Abstract
The Val158Met rs4680 polymorphism in the COMT gene regulates dopamine catabolism in the prefrontal cortex (PFC). Dopamine’s involvement in reward experience suggests those with the Met variant may exhibit trait-level sensitivity to reward due to more post-synaptic dopamine in the PFC. A physiological mediator of this association may be greater relative left asymmetry in the PFC, a putative biomarker for trait positive emotionality. Electroencephalograms of 120 participants were measured during a task that assesses two aspects of reward processing: pre-reward anticipation and post-reward consummatory affect. Participants provided genetics samples and completed the Temporal Experience of Pleasure Scale, which assesses trait-level anticipatory and consummatory positive affect. Met carriers had higher TEPS-Consummatory scores. This effect was mediated by greater relative left activation in the post-reward phase of the task. No effects were observed for the pre-reward phase. Results suggest that frontal asymmetry is an endophenotype between COMT genotype and trait reward responsivity.
Keywords: COMT, consummatory positive affect, EEG, reward processing, dopamine
One of the major functions of dopamine is to mediate reward-related processing in the prefrontal cortex (Miller, 2000). For example, dopaminergic neurons in the ventral tegmental area of the midbrain that innervate the prefrontal cortex may generate action potentials in response to unpredicted rewards (Mirenowicz & Schultz, 1994, 1996; Williams & Goldman-Rakic, 1993; Haber and Fudge, 1997). It is noteworthy that with more exposure to a particular reward, these neurons fire in response to cues that predict reward, rather than the reward itself (Schultz, 1998). The evidence for dopamine’s action before and after receipt of reward makes it unclear if dopamine is primarily associated with anticipation of reward, consummation of reward, or both processes (Wise, 2004), and there have been many shifts in the view of dopamine’s role in reward processing (Berridge, 2007).
Dopamine’s role in reward processing may be moderated by the amount of dopamine transmitted by neurons in the prefrontal cortex. Many genes affect dopamine transmission in the brain, including the COMT gene. COMT codes for catechol-O-methyltranferase (COMT), an enzyme that degrades extracellular dopamine in the synapse (Männistö & Kaakkola, 1999). Most importantly, variability in this gene contributes to differential efficiency of this enzyme. One allelic variant in COMT, Val158Met, is a single-nucleotide polymorphism (SNP) that replaces guanine with adenine, which results in an amino acid change from valine (Val) to methionine (Met) at codon 158. This change yields a more thermally unstable form of COMT, resulting in significantly lower enzymatic activity in the prefrontal cortex (Lachman et al., 1996; Chen et al., 2004). Thus, individuals with the methionine allele have higher levels of synaptic dopamine in their prefrontal cortex, resulting in more dopaminergic stimulation of post-synaptic neurons.
Given that the effect of this polymorphism on the amount of synaptic dopamine in the brain is fairly well understood, studies have also examined its psychological and behavioral associations. For example, there is evidence that people with the Met variant exhibit significantly higher reward responsiveness and reward-seeking behavior during reward tasks and report higher subjective ratings of pleasure in response to positive events than those with the Val polymorphism (Lancaster, Linden & Heerey, 2012; Wichers et al., 2007).
The positive affect associated with reward has two phases: anticipatory positive affect (APA) and consummatory positive affect (CPA). APA is experienced pre-reward and reflects motivation and/or a desire for a stimulus, whereas CPA occurs post-reward and reflects the in-the-moment experience of pleasure or the hedonic effect of a stimulus. The two affective states also parallel Berridge and Robinson’s (2003) distinction between ‘wanting’ and ‘liking’ and have been further delineated by Klein (1974; see also Sherdell, Waugh, & Gotlib, 2012), who described the clinical impact of deficits in APA versus CPA in subtypes of depression.
Although APA and CPA both occur during reward processing, people vary on how much APA and CPA they experience during each phase. Gard and colleagues (2006) developed a self-report inventory to measure individual differences in trait-level APA, CPA, and overall experiences of pleasure, the Temporal Experience of Pleasure Scale (TEPS). Studies with the TEPS suggest that the three subscales correlate with each other; but nevertheless demonstrate discriminant validity in their association with other measures of personality and in distinguishing various psychopathologies (Gard, Gard, Kring & John, 2006; Gard, Kring, Gard, Horan, & Green, 2007 Favrod, Ernst, Giuliani, Bosnack, 2009; Chan et al., 2010; Strauss, Wilbur, Warren, August, & Gold, 2011).
Given that dopamine is a key neurotransmitter involved in reward processing, individual differences in dopamine levels in the brain might predict individual differences in APA and/or CPA. One way to investigate this question is to examine whether COMT genotype predicts scores on the TEPS. If people with the Met allele, who have less COMT enzymatic activity, regularly retain more dopamine in their prefrontal cortex, their subjective ratings of APA and/or CPA would be hypothesized to be higher than those with the Val allele.
However, because the subjective experiences of APA and CPA occur quite downstream from the COMT genotype, it is important to consider physiological mediators of this relationship (Sanislow et al., 2010), or endophenotypes (Gottesman & Gould, 2003). A potential endophenotype of reward processing is asymmetric EEG activity in the prefrontal cortex (Stewart et al., 2010). For several decades, affective scientists have theorized that asymmetric activity in the frontal brain reflects differences in affective reactivity (e.g., Davidson, 2004; Shankman & Klein, 2003). Specifically, a pattern of greater relative left activity recorded during a resting state has repeatedly been associated with approach-related tendencies as observed in behavioral tasks (Tomarken, Davidson & Henriques, 1990) self-report (Wheeler, Davidson & Tomarken, 1993; Sutton & Davidson, 1997), and reward learning tasks (Pizzagalli, Sherwood, Henriques & Davidson, 2005). Deviation from this resting pattern of prefrontal asymmetry may also be a biological marker of an affective style that predisposes individuals to major depression (Gotlib, Ranganath & Rosenfeld, 1998; Henriques & Davidson 1990).
EEG asymmetry has typically been recorded during a resting state, but recently, researchers have argued that it yields better associations with affective style if it is recorded during appetitive and other emotional tasks (Coan, Allen & McKnight, 2006; Dennis & Solomon, 2010). As such, measuring prefrontal asymmetry during a reward task might also represent a psychophysiological mediator between COMT genotype and trait differences in APA and CPA. The present study therefore measures EEG asymmetry during a slot machine task developed by Shankman and colleagues (2007; 2013) that separates the anticipatory and consummatory phases of reward processing (Shankman, Sarapas & Klein, 2011).
To the authors’ knowledge, only one prior study has examined the relationship between trait approach motivation, prefrontal asymmetry, and COMT genotype (Wacker, Mueller, Pizagalli, Hennig & Stemmler, 2013). Wacker and colleagues found an association between greater relative left prefrontal asymmetry (as measured with resting EEG in a quasi-experimental manipulation of the emotional context – the presence of an attractive v. non-attractive female experimenter), greater self-reported levels of trait behavioral approach, and the G/G (i.e. Val/Val) allele. The present study extends this work in two important ways. First, it measures prefrontal asymmetry during a reward task, rather than in a subtle positively valenced context. Second, it utilizes a measure specific to the phases of reward processing.
