Abstract
Purpose
To study how excess body weight influences the energy cost of walking (Cw) and determine if overweight and obese older adults self-select stride frequency to minimize Cw.
Methods
Using body mass index (BMI) men and women between the ages of 65–80 yr were separated into normal weight (NW, BMI ≤ 24.9 kg m−2, n = 13) and overweight-obese groups (OWOB, BMI ≥25.0 kg m−2, n = 13). Subjects walked at 0.83 m s−1 on an instrumented treadmill that recorded gait parameters, and completed three, six-minute walking trials; at preferred stride frequency (PSF), at +10% PSF, and at −10% PSF. Cw was determined by indirect calorimetry. Repeated measures analysis of variance was used to compare groups, and associations were tested with Pearson correlations, α = 0.05.
Results
OWOB had 62% greater absolute Cw (301 ± 108 vs. 186 ± 104 J m−1, P < 0.001) and 20% greater relative Cwkg (3.48 ± 0.95 vs. 2.91 ± 0.94 J kg−1 m−1, P = 0.046) than NW. Although PSF was not different between OWOB and NW (P = 0.626), Cw was 8% greater in OWOB at +10% PSF (P < 0.001). At PSF OWOB spent less time in single-limb support (33.1 ± 1.5 vs. 34.9 ± 1.6 %GC, P = 0.021) and more time in double-limb support (17.5 ± 1.6 vs. 15.4 ± 1.4 %GC, P = 0.026) than NW. In OWOB, at PSF, Cw was correlated to impulse (r = −0.57, P = 0.027) and stride frequency (r = 0.51, P = 0.046).
Conclusions
Excess body weight is associated with greater Cw in older adults, possibly contributing to reduced mobility in overweight and obese older persons.
Keywords: Obesity, Aging, Mobility, Efficiency, Adiposity, Cadence
INTRODUCTION
In the United States 78% of males and 69% of females age 60 years or older are overweight or obese, which has increased by more than 20% in ten years (10). Being overweight or obese is not only related to increased risk of chronic disease, but has been associated with poor physical performance that increases older persons’ risk for dependency (12, 24). For example, being overweight in old age has been associated with an increased risk of falling (15, 28, 37) and older adults who are overweight or obese have a 1.5 – 5 fold risk of developing a walking limitation in comparison to those of normal weight (35).
Low muscle strength, power, and cardiorespiratory capacity have each been linked to reduced walking performance in older subjects who are overweight (2, 24). A high energy cost of walking (Cw) is another factor that potentially increases perceived exertion, could elicit early fatigue, and reduce mobility in this population (39). Cw is the oxygen or energy cost to walk a given distance (i.e. joules of energy, per kilogram of body mass, per meter of distance walked). While Cw has been shown to be 23% greater in older persons than in young (30), and Cw has been shown to be 10% greater in obese young adults compared with normal weight young adults (3), the difference in Cw between normal weight and overweight and obese older adults is currently unknown. It is likely that excess body weight alters walking gait and exacerbates the age-associated increase in Cw, placing overweight and obese older individuals at greater risk of mobility disability.
Gait analysis has identified a number of differences in spatial, temporal, and kinetic gait parameters between normal weight and overweight or obese walkers, however most research in this area has been completed in younger subjects. Generally, overweight and obese individuals prefer slower walking speeds, walk with wider stride widths, have shorter strides, slower stride frequencies, produce greater ground reaction forces at a given speed, spend more time in double-limb support, and less time in single-limb support phases of gait (18, 21, 23, 24, 33). These gait alterations may allow overweight and obese individuals to operate within their strength and cardiorespiratory functional capacities, increase postural stability, act as a strategy to minimize Cw, or, result from differences in body segment proportions, mass, and subsequent inertial properties.
