Abstract
Objective
To evaluate the frequency of adverse events (AEs) across four treatment conditions in the Child/Adolescent Anxiety Multimodal Study (CAMS), and to compare the frequency of AEs between children and adolescents.
Method
Participants ages 7-17 years (M=10.7) meeting the DSM-IV criteria for one or more of the following disorders: separation anxiety disorder, generalized anxiety disorder, or social phobia were randomized (2:2:2:1) to cognitive-behavioral therapy (CBT, n=139), sertraline (SRT, n=133), combination of both (COMB, n=140), or pill placebo (PBO, n=76). AEs were collected via a standardized inquiry method plus a self-report Physical Symptom Checklist (PSC).
Results
There were no differences between the double-blinded conditions (SRT vs. PBO) for total physical and psychiatric AEs or any individual physical or psychiatric AEs. The rates of total physical AEs were greater in the SRT-alone treatment condition when compared to CBT (p<.01) and COMB (p<.01). Moreover, those who received SRT alone reported higher rates of several physical AEs when compared to COMB and CBT. The rate of total psychiatric AEs was higher in children (≤12 years) across all arms (31.7% vs. 23.1%, p<.05). Total PSC scores decreased over time with no significant differences between treatment groups.
Conclusion
The results support the tolerability/safety of selective serotonin reuptake inhibitor (SSRI) treatment for anxiety disorders even after adjusting for the number of reporting opportunities leading to no differences in overall rates of AEs. Few differences occurred on specific items. Additional monitoring of psychiatric AEs is recommended in children (≤12 years).
Keywords: child, adolescent, anxiety, adverse events
Introduction
Anxiety disorders are among the most common childhood psychiatric disorders, with lifetime prevalence rates ranging from 15-20%.1,2 If left untreated, childhood anxiety disorders can lead to poor academic performance and social functioning,3,4 substance use problems,5 and an increased risk of developing additional psychiatric disorders in early adulthood.6 Fortunately, research has provided evidence to support the efficacy and safety of cognitive-behavioral therapy (CBT)7-9 and medication, specifically the selective serotonin reuptake inhibitors (SSRIs)10-13 and more recently duloxetine14 for the treatment of childhood anxiety disorders.
Despite robust evidence for the efficacy of SSRIs in the treatment of pediatric anxiety disorders, these compounds have also been associated with treatment-emergent adverse events (AEs). Previous studies have found SSRIs, as compared to pill placebos, to be associated with increased physical symptoms such as headache, nausea, stomach pain, diarrhea, fatigue, diaphoresis, dry mouth, restlessness, insomnia, drowsiness, increased psychomotor activity, change in appetite, sweating, and tremor.11,15-17 However, these AEs are most frequently rated in the mild to moderate range of severity and often decrease over time. Underscoring the importance of assessing changes in overt behavior as well as physical symptoms, a recent Cochrane review10 of 15 SSRI pediatric anxiety clinical trials found that increased irritability, hostility, insomnia, disinhibition, impulsivity, and restlessness occurred in greater frequency among children and adolescents randomized to SSRI treatment as compared to pill placebo and that these AEs often led to study discontinuation.15,18 Importantly, preadolescent children may be particularly vulnerable to SSRI AEs such as activation, restlessness, and gastric distress.19
Among other AEs associated with SSRI use, the development of suicidal thoughts/behaviors is perhaps the most troubling for patients, families, and treating clinicians. In 2004, the Food and Drug Administration (FDA) advisory committee reviewed the results of 24 controlled trials of 9 antidepressants (N=4,400). Although there were no reported suicides and no between-group differences in suicide attempts, when changes in suicidal ideation (i.e., thoughts of suicide) were included in the analyses, a small yet significant increase in suicidal behavior among children receiving medication (4%) as compared to those receiving pill placebo (2%) was observed. This ∼2% difference in attributable risk translates into a number needed to harm (NNH) of ∼50.20 A subsequent and larger meta-analysis reviewed 39 pediatric antidepressant trials (including indications in depression, obsessive-compulsive disorder and anxiety disorders) and found no suicides and a smaller risk difference between antidepressant medication and placebo for suicidal behavior (0.7%; 95% CI, 0.1% to 1.3%) with the NNH=143 (95% CI, 77 to 1000).21 Given the large number of studies and the inclusion of multiple psychiatric disorders, this meta-analysis suggests that the benefit of SSRI treatment may outweigh the possible risks of developing suicidal behavior among youth with anxiety. Despite a clear picture about short-term adverse events associated with antidepressant use, data regarding the potential negative effects of long-term use of these compounds in children and adolescents is limited.
One challenge with respect to estimates of AEs relates to how AE data are collected. Historically, most AE monitoring used the general inquiry method (e.g., “Has anything changed since last visit?”). Currently, greater emphasis is being placed on systematically inquiring about both short-term and long-term AEs.22,23 The few studies comparing general vs. systematic methods of inquiry found that systematic method results in more AEs being identified when compared to the general method.24 Despite the potential clinical utility of a systematic inquiry around AEs, there is no consensus about which approach to use. Given children's unique developmental status, AE monitoring via systematic inquiry is likely to be especially critical when evaluating medications for the treatment of children diagnosed with psychiatric disorders.25
As AEs collected in clinical trials include any new or worsening event during the intervention, AEs will likely include those events that may or may not be intervention related. The standard approach to assessing whether an AE is intervention related is to compare rates of AEs in the active as compared to the control group (e.g. medication vs. placebo). Ascertaining and interpreting between-group differences in AE rates is particularly daunting in comparative treatment trials that include both medication and non-medication treatment arms and trials that include both open and masked treatment assignment. For example, in the Treatment of Adolescents with Depression Study (TADS), there were substantial between-group differences in AE reporting.26 CBT therapists in TADS were charged with identifying AEs similar to pharmacotherapists, yet very few AEs were identified via the general inquiry by CBT therapists as compared to the pharmacotherapists. Whether this difference was due to lower patient and therapist expectations for AEs in open CBT or the relative lack of experience among CBT therapists as compared to pharmacotherapists in assessing AEs is not known.
To add to the growing literature examining the safety of medication use in pediatric populations, specifically the use of SSRIs to treat children and adolescents suffering from anxiety disorders, we present AE data from the Child/Adolescent Anxiety Multimodal Study (CAMS). 18,27,28 CAMS was a multi-site, randomized placebo-controlled study comparing 12 weeks of sertraline (SRT), CBT, their combination (COMB), and pill placebo (PBO) in 488 children and adolescents diagnosed with separation anxiety disorder (SAD), generalized anxiety disorder (GAD), or social phobia (SoP). Week-12 response rates in CAMS revealed that COMB was superior (80.7%) to both CBT and SRT (59.7% and 54.9%, respectively), and all active treatments were found to be more beneficial than PBO (23.7%).18 An analysis of AEs in the primary outcome paper focused on comparisons between SRT vs. PBO (the only double-blinded treatment conditions in CAMS and the only comparison that could be readily interpreted) and SRT vs. CBT (a non-medication open treatment), finding no differences in the frequency of moderate to severe AEs, including suicidal and homicidal ideation in SRT vs. PBO comparisons.18 However, rates of insomnia, fatigue, and restlessness were reported less frequently in CBT as compared to SRT. Comparisons between SRT and COMB were not included, as outcomes might be difficult to interpret because COMB was an open treatment condition. Specifically, participants knew they were taking active medication and may therefore have higher expectations for or more certainty about attributing AEs to medication than would otherwise be the case. Moreover, in COMB, participants were assessed by two clinicians (CBT therapist and pharmacotherapist), providing more opportunities to report AEs.
