Abstract
Purpose
The purpose of this study was to examine the role of induction chemoradiation in the treatment of potentially-resectable locally advanced (T2-3N0 and T1-3N+) esophageal cancer utilizing a large national database.
Methods
The National Cancer Data Base (NCDB) was queried for all patients undergoing esophagectomy for clinical T2-3N0 and T1-3N+ esophageal cancer of the mid- or lower-esophagus. Patients were stratified by the use of induction chemoradiation therapy versus surgery-first. Trends were assessed with the Cochran-Armitage test. Predictors of receiving induction therapy were evaluated with multivariable logistic regression. A propensity-matched analysis was conducted to compare outcomes between groups, and the Kaplan-Meier method was used to estimate long-term survival.
Results
Within the NCDB, 7,921 patients were identified, of which 6,103 (77.0%) were treated with chemoradiation prior to esophagectomy, while the remaining 1,818 (23.0%) were managed with surgery-first. Use of induction therapy increased over time, with an absolute increase of 11.8% from 2003-2011 (p<0.001). As revealed by the propensity model, induction therapy was associated with higher rates of negative margins and shorter hospital length of stay, but no differences in unplanned readmission and 30-day mortality rates. In unadjusted survival analysis, induction therapy was associated with better long-term survival compared to a strategy of surgery-first, with 5-year survival rates of 37.2% versus 28.6%, p<0.001. Following propensity score matching analysis, the use of induction therapy maintained a significant survival advantage over surgery-first (5-year survival: 37.9% versus 28.7%, p<0.001).
Conclusions
Treatment with induction chemoradiation therapy prior to surgical resection is associated with significant improvement in long-term survival, even after adjusting for confounders with a propensity model. Induction therapy should be considered in all medically-appropriate patients with resectable cT2-3N0 and cT1-3N+ esophageal cancer, prior to esophagectomy.
Keywords: Esophageal cancer, esophageal surgery, induction therapy, outcomes, National Cancer Database
INTRODUCTION
The National Comprehensive Cancer Network (NCCN) guidelines recommend esophagectomy as an option for patients with resectable esophageal cancer who are medical candidates.1 While definitive chemoradiation is often reserved only for those individuals who are not surgical candidates or who decline major surgery, the NCCN guidelines reflect a lack of definitive evidence and allow for a wide spectrum of treatment options that combine surgery with chemotherapy or radiation therapy. The use of induction chemoradiation therapy prior to esophagectomy has been the focus of much interest but early single-center clinical trials had inconclusive results.2-4 However, a recently published randomized trial demonstrated a survival benefit to induction chemoradiation followed by surgery compared to surgery alone for esophageal or esophagogastric junction cancer.5 Despite the important results of this trial showing a long-term survival advantage with the use of induction therapy, other trials have closed early due to poor accrual6 and there remains a paucity of Level 1 evidence confirming these findings and supporting this treatment strategy. The purpose of this study was to supplement the available evidence that guides esophageal cancer treatment by utilizing a large national cancer database to examine the role of induction chemoradiation in the treatment of potentially resectable locally advanced (clinical T2-3N0 and T1-3N+) esophageal cancer, among whom induction therapy is recommended as an appropriate option by NCCN guidelines.
METHODS
This analysis of the National Cancer Data Base (NCDB) was approved by the Institutional Review Board at Duke University. The NCDB is maintained as a cooperative effort by the American College of Surgeons Commission on Cancer (CoC) and the American Cancer Society, and gathers data from more than 1,500 CoC-approved facilities across the United States and Puerto Rico. The NCDB is estimated to capture approximately 70% of all newly diagnosed U.S. cases of cancer annually, and currently contains more than 30 million records. Clinical stage is recorded in the NCDB using the Facility Oncology Registry Data Standards (FORDS), and is defined as the clinical stage based on the best available information and as documented by the managing physician, however specific staging modality data are not available.