Present Study
The present study examined the relationship between COMT genotype and individual differences in APA and CPA and whether prefrontal asymmetry during a reward task mediated this relationship. We hypothesized that individuals possessing the Met allele, known to be related to more post-synaptic dopamine in their prefrontal cortex, would have higher scores on the TEPS. We expected prefrontal asymmetry recorded during a reward task (i.e., the slot machine paradigm described above) to mediate this relationship, such that possession of the Met allele would predict greater relative left activation during the task, which would in turn contribute to higher scores on the TEPS. Because we examined the anticipatory and consummatory phases separately, our results may elucidate the role of dopamine during these two phases of reward processing.
Method
Participants
Participants were 131 students enrolled in Introduction to Psychology at the University of Illinois at Chicago who received course credit for participation. Eleven people were excluded due to unusable EEG data (see below), which left a sample of 120 for analyses described herein. Given the associations between hemispheric asymmetry, emotional reactivity, and handedness, all participants were right-handed. Participants were mean age of 19.4 (SD = 2.05) years old. The sample contained 58.8% females. The ethnic composition was quite diverse: 37.4% Caucasian, 28.2% Latino, 22.9% Asian, and 11.5% African America. As shown in Table 1, none of these variables were associated with COMT genotype.
Table 1.
Sample Characteristics and Relationship to COMT Genotype
| Variable | COMT Genotype | Relation to COMT Genotype | |
|---|---|---|---|
| G/G | A-carrier | ||
| Gender (n) | |||
| Female | 26 | 51 | χ2 female = 0.44, ns |
| Male | 16 | 27 | χ2 male = 0.046, ns |
|
| |||
| Race (n) | |||
| Caucasian | 11 | 33 | χ2 = 0.00, ns |
| Latino | 12 | 22 | χ2 = 0.027, ns |
| Asian | 14 | 14 | χ2 = 0.14, ns |
| African American | 5 | 9 | χ2 = 0.03, ns |
|
| |||
| Hemispheric Asymmetry (M) | |||
| Pre-goal | .001 | −.004 | t(118) = −0.037, ns |
| Post-goal | −.013 | .024 | t(118) = 1.83, p = .07 |
Measures
Anticipatory and Consummatory Positive Affect
The 18-item TEPS was used to measure anticipatory and consummatory positive affective tendencies (Gard et al., 2006). Participants rated each item from 1 (very false for me) to 6 (very true for me); from their responses, TEPS-Anticipatory and TEPS-Consummatory scores were determined. Consistent with previous research (Gard et al., 2006), this scale had acceptable reliability (TEPS-Ant: α = .69, TEPS-Con: α = .69, TEPS-Total: α = .81).
Handedness
Although participants were pre-screened for handedness during recruitment, the Edinburgh Handedness Scale (Oldfield, 1971) was used to confirm laterality (range of laterality quotient +30 to +100).
Procedure
Slot Machine Task
Each participant played a computerized slot machine game (Shankman et al., 2007; 2013) that consisted of three reels that displayed fruit and numbers. The reels spun simultaneously for 11 s (anticipatory phase) and displayed the results for 11 s (consummatory phase; see Table 2). The game consisted of 72 spins, which were divided into three different pay-off situations: reward (R, 30 trials), during which participants won money if the reels landed on 3 pieces of fruit; no incentive (NI, 30 trials), in which participants were ineligible to win money regardless of outcome; and loss (L, 12 trials), in which participants lost money when the reels landed on 3 pieces of fruit. Although only the R and NI conditions are necessary to examine online reward processing, results from pilot testing revealed that a win in the R condition was more exciting if there were L trials in the game (Shankman et al., 2013). Thus, the L condition was included to keep the participant engaged in the task. The amount of money won or lost for each trial varied from $0.50 to $3.00. The game was divided into 3 blocks of 24 spins, and participants had a short break in between blocks during which they rated their emotions (see below).
Table 2.
Design of Slot Machine Paradigm (adapted from Shankman et al., 2007; 2013)
| Condition | No. of trials | Time in trial | Result and outcome | No. of trials | Time in trial |
|---|---|---|---|---|---|
| Reward | 30 | 11 s | Win – win $ 
|
15 | 11 s |
Lose – no win $ 
|
15 | 11 s | |||
| No Incentive | 30 | 11 s | Win – no win $ 
|
15 | 11 s |
Lose – no lose $
|
15 | 11 s | |||
| Loss | 12 | 11 s | Lose – lose $ 
|
6 | 11 s |
No lose – no lose $
|
6 | 11 s | |||
|
| |||||
| Totals | 72 |
792 s in ‘pre-
goal phase’ |
72 |
792 s in ‘post-
goal phase’ |
|
Participants were told that the trial order was random to generate authentic anticipatory and consummatory positive affect; however, unbeknownst to the participant, the trials were fixed, in that half of the spins landed on 3 pieces of fruit. They received their winnings (~$12.00) in cash after completing all 72 trials.
Data Processing
EEG recording and processing
EEG data were recorded from Ag/AgCl electrodes in a 64-channel stretch-lycra electrode cap (Compumedics Neuroscan 4.4, Charlotte, NC). The ground electrode was at the frontal pole (AFZ) and the online reference was near the vertex (between CZ and CPZ). Electrodes placed at the right supra- and infraorbital sites were used to monitor vertical eye movements (VEOG) and electrodes placed at the right and left outer canthi were used to monitor horizontal eye movements (HEOG). Electrode impedances were under 5,000 ohms, and homologous sites (e.g., F3/F4) were within 1,500 ohms of each other. Data were recorded through a Neuroscan Synamp2 data acquisition system at a gain of 10K (5K for eye channels) with a bandpass of DC-200 Hz. Data were acquired and digitized continuously at a rate of 1000 Hz.
Continuous EEG during the pre and post-goal phases were segmented into consecutive 1.024 s epochs every 0.512 s (50% overlap). After re-referencing to a digitally derived “linked mastoid” reference offline and then applying a baseline correction, epochs contaminated by blinks, eye movements, and movement-related artifacts were excluded from analyses manually, by direct visual inspection of the data. The EEG was tapered over the entire 1.024 s epoch by a Hanning window to suppress spectral side lobes. After removal of contaminated epochs, the average activity in the alpha power band for each electrode in each condition of the task were calculated and log transformed to normalize the skewed and kurtotic distributions. Consistent with previous studies (e.g., Bruder, et al., 1997), the alpha band was defined as activity between 8-13 Hz and was used as an inverse measure of brain activation at a particular site.
Hemispheric asymmetry
To examine hemispheric asymmetry as a mediator, we first subtracted alpha power on the left from alpha power in the homologous right electrode (e.g., F4 – F3) for each condition. Higher values on these asymmetry scores reflect greater activity in the left relative to right prefrontal regions and this measure controls for individual differences in overall alpha power and scalp thickness (Allen, Coan & Nazarian, 2004). Similar to prior studies with this sample (Sarapas et al., 2013; see also Shankman et al., 2013 for other studies with the task), to examine the change in asymmetry between the experimental (R) and control (NI) conditions, we subtracted the asymmetry scores in the NI condition from asymmetry scores in the R condition for each phase of the task. Thus, the variable that remains is the relative difference in asymmetry between the R and NI condition (i.e., ‘reward potentiated asymmetry’).