CAMS offers a unique opportunity to further evaluate the safety of an SSRI medication and other CAMS treatments given its relative large sample, large proportion of prepubertal children, and multiple treatment conditions. Also, CAMS augmented the AE assessment by including systematic inquiry of harm-related adverse events following the FDA black box warning regarding suicidal behavior on antidepressants in 2004.27 The current paper extends previous analyses of AEs by (a) comparing the frequency of AEs across the four treatment conditions evaluated in CAMS, including COMB; (b) comparing the frequency of AEs between children and adolescents; (c) examining the regular use of a self-report physical symptom checklist for eliciting changes in AEs over time; and d) addressing the greater opportunity for AE reporting in COMB treatment by statistically adjusting AE rates for the number of reporting opportunities.
Method
Descriptions of the CAMS rationale, design, and methods,27 participant baseline characteristics,28 and 12-week outcomes18 have been reported previously. Briefly, participants were ages 7 to 17 years old (M=10.7) and met DSM-IV criteria for one or more of the following: SAD, GAD, or SoP. Although participants with a wide range of comorbidities were included, those meeting criteria for major depressive disorder (MDD) or substance abuse (SA) disorder were excluded. The protocol was approved and monitored by institutional review boards at each center. Participants and at least one parent provided written informed consent. Participants were randomized (2:2:2:1) to the following treatments: CBT (n=139), SRT (n=133), COMB (n=140), or PBO (n=76). Only the SRT and PBO treatment conditions were double-blind, and this was made possible by using matching medication and pill placebo. CAMS used a “fixed-flexible” dosing schedule in which dose changes were tied to clinical response and tolerability. The maximum target dose of SRT was 200 mg/day.
Assessment of Adverse Events
To address limitations in previous studies and to assure uniform assessment of AEs across all treatment arms, CAMS developed a scripted AE inquiry procedure. This procedure involved two stages. In stage 1, study coordinators—and not treating clinicians—met with participants and family members prior to each treatment session. Study coordinators used a standardized script to elicit AEs that assessed whether the participant had experienced any medical or behavioral change since the last treatment visit. The script asked the participant and his or her parent/legal guardian, “Have you (or has your child) had any health or other problems since your last visit and today?” Responses to this question were recorded and passed to the child's primary clinician (either the pharmacotherapist or CBT therapist) for review (stage 2). At the beginning of each treatment session, the study clinician reviewed the AE information collected and asked additional follow-up questions to determine frequency, severity, impairment, and any treatment used to manage the AE (e.g. analgesics for headache). For participants in the COMB treatment condition, the pharmacotherapist reviewed AEs when the pharmacotherapy and CBT visit occurred on the same day. When the pharmacotherapy and CBT visit occurred on different days, the CBT therapist would also record the characteristics of any AEs reported by the participant or family member(s) since the last treatment visit.
Assessment of Harm-Related Adverse Events (Suicidal/Homicidal Ideation and/or behaviors)
Consistent with good practice, information about suicidal and homicidal ideation and related behaviors was collected at each visit. This information was only collected during stage 2 by the treating clinician and not by the study coordinator. A standardized script was used that included 3 questions: “Since your last visit, did you have any thoughts about not wanting to be alive?” “Since your last visit, did you have any thoughts about hurting yourself?” and “Since your last visit, did you have any thoughts about hurting someone else?” If participants answered “yes” to any of these questions, additional information was elicited to determine the content of thought (e.g., non-suicidal self-harm, passive death wish, suicidal ideation without plan, and suicidal ideation with plan) and whether any behavior (e.g., non-suicidal self-harm, suicide attempt, or harm to others) had taken place. Reports of these harm-related events were discussed on the weekly cross-site steering committee call to review severity and the event category. At the end of the entire study, the pharmacotherapy committee blindly reviewed the harm-related AEs for coding accuracy.
Assessment of Physical Symptoms
The Physical Symptom Checklist (PSC)26 was used to track somatic symptoms throughout the course of acute treatment (at baseline and weeks 4, 8, and 12). This data was not shared with the treating clinician but was used in data analyses as an alternative method of assessing changes in somatic and central nervous system symptoms across time. The PSC is a 47-item child self-report measure that includes a list of general health problems. Participants rate each item using a 4-point Likert scale (0=not at all to 3=very much) to indicate how much he or she has been bothered by the given symptom during the past week. A total physical symptom score was obtained by summing the items.
At baseline and before randomization was revealed, physical complaints rated as a 2 or 3 (“pretty much” or “very much”) on the PSC and occurring in at least 10% of the participants included the following: allergies (14.4%), head cold (12.8%), headache (20%), feeling drowsy (20.5%), dry skin (10.5%), stomach pain (18.8%), restless or uncomfortable urge to move (12.6%), trouble sleeping (23.8%), sleeplessness (16.2%), and nightmares (14.7%). The 10% cut off was chosen to ensure that only the most prevalent physical complaints were reported. Emergence of a new physical symptom or worsening physical symptom status after the baseline assessment was operationalized as a change in baseline score from 0 or 1 to 2 or 3 at weeks 4, 8, and 12 (these were classified as a new physical symptom) or a change in baseline score from 2 to 3 at weeks 4, 8, and 12 (these were classed as a worsening physical symptom).
Assessment of Clinical Improvement
The relationship between AEs and the Clinician Global Impression-Improvement/Severity scale (CGI-I/S)29 was examined across study time points. The CGI-S provides a global rating of symptom severity ranging from 1 (“not at all ill”) to 7 (“extremely ill”). The CGI-I provides a global rating of clinical improvement ranging from 1 (“very much improved”) to 7 (“very much worse”). The independent evaluators (IEs) completed the CGI measures at baseline (only the CGI-S) and then at weeks 4, 8, and 12. Clinicians also completed the CGI measures after each visit. These data are not provided. The pharmacotherapist used the CGI-S ratings to adjust medication dose during acute treatment.
Assessment of Adherence to Medication
Medication adherence was calculated based on medication returned and defined as the ratio of medication taken divided by what was prescribed (pills taken/pills prescribed). Missing adherence data by treatment condition (i.e., participants who forgot to return their unused pills) at any visit was 12.6% for COMB, 10.4% for SRT, and 13.0% for PBO.
Statistical Methods
Data for the present analyses include participants who, at the time of their assessment, remained in their active study arm (i.e., an observed cases [OC] analysis that set to missing future data from all participants who were no longer participating in their assigned treatment arm). To compare AE rates across study arms and across ages, Pearson's chi-square test or Fisher's exact test was used. As indicated earlier, participants in the COMB knew they were taking active medication and had more opportunities to be questioned about AEs than participants who only saw one clinician. Therefore, to control for possible ascertainment bias that this increase in contact may have caused, logistic regression analyses (using SAS PROC LOGISTIC) were conducted with the number of reporting opportunities added as a covariate. A generalized linear model (using GEE) for dichotomous outcomes (using SAS PROC GENMOD) was conducted to evaluate the relationship between treatment condition and AEs across time, with treatment condition, time, and their interaction included in the model. Similar analyses were performed and stratified by age and gender for the PSC to examine the effect of treatment condition on changes in the total mean severity of physical symptoms across time. In these models, we included linear time, treatment condition, and their interaction as fixed effects, with intercept and time as random effects. To test the treatment effect on change in CGI-I or CGI-S across time, similar analyses were performed. All analyses were performed in SAS 9.3 with significance level set at 0.05. Because all analyses were exploratory, no corrections for type-I error rates were conducted.
Results
Participants
Of the 488 participants, 74% were 12 years or younger (mean 10.7 ±2.8 years), and 431 (88.3%) completed the acute phase (through week 12 assessment). The proportion of participants who remained in their assigned treatment condition (completed treatment and all assessments) was 95.6% for CBT, 90.7% for COMB, 82.7% for SRT, and 80.3% for PBO. Participants who withdrew consent for both treatment and assessments were defined as study dropouts. The PBO treatment condition had 12 study dropouts (19.7%), SRT had 16 (17%), COMB had 12 (11%), and CBT had 6 (4.3%). Pairwise comparisons revealed that participants in the SRT and PBO treatment arms were significantly more likely to drop out of treatment than participants in the two CBT-containing conditions (p=.03, p=.006, respectively). There were no statistically significant differences in rates of dropout between participants in CBT and COMB.