It has been observed in normal weight, healthy, young people that Cw is minimized at the preferred stride frequency (PSF) following the principle of self-optimization (16). A U-shaped relationship between Cw and stride frequency has been reported in several studies that indicates energy expenditure is greater when stride frequency is increased or decreased from PSF (7, 16). It should be considered that in studies where walking speed is kept constant, increasing stride frequency elicits a reduction in stride length, and decreasing stride frequency increases stride length. Holt et al. (16) showed that in healthy, young adults that Cw was 46% greater when stride frequency was reduced by 15 strides min−1 from PSF and 18% greater when stride frequency was increased by the same amount. Delextrat et al. also demonstrated a U-shaped relationship between stride frequency and Cw in obese and non-obese teenagers (7). The obese subjects chose PSF that were 12% slower, and walking speeds that were nearly 30% slower than non-obese teens while exhibiting a 5% greater Cw per kilogram, and 32% greater Cw per kilogram of lean mass. Huang and colleagues showed that obese children walked with a 10% slower PSF, and spent 31% more time in double-limb support than their normal weight peers although they did not demonstrate a difference in Cw between groups (18). To the authors’ knowledge, no study has yet tested whether overweight older adults self-select stride frequencies that minimize Cw.
Therefore, the purposes of this study were to determine if there are differences in Cw between normal weight (NW) and overweight or obese (OWOB) older adults, to determine if OWOB self-select stride frequencies that minimize Cw, and to examine which anthropometric, gait, and metabolic parameters are associated with Cw in older walkers. Our objective was to study how gait and metabolic factors interact during walking in OWOB to better understand the greater prevalence of mobility limitation seen in these groups. We hypothesized that OWOB would choose slower PSF than NW that minimized their Cw, however, it was expected that the altered gait of OWOB would result in a greater Cw than seen in NW older subjects. We also hypothesized that Cw per kilogram of body mass would be correlated with body mass index (BMI), percent body fat, a faster stride frequency, a shorter stride length, a wider stride width, and a greater percentage of the gait cycle spent in double-limb support.
METHODS
Subjects
Twenty six, community-dwelling, male (n=11) and female (n=15) older adults between the ages of 65 – 80 years were recruited from the area surrounding the university. Using body mass index (BMI), participants were separated into NW (BMI ≤ 24.9 kg m−2, n = 13, 5 males, 8 females) and OWOB groups (BMI ≥25.0 kg m−2, n =13, 6 males, 7 females). Participants had to furnish primary care provider’s consent to participate in the study and must have been free from cardiopulmonary, musculoskeletal, or metabolic disease that would have prevented their safe completion of the research protocol. The use of human subjects in this study was approved by the University of New Hampshire Institutional Review Board and all subjects gave their written informed consent prior to participation.
Experimental Protocol
The study required two visits to the laboratory. On the first visit anthropometric measures were obtained including height, mass, waist and hip circumferences, and skinfold thicknesses. Skinfold measurements were taken using skinfold calipers (Harpenden, Baty International, West Sussex, England) on three sites: triceps, suprailiac and thigh for women; pectoral, abdominal and thigh for men. The sum of the skinfolds was used to calculate body density (20) and density was used to estimate percent body fat using equations specific for gender, ethnicity (Caucasian), and age (60 – 90 years) (14). The participants then completed the Rapid Assessment of Physical Activity (RAPA) questionnaire to assess the duration and intensity of their usual physical activity (36). Next, lower extremity functional mobility was assessed using the Short Physical Performance Battery (SPPB) which included tests of standing balance, the time to walk four meters at the usual pace, and the time to complete five chair stands (12). On the first visit subjects were habituated to treadmill walking for ten minutes at 0.83 m s −1 on an instrumented, gait analysis treadmill (Gaitway II, Kistler Instrument Corp., Amherst, NY, USA) equipped with an overhead support system and an upper-body harness to support body weight in the event of a fall. During this walk, participants wore the face mask and headgear of the metabolic system to gain comfort with the research protocol. In the final minute of the ten-minute walk, 15 seconds of gait data were recorded to determine PSF.