Genotyping
Each participant provided a saliva sample for genotype analyses using Oragene collection kits (DNA Genotek, Kanata, Ontario, Canada). Genomic DNA was isolated according to the manufacturer’s specification. A sequence validated TaqMan (Applied Biosystems) assay was utilized for genotyping which was done blind to behavioral data. We compared G/G homozygotes (i.e., individuals homozygous for the Val allele) to A/A homozygotes and A/G heterozygotes (i.e., carriers of the Met allele). Examining genotype as a three-level variable indicated that A/A homozygotes and heterozygotes displayed nearly identical patterns of results for both TEPS-Consummatory scores and asymmetry, and both groups differed from the G/G homozygotes at a trend level. Therefore, we collapsed across A-carriers and A/A homozygotes to increase statistical power.
Data Analysis
All statistical analyses were conducted using SPSS 20.0. To examine the relationship between COMT genotype and personality (i.e., c path of the mediation model), we conducted a one-way analysis of variance (ANOVA) for comparing GG homozygotes to A-carriers on TEPS-Anticipatory and TEPS-Consummatory scores. We performed another one-way ANOVA to look at the relationship between COMT genotype and prefrontal asymmetry during each phase of the task (i.e., a path of the mediation model). We conducted linear regression analyses to examine whether prefrontal asymmetry in each phase of the task predicted TEPS Anticipatory and Consummatory scores, respectively (i.e., b path of the mediation model).
We took two approaches to determining the effect of ethnicity on COMT genotype. First, we employed a chi-square analysis to examine allelic distributions within each ethnicity. Second, we used hierarchical linear regression to look at the effects of specific ethnicities and genotype-by-ethnicity interactions on TEPS-Consummatory scores (See Table 3). Three dummy-codes for ethnicity were created to reflect membership in African-American, Latino, and Asian ethnic groups, with Caucasian serving as the reference group. Any significant main effects of ethnicity or genotype-by-ethnicity interactions would be included as covariates in the primary analyses.
Table 3.
Effects of Ethnicity and Genotype-by-Ethnicity Interaction on TEPS-Consummatory Scores
| Variables Entered | β | p |
|---|---|---|
| Model 1 | ||
| African American | −.181 | .048* |
| COMT genotype | −.172 | .058+ |
| Af. Am X COMT | −.255 | .356 |
| Model 2 | ||
| Latino | −.535 | .052+ |
| COMT genotype | −.261 | .017* |
| Latino X COMT | .442 | .117 |
| Model 3 | ||
| Asian | .131 | .654 |
| COMT genotype | −.180 | .054+ |
| Asian X COMT | −.097 | .750 |
To examine whether hemispheric asymmetry mediated the relationship between genotype and trait differences in anticipatory and consummatory positive affect, we used Hayes and Preacher’s (2013) MEDIATE macro for SPSS in two mediation models. In each model, dichotomous COMT genotype was the independent variable. In the APA model, TEPS-Anticipatory score was the dependent variable, and asymmetry during the pre-goal phase was the mediator. In the CPA model, TEPS-Consummatory score was the dependent variable, and asymmetry during the post-goal phase was the mediator. For all analyses, age and gender were also included as covariates. Effects of ethnicity (a critical variable in genetics studies) were examined thoroughly and are described below.
Results
Genetic Distribution
Genotype counts for COMT Val158Met were 22 A/A, 56 A/G and 42 G/G. Genotypes did not deviate from Hardy-Weinberg Equilibrium in the entire sample or by ethnic group (all ps > .25).
EEG Descriptives
A mean of 1,530 (SD = 513) epochs were kept and used for analyses. Bivariate correlations revealed that the total number of accepted epochs was not systematically related to post-goal asymmetry or TEPS scores (all ps > .18), and results from one-way ANOVAs showed that the total number of accepted epochs did not differ across COMT genotype or by condition (all ps > .29).
CPA Model
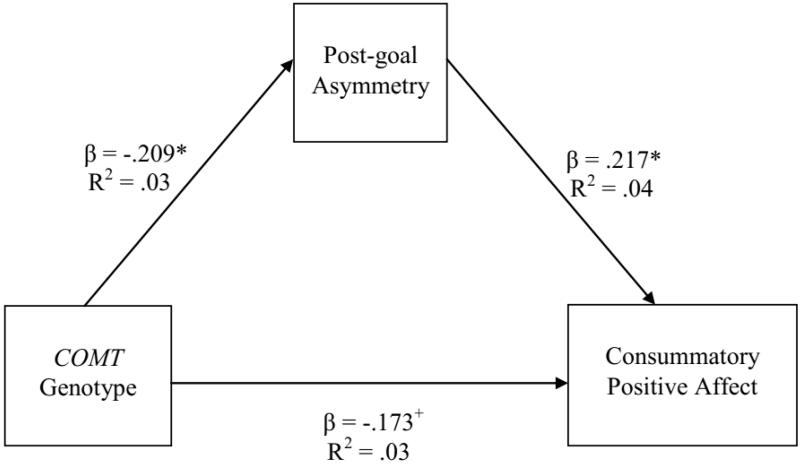
Three pathways were examined in the CPA mediation model (See Fig. 1). First, we examined the relationship between COMT genotype and TEPS-Consummatory scores (i.e., the c path). Although A-carriers had higher TEPS-Consummatory scores than GG-homozygotes, this result only approached significance, F(1, 117) = 3.68, p = .058. COMT genotype did predict differences in prefrontal asymmetry1 during the consummatory phase of the task (i.e., the a path), such that A-carriers exhibited greater reward-potentiated asymmetry than GG-homozygotes, F(1, 117) = 4.41, p < .05. Finally, prefrontal asymmetry during the consummatory phase of the task predicted TEPS-Consummatory scores (i.e., the b path), with greater reward-potentiated asymmetry during the consummatory phase of the task scored higher on the TEPS-Consummatory scale (β = .217, p < .05).
Figure 1.
Mediation model between COMT genotype, asymmetry in the post-goal phase of slot machine task, and self-reported consummatory positive affect with statistical significance and effect sizes for each path.
Most importantly, mediational analyses indicated that COMT genotype exerted a significant indirect effect on TEPS-Consummatory scores, as the c path between COMT and TEPS-Consummatory was mediated by asymmetry in the post-goal phase of the task (B = −0.73, 95% Confidence Interval [CI]: −1.82 to −.05)2. To see if either the R or NI condition was driving the mediation effect, we also conducted separate mediation analyses with asymmetry in the R and NI conditions as the mediator, and neither was significant (CIs contained 0). Rather, it was reward-potentiated asymmetry (i.e., the relative difference in asymmetry between the R and NI conditions) driving the mediation effect. Because these data are cross-sectional, we also examined whether TEPS-Consummatory scores mediated the relationship between COMT genotype and prefrontal asymmetry. However, the model did not reach significance (CI contained 0). As indicated above, gender and age were included as covariates in all regression analyses and contributed no unique variance t0 the dependent variable (all ps > .17).