Average medication adherence was 91.3% for COMB participants (based on n=135 or 96.4% of possible COMB participants), 90.2% for SRT (based on n=122 or 91.7% of possible SRT participants), and 91.0% for PBO (based on n=64 or 84.2% of possible PBO participants). There was no significant difference in medication adherence between the three medication treatment groups (p=.87). With respect to opportunities to report AEs, the number of opportunities for the elicitation of AEs differed across treatment arms with the greatest mean number occurring in COMB (12.8 ±4.0) as compared to the other treatments: SRT (9.9 ±3.6), CBT (10.6 ±2.0), and PBO (9.7 ±4.2). Pairwise comparisons revealed that COMB had significantly more reporting opportunities than the other treatment groups (all p values < .001), with no significant difference between SRT, CBT, or PBO.29
The average medication dose, among those participants who completed the full course of medication treatment, was not statistically different between COMB and SRT (t=1.48, p=.10) (COMB 133.7 ±59.8 mg/day; SRT 146.0 ±60.8 mg/day). However, the average medication dose for both active treatments was significantly lower than PBO (COMB vs. PBO, t=3.89, p<.001; SRT vs. PBO, t=2.58, p<.02; average dose for PBO was 175.8 ±43.7 mg/day). However, a significant difference in average medication dose (F=7.55, p<.001) was found by age with children having, on average, a lower medication dose (137.1 ±61.6 mg/day; n=211) when compared to adolescents (165.9 ±53.4 mg/day; n=80).
Physical Adverse Events
Elicited AEs
A comparison of elicited physical events is presented in Table 1. Only those AEs rated as moderate to severe, resulted in functional impairment, and occurred in at least 3% or more of the participants are listed. A post hoc analysis was performed adjusting for the number of elicitation opportunities across the four treatment arms. Results from between-group pairwise comparisons (both unadjusted and adjusted p values) are presented.
Table 1. Spontaneously Reported Physical Adverse Events in at Least 3% of the Treatment Groups a.
| Physical Symptoms | COMB (n =140) No. (%) | SRT (n = 33) No. (%) | CBT (n = 139) No. (%) | PBO (n = 76) No. (%) | Total (N = 488) No. (%) | Unadjusted Comparison | Adjusted Comparison |
|---|---|---|---|---|---|---|---|
| Total Physical Symptom | 58 (41.4) | 67 (50.4) | 51 (36.7) | 35 (46.1) | 211 (43.2) | SRT≈COMB; SRT>CBT* | SRT>COMB**; SRT>CBT** |
| Headaches | 18 (12.9) | 21 (15.8) | 12 (8.6) | 6 (7.9) | 57 (11.7) | ||
| Gastric Distress | 14 (10.0) | 15 (11.3) | 11 (7.9) | 6 (7.9) | 46 (9.4) | ||
| Sore Throat | 10 (7.1) | 6 (4.5) | 12 (8.6) | 6 (7.9) | 34 (7.0) | ||
| Cold Symptoms | 8 (5.7) | 9 (6.8) | 10 (7.2) | 3 (3.9) | 30 (6.1) | ||
| Vomiting | 8 (5.7) | 6 (4.5) | 5 (3.6) | 4 (5.3) | 23 (4.7) | ||
| Insomnia | 7 (5.0) | 11 (8.3) | 2 (1.4) | 3 (4.0) | 23 (4.7) | SRT>CBT** | SRT>CBT** |
| Fever | 6 (4.3) | 1 (0.8) | 8 (5.8) | 3 (4.0) | 18 (3.7) | CBT>SRT* | SRT≈CBT |
| Upper Respiratory Infection | 5 (3.6) | 3 (2.3) | 7 (5.0) | 3 (4.0) | 18 (3.7) | ||
| Diarrhea | 6 (4.3) | 5 (3.8) | 4 (2.9) | 2 (2.6) | 17 (3.5) | ||
| Interrupted sleep | 6 (4.3) | 6 (4.5) | 2 (1.4) | 2 (2.6) | 16 (3.3) | ||
| Nausea | 5 (3.6) | 4 (3.0) | 3 (2.2) | 3 (4.0) | 15 (3.1) | ||
| Body Ache | 5 (3.6) | 4 (3.0) | 3 (2.2) | 2 (2.6) | 14 (2.9) | ||
| Fatigue | 3 (2.1) | 8 (6.0) | 0 | 3 (4.0) | 14 (2.9) | SRT>CBT**; PBO>CBT* | SRT>CBT**; PBO>CBT* |
| Accidental injury | 4 (2.9) | 4 (3.0) | 4 (2.9) | 1 (1.3) | 13 (2.7) | ||
| Allergies | 5 (3.6) | 2 (1.5) | 3 (2.2) | 2 (2.6) | 12 (2.5) | ||
| Asthma | 3 (2.1) | 5 (3.8) | 2 (1.4) | 0 | 10 (2.0) | ||
| Other Infection | 5 (3.6) | 0 | 4 (2.9) | 1 (1.3) | 10 (2.0) | ||
| Ear Pain | 5 (3.6) | 2 (1.6) | 2 (1.4) | 0 | 9 (1.8) | ||
| Sedation | 0 | 6 (4.5) | 0 | 1 (1.3) | 7 (1.4) | SRT>COMB**; SRT>CBT** | SRT>COMB*; SRT>CBT* |
| Medical Procedure | 1 (0.7) | 1 (0.8) | 1 (0.7) | 3 (4.0) | 6 (1.2) |
Note: CBT = cognitive-behavioral therapy; COMB = combination therapy; PBO = pill placebo; SRT = sertraline.
Adjusting for number of reporting opportunities.
p<0.05,
p<0.01
There were no differences between the double-blinded conditions (SRT vs. PBO) for either total physical AEs or any individual physical AE. When controlling for the number of reporting opportunities, the rates of total physical AEs were greater in the SRT treatment condition when compared to CBT (p<.01) and COMB (p<.01) treatment conditions. Regarding individual physical AEs, participants in SRT reported higher rates of insomnia (p<.01), fatigue (p<.01), and sedation (p<.05) when compared to participants in CBT or COMB, but not PBO.
Physical Symptom Checklist
The PSC was used to evaluate worsening and emergence of new self-reported physical symptoms during acute treatment. When comparing the 2 double-blind treatment arms (Table 2), worsening or emergence of new physical symptoms occurred in the PBO treatment condition more often than in the SRT treatment condition. These included increased symptoms of stomach pain (21.4% vs. 9.6%, p<.05), difficulty breathing (9.1% vs. 1.1%, p<.05), and numbness or tingling in arms or legs (9.1% vs. 0%, p<.01). The only exception was trouble sleeping, where participants receiving SRT reported worsening or new onset of sleep difficulty when compared to PBO participants (27.7% vs. 13.0%, p<.05).