Energy Cost of Walking
The second visit to the laboratory was completed within one week of the first visit and involved measuring Cw via indirect calorimetry while walking for six minutes each at one of three stride frequencies: PSF, 10% slower than PSF (−10% PSF), and 10% faster than PSF (+10% PSF). Subjects were instructed to refrain from activity on the day of testing. Immediately before each data collection, the oxygen and carbon dioxide analyzers of an indirect calorimeter (Vmax 229LV Lite, SensorMedics Corp., Yorba Linda, CA, USA) were calibrated to known gas concentrations and the ventilatory flow sensor (Vmax Sensor 770279, SensorMedics Corp., Yorba Linda, CA, USA) was calibrated with a three-liter calibration syringe (763722, SensorMedics Corp., Yorba Linda, CA, USA). The participants were fit with a mouth breathing face mask and head cap (7930 series, Hans Rudolph Inc., Kansas City, MO, USA) and care was taken to assure that the mask was completely sealed around the nose and mouth prior to connecting the flow sensor and gas sample tubing. Expired gases and ventilatory flow were recorded breath-by-breath, which were averaged over the final two minutes of each walk. It was assumed that at this point the subjects had reached a metabolic steady-state. Oxygen uptake (VO2, L min−1) and the respiratory exchange ratio (RER) were used to determine the caloric cost per minute according to the methods of Weir (38) using thefollowing equation:
| Eq. 1 |
The rate of energy expenditure in kCal min−1 was converted to joules min−1 through a unit conversion and was then divided by velocity (m min−1) to obtain the gross energy cost per meter of distance walked in both absolute (Cw, in J m−1) and relative terms (Cwkg, in J kg−1 m−1).
Manipulation of Stride Frequency
During visit two, participants first completed a two-minute warm-up on the treadmill at the test speed of 0.83 m s −1, which was followed by a two-minute seated rest period. The participants then completed three six-minute walking trials at 0.83 m s −1 at PSF, −10% PSF, and +10% PSF in a stratified random order. This standard walking speed was chosen such that all participants could complete the six-minute walk without stopping based on previous research with OWOB older adults in our laboratory (24). A fixed speed was chosen over preferred speed to eliminate differences in speed between NW and OWOB as a confounding factor. Stride frequency was paced by a metronome and subjects were blinded to the stride frequency condition. Subject compliance with the prescribed stride frequency was monitored by investigators and verbal feedback was provided to the subjects if they began to drift from the target stride frequency. Between each six-minute walking trial the participant sat in a chair placed on the treadmill for five minutes to allow metabolic rate to approach resting levels before the next trial and to prevent fatigue during the research protocol.
Gait Assessment
Vertical ground reaction force (vGRF) and center of pressure (COP) data were sampled from the treadmills force plates at 100 Hz by the treadmill software (Gaitway v. 2.0.8.50, Kistler Instrument Corp., Amherst, NY, USA). Data were collected for a period of 15 seconds, in the fifth minute of exercise, to coincide with the metabolic measurements. This provided an average of 11 strides per trial at −10% PSF, 12 strides at PSF, and 13 strides at +10% PSF. The deck of the treadmill consists of two force plates arranged front to back such that foot strike of one limb occurs on the front force plate while toe off of the contralateral limb occurs on the back plate. Therefore during double-limb stance the forces are being measured independently for each foot, and the manufacturer’s proprietary mathematical algorithm easily separates the vGRF and COP data between feet. During single-limb support all the vGRF and COP data collected by both plates are attributed to the support limb and no data are attributed to the swing limb.
For each step, an automated analysis identified the peak vGRF for both the weight acceptance and push-off force peaks and impulse was calculated as the integral of the vGRF vs. time curve. Using the contact time data obtained from each foot’s force measurements, stride frequency, single-limb support time, and double-limb support time were determined. Using COP data, stride length was measured as the anteroposterior distance from the initial COP at foot contact to the COP at the next contact of the same foot, with the software accounting for the stationary nature of treadmill walking by accurate measurement of treadmill belt velocity. Stride width was calculated as the average distance between the right and left foot COPs in the mediolateral direction. The reliability of these kinetic, spatial, and temporal variables in our laboratory has been described previously (25). Gait variables were recorded independently for each leg and were averaged across both strides and legs for analysis.
Statistical Analysis
Differences in subject characteristics between NW and OWOB were compared using a one-way analysis of variance test (IBM SPSS Statistics, v. 20, IBM Corporation, Armonk, NY, USA). The normality of the data were confirmed with the Shapiro-Wilk test and homogeneity of variance was confirmed with Levene’s test of equality of error variances. Repeated measures analysis of variance was used to compare NW and OWOB for Cw (both absolute and relative to body mass), minute ventilation (VE), ventilatory equivalent for oxygen (VE/VO2), and respiratory exchange ratio (RER) across stride frequency conditions. A second repeated measures analysis of variance was used to compare groups for weight acceptance peak force, push-off peak force, impulse, stride length, stride width, stride frequency, double-limb support time, and single-limb support time across stride frequency conditions. Paired t-tests were used within groups to determine if the Cw differed from the PSF condition and independent t-tests were used to investigate the source of significant group by condition interactions. The Pearson product moment statistic was used to explore which subject characteristics and gait parameters were related to the Cw. All data are reported as mean (standard deviation) and the rejection criterion was set at P ≤ 0.05 for all statistical tests.