APA Model
Results from analogous analyses in the anticipatory phase of the task did not yield significant results for any path involved in the mediation model. Specifically, the c path for the effect of COMT genotype on TEPS-Anticipatory scores was not significant, F(1, 117) = 0.00, n.s. The component parts (i.e., the a and b paths) of the mediation model were not significant either. That is, COMT genotype did not predict prefrontal asymmetry during the anticipatory phase of the task, F(1, 117) = 0.14, n.s. , and prefrontal asymmetry did not predict TEPS-anticipatory scores (β = −.02, n.s.). Because none of the proposed paths of the mediation model were significant, test of mediation were not performed.
Effect of Ethnicity on TEPS Scores
We took several approaches to examining whether the observed phenotypic differences associated with COMT did not merely reflect different ethnic groups’ genetic distributions.
First, we examined the allelic distributions that were associated with each ethnic group. A chi-square analysis comparing the allelic ratios across ethnic groups indicated that the ratios of A/A, A/G and G/G did not differ by ethnic group, χ2 = 4.71, n.s. Results from the regression analyses indicated significant main effects of Latino and African American group membership on TEPS-Consummatory scores, but no significant genotype-by-ethnicity interactions. Additionally, when Latino and African American group membership were included as covariates, COMT still predicted TEPS-Consummatory scores (c-path coefficient: β = −0.243, p < .05), and the mediation effect was still observed (i.e., the CI did not contain 0).
Discussion
Results from this study indicate that prefrontal hemispheric asymmetry mediated the relationship between COMT genotype and trait differences in positive affectivity. Specifically, the Met variant was associated with higher TEPS-Consummatory scores, and that effect was mediated by greater relative activation in the left hemisphere of the prefrontal cortex after receipt of a reward.
These results support previous findings of positive associations between the Met polymorphism and reward responsiveness (Lancaster, Linden & Heerey, 2012; Wichers et al., 2007) and may have important implications for the development of psychopathology. Reward responsivity is one of the transdiagnostic domains outlined in NIMH’s Research Domain Criteria (Insel et al., 2010; Sanislow et al., 2010). Indeed, associations between COMT genotype and psychiatric disorders characterized by deficits in reward responsiveness, such as major depressive disorder (MDD) and substance use disorders (SUDs), have been examined. For example, the reward deficiency hypothesis (Blum, Cull, Braverman, & Comings, 1996) posits that individuals who experience less pleasure in response to natural rewards (e.g., food, sex, and social interaction) are more likely to seek sensations of pleasure from unnatural rewards (i.e., addictive substances), thereby incurring greater risk for developing substance use disorders. Genetic studies that have tested this theory have implicated genes involved in dopamine synthesis, degradation, and transport, such as COMT and DRD4 (Comings & Blum, 2000), and indeed reported links between the Val allele and nicotine dependence (Beuten, Payne, Ma, & Li, 2006), methamphetamine abuse (Li et al., 2004), and polysubstance abuse (Vandebergh, Miller, Uhl, & Lachman 1997). Thus, the increased consummatory pleasure associated with the COMT Met allele may be protective against development of substance use disorders. However, several studies have also found the Met allele to be associated with risk-taking (Lancaster et al., 2012; Heitland et al., 2012) or higher levels of sensation seeking (at least in females: Amstadter et al., 2012; Lang, Bajbouj, Sander, & Gallinat, 2007) and impulsivity (Soeiro-De-Souza, Stanford, Bio, Machado-Viera, Moreno, 2013), which may contribute to risk for development of SUDs. Studies have also differed on the contribution of COMT genotype to the development of MDD (Funke et al., 2005; Smolka et al., 2005; Henderson et al., 2000). As such, more research is necessary to elucidate the likely complex relationship between COMT genotype and development of psychopathology.
The current findings are further supported by evidence for the localization of COMT to activity in the prefrontal cortex. Both the animal and human literature have demonstrated that COMT moderates dopamine neurotransmission in the prefrontal cortex (Karoum, Chrapusta & Egan, 1994), and that COMT is expressed more in neurons found in the prefrontal cortex (Matsumuto et al., 2003). For example, an imaging genetics study found that the Met variant of the COMT genotype was associated with greater activation in the orbitofrontal cortex upon receipt of reward (Dreher, Kohn, Kolachana, Weinberger, & Berman,, 2009). Taken together, this evidence corroborates the present study’s demonstrated link between COMT genotype, prefrontal asymmetry in the post-goal phase, and self-reported consummatory positive affect.
Our findings indicate that COMT genotype is associated specifically with the consummatory phase of reward processing—seen both in its relationship with prefrontal asymmetry during the post-goal phase of the slot machine task and trait differences in consummatory positive affect reported on the TEPS-Consummatory scale. Other psychophysiological examinations of dopamine and reward have also suggested dopamine’s association with consummatory processes. For example, studies of nonhuman primates have demonstrated that dopaminergic neurons in the midbrain fire both during anticipation and upon receipt of reward (Fiorillo, Tobler & Schultz, 2003). Similarly, fMRI studies in humans have shown activation of the prefrontal cortex in response to receipt of a reward (Knutson et al., 2003; Dreher, Kohn & Berman, 2006), whereas dopaminergic neurons in the ventral striatum are more responsive to anticipation of reward (Knutson, Fong, Bennet, Adams, & Hommer, 2003; O’Doherty, Diechmann, Critchley, & Dolan, 2002). Although others have postulated that dopamine may be specifically involved in anticipatory processes (see Berridge, 2007 for review), the positive associations between dopamine functioning and consummatory processes observed in the current study could be explained by the fact that we were examining prefrontal asymmetry in the context of reward processing, where COMT has been implicated.
One possible mechanism to explain the relationship between COMT genotype, prefrontal asymmetry in the post-goal phase of reward processing, and trait consummatory positive affect is the opioid system. μ-opioids have been linked to hedonic experience, or ‘liking’ (Barbano & Cador, 2007; Berridge, 2003), and COMT genotype has been linked to the μ-opioid receptor system. For example, post-mortem studies of the human brain have demonstrated that the Met variant of COMT is linked to increased numbers of μ-opioid receptor binding sites (Kowarik et al., 2012; Berthele et al., 2005). Most of the studies examining the effect of COMT genotype on the μ-opioid receptor system have focused on pain and have found the Met allele to be associated with increased experience of pain (e.g., Zubieta et al., 2003). Given the overlapping neurobiology of pleasure and pain (Leknes & Tracey, 2008), it is possible that individuals with the Met allele of the COMT are more sensitive and reactive to environmental stimuli, which may help explain why they are more responsive to reward and more sensitive to pain.