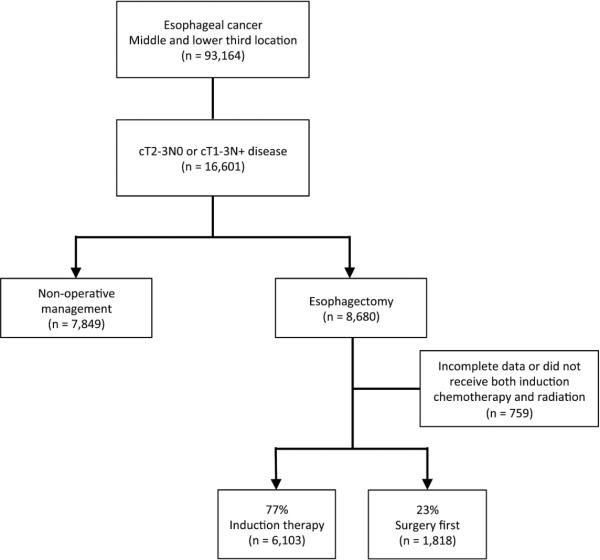
Patients diagnosed with esophageal tumors located in the middle- and distal-third of the esophagus from 1998 to 2011 were included for analysis (Figure 1). Patients with cervical tumors were excluded, as definitive chemoradiation is the preferred treatment in this patient population. All survival analyses were limited to patients diagnosed prior to 2007, as long-term survival data is only available for patients diagnosed up through 2006 in the NCDB. Patients were identified using International Classification of Diseases for Oncology, 3rd Edition (ICD-O-3) codes for adenocarcinoma and squamous cell carcinoma tumor histologies, and patients undergoing esophagectomy were identified for inclusion using FORDS procedure codes. Tumor-node-metastasis (TNM) stage was extracted directly from the NCDB, and only patients with clinical T2-3N0 and T1-3N+ disease were included for analysis. Patients with clinical evidence of metastatic disease (cM+) prior to treatment were excluded.
Figure 1.
Study population and patient selection scheme
The primary predictor variable was induction chemoradiation therapy use, and patients were therefore grouped by the use of induction therapy. For the purposes of this study, induction therapy was defined as the use of both chemotherapy and radiation therapy prior to esophagectomy; patients treated with only one modality (radiation or chemotherapy but not both) were excluded, as were patients lacking data on the timing of therapy. Trends in induction therapy use were assessed over time with the Cochran-Armitage trend test. Baseline characteristics and short-term outcomes were compared using the Mann-Whitney U test for continuous variables, and Pearson’s chi-square test for discrete variables.
To address potential bias induced by the treatment decision, we estimated propensity scores, defined as the conditional probability of a patient being treated with induction therapy prior to esophagectomy, versus an approach of surgery first. The propensity scores of the patients were estimated using a logistic regression model with the following predictors: patient age, sex, race, Charlson/Deyo comorbidity index, tumor histology (squamous cell versus adenocarcinoma), clinical T stage, presence of nodal disease prior to treatment, patient census tract education and income levels, and facility volume and type (academic versus community program). Patients were then matched based on propensity score using a nearest neighbor algorithm (MatchIt: Nonparametric Preprocessing for Parametric Casual Inference).7 Model balance was assessed by comparing group characteristics (both matched and non-matched variables) with standardized differences, and a difference of less than 20% was taken to indicate a negligible difference for a particular covariate between groups.
Long-term survival was estimated using the Kaplan-Meier method, comparing survival curves with the log-rank test both among the unadjusted and propensity-matched patient cohorts. All analyses assessed overall survival, defined by the NCDB as the time from date of diagnosis to date of death or censoring. Predictors of long term survival for patients who had esophagectomy were evaluated using a multivariable Cox proportional hazards model that included age, sex, race, Charlson/Deyo comorbidity index, final pathologic stage, tumor histology (squamous versus adenocarcinoma), patient census tract education and income levels, treatment facility volume, and use of induction therapy.
Model diagnostics and balance were assessed, and no major model assumptions were violated (Supplemental Figures A and B). Missing data was handled with complete case analysis in light of the substantial completeness (≥95%) of the NCDB with respect to the patient population and variables of interest. We made an affirmative decision to control for type I error at the level of the comparison. A p-value of ≤0.05 was used to indicate statistical significance for all comparisons. All statistical analyses were performed using R version 3.0.2 (R Foundation for Statistical Computing, Vienna, Austria).
RESULTS
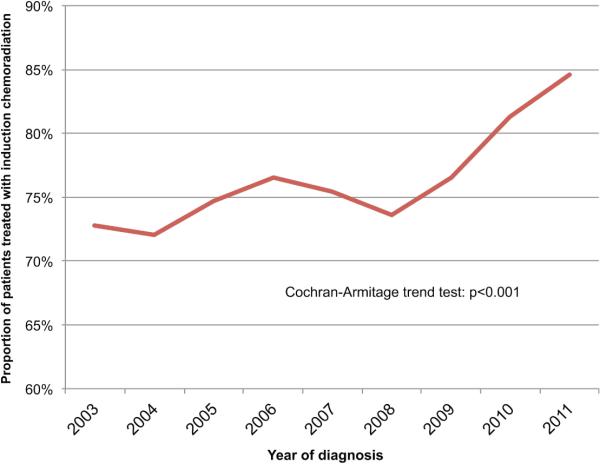
Of a total of 93,164 patients with mid- or lower-third esophageal cancer in the NCDB, there were 16,601 patients who met study criteria based on having clinical stage of T2-3N0 or cT1-3N+ adenocarcinoma or squamous cell carcinoa. Of these 16,601 patients with these specific stages, there were 8,680 patients identified as having undergone esophagectomy as part of their treatment (Figure 1). After excluding 759 patients with incomplete data or who received only induction chemotherapy or radiation therapy but not both, 6,103 (77.0%) were treated with chemoradiation prior to esophagectomy, while the remaining 1,818 (23.0%) were managed with an approach of surgery-first. Use of induction therapy increased over time, with an absolute increase of 11.8% from 2003-2011 (p<0.001; Figure 2). Comparison of the two groups revealed somewhat similar patient populations, although the induction therapy group tended to be slightly younger with less comorbidities, but with substantially higher T-stage and rates of nodal disease, as well worse overall tumor grade (Table 1). Following multivariable adjustment, younger age, male sex, fewer comorbidities, higher T-stage, nodal disease, facility type and higher facility volume were all independently associated with use of induction therapy (Table 2).