RESULTS
The OWOB group was 21 kg heavier, had a greater BMI, a greater percent body fat, carried 12 kg more fat mass, and had greater waist circumference and waist-hip ratio than the NW group (Table 1). NW and OWOB did not differ in age, height, activity level, or in measures of lower-extremity function as determined by the SPPB.
Table 1.
Participant descriptive characteristics.
| Normal Weight | Overweight - Obese | P - value | |
|---|---|---|---|
| Age (yr) | 71.7 (4.7) | 71.9 (4.1) | 0.929 |
| Mass (kg) | 63.4 (9.3) | 84.3 (13.3) | <0.001* |
| Height (m) | 1.68 (0.10) | 1.69 (0.11) | 0.731 |
| Body Mass Index (kg m−2) | 22.4 (1.8) | 29.5 (4.1) | <0.001* |
| Percent Body Fat (%) | 21.7 (4.7) | 30.5 (9.5) | 0.007* |
| Fat Mass (kg) | 13.6 (3.2) | 26.2 (10.2) | <0.001* |
| Waist Circumference (cm) | 76.9 (5.3) | 98.7 (8.6) | <0.001* |
| Waist-Hip Ratio | 0.81 (0.04) | 0.91 (0.10) | 0.004* |
| Aerobic Physical Activity Score | 6.1 (1.1) | 5.1 (1.8) | 0.096 |
| Strength Physical Activity Score | 2.1 (1.1) | 2.2 (1.3) | 0.872 |
| SPPB Total Score | 11.5 (0.5) | 11.3 (0.9) | 0.449 |
| SPPB Balance Score | 3.9 (0.3) | 3.8 (0.6) | 0.659 |
| SPPB Walking Speed (m s−1) | 1.06 (0.17) | 1.07 (0.19) | 0.885 |
| SPPB Chair Rise Time (s) | 10.07 (2.45) | 10.78 (2.8) | 0.493 |
Values are mean (SD)
= difference between groups, P ≤ 0.05
SPPB = Short Physical Performance Battery
Metabolic Measures
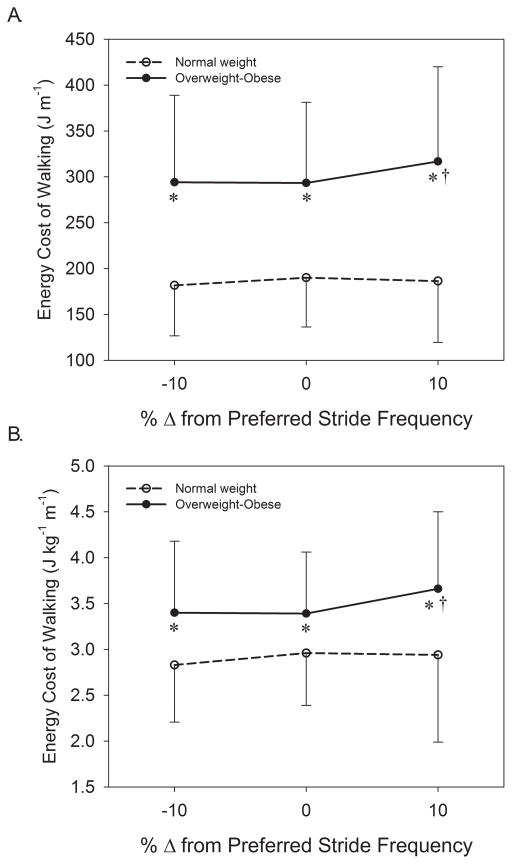
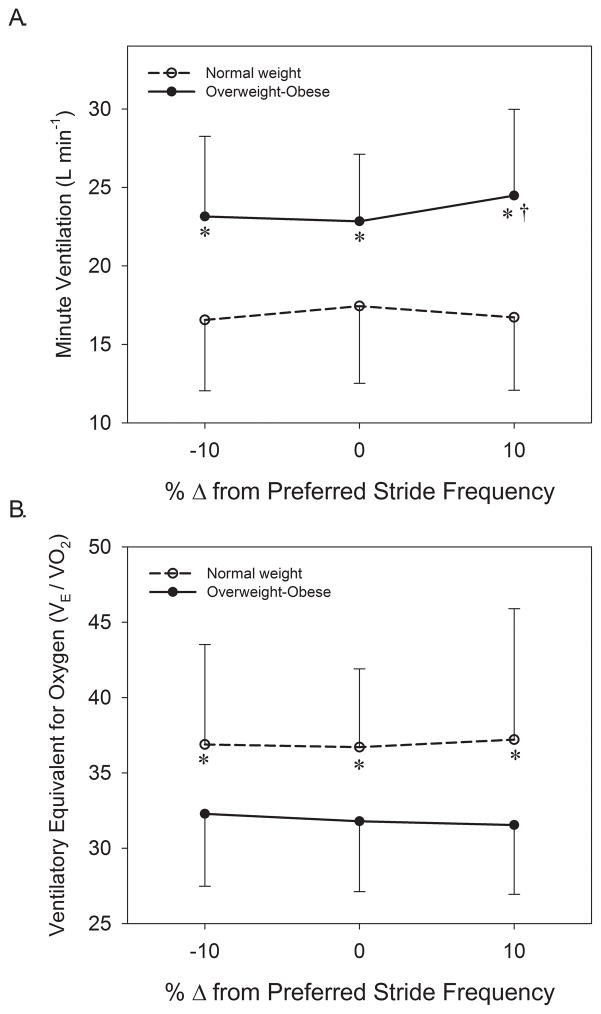
The repeated measures analysis for the metabolic variables showed that there were no main effects of stride frequency (P > 0.2), nor were there significant stride frequency by group interactions (P > 0.05) for Cw, Cwkg, RER, or VEVO2. Significant main effects of group existed for Cw (P = 0.001) and Cwkg (P = 0.046) that indicated Cw