Several prior studies have failed to link dopamine neurotransmission and prefrontal asymmetry (e.g., Schmidt, Fox, Perez-Edgar & Hamer, 2009; Wacker & Stemmler, 2006). These studies, however, measured prefrontal asymmetry during a resting condition. Interestingly, recent evidence suggests that relationships between the two variables may be more observable during an experimental context that manipulates approach motivation, similar to other capability models postulated by affective scientists (Coan, Allen & McKnight, 2006; Dennis & Solomon, 2010). For example, as previously discussed, one study found that the associations between prefrontal asymmetry, behavioral approach tendencies, and COMT genotype depended on the situational context in which the resting EEG was recorded – specifically, the attractiveness of the experimenter (Wacker et al., 2013).
The present study contained important methodological distinctions from the Wacker and colleagues’ (2013) investigation that point to specific aspects of dopaminergic functioning in the context of reward. First, instead of quasi-experimentally manipulating the affective context of the EEG recording situation (i.e., asking participants to retrospectively rate the attractiveness of the experimenter one year after testing and using that rating as measure of experimental context), we measured EEG asymmetry during a reward task. This not only allowed us to examine prefrontal asymmetry associated with reward, but also the separate patterns of asymmetry that occur during the anticipatory and consummatory phases of reward processing. Furthermore, rather than broadly measuring positive affectivity or behavioral approach (e.g., positive emotionality scale of the General Temperament Survey, Clark & Watson, 1990; or the BIS/BAS Scale, Carver & White, 1994), the present study used the TEPS which closely aligns with the two phases of the reward task. The present results also diverge from those of Wacker et al. (2013), who found that the G/G variant of COMT Val158Met was associated with greater prefrontal asymmetry, whereas we found that the A-carrier variants predicted greater prefrontal asymmetry. Given the relative infancy of the work linking genetics, psychophysiological endophenotypes, and personality phenotypes associated with reward processing, more research is needed to clarify the relationship between these variables.
Although the a- and b-paths of the CPA model, as well as the full mediational model, were significant, the overall c-path (i.e., the direct effect of COMT genotype on TEPS-Consummatory scores), only approached significance. This may be due to the fact that consummatory positive affect, based on responses to the self-report TEPS-Consummatory scale, occurs quite “downstream” from the effects of COMT genotype. In other words, all of the intermediate steps between genotype and self-reported personality phenotype (e.g., genotype, proteins, cellular activity, brain structures, brain systems, behaviors and cognition, personality) may reduce the power of a direct effect from being observed. Therefore, it is important to investigate intermediate phenotypes, such as prefrontal hemispheric asymmetry, whose intermediate effects can elucidate these complex pathways (Rasetti & Weinberger, 2011; Sanislow et al., 2010; Kendler & Neale et al., 2010).
Contemporary views of statistical mediation can also help explain why a significant effect of COMT genotype on TEPS-Consummatory scores was not observed. Modern conceptualizations of mediation do not require the c-path to reach significance to proceed with tests of mediation (MacKinnon, Lockwood, Hoffman, West & Sheets, 2002; Shrout & Bolger, 2002; Hayes, 2009). In some cases, such as instances of partial mediation, there may other (i.e., unmeasured) mediators exercising the opposite effect of the observed mediator, essentially “canceling out” the direct effect.
Strengths and Limitations
This study contained several strengths. First, the sample was relatively large for a psychophysiological study and had a broad range of positive affective responses, thereby allowing us to use the task to examine individual differences in personality. Second, the within-subjects design of the slot machine game afforded greater power to look at hemispheric asymmetry and its relationship to COMT genotype and personality variables. Third, this study examined reward responsiveness at three levels of analysis—genotype, psychophysiology, and personality—and found a significant meditational effect for psychophysiology, highlighting a potential endophenotype for consummatory positive affect. Finally, this study provided psychophysiological validation for the TEPS-Consummatory scale (Gard et al., 2007). Since its inception, the scale has been widely used in assessing anticipatory and consummatory pleasure in clinical populations, particularly individuals with schizophrenia (Gard et al., 2007; Horan, Kring & Blanchard, 2006) and major depression (Liu et al., 2011; Pizzagalli et al., 2009). This study demonstrates that this scale is useful for the study of personality in nonclinical samples, and that the scale maps onto genetic and psychophysiological correlates of reward processing.
Despite these strengths, this study contained limitations as well. The sample size and ethnic diversity of the sample were not ideal for genetic analyses, although we still found effects consistent with our hypothesis. A second limitation is that the mediation analysis was cross-sectional which eliminates concluding causality of the model (MacKinnon, Fairchild, & Fritz, 2007). A third limitation is that the sample was obtained from an undergraduate Introduction to Psychology course, which may call into question the generalizability of the results.
Conclusion
This study demonstrated important links between COMT genotype, prefrontal asymmetry during the consummatory phase of reward processing, and self-reported trait-level consummatory positive affect. The present findings provide psychophysiological validation of the TEPS and may help explain how COMT genotype contributes to risk for psychopathology, specifically major depression and substance use disorders. Finally, the results described here highlight the role of dopamine in consummatory processes in the prefrontal cortex.
Acknowledgements
This work was supported by the National Institutes of Health under Grants R21MH080689 and R01MH098093 and the UIC Chancellor's Discovery Fund for Multidisciplinary Pilot Research.
Footnotes
We also examined the relationship between COMT genotype, TEPS-Consummatory scores and hemispheric asymmetry in the post-goal phase in posterior electrodes, focusing on the parietal region. We did not find an association between posterior asymmetry in the post-goal phase and TEPS-Consummatory scores (all p > .52). Additionally, there was no significant association between COMT genotype and posterior asymmetry (all p > .65)
When TEPS-Anticipatory score was included as a covariate in the CPA mediation model, the model became insignificant (i.e., the confidence interval contained 0). This is likely due to the high correlation between the TEPS-Anticipatory and Consummatory scores (r = .60), which impedes finding statistical specificity when both variables are included in the model. That being said, when TEPS-Anticipatory score was included as a covariate in the univariate ANOVA testing the c path (i.e., COMT genotype predicting TEPS-Consummatory score) and regression testing the b path (i.e., prefrontal asymmetry in the post-goal phase predicting TEPS-Consummatory score) the effects remained significant.