Figure 2.
Trends in the use of induction chemoradiation therapy, 2003-2011
Table 1.
Baseline patient characteristics, unadjusted and following propensity-matching
| Unadjusted | Propensity-matched | ||||||
|---|---|---|---|---|---|---|---|
| Patient characteristic | Overall (n = 7,921) | Surgery first (n = 1,818) | Induction therapy (n = 6,103) | P-value | Surgery first (n = 1,681) | Induction therapy (n = 1,681) | Std Diff* |
| Age, yrs (IQR) | 62 (55, 69) | 65 (58, 73) | 61 (54, 67) | < 0.001 | 66 (58, 73) | 65 (58, 71) | 9% |
| Female | 1,120 (14.1%) | 315 (17.3%) | 805 (13.2%) | < 0.001 | 296 (17.6%) | 258 (15.3%) | 6% |
| Race | 0.004 | 3% | |||||
| White | 7,346 (94%) | 1,660 (92.5%) | 5,686 (94.4%) | 1,560 (92.8%) | 1,572 (93.5%) | ||
| Black | 344 (4.4%) | 104 (5.8%) | 240 (4%) | 93 (5.5%) | 84 (5%) | ||
| Other | 125 (1.6%) | 30 (1.7%) | 95 (1.6%) | 28 (1.7%) | 25 (1.5%) | ||
| Charlson Comorbidity Score | < 0.001 | 7% | |||||
| 0 | 5,996 (75.7%) | 1,252 (68.9%) | 4,744 (77.7%) | 1,156 (68.8%) | 1,211 (72%) | ||
| 1 | 1,562 (19.7%) | 425 (23.4%) | 1,137 (18.6%) | 395 (23.5%) | 364 (21.7%) | ||
| ≥2 | 363 (4.6%) | 141 (7.8%) | 222 (3.6%) | 130 (7.7%) | 106 (6.3%) | ||
| Income quartile | 0.004 | 10% | |||||
| Bottom | 813 (10.9%) | 221 (12.9%) | 592 (10.3%) | 218 (13%) | 185 (11%) | ||
| Second | 1,453 (19.4%) | 352 (20.5%) | 1,101 (19.1%) | 340 (20.2%) | 388 (23.1%) | ||
| Third | 2,188 (29.3%) | 471 (27.5%) | 1,717 (29.8%) | 464 (27.6%) | 474 (28.2%) | ||
| Top | 3,024 (40.4%) | 669 (39.1%) | 2,355 (40.8%) | 659 (39.2%) | 634 (37.7%) | ||
| Education quartile | 0.19 | 7% | |||||
| Bottom | 952 (12.7%) | 243 (14.2%) | 709 (12.3%) | 240 (14.3%) | 220 (13.1%) | ||
| Second | 1,682 (22.5%) | 389 (22.7%) | 1,293 (22.4%) | 379 (22.5%) | 405 (24.1%) | ||
| Third | 2,056 (27.5%) | 455 (26.6%) | 1,601 (27.8%) | 447 (26.6%) | 464 (27.6%) | ||
| Top | 2,788 (37.3%) | 626 (36.5%) | 2,162 (37.5%) | 615 (36.6%) | 592 (35.2%) | ||
| cT stage element | < 0.001 | 15% | |||||
| T1 | 268 (3.4%) | 108 (5.9%) | 160 (2.6%) | 97 (5.8%) | 113 (6.7%) | ||
| T2 | 2,259 (28.5%) | 907 (49.9%) | 1,352 (22.2%) | 845 (50.3%) | 806 (47.9%) | ||
| T3 | 5,394 (68.1%) | 803 (44.2%) | 4,591 (75.2%) | 739 (44%) | 762 (45.3%) | ||
| cN stage element | < 0.001 | 12% | |||||
| NO | 3,160 (39.9%) | 1,062 (58.4%) | 2,098 (34.4%) | 989 (58.8%) | 887 (52.8%) | ||
| N1 | 4,761 (60.1%) | 756 (41.6%) | 4,005 (65.6%) | 692 (41.2%) | 794 (47.2%) | ||
| Histology | < 0.001 | 3% | |||||
| Adenocarcinoma | 6,636 (83.8%) | 1,466 (80.6%) | 5,170 (84.7%) | 1,363 (81.1%) | 1,385 (82.4%) | ||
| Squamous | 1,285 (16.2%) | 352 (19.4%) | 933 (15.3%) | 318 (18.9%) | 296 (17.6%) | ||
| Grade | 0.037 | 6% | |||||
| Well differentiated | 349 (5.1%) | 108 (6.3%) | 241 (4.7%) | 95 (6%) | 87 (6.3%) | ||
| Moderately differentiated | 2,871 (42.2%) | 733 (42.8%) | 2,138 (42%) | 689 (43.6%) | 591 (42.6%) | ||
| Poorly differentiated | 3,436 (50.5%) | 840 (49%) | 2,596 (51%) | 769 (48.6%) | 679 (48.9%) | ||
| Undifferentiated/anaplastic | 150 (2.2%) | 32 (1.9%) | 118 (2.3%) | 28 (1.8%) | 31 (2.2%) | ||
| Treatment facility | 0.01 | 8% | |||||