was 62% and Cwkg 20% greater in OWOB than NW when averaged across stride frequency conditions (Figure 1). In comparison to PSF, results showed that in OWOB Cw and Cwkg were 8% greater at +10% PSF (P = 0.004). The main effect of group for RER was not significant (P = 0.700). A main effect of group existed for VE (P = 0.002) that indicated OWOB had a 39% greater VE than NW (Figure 2A), and for VE/VO2 (P = 0.035) that demonstrated a 16% greater ventilatory equivalent for oxygen in NW than OWOB (Figure 2B). There was a significant stride frequency by group interaction for VE (P = 0.025) whereby ventilation was disproportionately greater with an increase in stride frequency in OWOB. Within OWOB, VE was 7% greater at +10% PSF than at PSF (P = 0.005; Figure 2A). A post-hoc power analysis revealed that the study was powered at 0.72 to detect differences in the metabolic variables across stride frequencies, 0.88 to detect differences between groups for metabolic parameters, and 0.81 to detect stride frequency by group interactions for metabolic parameters.
Figure 1.
Comparison of the gross energy cost of walking between normal weight and overweight-obese older adults in absolute terms (A) and normalized to body mass (B) while walking at preferred stride frequency, 10% slower than preferred, and 10% faster than preferred.
* = significant difference between groups, P < 0.05.
† = significantly different from preferred stride frequency, P < 0.05.
Figure 2.
Comparison of minute ventilation (A) and ventilatory equivalent for oxygen between normal weight and overweight-obese older adults walking at preferred stride frequency, 10% slower than preferred, and 10% faster than preferred.
* = significant difference between groups, P < 0.05.
† = significantly different from preferred stride frequency, P < 0.05.