References
- Allen JJ, Coan JA, Nazarian M. Issues and assumptions on the road from raw signals to metrics of frontal EEG asymmetry in emotion. Biological Psychology. 2004;67(1):183–218. doi: 10.1016/j.biopsycho.2004.03.007. [DOI] [PubMed] [Google Scholar]
- Amstadter AB, MacPherson L, Wang F, Banducci AN, Reynolds EK, Potenza MN, Lejuez CW. The relationship between risk-taking propensity and the COMT Val158Met polymorphism among early adolescents as a function of sex. Journal of Psychiatric Research. 2012;46(7):940–945. doi: 10.1016/j.jpsychires.2012.04.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barbano MF, Cador M. Opioids for hedonic experience and dopamine to get ready for it. Psychopharmacology. 2007;191(3):497–506. doi: 10.1007/s00213-006-0521-1. [DOI] [PubMed] [Google Scholar]
- Berridge KC. The debate over dopamine’s role in reward: the case for incentive salience. Psychopharmacology. 2007;191(3):391–431. doi: 10.1007/s00213-006-0578-x. [DOI] [PubMed] [Google Scholar]
- Berridge KC, Robinson TE. Parsing reward. Trends In Neurosciences. 2003;26(9):507–513. doi: 10.1016/S0166-2236(03)00233-9. [DOI] [PubMed] [Google Scholar]
- Berthele A, Platzer S, Jochim B, Boecker H, Buettner A, Conrad B, Toelle TR. COMT Val108/158Met genotype affects the mu-opioid receptor system in the human brain: Evidence from ligand-binding, G-protein activation and preproenkephalin mRNA expression. Neuroimage. 2005;28(1):185–193. doi: 10.1016/j.neuroimage.2005.05.030. [DOI] [PubMed] [Google Scholar]
- Beuten J, Payne TJ, Ma JZ, Li MD. Significant association of catechol-O-methyltransferase (COMT) haplotypes with nicotine dependence in male and female smokers of two ethnic populations. Neuropsychopharmacology. 2006;31(3):675–684. doi: 10.1038/sj.npp.1300997. [DOI] [PubMed] [Google Scholar]
- Blum K, Cull JG, Braverman ER, Comings DE. Reward deficiency syndrome. American Scientist. 1996:132–145. [Google Scholar]
- Chan RC, Wang Y, Huang J, Shi Y, Wang Y, Hong X, Kring AM. Anticipatory and consummatory components of the experience of pleasure in schizophrenia: cross-cultural validation and extension. Psychiatry Research. 2010;175(1):181–183. doi: 10.1016/j.psychres.2009.01.020. [DOI] [PubMed] [Google Scholar]
- Clark LA, Watson D. The General Temperament Survey. Southern Methodist University; Dallas, TX: 1990. Unpublished manuscript. [Google Scholar]
- Coan JA, Allen JJ, McKnight PE. A capability model of individual differences in frontal EEG asymmetry. Biological Psychology. 2006;72(2):198–207. doi: 10.1016/j.biopsycho.2005.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Comings DE, Blum K. Reward deficiency syndrome: Genetic aspects of behavioral disorders. Progress in Brain Research. 2000;126:325–341. doi: 10.1016/S0079-6123(00)26022-6. [DOI] [PubMed] [Google Scholar]
- Davidson RJ. What does the prefrontal cortex “do” in affect: perspectives on frontal EEG asymmetry research. Biological Psychology. 2004;67(1):219–234. doi: 10.1016/j.biopsycho.2004.03.008. [DOI] [PubMed] [Google Scholar]
- Dennis TA, Solomon B. Frontal EEG and emotion regulation: Electrocortical activity in response to emotional film clips is associated with reduced mood induction and attention interference effects. Biological Psychology. 2010;85(3):456–464. doi: 10.1016/j.biopsycho.2010.09.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dreher JC, Kohn P, Kolachana B, Weinberger DR, Berman KF. Variation in dopamine genes influences responsivity of the human reward system. Proceedings of the National Academy of Sciences. 2009;106(2):617–622. doi: 10.1073/pnas.0805517106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Favrod J, Ernst F, Giuliani F, Bonsack C. Validation of the Temporal Experience of Pleasure Scale (TEPS) in a French-speaking environment] L'Encéphale. 2009;35(3):241. doi: 10.1016/j.encep.2008.02.013. [DOI] [PubMed] [Google Scholar]
- Fiorillo CD, Tobler PN, Schultz W. Discrete coding of reward probability and uncertainty by dopamine neurons. Science. 2003;299(5614):1898–1902. doi: 10.1126/science.1077349. [DOI] [PubMed] [Google Scholar]
- Funke B, Malhotra AK, Finn CT, Plocik AM, Lake SL, Lencz T, Kucherlapati R. COMT genetic variation confers risk for psychotic and affective disorders: a case control study. Behavioral and Brain Functions. 2005;1(1):19. doi: 10.1186/1744-9081-1-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gard DE, Gard MG, Kring AM, John OP. Anticipatory and consummatory components of the experience of pleasure: a scale development study. Journal of Research in Personality. 2006;40(6):1086–1102. [Google Scholar]
- Gard DE, Kring AM, Gard MG, Horan WP, Green MF. Anhedonia in schizophrenia: distinctions between anticipatory and consummatory pleasure. Schizophrenia Research. 2007;93(1):253–260. doi: 10.1016/j.schres.2007.03.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gotlib IH, Ranganath C, Rosenfeld JP. Frontal EEG alpha asymmetry, depression, and cognitive functioning. Cognition and Emotion. 1998;12(3):449–478. [Google Scholar]
- Gottesman II, Gould TD. The endophenotype concept in psychiatry: etymology and strategic intentions. American Journal of Psychiatry. 2003;160(4):636–645. doi: 10.1176/appi.ajp.160.4.636. [DOI] [PubMed] [Google Scholar]
- Haber SN, Fudge JL. The interface between dopamine neurons and the amygdala. Schizophrenia Bulletin. 1997;23(3):471–482. doi: 10.1093/schbul/23.3.471. [DOI] [PubMed] [Google Scholar]
- Hayes AF. Beyond Baron and Kenny: Statistical mediation analysis in the new millennium. Communication Monographs. 2009;76(4):408–420. [Google Scholar]
- Hayes AF, Preacher KJ. Statistical mediation analysis with a multicategorical independent variable. British Journal of Mathematical and Statistical Psychology. 2013 doi: 10.1111/bmsp.12028. [DOI] [PubMed] [Google Scholar]
- Heitland I, Oosting RS, Baas JMP, Massar SAA, Kenemans JL, Böcker KBE. Genetic polymorphisms of the dopamine and serotonin systems modulate the neurophysiological response to feedback and risk taking in healthy humans. Cognitive, Affective, & Behavioral Neuroscience. 2012;12(4):678–691. doi: 10.3758/s13415-012-0108-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henderson AS, Korten AE, Jorm AF, Jacomb PA, Christensen H, Rodgers B, Easteal S. COMT and DRD3 polymorphisms, environmental exposures, and personality traits related to common mental disorders. American Journal of Medical Genetics. 2000;96(1):102–107. doi: 10.1002/(sici)1096-8628(20000207)96:1<102::aid-ajmg20>3.0.co;2-3. [DOI] [PubMed] [Google Scholar]