Table 2. Worsening or Emergence of Symptoms by Self-Report (Physical Symptom Checklist).
| Physical Symptoms | COMB (n = 108) No. (%) | SRT (n = 97) No. (%) | CBT (n = 117) No. (%) | PBO (n = 55) No. (%) | Total (n=377) No. (%) | Comparisons |
|---|---|---|---|---|---|---|
| Head cold or sniffles | 20 (18.9) | 29 (29.9) | 20 (17.4) | 22 (39.3) | 91 (24.3) | PBO>COMB**; PBO>CBT* |
| Fever | 3 (2.9) | 5 (5.3) | 8 (7.1) | 7 (13.0) | 23 (6.3) | PBO>COMB* |
| Hives | 4 (3.8) | 2 (2.1) | 0 | 3 (5.5) | 9 (2.5) | COMB>CBT**; PBO>CBT* |
| Stomach pain or ache | 15 (14.0) | 9 (9.6) | 18 (15.5) | 12 (21.4) | 54 (14.5) | PBO>SRT* |
| Pain with urination | 0 | 1 (1.1) | 0 | 3 (5.5) | 4 (1.1) | PBO>COMB*; PBO>CBT* |
| Trouble sleeping | 16 (15.4) | 28 (27.7) | 18 (16.1) | 7 (13.0) | 69 (18.4) | SRT>COMB*; SRT>CBT*; SRT>PBO* |
| Difficulty breathing | 3 (2.9) | 1 (1.1) | 7 (6.2) | 5 (9.1) | 16 (4.4) | PBO>SRT* |
| Coughing or wheezing | 12 (11.5) | 12 (12.6) | 9 (8.0) | 13 (23.6) | 46 (12.6) | PBO>COMB*; PBO>CBT** |
| Dental problems | 8 (7.8) | 4 (4.2) | 2 (1.8) | 3 (5.5) | 17 (4.6) | COMB>CBT** |
| Numbness or tingling in arms or legs | 0 | 0 | 2 (1.8) | 5 (9.1) | 7 (1.9) | PBO>COMB**; PBO>SRT** |
| Feeling bloated or gassy | 7 (6.7) | 7 (7.5) | 1 (0.9) | 4 (7.4) | 19 (5.3) | COMB>CBT*; SRT>CBT*; PBO>CBT* |
Note: CBT = cognitive-behavioral therapy; COMB = combination therapy; PBO = pill placebo; SRT = sertraline.
p < 0.05,
p < 0.01
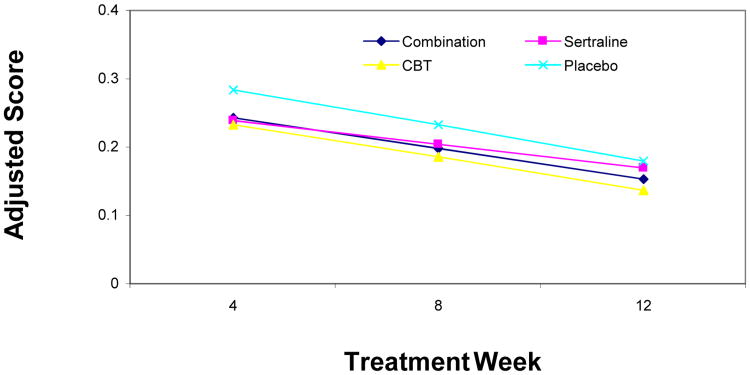
Regression analyses revealed an overall decrease in the mean total PSC score across time (p<.01), but no significant differences in the rate of change (i.e., slope) between treatment conditions (p=.47). Furthermore, there were no significant between-group differences in the total mean PSC score at week 12 (Figure 1). There were also no gender or age differences on PSC mean change scores over time.
Figure 1.
Change of average Physical Symptom Checklist (PSC) scores over time. Note: CBT = cognitive-behavioral therapy.
Clinical Global Impression-Improvement and Severity Scales (CGI-I/S)
Within the CBT treatment condition, children who reported at least one physical AE were more likely to be rated as a Week-12 treatment responder (CGI-I ≤ 2) when compared to children who did not report any physical AEs (p < .02). There were no significant differences in rates of treatment response between those who did and did not report physical AEs among the remaining three treatment conditions. Across each treatment condition, there were no significant differences in the percentage of children who received a Week-12 CGI-S score of ≤ 2 between those who reported at least one psychiatric AE when compared to those who endorsed no psychiatric AEs. There were no significant pairwise differences between treatment conditions on the CGI-I or CGI-S in the percentage of children who reported at least one physical or psychiatric AE when compared to those who did not endorse any AEs during acute treatment.
Psychiatric and Harm-Related Adverse Events
There were no differences between the double-blind treatments (SRT vs. PBO) for either total psychiatric AEs or any individual psychiatric AE (Table 3). When assessing the total number of psychiatric AEs (adjusting for the number of reporting opportunities), the treatment arms containing SRT showed significantly higher rates of psychiatric AEs when compared to CBT (p<.05). Individual psychiatric AEs were greater among COMB participants when compared to CBT participants for disinhibition (p<.05) and increased motor activity (p<.05). Rates for the restless/fidgety AEs were greater for SRT when compared to CBT (p<.05). There were more total harm-related events (suicidal/homicidal ideation and/or behaviors) among COMB participants than SRT or PBO participants (p<.05, p<.05, respectively). There were no suicide attempts in any treatment condition.
Table 3. Spontaneously Reported Psychiatric Adverse Events in at Least 3% of the Treatment Groups a.
| Psychiatric Symptoms | COMB (n = 140) No. (%) | SRT (n = 133) No. (%) | CBT (n = 139) No. (%) | PBO (n = 76) No. (%) | Total (N=488) No. (%) | Unadjusted Comparisons | Adjusted Comparisons |
|---|---|---|---|---|---|---|---|
| Total Psychiatric Symptoms | 41 (29.3) | 23 (17.3) | 13 (9.4) | 10 (13.2) | 87 (17.8) | COMB>ALL*; SRT>CBT* | COMB>CBT***; SRT>CBT* |
| Disinhibition | 12 (8.6) | 6 (4.5) | 2 (1.4) | 1 (1.3) | 21 (4.3) | COMB>CBT**; COMB>PBO* | COMB>CBT* |
| Increased Motor Activity | 10 (7.1) | 4 (3.0) | 1 (0.7) | 1 (1.3) | 16 (3.3) | COMB>CBT** | COMB>CBT* |
| Disobedient/ Defiant | 9 (6.4) | 4 (3.0) | 2 (1.4) | 0 | 15 (3.1) | COMB>PBO* | |
| Emotional Outburst/Tantrums | 1 (0.7) | 4 (3.0) | 4 (2.9) | 3 (4.0) | 12 (2.5) | ||
| Restless/ Fidgety | 5 (3.6) | 5 (3.8) | 0 | 2 (2.6) | 12 (2.5) | SRT>CBT* | SRT>CBT* |
| Anxiety/ Nervousness | 5 (3.6) | 1 (0.8) | 1 (0.7) | 4 (5.3) | 11 (2.3) | PBO>CBT* | |
| Irritability | 3 (2.1) | 4 (3.0) | 3 (2.2) | 1 (1.3) | 11 (2.3) | ||
| Agitation | 7 (5.0) | 1 (0.8) | 1 (0.7) | 0 | 9 (1.8) | COMB>PBO* | |
| Impulsivity | 5 (3.6) | 2 (1.5) | 1 (0.7) | 1 (1.3) | 9 (1.8) | ||
| Total Harm-Related | 14 (10.0) | 3 (2.3) | 8 (5.8) | 1 (1.3) | 26 (5.3) | COMB>SRT**; COMB>PBO* | COMB>SRT*; COMB>PBO* |
| Aggression | 8 (5.7) | 1 (0.8) | 2 (1.4) | 0 | (2.3) | COMB>SRT**; COMB>PBO* | |
| Self-Harm Behavior | 2 (1.4) | 1 (0.8) | 1 (0.7) | 0 | 4 (0.8) | ||
| Suicidal Ideation | 5 (3.6) | 0 | 5 (3.6) | 1 (1.3) | 11 (2.3) | ||
| Suicide Attempt | 0 | 0 | 0 | 0 | 0 | ||
| Homicidal Ideation | 0 | 2 (1.5) | 0 | 0 | 2 (0.4) |
Note: ALL = sertraline (SRT), cognitive-behavioral therapy (CBT), and pill placebo (PBO); COMB = combination therapy.