Gait Measures
For all gait measures, with the exception of stride width, a significant main effect of stride frequency was found (P < 0.05, Table 2). Averaged across groups, weight acceptance peak force was 4% greater in the +10% PSF than in the −10% PSF condition (P < 0.001). Push-off peak force (−1%, P = 0.030), impulse (−16%, P < 0.001), stride length (−15%, P < 0.001), and single support time (−5%, P = 0.002) were lower in the +10% PSF than in the −10% PSF. Double support time was 6% longer in the +10% PSF condition than in the −10% PSF condition (P = 0.013).
Table 2.
Comparison of ground reaction forces, temporal, and spatial gait parameters between normal weight and overweight-obese subjects at preferred, 10% slower than preferred, and 10% faster than preferred stride frequency.
| −10% of preferred stride frequency | Preferred cadence | +10% of preferred stride frequency | ||||
|---|---|---|---|---|---|---|
|
| ||||||
| Variable | Normal Weight Group | Overweight Group | Normal Weight Group | Overweight Group | Normal Weight Group | Overweight Group |
| Stride frequency (strides min−1) | 43.4 (5.5) | 45.1 (4.7) | 47.6 (6.3) | 48.7 (5.4) | 52.4 (6.9) | 51.6 (5.9) |
| Stride length (m) | 1.19 (0.14) | 1.14 (0.11) | 1.09 (0.14) | 1.05 (0.11) | 0.98 (0.13) | 0.97 (0.1) |
| Stride width (cm) | 13.94 (2.63) | 14.88 (4.08) | 13.14 (2.8) | 15.42 (3.43) | 13.71 (3.98) | 14.92 (2.99) |
| Weight acceptance peak force (BW) | 1.03 (0.05) | 1.03 (0.04) | 1.04 (0.05) | 1.05 (0.04) | 1.07 (0.04) | 1.07 (0.07) |
| Push off peak force (BW) | 1.01 (0.03) | 1.01 (0.03) | 1.01 (0.03) | 0.99 (0.02) | 1.01 (0.04) | 0.98 (0.03) |
| Impulse (BW s) | 0.70 (0.09) | 0.67 (0.06) | 0.64 (0.08) | 0.62 (0.06) | 0.58 (0.08) | 0.57 (0.06) |
| Double support time (% gait cycle) | 14.64 (1.62) | 16.73 (1.91) | 15.42 (1.42) | 17.5 (1.64) * | 15.98 (1.54) | 17.17 (2.4) |
| Single support time (% gait cycle) | 35.65 (1.41) | 33.92 (1.87) | 34.92 (1.55) | 33.12 (1.45) * | 34.41 (1.41) | 32.06 (3.58) * |
BW = body weights
Values are mean (SD)
= different from normal weight group, P ≤ 0.05.
Significant main effects of group existed for double support time (P = 0.008) and single support time (P = 0.007; Table 2). OWOB spent 10% more time in double support and 6% less time in single limb support than did NW when averaged across stride frequency conditions. There were no statistically significant group by stride frequency interactions for any of the gait variables. The study was powered at 1.0 to detect differences in gait across the stride frequency conditions, 0.55 to detect group differences in gait, and 0.74 to detect stride frequency by group interactions.
Correlations to Energy Cost of Walking
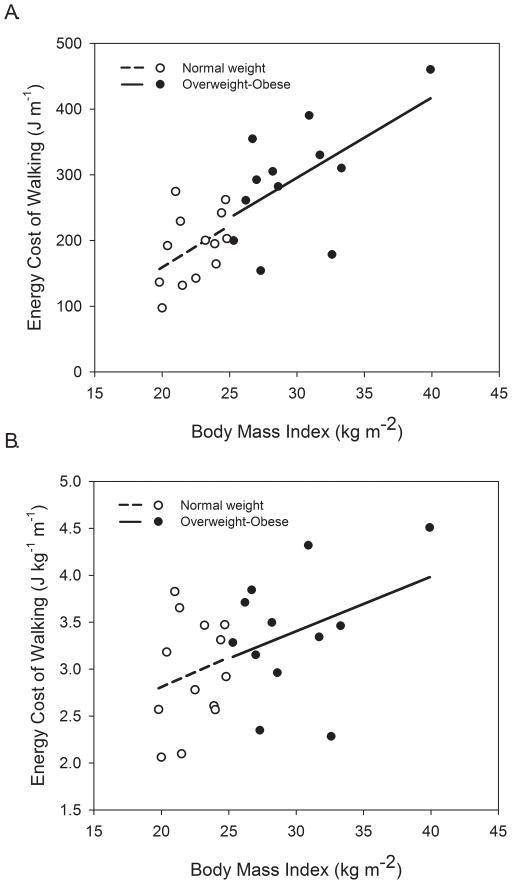