- Henriques JB, Davidson RJ. Regional brain electrical asymmetries discriminate between previously depressed and healthy control subjects. Journal of Abnormal Psychology. 1990;99(1):22–31. doi: 10.1037//0021-843x.99.1.22. [DOI] [PubMed] [Google Scholar]
- Horan WP, Kring AM, Blanchard JJ. Anhedonia in schizophrenia: a review of assessment strategies. Schizophrenia Bulletin. 2006;32(2):259–273. doi: 10.1093/schbul/sbj009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Insel T, Cuthbert B, Garvey M, Heinssen R, Pine DS, Quinn K, Wang P. Research domain criteria (RDoC): toward a new classification framework for research on mental disorders. American Journal of Psychiatry. 2010;167(7):748–751. doi: 10.1176/appi.ajp.2010.09091379. [DOI] [PubMed] [Google Scholar]
- Karoum F, Chrapusta SJ, Egan MF. 3-Methoxytyramine is the major metabolite of released dopamine in the rat frontal cortex. Journal of Neurochemistry. 1994;63:972–979. doi: 10.1046/j.1471-4159.1994.63030972.x. [DOI] [PubMed] [Google Scholar]
- Kendler KS, Neale MC. Endophenotype: a conceptual analysis. Molecular Psychiatry. 2010;15(8):789–797. doi: 10.1038/mp.2010.8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klein DF. Endogenomorphic depression: a conceptual and terminological revision. Archives of General Psychiatry. 1974;31(4):447. doi: 10.1001/archpsyc.1974.01760160005001. [DOI] [PubMed] [Google Scholar]
- Knutson B, Fong GW, Bennett SM, Adams CM, Hommer D. A region of mesial prefrontal cortex tracks monetarily rewarding outcomes: characterization with rapid event-related fMRI. Neuroimage. 2003;18(2):263–272. doi: 10.1016/s1053-8119(02)00057-5. [DOI] [PubMed] [Google Scholar]
- Kowarik MC, Einhäuser J, Jochim B, Büttner A, Tölle TR, Riemenschneider M, Berthele A. Impact of the COMT Val108/158Met polymorphism on the mu-opioid receptor system in the human brain: Mu-opioid receptor, met-enkephalin and beta-endorphin expression. Neuroscience Letters. 2012;506(2):214–219. doi: 10.1016/j.neulet.2011.11.008. [DOI] [PubMed] [Google Scholar]
- Lachman HM, Papolos DF, Saito T, Yu YM, Szumlanski CL, Weinshilboum RM. Human catechol-O-methyltransferase pharmacogenetics: description of a functional polymorphism and its potential application to neuropsychiatric disorders. Pharmacogenetics and Genomics. 1996;6(3):243–250. doi: 10.1097/00008571-199606000-00007. [DOI] [PubMed] [Google Scholar]
- Lancaster TM, Linden DE, Heerey EA. COMT val158met predicts reward responsiveness in humans. Genes, Brain and Behavior. 2012;11(8):986–992. doi: 10.1111/j.1601-183X.2012.00838.x. [DOI] [PubMed] [Google Scholar]
- Lang UE, Bajbouj M, Sander T, Gallinat J. Gender-dependent association of the functional catechol-O-methyltransferase Val158Met genotype with sensation seeking personality trait. Neuropsychopharmacology. 2007;32(9):1950–1955. doi: 10.1038/sj.npp.1301335. [DOI] [PubMed] [Google Scholar]
- Leknes S, Tracey I. A common neurobiology for pain and pleasure. Nature Reviews Neuroscience. 2008;9(4):314–320. doi: 10.1038/nrn2333. [DOI] [PubMed] [Google Scholar]
- Li T, Chen CK, Hu X, Ball D, Lin SK, Chen W, Collier DA. Association analysis of the DRD4 and COMT genes in methamphetamine abuse. American Journal of Medical Genetics Part B: Neuropsychiatric Genetics. 2004;129(1):120–124. doi: 10.1002/ajmg.b.30024. [DOI] [PubMed] [Google Scholar]
- Liu WH, Chan RC, Wang LZ, Huang J, Cheung EF, Gong QY, Gollan JK. Deficits in sustaining reward responses in subsyndromal and syndromal major depression. Progress in Neuro-Psychopharmacology and Biological Psychiatry. 2011;35(4):1045–1052. doi: 10.1016/j.pnpbp.2011.02.018. [DOI] [PubMed] [Google Scholar]
- MacKinnon DP, Fairchild AJ, Fritz MS. Mediation analysis. Annual Review of Psychology. 2007;58:593. doi: 10.1146/annurev.psych.58.110405.085542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacKinnon DP, Lockwood CM, Hoffman JM, West SG, Sheets V. A comparison of methods to test mediation and other intervening variable effects. Psychological Methods. 2002;7(1):83. doi: 10.1037/1082-989x.7.1.83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Männistö PT, Kaakkola S. Catechol-O-methyltransferase (COMT): biochemistry, molecular biology, pharmacology, and clinical efficacy of the new selective COMT inhibitors. Pharmacological Reviews. 1999;51(4):593–628. [PubMed] [Google Scholar]
- Matsumoto M, Weickert CS, Beltaifa S, Kolachana B, Chen J, Hyde TM, Kleinman JE. Catechol O-methyltransferase (COMT) mRNA expression in the dorsolateral prefrontal cortex of patients with schizophrenia. Neuropsychopharmacology: Official Publication of the American College of Neuropsychopharmacology. 2003;28(8):1521–1530. doi: 10.1038/sj.npp.1300218. [DOI] [PubMed] [Google Scholar]
- Miller EK. The prefontral cortex and cognitive control. Nature Reviews Neuroscience. 2000;1(1):59–65. doi: 10.1038/35036228. [DOI] [PubMed] [Google Scholar]
- Mirenowicz J, Schultz W. Importance of unpredictability for reward responses in primate dopamine neurons. Journal of Neurophysiology. 1994;72(2):1024–1027. doi: 10.1152/jn.1994.72.2.1024. [DOI] [PubMed] [Google Scholar]
- Mirenowicz J, Schultz W. Preferential activation of midbrain dopamine neurons by appetitive rather than aversive stimuli. Nature. 1996;379(6564):449–451. doi: 10.1038/379449a0. [DOI] [PubMed] [Google Scholar]
- O'Doherty JP, Deichmann R, Critchley HD, Dolan RJ. Neural responses during anticipation of a primary taste reward. Neuron. 2002;33(5):815–826. doi: 10.1016/s0896-6273(02)00603-7. [DOI] [PubMed] [Google Scholar]
- Oldfield RC. Assessment and analysis of handedness: Edinburgh Inventory. Neuropsychologia. 1971;9:97–113. doi: 10.1016/0028-3932(71)90067-4. [DOI] [PubMed] [Google Scholar]
- Pizzagalli DA, Sherwood RJ, Henriques JB, Davidson RJ. Frontal Brain Asymmetry and Reward Responsiveness A Source-Localization Study. Psychological Science. 2005;16(10):805–813. doi: 10.1111/j.1467-9280.2005.01618.x. [DOI] [PubMed] [Google Scholar]
- Rasetti R, Weinberger DR. Intermediate phenotypes in psychiatric disorders. Current Opinion in Genetics & Development. 2011;21(3):340–348. doi: 10.1016/j.gde.2011.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanislow CA, Pine DS, Quinn KJ, Kozak MJ, Garvey MA, Heinssen RK, Cuthbert BN. Developing constructs for psychopathology research: Research domain criteria. Journal of Abnormal Psychology. 2010;119(4):631. doi: 10.1037/a0020909. [DOI] [PubMed] [Google Scholar]
