CD36 has clearly been shown to play an important functional role in oxidized LDL processing and in disease progression (27). CD36 may also be involved in uptake of necrotic cells by macrophages (28). Pathological processes in the plasma of patients with SLE reveal mechanisms that impede normal cholesterol flow via upregulation of CD36. The current study provides a biochemical rationale for the high incidence of premature coronary artery disease persistently observed in SLE patients. Increased CD36 expression may be implicated in the etiology of accelerated atherosclerosis in some autoimmune disease states. Although enhanced CD36 expression may be a contributing factor, atherosclerosis is a multifactorial disorder and transport of lipids is orchestrated by a multitude of molecular factors (29). A number of receptors in addition to CD36 are known to be involved in mediating cholesterol and lipid uptake by monocytes/macrophages, including the class A type I/II scavenger receptor, the lectin-like oxidized LDL receptor-1, and scavenger receptor that binds phosphatidyl serine and oxidized lipoprotein (30). Autoantibodies to oxidized LDL and anti-beta2 glycoprotein I have proatherogenic properties and titers of these antibodies are elevated in SLE as are the levels of oxidized LDL itself (31–33). Antibodies to oxidized LDL can bind to oxidized LDL on endothelium, resulting in the formation of oxidized LDL immune complexes that are able to recruit monocytes via either Fc gamma receptor or CD36 (34).
Of the 13 patients studied, plasma from 12 (92%) of the patients increased CD36 expression. This study involves only a small sample, so that possible effects of comorbidities such as diabetes, hypertension and renal disease could not be accounted for with statistical rigor. The influence of therapeutic regimens on the system is also unknown. Now that these initial results have shown a significant impact of lupus plasma on CD36 in this sample, expanded studies will be designed to permit covariate analysis of the relationship within more narrow subgroups. The results reported here do not reveal which specific immune mediators participate in promoting CD36 expression nor have we determined which plasma fraction or fractions are responsible for the effect. Further studies are needed to define the plasma components and pathways involved and to determine whether potency of individual SLE patient plasma in affecting CD36 expression may have prognostic value in evaluating cardiovascular risk. The upregulation of CD36 by lupus plasma provides an insight into a contributing factor in the pathogenesis of atheroma in SLE. Strategies to prevent atherosclerosis aimed at blocking CD36 activity, specifically in macrophages, may provide a novel therapeutic approach to atherosclerotic cardiovascular disease in SLE. CD36 may be an attractive target for pharmacologic intervention.
Acknowledgments
This work was supported by an Innovative Research Grant from the Arthritis Foundation, National Center and a grant from the National Institutes of Health/National Heart, Lung and Blood Institute HL073814 (Reiss). Additional support was provided by the Arthritis Foundation, New York Chapter (Chan), the National Institutes of Health (AR41911, AA13336 and GM56268), King Pharmaceuticals, and the General Clinical Research Center (M01RR00096) (Cronstein).
References
- 1.Asanuma Y, Oeser A, Shintani AK, Turner E, Olsen N, Fazio S, Linton MF, Raggi P, Stein CM. Premature coronary-artery atherosclerosis in systemic lupus erythematosus. N Engl J Med. 2003;349:2407–2415. doi: 10.1056/NEJMoa035611. [DOI] [PubMed] [Google Scholar]
- 2.Hahn B, McMahon M. Atherosclerosis and systemic lupus erythematosus: the role of altered lipids and of autoantibodies. Lupus. 2008;17:368–370. doi: 10.1177/0961203308089989. [DOI] [PubMed] [Google Scholar]
- 3.Frostegard J. Systemic lupus erythematosus and cardiovascular disease. Lupus. 2008;17:364–367. doi: 10.1177/0961203308089988. [DOI] [PubMed] [Google Scholar]
- 4.Esdaile JM, Panaritis C, Abrahamowicz M. Traditional Framingham risk factors fail to fully account for accelerated atherosclerosis in systemic lupus erythematosus. Arthritis Rheum. 2001;44:2331–2337. doi: 10.1002/1529-0131(200110)44:10<2331::aid-art395>3.0.co;2-i. [DOI] [PubMed] [Google Scholar]