When data were pooled between groups BMI was positively associated with both Cw (r = 0.79, P < 0.001, Figure 3A) and Cwkg (r = 0.51, P = 0.005, Figure 3B) at PSF, with similar trends evident within groups (regression lines). Other subject characteristics that were positively correlated with Cwkg at PSF included body mass (r = 0.87, P < 0.001), percent body fat (r = 0.42, P = 0.018), fat mass (r = 0.58, P = 0.001), and waist circumference (r = 0.48, P = 0.008). When data were pooled between groups VE was positively and strongly related to Cwkg at PSF (r = 0.85, P < 0.001), with similar relationships existing within each group (see Figure A, Supplemental Digital Content 1, relationship between minute ventilation and the gross energy cost of walking). VE/VO2 was inversely correlated to Cwkg at PSF (r = −0.50, P = 0.003) but this relationship was stronger for OWOB than for NW (see Figure B, Supplemental Digital Content 1, relationship between the ventilatory equivalent for oxygen and gross energy cost of walking). Table 3 reports correlations between gait variables and Cw for each group as well as for data pooled between groups.
Figure 3.
Relationships between body mass index (BMI) and the gross energy cost of walking in absolute terms (A) and normalized to body mass (B). Regression lines are shown for normal weight (dashed, r = 0.44, P = 0.068) and overweight-obese (r = 0.57, P = 0.026) for energy cost of walking (A) and for energy cost of walking per kilogram of body mass (r = 0.20, P = 0.25 for normal weight, r = 0.36, P = 0.128 for overweight-obese).
Table 3.
Correlation coefficients between the energy cost of walking and gait parameters.
| −10% of preferred stride frequency | Preferred stride frequency | +10% of preferred stride frequency | |||||||
|---|---|---|---|---|---|---|---|---|---|
|
| |||||||||
| Variable | NW | OWOB | Pooled | NW | OWOB | Pooled | NW | OWOB | Pooled |
| Stride frequency | −0.19 | 0.14 | 0.04 | −0.01 | 0.51 * | 0.24 | 0.19 | 0.00 | 0.09 |
| Stride length | 0.20 | −0.15 | −0.04 | 0.01 | −0.49 | −0.26 | −0.20 | −0.18 | −0.19 |
| Stride width | 0.38 | 0.18 | 0.27 | 0.31 | 0.11 | 0.29 | 0.59 * | 0.01 | 0.37 * |
| Weight acceptance peak force | 0.35 | 0.03 | 0.18 | 0.26 | 0.21 | 0.26 | 0.53 * | 0.42 | 0.43 * |
| Push off peak force | −0.33 | −0.45 | −0.44 * | −0.17 | −0.35 | −0.34 * | −0.18 | −0.24 | −0.30 |
| Impulse | 0.16 | −0.21 | −0.08 | −0.01 | −0.57 * | −0.29 | −0.19 | −0.51 * | −0.32 |
| Double support time | −0.34 | 0.34 | 0.11 | −0.08 | 0.33 | 0.22 | −0.29 | −0.33 | −0.16 |
| Single support time | 0.37 | −0.31 | −0.08 | 0.12 | −0.36 | −0.22 | 0.41 | −0.41 | −0.28 |
NW = normal weight group
OWOB = overweight-obese group
= significant, P ≤ 0.05.
DISCUSSION
We examined whether overweight and obese older adults exhibit a greater energy cost of walking and tested whether they self-select stride frequencies that minimize the cost of walking. Additionally, this study identified biomechanical and ventilatory factors related to the cost of walking in older persons. Important findings of this study were that both Cw and Cwkg were significantly greater in OWOB than in NW, and Cw and Cwkg were greater in the faster stride frequency condition only in OWOB. In OWOB subjects walking at PSF, a greater Cwkg was related to a smaller impulse and faster stride frequency, whereas gait parameters were not closely associated with Cwkg in NW. A greater minute ventilation and lower ventilatory equivalent for oxygen were also associated with a greater Cwkg in older walkers.