| Community Program | 3,514 (44.6%) | 758 (41.9%) | 2,756 (45.4%) | 707 (42.1%) | 774 (46%) | ||
| Academic/Research | 4,371 (55.4%) | 1,051 (58.1%) | 3,320 (54.6%) | 974 (57.9%) | 907 (54%) | ||
Standardized differences of less than 20% indicate a negligible difference for a particular covariate between groups
Table 2.
Independent predictors of patients being treated with induction therapy
| Predictor | Odds ratio | Upper 95% CI | Lower 95% CI | p-value |
|---|---|---|---|---|
| Age (per decade) | 0.74 | 0.71 | 0.78 | <0.001 |
| Female sex | 0.79 | 0.69 | 0.90 | 0.001 |
| Race (ref = White) | ||||
| Black | 1.00 | 0.77 | 1.28 | 0.97 |
| Other | 0.75 | 0.52 | 1.08 | 0.12 |
| Charlson/Deyo comorbidity (ref = 0) | ||||
| 1 | 0.81 | 0.72 | 0.91 | <0.001 |
| ≥2 | 0.54 | 0.44 | 0.67 | <0.001 |
| Education above median | 1.01 | 0.90 | 1.13 | 0.85 |
| Income above median | 1.01 | 0.90 | 1.14 | 0.87 |
| Clinical T stage | ||||
| T2 | 4.38 | 3.79 | 5.05 | <0.001 |
| T3 | 9.86 | 8.61 | 11.29 | <0.001 |
| Clinical N+ | 2.15 | 1.95 | 2.37 | <0.001 |
| Squamous pathology (ref = Adenocarcinoma) | 1.07 | 0.93 | 1.24 | 0.35 |
| Academic/Research Facility (ref = Community) | 0.70 | 0.63 | 0.78 | <0.001 |
| Facility volume (per 5 cases) | 1.01 | 1.00 | 1.02 | 0.012 |
Among patients treated with surgery first, 560 (30.8%) went on to receive some form of adjuvant therapy, with 529 of these patients receiving postoperative chemotherapy and 389 patients receiving postoperative radiation therapy. Due to limitations with how chemoradiation information is structured in the NCDB, only 4,744 (77.7%) of patients who received induction therapy had data available regarding adjuvant therapy use. Of these, 353 (8%) were subsequently treated with adjuvant chemotherapy, and 31 (0.7%) were treated with adjuvant radiation therapy. Among patients treated with induction therapy, median radiation dose was 45 Gy (IQR: 45,50.4 Gy). Specific chemotherapy regimen used consisted of multi-agent administration for 3,894 (82%), single-agent chemotherapy for 265 (6%) and was not known for 585 (12%).
Following propensity matching, baseline characteristics between groups were highly similar, with all standardized differences < 20% (Table 1). In unadjusted comparisons between groups, patients treated with induction therapy had substantially higher rates of negative margins following surgery, as well as shorter hospital length of stay, lower readmission rates, and lower 30-day mortality rates (Table 3). Following propensity-adjustment, the induction therapy group maintained a significant advantage with respect to negative margins (95.5 vs. 85.7%, p<0.001) and hospital length of stay (median 10 vs. 11 days, p<0.001), however the differences in adjusted unplanned readmission and 30-day mortality rates were no longer statistically significant (p=0.07 and 0.21, respectively; Table 3). Interestingly, the number of lymph nodes procured was significantly less in among the patients treated with induction therapy compared to surgery-first in both unadjusted and propensity-matched comparisons (median 10 vs. 13 nodes, p<0.001, for both comparisons).
Table 3.