Adjusting for number of reporting opportunities.
p < 0.05;
p < 0.01;
p < 0.001
Examination of AEs in Children and Adolescents
Among Children
Table 4 shows moderate to severe AEs that occurred in at least 3% of the participants in any treatment condition by age (results for both unadjusted and adjusted for number of reporting opportunities are presented). Children ages 7 to 12 showed a higher rate of total physical AEs in SRT treatment condition as compared to COMB or CBT (p<.01); specifically, headache (SRT > CBT, p<.05), insomnia (SRT > CBT, p<.05), and sedation (SRT > COMB, p<.05; SRT > CBT, p<.05). Children in SRT group also showed a higher rate of headaches than those in PBO (16.2% vs. 3.7%, p<.05). Additionally, children exhibited a higher rate of total psychiatric AEs in the COMB and SRT treatment conditions when compared to CBT (p<.05, p<.01, respectively). Children in the COMB group showed higher rates of total harm-related AEs when compared to SRT and PBO groups (p<.05, p<.01, respectively) but not when compared to CBT group. However, these differences in total harm-related AEs were not significant when comparisons were adjusted for reporting opportunities.
Table 4. Age Subgroup Analysis for Spontaneously Reported Adverse Events a.
| Children | |||||||
|---|---|---|---|---|---|---|---|
|
| |||||||
| COMB (n = 101) No. (%) | SRT (n = 99) No. (%) | CBT (n = 108) No. (%) | PBO (n = 54) No. (%) | Total (n = 362) No. (%) | Unadjusted Comparisons | Adjusted Comparisons | |
| Total Physical | 41 (40.6) | 55 (55.6) | 40 (37.0) | 24 (44.4) | 160 (44.2) | SRT>COMB*; SRT>CBT** | SRT>COMB**; SRT>CBT** |
| Headaches | 10 (9.9) | 16 (16.2) | 8 (7.4) | 2 (3.7) | 36 (9.9) | SRT>PBO*; SRT>CBT* | SRT>PBO*; SRT>CBT* |
| Insomnia | 4 (4.0) | 9 (9.1) | 2 (1.9) | 2 (3.7) | 17 (4.7) | SRT>CBT* | SRT>CBT* |
| Fatigue | 2 (2.0) | 4 (4.0) | 0 | 2 (3.7) | 8 (2.2) | SRT>CBT* | |
| Sedation | 0 | 6 (6.1) | 0 | 1 (1.9) | 7 (1.9) | SRT>COMB*; SRT>CBT* | SRT>COMB**; SRT>CBT* |
| Medical/Surgical Procedure | 0 | 1 (1.0) | 1 (0.9) | 3 (5.6) | 5 (1.4) | PBO>CBT* | |
| Total Psychiatric | 32 (31.7) | 21 (21.2) | 11 (10.2) | 9 (16.7) | 73 (20.2) | COMB>CBT***; COMB>PBO*; SRT>CBT* | COMB>CBT**; SRT>CBT* |
| Disinhibition | 12 (11.9) | 6 (6.1) | 2 (1.9) | 1 (1.9) | 21 (5.8) | COMB>CBT**; COMB>PBO* | COMB>CBT* |
| Increased Motor Activity | 9 (8.9) | 4 (4.0) | 1 (0.9) | 1 (1.9) | 15 (4.1) | COMB>CBT** | COMB>CBT* |
| Restless/ Fidgety | 4 (4.0) | 4 (4.0) | 0 | 2 (3.7) | 10 (2.8) | COMB>CBT*; SRT>CBT* | PBO>CBT* |
| Anxiety/ Nervousness | 3 (3.0) | 1 (1.0) | 0 | 3 (5.6) | 7 (1.9) | PBO>CBT* | |
| Agitation | 4 (4.0) | 1 (1.0) | 0 | 0 | 5 (1.4) | COMB>CBT* | |
| Total Harm-Related | 11 (10.9) | 2 (2.0) | 5 (4.6) | 0 | 18 (5.0) | COMB>SRT*; COMB>PBO** | |
|
| |||||||
| Adolescents | |||||||
|
| |||||||
| COMB (n = 39) No. (%) | SRT (n = 39) No. (%) | CBT (n = 31) No. (%) | PBO (n = 22) No. (%) | Total (n = 126) No. (%) | Unadjusted Comparisons | Adjusted Comparisons | |
|
| |||||||
| Total Psychiatric | 9 (23.1) | 2 (5.9) | 2 (6.5) | 1 (4.6) | 14 (11.1) | COMB>SRT* | |
Note: CBT = cognitive-behavioral therapy; COMB = combination therapy; PBO = pill placebo; SRT = sertraline.
Adjusting for number of reporting opportunities.
p < 0.05;
p < 0.01;
p < 0.001
Among Adolescents
For adolescents aged 13 to 17 (n=126), there were no differences between treatment arms for physical AEs and psychiatric AEs (based on adjusted comparisons).
Children vs. Adolescents
Children who received SRT reported higher rates of AEs overall when compared to adolescents (16.2% vs. 3.7%, p<.05). Specifically, the rate of total psychiatric AEs was higher in children across all treatment arms (31.7 % vs. 23.1 %, p<.05). When compared to adolescents, children experienced more disinhibition (p<.01), and this was especially true in the COMB condition (p<.05). There were no differences between the two groups in the rate of total physical AEs across the entire sample. However, when compared to children, adolescents experienced more of the following physical AEs: headaches (p<.05), cold symptoms (p<.01), and body aches (p<.05).
Discussion
The findings reported in this paper support the mounting data suggesting the tolerability and safety of acute SSRI treatment for pediatric anxiety disorders. More specifically, no significant differences in the overall rate of physical or psychiatric AEs were found when comparing the two blinded treatment arms, SRT and PBO, using the systematic inquiry method. The assessment of emergent physical symptoms or worsening of physical symptoms using the self-report PSC revealed that trouble sleeping occurred more often in participants assigned to take SRT as compared to PBO. In the age subgroup analyses, children treated with SRT reported a higher frequency of headaches than those treated with PBO. When tolerability was compared between child and adolescents, there were no significant differences in the overall rate of physical AEs. However, children showed increased rates of psychiatric AEs, specifically, disinhibition. The combination of CBT and SRT (i.e., COMB) treatments, taking into account the psychiatric adverse events, may not offer a more favorable adverse event profile compared to SRT alone.for youth with anxiety disorders. This study highlights the importance of carefully measuring baseline physical and psychiatric symptoms, systematically monitoring AEs, and using this information to guide medication adjustment, especially in the younger age group.
The purpose of adjusting for the number of reporting opportunities was to control for the possibility of bias resulting from being asked about the presence of AEs more frequently, as was the case for COMB participants. However, this adjustment had little impact on the overall pattern of AEs reported. Adjusting for the number of reporting opportunities resulted in differences in outcomes mainly when the base rates of AEs were low (e.g., fever, disobedient behavior, impulsivity, and aggression).
Few studies to date have examined developmental differences in the rates of AEs between children and adolescents. Subgroup analysis by age showed that children reported no significant difference in overall physical or psychiatric AEs when comparing SRT vs. PBO. However, a greater number of children experienced physical symptoms in the SRT condition when compared to COMB or CBT. In adolescents, no differences were found when comparing frequency of physical and psychiatric AEs across treatment conditions. However, comparisons between children and adolescents showed that children in the SRT arm reported more AEs overall when compared to adolescents. Similarly, total psychiatric AEs were higher in children across all treatment arms. The present analyses suggest children may be at greater risk for experiencing overall AEs and psychiatric AEs, which often leads to study discontinuation.30 The “fixed-flexible” dosing schedule used in CAMS was designed to maximize efficacy, and it was linked to the participant's clinical response and tolerability. Although the protocol dosing strategy did not differ for children and adolescents, children received a lower SRT dose on average across both COMB and SRT treatment groups. The differences between the age groups in the rate of AEs may have been driven by the particular dosing strategy used in CAMS as compared to the slower and lower dosing in clinical practice.