- 5.McMahon M, Hahn B. Atherosclerosis and systemic lupus erythematosus: mechanistic basis of the association. Curr Opin Immunol. 2007;19:633–639. doi: 10.1016/j.coi.2007.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.de Carvalho JF, Bonfa E, Borba EF. Systemic lupus erythematosus and “lupus dyslipoproteinemia.”. Autoimmun Rev. 2008;7:246–250. doi: 10.1016/j.autrev.2007.11.016. [DOI] [PubMed] [Google Scholar]
- 7.Delgado-Alves J, Ames PRJ. Atherosclerosis, oxidative stress and autoantibodies in systemic lupus erythematosus and primary antiphospholipid syndrome. Immunobiology. 2003;207:23–28. doi: 10.1078/0171-2985-00215. [DOI] [PubMed] [Google Scholar]
- 8.Nicholson AC, Pearce SFA, Silverstein RL. Oxidized LDL binds to CD36 on human monocyte-derived macrophages and transfected cell lines. Evidence implicating the lipid moiety of the lipoprotein as the binding site. Arterioscler Thromb Vasc Biol. 1995;15:269–275. doi: 10.1161/01.atv.15.2.269. [DOI] [PubMed] [Google Scholar]
- 9.Reiss AB, Merrill JT, Rahman MM, Hasneen K, Chan ESL, Belmont HM, Khoa ND, Cronstein BN. Inhibition of expression of the antiatherogenic cholesterol 27-hydroxylase in human monocytoid cells exposed to SLE patient serum is abrogated by blocking the interferon-gamma receptor. Arthritis Rheum. 2003;48:S196. [Google Scholar]
- 10.Matsuura E, Kobayashi K, Hurley BL, Lopez LR. Atherogenic oxidized low-density lipoprotein/beta2-glycoprotein I (oxLDL/beta2GPI) complexes in patients with systemic lupus erythematosus and antiphospholipid syndrome. Lupus. 2006;15:478–483. doi: 10.1191/0961203306lu2337oa. [DOI] [PubMed] [Google Scholar]
- 11.Gerasimova EV, Alekberova ZS, Popkova TV, Sobenin IA. Atherogenic cholesterol-containing circulating immune complexes—one of the components of the serum in patients with systemic lupus erythematosus. Klin Med (Mosk) 2003;81:39–41. [PubMed] [Google Scholar]
- 12.Tan EM, Cohen AS, Fries JF, Masi AT, McShane DJ, Rothfield NF, Schaller JG, Talal N, Winchester RJ. The 1982 revised criteria for the classification of systemic lupus erythematosus. Arthritis Rheum. 1982;25:1271–1277. doi: 10.1002/art.1780251101. [DOI] [PubMed] [Google Scholar]
- 13.Han S, Sidell N. Peroxisome-proliferator-activated-receptor gamma (PPARgamma) independent induction of CD36 in THP-1 monocytes by retinoic acid. Immunology. 2002;106:53–59. doi: 10.1046/j.1365-2567.2002.01404.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Reiss AB, Patel CA, Rahman MM, Chan ES, Hasneen K, Montesinos MC, Trachman JD, Cronstein BN. Interferon-gamma impedes reverse cholesterol transport and promotes foam cell transformation in THP-1 human monocytes/macrophages. Med Sci Monit. 2004;10:BR420–BR425. [PubMed] [Google Scholar]
- 15.Rahman EU, Ruan XZ, Chana RS, Brunskill NJ, Powis SH, Varghese Z, Moorhead JF, Wheeler DC. Mesangial matrix-activated monocytes express functional scavenger receptors and accumulate intracellular lipid. Nephrol Dial Transplant. 2008;23:1876–1885. doi: 10.1093/ndt/gfm901. [DOI] [PubMed] [Google Scholar]
- 16.Kotzin BL. Systemic lupus erythematosus. Cell. 1996;85:303–306. doi: 10.1016/s0092-8674(00)81108-3. [DOI] [PubMed] [Google Scholar]
- 17.Reid HL, De Ceulaer K. Abnormal plasma and serum viscosity in systemic lupus erythematosus (SLE): a Jamaican study. Clin Hemorheol Microcirc. 1999;20:175–180. [PubMed] [Google Scholar]
- 18.Cottiero RA, Madaio MP, Levey AS. Glomerular filtration rate and urinary albumin excretion rate in systemic lupus erythematosus. Nephron. 1995;69:140–146. doi: 10.1159/000188429. [DOI] [PubMed] [Google Scholar]
- 19.Kim YG. Serum cholesterol in idiopathic and lupus-related protein-losing enteropathy. Lupus. 2008;17:575–579. doi: 10.1177/0961203307087407. [DOI] [PubMed] [Google Scholar]
- 20.Nicholson AC, Han J, Febbraio M, Silverstein RL, Hajjar DP. Role of CD36, the macrophage class B scavenger receptor, in atherosclerosis. Ann N Y Acad Sci. 2001;947:224–228. doi: 10.1111/j.1749-6632.2001.tb03944.x. [DOI] [PubMed] [Google Scholar]