Short-term outcomes and endpoints, unadjusted and following propensity-matching
| Unadjusted | Propensity-matched | ||||||
|---|---|---|---|---|---|---|---|
| Outcome / endpoint | Overall | Surgery first (n = 1,818) | Induction therapy (n = 6,103) | P-value | Surgery first (n = 1,681) | Induction therapy (n = 1,681) | P-value |
| Nodes removed (IQR) | 11 (5, 17) | 13 (7, 20) | 10 (4, 16) | < 0.001 | 13 (7, 20) | 10 (4, 16) | < 0.001 |
| Surgical margins | < 0.001 | < 0.001 | |||||
| Negative | 7,054 (92.6%) | 1,522 (85.7%) | 5,532 (94.7%) | 1,407 (85.7%) | 1,541 (95.5%) | ||
| Positive margin - microscopic | 348 (4.6%) | 150 (8.5%) | 198 (3.4%) | 139 (8.5%) | 47 (2.9%) | ||
| Positive margin - macroscopic | 217 (2.8%) | 103 (5.8%) | 114 (2%) | 95 (5.8%) | 25 (1.5%) | ||
| Length of stay (days) | 10 (8, 15) | 11 (8, 18) | 10 (8, 14) | < 0.001 | 11 (8, 18) | 10 (8, 15) | < 0.001 |
| Unplanned readmission | 468 (6.2%) | 134 (7.6%) | 334 (5.8%) | 0.006 | 127 (7.8%) | 98 (6.1%) | 0.067 |
| Perioperative (30d) mortality | 263 (3.3%) | 75 (4.1%) | 188 (3.1%) | 0.035 | 72 (4.3%) | 57 (3.4%) | 0.21 |
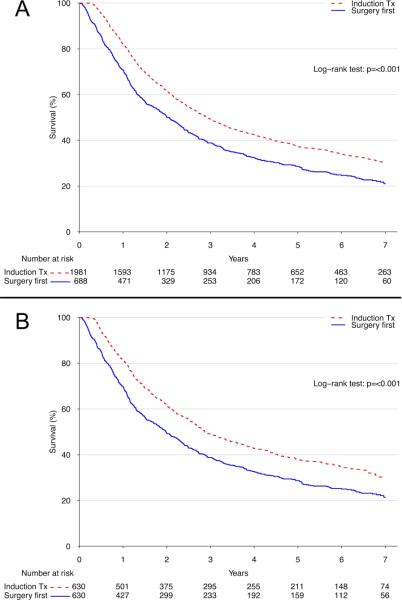
Upon unadjusted comparison, induction therapy was associated with significantly better long-term survival compared to a strategy of surgery-first, with 5-year survival rates of 37.2% (95% confidence interval [CI]: 35.0-39.4%) versus 28.6% (95% CI: 25.4-32.3%) for surgery-first, p<0.001 (Figure 3A). Following propensity-adjustment, the use of induction therapy maintained a significant survival advantage over surgery-first (5-year survival: 37.9% [95% CI: 34.2-42.0%] versus 28.7% [95% CI: 25.3-32.6%], p<0.001) (Figure 3B).
Figure 3.
(A) Unadjusted and (B) propensity-adjusted Kaplan-Meier survival estimates for induction therapy versus surgery first.
In light of known inaccuracies in clinical staging and for comparison of final disease stage between groups, final pathologic TNM staging data are shown in Table 4. Patients in the induction therapy group were much more likely to be ypT0, ypN0 and overall ypStage 0 compared to their surgery-first counterparts. Of the patients treated with induction therapy, 65.6% were clinically node positive prior to treatment (compared to 41.6% of the surgery first group) while only 37.8% of the induction therapy group were pathologically node positive following esophagectomy (compared to 51.2% of the surgery first group). Similarly, while 75.2% of the tumors in the induction therapy group were clinically staged as T3 (compared to 44.2% of the surgery first group), only 41.5% of patients were subsequently found to have T3 or greater disease pathologically (compared with 52.3% of the non-induction group).
Table 4.
Summary of pathologic stage following esophagectomy, stratified by use of induction therapy
| Stage | Surgery first | Induction therapy |
|---|---|---|
| Overall stage | ||
| Stage 0 | 6 (0.4%) | 201 (5.7%) |
| Stage I | 290 (18.4%) | 560 (15.8%) |
| Stage II | 685 (43.6%) | 1616 (45.5%) |
| Stage III | 555 (35.3%) | 1086 (30.5%) |
| Stage IV | 36 (2.3%) | 92 (2.6%) |
| T-stage | ||
| T0/IS | 22 (1.3%) | 964 (22.2%) |
| T1 | 376 (22.4%) | 667 (15.4%) |
| T2 | 403 (24%) | 903 (20.8%) |
| T3 | 853 (50.8%) | 1752 (40.4%) |
| T4 | 25 (1.5%) | 48 (1.1%) |
| Nodal status | ||
| N0 | 809 (48.8%) | 2818 (62.2%) |
| N1 | 849 (51.2%) | 1713 (37.8%) |
DISCUSSION
In this study of nearly 8,000 patients undergoing esophagectomy for cT1-3N+ and cT2-3N0 esophageal cancer, we found that treatment with induction chemoradiation therapy prior to surgical resection is associated with a substantial and significant improvement in long-term survival, supporting current NCCN guideline recommendations. Use of induction therapy in this population was also found to be increasing over time, rising from 72.8% of patients in 2003 to 84.6% in 2011. We identified a number of factors that were independently associated with not receiving induction therapy, which included older age, female sex, comorbidity burden, lower stage disease (T- and N- status), and treatment facility characteristics. Even after adjusting for all of these factors with a robust propensity model, induction therapy maintained a significant survival advantage.