- Sarapas C, Katz AC, Nelson BD, Campbell ML, Bishop JR, Robison-Andrew EJ, Shankman SA. Are individual differences in appetitive and defensive motivation related? A psychophysiological examination in two samples. Cognition & Emotion. 2013:1–20. doi: 10.1080/02699931.2013.848787. ahead-of-print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmidt LA, Fox NA, Perez-Edgar K, Hamer DH. Linking gene, brain, and behavior DRD4, frontal asymmetry, and temperament. Psychological Science. 2009;20(7):831–837. doi: 10.1111/j.1467-9280.2009.02374.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schultz W. Predictive reward signal of dopamine neurons. Journal of Neurophysiology. 1998;80(1):1–27. doi: 10.1152/jn.1998.80.1.1. [DOI] [PubMed] [Google Scholar]
- Shankman SA, Klein DN. The relation between depression and anxiety: an evaluation of the tripartite, approach-withdrawal and valence-arousal models. Clinical Psychology Review. 2003;23(4):605–637. doi: 10.1016/s0272-7358(03)00038-2. [DOI] [PubMed] [Google Scholar]
- Shankman SA, Klein DN, Tenke CE, Bruder GE. Reward sensitivity in depression: A biobehavioral study. Journal of Abnormal Psychology. 2007;116(1):95–104. doi: 10.1037/0021-843X.116.1.95. [DOI] [PubMed] [Google Scholar]
- Shankman SA, Nelson BD, Sarapas C, Robison-Andrew E, Campbell ML, Altman SE, Gorka SM. A psychophysiological investigation of threat and reward sensitivity in individuals with panic disorder and/or major depressive disorder. Journal of Abnormal Psychology. 2013;122(2):322–338. doi: 10.1037/a0030747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shankman SA, Sarapas C, Klein DN. The effect of pre-vs. post-reward attainment on EEG asymmetry in melancholic depression. International Journal of Psychophysiology. 2011;79(2):287–295. doi: 10.1016/j.ijpsycho.2010.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sherdell L, Waugh CE, Gotlib IH. Anticipatory pleasure predicts motivation for reward in major depression. Journal of Abnormal Psychology. 2012;121(1):51–60. doi: 10.1037/a0024945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smolka MN, Schumann G, Wrase J, Grüsser SM, Flor H, Mann K, Heinz A. Catechol-O-methyltransferase val158met genotype affects processing of emotional stimuli in the amygdala and prefrontal cortex. The Journal of Neuroscience. 2005;25(4):836–842. doi: 10.1523/JNEUROSCI.1792-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shrout PE, Bolger N. Mediation in experimental and nonexperimental studies: New procedures and recommendations. Psychological Methods. 2002;7(4):422. [PubMed] [Google Scholar]
- Soeiro-De-Souza MG, Stanford MS, Bio DS, Machado-Vieira R, Moreno RA. Association of the COMT Met158 allele with trait impulsivity in healthy young adults. Molecular Medicine Reports. 2013;7(4):1067–1072. doi: 10.3892/mmr.2013.1336. [DOI] [PubMed] [Google Scholar]
- Starkstein SE, Brockman S. Apathy and Parkinson’s disease. Current Treatment Options in Neurology. 2011;13(3):267–273. doi: 10.1007/s11940-011-0118-9. [DOI] [PubMed] [Google Scholar]
- Strauss GP, Wilbur RC, Warren KR, August SM, Gold JM. Anticipatory vs. consummatory pleasure: What is the nature of hedonic deficits in schizophrenia? Psychiatry Research. 2011;187(1):36–41. doi: 10.1016/j.psychres.2011.01.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stewart JL, Bismark AW, Towers DN, Coan JA, Allen JJ. Resting frontal EEG asymmetry as an endophenotype for depression risk: sex-specific patterns of frontal brain asymmetry. Journal of Abnormal Psychology. 2010;119(3):502. doi: 10.1037/a0019196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sutton SK, Davidson RJ. Prefrontal brain asymmetry: A biological substrate of the behavioral approach and inhibition systems. Psychological Science. 1997;8(3):204–210. [Google Scholar]
- Tomarken AJ, Davidson RJ, Henriques JB. Resting frontal brain asymmetry predicts affective responses to films. Journal of Personality and Social Psychology. 1990;59(4):791. doi: 10.1037//0022-3514.59.4.791. [DOI] [PubMed] [Google Scholar]
- Tobler PN, Fiorillo CD, Schultz W. Adaptive coding of reward value by dopamine neurons. Science. 2005;307(5715):1642–1645. doi: 10.1126/science.1105370. [DOI] [PubMed] [Google Scholar]
- Vandenberg DJ, Miller I, Uhl G, Lachman HM. A high activity COMT allele is associated with substance abuse vulnerablility. American Journal of Medical Genetics Part B: Neuropsychiatric Genetics. 1997;74:439–442. [PubMed] [Google Scholar]
- Volkow ND, Wang GJ, Telang F, Fowler JS, Logan J, Childress AR, Wong C. Cocaine cues and dopamine in dorsal striatum: Mechanism of craving in cocaine addiction. The Journal of Neuroscience. 2006;26(24):6583–6588. doi: 10.1523/JNEUROSCI.1544-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wacker J, Mueller EM, Pizzagalli DA, Hennig J, Stemmler G. Dopamine-D2-receptor blockade reverses the association between trait approach motivation and frontal asymmetry in an approach-motivation context. Psychological Science. 2013;24(4):489–497. doi: 10.1177/0956797612458935. [DOI] [PubMed] [Google Scholar]
- Wacker J, Stemmler G. Agentic extraversion modulates the cardiovascular effects of the dopamine D2 agonist bromocriptine. Psychophysiology. 2006;43(4):372–381. doi: 10.1111/j.1469-8986.2006.00417.x. [DOI] [PubMed] [Google Scholar]
- Wheeler RE, Davidson RJ, Tomarken AJ. Frontal brain asymmetry and emotional reactivity: A biological substrate of affective style. Psychophysiology. 1993;30(1):82–89. doi: 10.1111/j.1469-8986.1993.tb03207.x. [DOI] [PubMed] [Google Scholar]
- Wichers M, Aguilera M, Kenis G, Krabbendam L, Myin-Germeys I, Jacobs N, van Os J. The catechol-O-methyl transferase Val158Met polymorphism and experience of reward in the flow of daily life. Neuropsychopharmacology. 2007;33(13):3030–3036. doi: 10.1038/sj.npp.1301520. [DOI] [PubMed] [Google Scholar]
- Williams SM, Goldman-Rakic PS. Characterization of the dopaminergic innervation of the primate frontal cortex using a dopamine-specific antibody. Cerebral Cortex. 1993;3(3):199–222. doi: 10.1093/cercor/3.3.199. [DOI] [PubMed] [Google Scholar]
- Wise RA. Dopamine, learning and motivation. Nature Reviews Neuroscience. 2004;5(6):483–494. doi: 10.1038/nrn1406. [DOI] [PubMed] [Google Scholar]
- Zubieta JK, Heitzeg MM, Smith YR, Bueller JA, Xu K, Xu Y, Goldman D. COMT val158met genotype affects μ-opioid neurotransmitter responses to a pain stressor. Science. 2003;299(5610):1240–1243. doi: 10.1126/science.1078546. [DOI] [PubMed] [Google Scholar]