- 21.Collot-Teixeira S, Martin J, McDermott-Roe C, Poston R, McGregor JL. CD36 and macrophages in atherosclerosis. Cardiovasc Res. 2007;75:468–477. doi: 10.1016/j.cardiores.2007.03.010. [DOI] [PubMed] [Google Scholar]
- 22.Nozaki S, Kashiwagi H, Yamashita S, Nakagawa T, Kostner B, Tomiyama Y, Nakata A, Ishigamia M, Miyagawa J, Kameda-Takemura K, Kurata Y, Matsuzawa Y. Reduced uptake of oxidized low density lipoprotein in monocyte-derived macrophages from CD36-deficient subjects. J Clin Invest. 1995;96:1859–1865. doi: 10.1172/JCI118231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Nakata A, Nakagawa Y, Nishida M, Nozaki S, Miyagawa J, Nakagawa T, Tamura R, Matsumoto K, Kameda-Takemura K, Yamashita S, Matsuzawa Y. CD36, a novel receptor for oxidized low-density lipoproteins, is highly expressed on lipid-laden macrophages in human atherosclerotic aorta. Arterioscler Thromb Vasc Biol. 1999;19:1333–1339. doi: 10.1161/01.atv.19.5.1333. [DOI] [PubMed] [Google Scholar]
- 24.Febbraio M, Podrez EA, Smith JD, Hajjar DP, Hazen SL, Hoff HF, Sharma K, Silverstein RL. Targeted disruption of the class B scavenger receptor CD36 protects against atherosclerotic lesion development in mice. J Clin Invest. 2000;105:1049–1056. doi: 10.1172/JCI9259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Febbraio M, Guy E, Silverstein RL. Stem cell transplantation reveals that absence of macrophage CD36 is protective against atherosclerosis. Arterioscler Thromb Vasc Biol. 2004;24:2333–2338. doi: 10.1161/01.ATV.0000148007.06370.68. [DOI] [PubMed] [Google Scholar]
- 26.Kuchibhotla S, Vanegas D, Kennedy DJ, Guy E, Nimako G, Morton RE, Febbraio M. Absence of CD36 protects against atherosclerosis in ApoE knock-out mice with no additional protection provided by absence of scavenger receptor A I/II. Cardiovasc Res. 2008;78:185–196. doi: 10.1093/cvr/cvm093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kunjathoor VV, Febbraio M, Podrez EA, Moore KJ, Andersson L, Koehn S, Rhee JS, Silverstein R, Hoff HF, Freeman MW. Scavenger receptors class A-I/II and CD36 are the principal receptors responsible for the uptake of modified low density lipoprotein leading to lipid loading in macrophages. J Biol Chem. 2002;277:49982–49988. doi: 10.1074/jbc.M209649200. [DOI] [PubMed] [Google Scholar]
- 28.Bottcher A, Gaipl US, Furnrohr BG, Herrmann M, Girkontaite I, Kalden JR, Voll RE. Involvement of phosphatidylserine, alphavbeta3, CD14, CD36, and complement C1q in the phagocytosis of primary necrotic lymphocytes by macrophages. Arthritis Rheum. 2006;54:927–938. doi: 10.1002/art.21660. [DOI] [PubMed] [Google Scholar]
- 29.Hansson GK, Robertson AK, Soderberg-Naucler C. Inflammation and atherosclerosis. Annu Rev Pathol. 2006;1:297–329. doi: 10.1146/annurev.pathol.1.110304.100100. [DOI] [PubMed] [Google Scholar]
- 30.Hofnagel O, Luechtenborg B, Weissen-Plenz G, Robenek H. Statins and foam cell formation: impact on LDL oxidation and uptake of oxidized lipoproteins via scavenger receptors. Biochim Biophys Acta. 2007;1771:1117–1124. doi: 10.1016/j.bbalip.2007.06.003. [DOI] [PubMed] [Google Scholar]
- 31.Bassi N, Ghirardello A, Iaccarino L, Zampieri S, Rampudda ME, Atzeni F, Sarzi-Puttini P, Shoenfeld Y, Doria A. OxLDL/beta2GPIanti-oxLDL/beta2GPI complex and atherosclerosis in SLE patients. Autoimmun Rev. 2007;7:52–58. doi: 10.1016/j.autrev.2007.06.003. [DOI] [PubMed] [Google Scholar]
- 32.Doria A, Shoenfeld Y, Wu R, Gambari PF, Puato M, Ghirardello A, Gilburd B, Corbanese S, Patnaik M, Zampieri S, Peter JB, Favaretto E, Iaccarino L, Sherer Y, Todesco S, Pauletto P. Risk factors for subclinical atherosclerosis in a prospective cohort of patients with systemic lupus erythematosus. Ann Rheum Dis. 2003;62:1071–1077. doi: 10.1136/ard.62.11.1071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.George J, Afek A, Gilburd B, Harats D, Shoenfeld Y. Autoimmunity in atherosclerosis: lessons from experimental models. Lupus. 2000;9:223–227. doi: 10.1191/096120300678828190. [DOI] [PubMed] [Google Scholar]
- 34.Nagarajan S. Anti-OxLDL IgG blocks OxLDL interaction with CD36, but promotes FcgammaR, CD32A-dependent inflammatory cell adhesion. Immunol Lett. 2007;108:52–61. doi: 10.1016/j.imlet.2006.09.008. [DOI] [PubMed] [Google Scholar]