Findings reported in this study bring attention to the presence of significant before-treatment physical symptoms in this anxious population, especially in the sleep domain, with approximately 44% of the sample having some sleep difficulty as measured by the PSC. Also, when examining worsening of physical symptoms, sleep emerged as the only physical symptom that occurred more frequently in the SRT-treated group when compared to PBO. However, it should be noted that overall physical symptoms, as measured by the PSC, improved across time in each of the active treatment conditions, as well as in the PBO condition. This may be explained by AEs diminishing over time and/or as the anxiety disorder improves, the physical symptoms decrease. Thus, SSRI treatment reduces sleep difficulties in some youth with anxiety while it may worsen an already present problem in others. These findings should alert practitioners to the importance of obtaining accurate measurement of baseline physical symptoms and underscore the importance of tracking physical symptoms over time.
Of interest is the observation that the absence of AEs leads to more rapid improvement within treatment conditions. The interesting exception was in CBT only, where the presence of an AE was associated with faster improvement. This may be a random finding or may reflect that the perception of an AE during CBT is experienced as less threatening. Or it could be that CBT, when done correctly via graduated exposure tasks, results in the experience of more anxiety-based physical symptoms as a natural byproduct of the treatment. The presence of psychiatric AEs over the course of 12 weeks did not impact the slope of change for the global improvement or severity for participants within treatment conditions, which suggests that if these types of AEs are successfully clinically managed, children and adolescents will still be able to achieve positive response.
The CAMS results highlight the challenge of assessing AEs in the pediatric population and point to the need for improved approaches or methods, particularly in the younger population. This area of work has continued to progress with the development of improved assessment tools such as the Pediatric Adverse Event Rating Scale (PAERS),31 which is completed by the child and parent, who each identify and rate the severity of the AEs listed. The responses are then reviewed by a clinician who makes a summary rating and uses this information to determine next treatment steps. Another approach that requires less clinician time and may decrease clinician bias is the computerized self-administered screen called Columbia Health and Adverse Reactions to Medication (CHARMS).32 The child and parent listen to questions through headphones and enter their own responses on the computer. The CHARMS has questions for the parent (about the youth) and child (about him/herself) and reviews all body symptom categories and generates a printed report for clinician review.32 Such structured approaches should be associated with improved identification and tracking of AEs in youth.
As is true with most randomized controlled medication trials, specific hypotheses regarding AEs were not preplanned or adequately powered; therefore, this study used post hoc analyses and may result in spurious association between treatment and AEs. As noted previously, the COMB and CBT treatment arms were not blinded and the children in COMB knew they were on active medication and had a greater number of sessions and greater contact with study personnel to report AEs. Although this paper adjusted for the number of opportunities for AEs reporting, the lack of blinding in the COMB treatment condition may have resulted in higher expectancy effects for AEs. Although CAMS used a 2-stage adverse event inquiry across treatment arms at every visit, it only used two structured assessments, a self-report for physical symptoms, and clinician-administered questions on harm to self or others. The study did not use a detailed body systems review or a specialized approach for assessing specific psychiatric AEs. In the future, it would be important to use such scales with each clinical visit. Given that no adjustments were made for multiple comparisons, the potential that any reported differences in rates of AEs might be due to type-I error is always a possibility. Lastly, while this study adds information relevant to AEs in youth with anxiety treated with SSRIs, these results may not generalize to youth receiving SSRIs for treatment of other psychiatric disorders. Most notably, youth diagnosed with comorbid major depression were excluded from the trial and limited the number of adolescents enrolled. Excluding teens with depression and anxiety may have resulted in fewer harm-related AEs and might explain the discrepancy between the current results and existing studies, suggesting a greater frequency of harm-related events for depressed youth on SSRIs.
Sertraline was well tolerated and the benefits outweigh the adverse effects associated with the use of SSRIs in youth with anxiety. The results from this secondary analysis highlight the need for careful assessment and monitoring of AEs with SSRI treatment for children and adolescents diagnosed with anxiety disorders. The frequency and types of AEs identified were consistent with the published literature. There were no attempted suicides. The treatment arms containing medication showed significantly greater rates of AEs as compared to the CBT treatment. Based on these data, it may be prudent especially for children under 12 to consider treatment with CBT first. However, this may be challenging given the paucity of community clinicians trained to deliver high-quality CBT treatment.33 In light of this and the potential for long-term negative consequences of not treating pediatric anxiety disorders, choosing an SSRI alone is a reasonable and safe first treatment option with careful monitoring for AEs. Additionally, as seen by the CAMS18 and the Pediatric OCD Treatment Study,34 COMB treatment acutely provides the best efficacy outcome along with favorable AE profile. It is the responsibility of the treatment provider to inform, educate, and help the child/adolescent and his/her family to assess the benefits versus the risk of all the treatment options and to understand which modality is the best initial choice for his/her particular need.
Acknowledgments
This research was supported by NIMH grants U01 MH64088 (J.P.), U01 MH064003 (S.C.), U01 MH63747 (P.K.), U01 MH64003 (B.B.), U01 MH64092 (A.M.A.), U01 MH64107 (J.M.), and U01 MH064089 (J.W.). Sertraline and matching placebo were supplied free of charge by Pfizer.
Drs. Iyengar, Shen, and Compton served as the statistical experts for this research.
The views expressed in this article represent those of the authors and are not intended to represent the position of NIMH, the National Institutes of Health (NIH), or the Department of Health and Human Services. The authors acknowledge all of the therapists, interviewers, research coordinators, co-investigators, and consultants who were part of this study. Special gratitude is also extended to the children and families who participated in this research.