Several studies have investigated the role of induction therapy prior to esophagectomy. Most early studies were somewhat inconclusive but in general suggested a survival benefit exists when both chemotherapy and radiation therapy are given pre-operatively.2, 8-11 More recent trials and retrospective studies have also supported the use of induction therapy.12-16 Importantly, a recently published randomized trial demonstrated a survival benefit to induction chemoradiation followed by surgery compared to surgery alone for esophageal or esophagogastric junction cancer.5 However, the performance of further studies to confirm the important findings of this trial may be unlikely considering the difficulties inherent in performing a randomized trial that involves a surgical procedure.6 Overall, the importance of our present study is that it provides strong collaboration to the currently available evidence that induction therapy prior to esophagectomy optimizes survival.
Some of the apparent survival benefit associated with induction therapy is likely due to pathologic down-staging after chemoradiation therapy prior to esophagectomy. Our results support this assertion, with substantially higher rates of T and N downstaging among the induction therapy group compared to the surgery first group. It should be noted that clinical staging modalities for esophageal cancer are somewhat unreliable, with significant percentages of patients being both under and over staged, particularly for patients clinically staged as T2N0.17-21 However while inaccuracies in clinical staging might explain some of this stage migration observed in our study, the majority of these changes are likely attributed to the effects of induction therapy.
Importantly, our results also confirm existing studies suggesting that pre-operative chemoradiation does not increase perioperative risks.22, 23 In unadjusted analysis, use of induction therapy was found to be associated with significantly lower rates of 30-day mortality and readmission, suggesting that perioperative risks are, at the very least, probably not increased by the use of induction therapy. While these advantages did not persist following propensity adjustment, advantages with respect to surgical margin status and hospital length of stay did remain. In any event, performing esophagectomy after induction therapy is clearly safe in appropriately selected patients.
In light of the ongoing debate regarding the role of induction therapy in the surgical management of esophageal cancer, it is perhaps not surprising that nearly a quarter of the patients in our study did not receive preoperative chemotherapy or radiation therapy. Nevertheless, this study does identify certain patient, tumor and treatment characteristics independently associated with the use of induction therapy. The most powerful predictors of induction therapy use were tumor characteristics, with T stage and clinical nodal status being the driving forces. Patient characteristics, such as younger age and lower comorbidity burden were also associated with use of induction therapy. Much of this is likely the result of provider bias to treat higher stage tumors more aggressively, with concomitant attempts to avoid potential complications among seemingly frail patients. While socioeconomic factors did not appear to be important in the decision to pursue induction therapy, facility characteristics were notably relevant. Higher-volume facilities were more likely to administer induction therapy than their lower-volume counterparts, and after adjusting for facility volume, academic/research facilities were in fact more likely to treat patients with surgery-first. These findings raise concerns that the management of esophageal cancer lacks standardization, and that certain centers are guiding management decisions based on factors other than patient and tumor characteristics.
The NCDB offers some particular advantages over both existing trials and other databases. The primary strength is derived from the population-based nature of the database, representing approximately 70% of annual esophageal cancer diagnoses in the United States. The inherent statistical power available as a result of this large sample size allows for comparisons to be drawn on cohorts that are an order of magnitude larger than either single or multi-institutional series. The NCDB also provides detailed and discrete clinical and pathological staging information as well as patient comorbidity and facility data, none of which are available in other smaller data sources such as the Surveillance, Epidemiology, and End Results (SEER) database.