She has received honoraria from the American Academy of Child and Adolescent Psychiatry (AACAP) and the American Society of Clinical Psychopharmacology (ASCP) workshop for pediatric clinical trials. She also receives fees for academic lectures and Ground Rounds. Dr. Walkup has received grant support from the Hartwell Foundation and the Tourette Syndrome Association. He has served as a consultant to Shire. He has received free medication and placebo from Eli Lilly and Co., Pfizer, and Abbott for NIMH-funded studies. He has served on the advisory board and speakers' bureau of the Tourette Syndrome Association. He has received royalties from Guilford Press and Oxford University Press for books on Tourette syndrome. He has received an honorarium and travel support for an educational meeting from the Tourette Syndrome Association. He also has received travel support for an unpaid position on the Medical Advisory Board of the Tourette Syndrome Association. He is an unpaid member of the Scientific Advisory Board of the Trichotillomania Learning Center, the Scientific Council of the Anxiety and Depression Association of America, and a Scientific Advisor to the American Foundation of Suicide Prevention. Dr. Compton has received research support from NIMH and has served as a consultant to Shire Pharmaceuticals. He has received honoraria from the Nordic Long-Term Obsessive-Compulsive Disorder (OCD)-Treatment Study Research Group and Journal of Consulting and Clinical Psychology (JCCP). He has provided expert testimony at Duke Forensic Group. Dr. Sakolsky has received research support from NIMH and the National Alliance for Research on Schizophrenia and Depression (NARSAD). She has received honoraria from AACAP for teaching at the 2012 Annual Review Course. She has served as an editorial board member of Child and Adolescent Psychopharmacology News and is a specialty consultant for the Prescriber Letter. Dr. Kendall has received grant support from NIMH and NICHD. He has received royalties from publication of the anxiety treatment materials and books on child mental health from Guilford Press, Ericsson, Workbook Publishing, and Oxford University Press. Dr. Kendall has received honoraria from lectures on the topic of anxiety in youth. Dr. McCracken has received research support from NIMH, NICHD, Maternal and Child Health Bureau, Seaside Therapeutics, and Roche. He has served as a consultant to BioMarin and Roche. He has received speaker honoraria from the Tourette Syndrome Association Speaker's Bureau, the Nevada Psychiatric Association, and AACAP. He has received study drug and placebo from Shire. Dr. Albano has received research grant support from NIMH, Duke University Research Institute, and private donors. She has received royalties from Oxford University Press, Lynn Sonberg Books, and Avery Press. She has received honoraria from the American Psychological Association and consultant fees from Brackett Global Data Safety and Monitoring (DSMB), IMPACT, and Cambridge University. Dr. Piacentini has received grant or research support from NIMH, Pfizer through the Duke University Child and Adolescent Psychiatry Trials Network (CAPTN), and the Tourette Syndrome Association. He has served as a consultant to the Coleman Research Group and NIMH. He has served on the speakers' bureau of the Tourette Syndrome Association and the International Obsessive Compulsive Disorder Foundation. He has received book royalties from Guilford Press and Oxford University Press. He is a coauthor of the Child OCD Impact Scale-Revised (COIS-R) and the Child Anxiety Impact Scale (CAIS), the Parent Tic Questionnaire (PTQ), and the Premonitory Urge for Tics Scale (PUTS) assessment tools, all of which are in the public domain, and therefore no royalties are received. He has received financial support from the Furlotti Family Foundation to develop a computerized child OCD treatment program. Dr. Riddle has received grant or research funding from NIMH and drug from Bristol-Meyers Squibb. Dr. Keeton has received grants or funding from NIMH and the Department of Education. Dr. Waslick has received research support from Pfizer (through the Duke University CAPTN), Sunovion, and Forest Laboratories. Dr. Chrisman has received funding from the Doris Duke Foundation Grant for teaching Advanced Practice Practitioners. Dr. March has received research support from Pfizer, NIMH, and the National Institute on Drug Abuse. He has served as a consultant or scientific advisor to Pfizer, Eli Lilly and Co., Bristol-Myers Squibb, and Attention Therapeutics. He has received study drug for an NIMH-funded study from Eli Lilly and Co. and Pfizer. Dr. March is an equity holder in MedAvante and has received royalties from Guilford Press, Oxford University Press, and MultiHealth Systems. Dr. Birmaher has received grant support from NIMH and book royalties from Random House, Inc., Lippincott Williams and Wilkins, and UpToDate.
Footnotes
Disclosure: Dr. Rynn has received research support from NIH, NIMH, the National Institute of Child Health and Human Development (NICHD), Eli Lilly and Co., Pfizer, Merck, and Shire. She has served as a consultant to Shire and has received royalties from American Psychiatric Publishing and a writing fee from Oxford University Press.
Drs. Sherrill, Shen, and Iyengar report no biomedical financial interests or potential conflicts of interest.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Contributor Information
Dr. Moira A. Rynn, Columbia University Medical Center (CUMC)/New York State Psychiatric Institute, New York.
Dr. John T. Walkup, Weill Cornell Medical College and New York Presbyterian Hospital, New York.
Dr. Scott N. Compton, Duke University Medical Center, Durham, NC.
Dr. Dara J. Sakolsky, Western Psychiatric Institute and Clinic–University of Pittsburgh Medical Center, Pittsburgh.
Dr. Joel T. Sherrill, Division of Services and Intervention Research at the National Institute of Mental Health (NIMH), Bethesda, MD.
Dr. Sa Shen, University of Illinois at Urbana–Champaign.
Dr. Philip C. Kendall, Temple University, Philadelphia.
Dr. James McCracken, University of California, Los Angeles (UCLA) Semel Institute for Neuroscience and Human Behavior.
Dr. Anne Marie Albano, Columbia University Medical Center (CUMC)/New York State Psychiatric Institute, New York.
Dr. John Piacentini, University of California, Los Angeles (UCLA) Semel Institute for Neuroscience and Human Behavior.
Dr. Mark A. Riddle, Johns Hopkins University School of Medicine, Baltimore, MD.
Dr. Courtney Keeton, Johns Hopkins University School of Medicine, Baltimore, MD.
Dr. Bruce Waslick, Baystate Medical Center, Springfield, MA.
Dr. Allan Chrisman, Duke University Medical Center, Durham, NC.
Dr. Satish Iyengar, Western Psychiatric Institute and Clinic–University of Pittsburgh Medical Center, Pittsburgh.
Dr. John S. March, Duke University Medical Center, Durham, NC.
Dr. Boris Birmaher, Western Psychiatric Institute and Clinic–University of Pittsburgh Medical Center, Pittsburgh.
References
- 1.Beesdo K, Knappe S, Pine DS. Anxiety and anxiety disorders in children and adolescents: Developmental issues and implications for DSM-V. Psychiatr Clin North Am. 2009;32(3):483–524. doi: 10.1016/j.psc.2009.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Costello EJ, Egger HL, Angold A. The Developmental Epidemiology of Anxiety Disorders: Phenomenology, Prevalence, and Comorbidity. Child Adolesc Psychiatr Clin N Am. 2005;14(4):631–648. doi: 10.1016/j.chc.2005.06.003. [DOI] [PubMed] [Google Scholar]
- 3.Dweck CS, Wortman CB. Series in Clinical and Community Psychology: Achievement, Stress, and Anxiety. Washington, DC: Hemisphere; 1982. Learned helplessness, anxiety, and achievement motivation: Neglected parallels in cognitive, affective, and coping responses; pp. 93–125. [Google Scholar]
- 4.Strauss CC, Lease CA, Kazdin AE, Dulcan MK, Last CG. Multimethod assessment of the social competence of children with anxiety disorders. J Clin Child Psychol. 1989;18(2):184–189. [Google Scholar]