Despite these advantages, there are clear limitations to our study. While our propensity models attempt to correct for selection bias in the treatment decision regarding induction therapy use, the possibility of unmeasured confounding cannot be excluded. As treatment intent is unknown, our results could be subject to an inherent stage migration bias. Induction therapy patients had a relative delay in time from diagnosis to surgery of 89 days compared to patients not treated with induction therapy, and some patients may have progressed and were restaged following induction therapy. Those who developed metastatic and/or unresectable disease were therefore not included in our study population, while patients with similar biology who were treated with surgery-first were most likely included. Furthermore, while we were able to characterize patients as having received induction chemoradiation therapy or not, we do not have specific data regarding radiation doses or chemotherapy regimens, nor information regarding perioperative nutrition and feeding strategies. Similarly, due to the way data is structured in the NCDB, we were not able to reliably determine if some of the induction therapy patients went on to get additional chemoradiation therapy adjuvantly, nor could we accurately differentiate between cases of planned esophagectomy after induction therapy versus salvage resection following definitive chemoradiation. Similarly, data regarding patient comorbidities and functional status is relatively limited. Lastly, other important endpoints including disease-specific survival, cancer recurrence, and 90-day survival data are not presently available in the NCDB.
Although the use of induction therapy for esophageal cancer has been increasing with time, we found that nearly one-quarter of these patients do not receive chemoradiation therapy prior to esophagectomy when it may be indicated. Using a large national cancer database, we confirm previous findings from smaller studies, and report that combined chemoradiation therapy prior to surgery is associated with meaningful survival advantages compared to a strategy of surgery-first. This study emphasizes the importance of multidisciplinary evaluations in the management of patients with esophageal cancer, and strongly suggests that induction therapy should be considered in all medically appropriate patients with resectable cT2-3N0 and cT1-3N+ esophageal cancer, prior to esophagectomy.
Supplementary Material
Supplemental Figure A: Histogram of propensity score distributions, pre- and post- match
Supplemental Figure B: Jitter plot showing individual patients included in propensity match, by propensity score
ACKNOWELDGEMENTS
The NCDB is a joint project of the Commission on Cancer of the American College of Surgeons and the American Cancer Society. The data used in the study are derived from a de-identified NCDB file. The American College of Surgeons and the Commission on Cancer have not verified and are not responsible for the analytic or statistical methodology employed, or the conclusions drawn from these data by the investigators.
Funding: This work was supported in part by the NIH-funded Cardiothoracic Surgery Trials Network (M.G.H. and M.F.B.).
Footnotes
Conflicts of interest: One of the authors (T.A.D.) serves as a consultant for Scanlan International, Inc.
REFERENCES
- 1.Ajani JA, Barthel JS, Bentrem DJ, et al. Esophageal and esophagogastric junction cancers. J Natl Compr Canc Netw. 2011;9:830–87. doi: 10.6004/jnccn.2011.0072. [DOI] [PubMed] [Google Scholar]
- 2.Bates BA, Detterbeck FC, Bernard SA, Qaqish BF, Tepper JE. Concurrent radiation therapy and chemotherapy followed by esophagectomy for localized esophageal carcinoma. J Clin Oncol. 1996;14:156–63. doi: 10.1200/JCO.1996.14.1.156. [DOI] [PubMed] [Google Scholar]
- 3.Forastiere AA, Orringer MB, Perez-Tamayo C, Urba SG, Zahurak M. Preoperative chemoradiation followed by transhiatal esophagectomy for carcinoma of the esophagus: final report. J Clin Oncol. 1993;11:1118–23. doi: 10.1200/JCO.1993.11.6.1118. [DOI] [PubMed] [Google Scholar]
- 4.Kavanagh B, Anscher M, Leopold K, et al. Patterns of failure following combined modality therapy for esophageal cancer, 1984-1990. Int J Radiat Oncol. 1992;24:633–42. doi: 10.1016/0360-3016(92)90708-p. [DOI] [PubMed] [Google Scholar]
- 5.van Hagen P, Hulshof MCCM, van Lanschot JJB, et al. Preoperative chemoradiotherapy for esophageal or junctional cancer. N Eng J Med. 2012;366:2074–84. doi: 10.1056/NEJMoa1112088. [DOI] [PubMed] [Google Scholar]
- 6.Tepper J, Krasna MJ, Niedzwiecki D, et al. Phase III trial of trimodality therapy with cisplatin, fluorouracil, radiotherapy, and surgery compared with surgery alone for esophageal cancer: CALGB 9781. J Clin Oncol. 2008;26:1086–92. doi: 10.1200/JCO.2007.12.9593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ho DE, Imai K, King G, Stuart EA. MatchIt : Nonparametric Preprocessing for Parametric Causal Inference. J Stat Soft. 2011;42:1–28. [Google Scholar]