- 5.Kendall PC, Safford S, Flannery-Schroeder E, Webb A. Child Anxiety Treatment: Outcomes in Adolescence and Impact on Substance Use and Depression at 7.4-Year Follow-Up. J Consult Clin Psychol. 2004;72(2):276–287. doi: 10.1037/0022-006X.72.2.276. [DOI] [PubMed] [Google Scholar]
- 6.Pine DS, Cohen P, Gurley D, Brook J, Ma Y. The risk for early-adulthood anxiety and depressive disorders in adolescents with anxiety and depressive disorders. Arch Gen Psychiatry. 1998;55(1):56–64. doi: 10.1001/archpsyc.55.1.56. [DOI] [PubMed] [Google Scholar]
- 7.Compton SN, March JS, Brent D, Albano AM, Weersing VR, Curry J. Cognitive-Behavioral Psychotherapy for Anxiety and Depressive Disorders in Children and Adolescents: An Evidence-Based Medicine Review. J Am Acad Child Adolesc Psychiatry. 2004;43(8):930–959. doi: 10.1097/01.chi.0000127589.57468.bf. [DOI] [PubMed] [Google Scholar]
- 8.Kendall PC, Settipani CA, Cummings CM. No need to worry: The promising future of child anxiety research. J Clin Child Adolesc Psychol. 2012;41(1):103–115. doi: 10.1080/15374416.2012.632352. [DOI] [PubMed] [Google Scholar]
- 9.Vidair HB, Fichter CN, Kunkle KL, Boccia AS. Targeting parental psychopathology in child anxiety. Child Adolesc Psychiatr Clin N Am. 2012;21(3):669–689. doi: 10.1016/j.chc.2012.05.007. [DOI] [PubMed] [Google Scholar]
- 10.Ipser JC, Stein DJ, Hawkridge S, Hoppe L. Pharmacotherapy for anxiety disorders in children and adolescents. Cochrane Database Syst Rev. 2009;(3):CD005170. doi: 10.1002/14651858.CD005170.pub2. [DOI] [PubMed] [Google Scholar]
- 11.Pine DS, Walkup JT, Labellarte MJ, et al. Fluvoxamine for the treatment of anxiety disorders in children and adolescents. N Engl J Med. 2001;344(17):1279–1285. doi: 10.1056/NEJM200104263441703. [DOI] [PubMed] [Google Scholar]
- 12.Rynn M, Puliafico A, Heleniak C, Rikhi P, Ghalib K, Vidair H. Advances in pharmacotherapy for pediatric anxiety disorders. Depress Anxiety. 2011;28(1):76–87. doi: 10.1002/da.20769. [DOI] [PubMed] [Google Scholar]
- 13.Velosa JF, Riddle MA. Pharmacologic treatment of anxiety disorders in children and adolescents. Child Adolesc Psychiatr Clin N Am. 2000;9(1):119–133. [PubMed] [Google Scholar]
- 14.Strawn JR, Prakash A, Zhang Q, et al. A randomized, placebo-controlled study of duloxetine for the treatment of children (7-11 Years) and adolescents (12-17 Years) with generalized anxiety disorder. Annual Meeting of the American Academy of Child and Adolescent Psychiatry; October 22-27, 2013; Orlando, FL. [DOI] [PubMed] [Google Scholar]
- 15.Birmaher B, Axelson DA, Monk K, et al. Fluoxetine for the treatment of childhood anxiety disorders. J Am Acad Child Adolesc Psychiatry. 2003;42(4):415–423. doi: 10.1097/01.CHI.0000037049.04952.9F. [DOI] [PubMed] [Google Scholar]
- 16.March JS, Biederman J, Wolkow R, et al. Sertraline in children and adolescents with obsessive-compulsive disorder: A multicenter randomized controlled trial. JAMA. 1998;280(20):1752–1756. doi: 10.1001/jama.280.20.1752. [DOI] [PubMed] [Google Scholar]
- 17.Wagner KD, Ambrosini P, Rynn M, et al. Efficacy of sertraline in the treatment of children and adolescents with major depressive disorder: two randomized controlled trials. JAMA. 2003;290(8):1033–1041. doi: 10.1001/jama.290.8.1033. [DOI] [PubMed] [Google Scholar]
- 18.Walkup JT, Albano AM, Piacentini J, et al. Cognitive behavioral therapy, sertraline, or a combination in childhood anxiety. N Engl J Med. 2008;359(26):2753–2766. doi: 10.1056/NEJMoa0804633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Safer DJ, Zito JM. Treatment-emergent adverse events from selective serotonin reuptake inhibitors by age group: children versus adolescents. J Child Adolesc Psychopharmacol. 2006;16(1-2):159–169. doi: 10.1089/cap.2006.16.159. [DOI] [PubMed] [Google Scholar]
- 20.Hammad TA. Review and Evaluation of Clinical Data: Relationship between psychotropic drugs and pediatric suicidality. [Accessed December 8, 2014];US Food and Drug Administration. website. http://www.fda.gov/ohrms/dockets/ac/04/briefing/2004-4065b1-10-TAB08-Hammads-Review.pdf. Published August 16, 2004.
- 21.Bridge JA, Iyengar S, Salary CB, et al. Clinical response and risk for reported suicidal ideation and suicide attempts in pediatric antidepressant treatment: a meta-analysis of randomized controlled trials. JAMA. 2007;297(15):1683–1696. doi: 10.1001/jama.297.15.1683. [DOI] [PubMed] [Google Scholar]
- 22.Correll CU, Penzner JB, Parikh UH, et al. Recognizing and monitoring adverse events of second-generation antipsychotics in children and adolescents. Child Adolesc Psychiatr Clin N Am. 2006;15(1):177–206. doi: 10.1016/j.chc.2005.08.007. [DOI] [PubMed] [Google Scholar]
- 23.Olfson M, Marcus SC, Weissman MM, Jensen PS. National trends in the use of psychotropic medications by children. J Am Acad Child Adolesc Psychiatry. 2002;41(5):514–521. doi: 10.1097/00004583-200205000-00008. [DOI] [PubMed] [Google Scholar]
- 24.Greenhill LL, Vitiello B, Fisher P, et al. Comparison of increasingly detailed elicitation methods for the assessment of adverse events in pediatric psychopharmacology. J Am Acad Child Adolesc Psychiatry. 2004;43(12):1488–1496. doi: 10.1097/01.chi.0000142668.29191.13. [DOI] [PubMed] [Google Scholar]
- 25.Zito JM, Safer DJ, dosReis S, Gardner JF, Boles M, Lynch F. Trends in the prescribing of psychotropic medications to preschoolers. JAMA. 2000;283(8):1025–1030. doi: 10.1001/jama.283.8.1025. [DOI] [PubMed] [Google Scholar]
- 26.TADS Study Team. Fluoxetine, Cognitive-Behavioral Therapy, and Their Combination for Adolescents With Depression: Treatment for Adolescents With Depression Study (TADS) Randomized Controlled Trial. JAMA. 2004;292(7):807–820. doi: 10.1001/jama.292.7.807. [DOI] [PubMed] [Google Scholar]
- 27.Compton SN, Walkup JT, Albano AM, et al. Child/Adolescent Anxiety Multimodal Study (CAMS): rationale, design, and methods. Child Adolesc Psychiatry Ment Health. 2010;4:1–15. doi: 10.1186/1753-2000-4-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kendall PC, Compton SN, Walkup JT, et al. Clinical characteristics of anxiety disordered youth. J Anxiety Disord. 2010;24(3):360–365. doi: 10.1016/j.janxdis.2010.01.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Guy W. The ECDEU Assessment Manual for Psychopharmacology-Revised. Rockville, MD: US Dept. of Health, Education, and Welfare, Public Health Service, Alcohol, Drug Abuse, and Mental Health Administration, National Institute of Mental Health, Psychopharmacology Research Branch, Division of Extramural Research Programs; 1976. [Google Scholar]
- 30.Geller DA, Wagner KD, Emslie G, et al. Paroxetine treatment in children and adolescents with obsessive-compulsive disorder: a randomized, multicenter, double-blind, placebo-controlled trial. J Am Acad Child Adolesc Psychiatry. 2004;43(11):1387–1396. doi: 10.1097/01.chi.0000138356.29099.f1. [DOI] [PubMed] [Google Scholar]
- 31.Wehmeier PM, Schacht A, Lehmann M, Dittmann RW, Silva SG, March JS. Emotional well-being in children and adolescents treated with atomoxetine for attention-deficit/hyperactivity disorder: findings from a patient, parent and physician perspective using items from the pediatric adverse event rating scale (PAERS) Child Adolesc Psychiatry Ment Health. 2008;2(1):11. doi: 10.1186/1753-2000-2-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Fisher PW, Lake AM, Hundt SR, et al. Columbia Health and Adverse Reactions to Medication Screen (CHARMS): A computerized measure for identifying possible adverse events. Poster presented at the 57th Annual Meeting of the American Academy of Child and Adolecent Psychiatry; October 26-31, 2010; New York, NY. [Google Scholar]
- 33.Taylor CB, Chang VY. Issues in the dissemination of cognitive-behavior therapy. Nord J Psychiatry. 2008;62:37–44. doi: 10.1080/08039480802315673. [DOI] [PubMed] [Google Scholar]
- 34.POTS Treatment Study Team. Cognitive-behavior therapy, sertraline, and their combination for children and adolescents with obsessive-compulsive disorder: the Pediatric OCD Treatment Study (POTS) randomized controlled trial. JAMA. 2004;292(16):1969–1976. doi: 10.1001/jama.292.16.1969. [DOI] [PubMed] [Google Scholar]