- 8.Bosset JF, Gignoux M, Triboulet JP, et al. Chemoradiotherapy followed by surgery compared with surgery alone in squamous-cell cancer of the esophagus. N Eng J Med. 1997;337:161–7. doi: 10.1056/NEJM199707173370304. [DOI] [PubMed] [Google Scholar]
- 9.Nygaard K, Hagen S, Hansen HS, et al. Pre-operative radiotherapy prolongs survival in operable esophageal carcinoma: a randomized, multicenter study of pre-operative radiotherapy and chemotherapy. The second Scandinavian trial in esophageal cancer. World J Surg. 1992;16:1104–9. doi: 10.1007/BF02067069. [DOI] [PubMed] [Google Scholar]
- 10.Urba SG, Orringer MB, Turrisi A, Iannettoni M, Forastiere A, Strawderman M. Randomized trial of preoperative chemoradiation versus surgery alone in patients with locoregional esophageal carcinoma. J Clin Oncol. 2001;19:305–13. doi: 10.1200/JCO.2001.19.2.305. [DOI] [PubMed] [Google Scholar]
- 11.Walsh TN, Noonan N, Hollywood D, Kelly A, Keeling N, Hennessy TP. A comparison of multimodal therapy and surgery for esophageal adenocarcinoma. N Engl J Med. 1996;335:462–7. doi: 10.1056/NEJM199608153350702. [DOI] [PubMed] [Google Scholar]
- 12.Burmeister BH, Smithers BM, Gebski V, et al. Surgery alone versus chemoradiotherapy followed by surgery for resectable cancer of the oesophagus: a randomised controlled phase III trial. Lancet Oncol. 2005;6:659–68. doi: 10.1016/S1470-2045(05)70288-6. [DOI] [PubMed] [Google Scholar]
- 13.Kountourakis P, Correa AM, Hofstetter WL, et al. Combined modality therapy of cT2N0M0 esophageal cancer: the University of Texas M. D. Anderson Cancer Center experience. Cancer. 2011;117:925–30. doi: 10.1002/cncr.25651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Schwer AL, Ballonoff A, McCammon R, Rusthoven K, D'Agostino RB, Schefter TE. Survival effect of neoadjuvant radiotherapy before esophagectomy for patients with esophageal cancer: a surveillance, epidemiology, and end-results study. Int J Radiat Oncol. 2009;73:449–55. doi: 10.1016/j.ijrobp.2008.04.022. [DOI] [PubMed] [Google Scholar]
- 15.Solomon N, Zhuge Y, Cheung M, Franceschi D, Koniaris LG. The roles of neoadjuvant radiotherapy and lymphadenectomy in the treatment of esophageal adenocarcinoma. Ann Surg Oncol. 2010;17:791–803. doi: 10.1245/s10434-009-0819-4. [DOI] [PubMed] [Google Scholar]
- 16.Worni M, Castleberry AW, Gloor B, et al. Trends and outcomes in the use of surgery and radiation for the treatment of locally advanced esophageal cancer: a propensity score adjusted analysis of the surveillance, epidemiology, and end results registry from 1998 to 2008. Dis Esophagus. 2013 doi: 10.1111/dote.12123. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Crabtree TD, Kosinski AS, Puri V, et al. Evaluation of the reliability of clinical staging of T2 N0 esophageal cancer: a review of the Society of Thoracic Surgeons database. Ann Thorac Surg. 2013;96:382–90. doi: 10.1016/j.athoracsur.2013.03.093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Crabtree TD, Yacoub WN, Puri V, et al. Endoscopic ultrasound for early stage esophageal adenocarcinoma: implications for staging and survival. Ann Thorac Surg. 2011;91:1509–15. doi: 10.1016/j.athoracsur.2011.01.063. [DOI] [PubMed] [Google Scholar]
- 19.Rice TW, Mason DP, Murthy SC, et al. T2N0M0 esophageal cancer. J Thorac Cardiovasc Surg. 2007;133:317–24. doi: 10.1016/j.jtcvs.2006.09.023. [DOI] [PubMed] [Google Scholar]
- 20.Stiles BM, Mirza F, Coppolino A, et al. Clinical T2-T3N0M0 esophageal cancer: the risk of node positive disease. Ann Thorac Surg. 2011;92:491–6. doi: 10.1016/j.athoracsur.2011.04.004. [DOI] [PubMed] [Google Scholar]
- 21.Zhang JQ, Hooker CM, Brock MV, et al. Neoadjuvant chemoradiation therapy is beneficial for clinical stage T2 N0 esophageal cancer patients due to inaccurate preoperative staging. Ann Thorac Surg. 2012;93:429–35. doi: 10.1016/j.athoracsur.2011.10.061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hamai Y, Hihara J, Taomoto J, Yamakita I, Ibuki Y, Okada M. Effects of neoadjuvant chemoradiotherapy on postoperative morbidity and mortality associated with esophageal cancer. Dis Esophagus. 2014 doi: 10.1111/dote.12207. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 23.Ruol A, Portale G, Castoro C, et al. Effects of neoadjuvant therapy on perioperative morbidity in elderly patients undergoing esophagectomy for esophageal cancer. Ann Surg Oncol. 2007;14:3243–50. doi: 10.1245/s10434-007-9455-z. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental Figure A: Histogram of propensity score distributions, pre- and post- match
Supplemental Figure B: Jitter plot showing individual patients included in propensity match, by propensity score