The current study is the first to demonstrate that Cwkg is 20% greater in OWOB older adults than in their NW peers. This result persisted even when excluding the most obese subject from the analysis (BMI = 39.9 kg m2). This finding is in accordance with several studies in younger people that have shown that the Cwkg is 10% greater in obese adults (3) and 25% greater in obese teenagers (31) in comparison to NW subjects. The current study compared healthy, older adults, who had normal mobility over a short distance, yet differed by BMI. At first glance, a greater Cw may seem advantageous with respect to caloric expenditure and the maintenance of body weight, but in effect it is likely to increase perceived exertion as a result of performing exercise at a higher relative intensity (3). This may elicit early fatigue in OWOB older persons with limited metabolic capacity, predisposing them to a greater risk of mobility limitation (2, 34). In fact, Wert and colleagues (39) have demonstrated that Cwkg was inversely correlated (r = −0.50, P < 0.001) with self-reported physical function across a spectrum of common daily activities performed by older adults. Despite the known relationship between excess body weight and reduced physical function, our OWOB group demonstrated similar performance in the SPPB to NW. It is possible that either the greater Cwkg in OWOB does not negatively affect lower extremity mobility, or that the short distance of the SPPB walk (4 m) was not long enough to be impacted by Cwkg. Future research should use a more appropriate test to evaluate the relationship between Cwkg and functional mobility, such as the long-distance corridor walk that is conducted over 400 m (29).
We hypothesized that OWOB would subconsciously self-select slower PSF than NW in order to minimize Cwkg. This hypothesis was not supported as the PSF did not differ between groups. Speed was controlled to eliminate it as a confounding factor, which may have mitigated the difference in gait performance between NW and OWOB. OWOB older adults have been shown to self-select walking speeds that are nearly 20% slower than NW (24), likely due to a low functional capacity relative to mass, but possibly as a strategy to prevent decreases in economy that would occur at faster speeds and stride frequencies (3). Had this study been conducted at a faster speed, requiring faster stride frequencies, it is possible that the PSF would have differed between OWOB and NW.
Within OWOB, a faster stride frequency was correlated with a greater Cwkg when walking at PSF. Moreover, increasing stride frequency by 10% from PSF (and subsequently reducing stride length) elicited an 8% greater Cwkg for OWOB, but not for NW. These findings support the theory that OWOB limit stride frequency in order to minimize Cwkg. These results are similar to the response reported by Russell et al. (32) who demonstrated a 5% greater Cw when stride frequency was increased by 15% in obese young adults. While limiting stride frequency may help minimize Cwkg, it may contribute to the reduced walking speed seen in OWOB individuals because speed is the product of stride frequency and stride length.
Decreasing stride frequency from PSF did not elicit an increase in Cw in either group. Thus, this study did not demonstrate the U-shaped relationship between stride frequency and Cw that others have shown (7, 16). One probable explanation is that the current study only altered stride frequency from preferred by ±10% whereas others have changed stride frequency by 20% or more (7, 16). We chose to increase stride frequency by only 10% because we observed that increasing stride frequency by more than 10% elicits an abnormal shuffling gait pattern in older adults who already walk with short strides.
When walking at the standard speed of 0.83 m s−1, at PSF, the only gait parameters that differed between NW and OWOB were single and double-limb support times. Like Cwkg, these results persisted even after exclusion of the most obese subject. OWOB older adults spent a greater portion of the gait cycle in double-limb support and less in single-limb support than did NW older adults, which agrees with previous studies (21, 24, 27). This may result from a reduced ability to support body weight with a single limb because of a low strength to weight ratio, necessitating sharing the work of weight support between limbs (24). Browning et al. (4) demonstrated that obese walkers spent more time in double-limb support, and performed more absolute external work during this phase, but performed similar external work per kilogram of body mass as normal weight walkers. They theorized that since body weight support accounts for approximately 25% of Cw (11), greater muscle activation of lower extremity muscles could contribute to the greater Cwkg seen in obese walkers. In support of this idea, data from our laboratory has shown that OWOB subjects, who possess a low strength to weight ratio, have a greater activation of the knee extensor and plantarflexor muscles than NW during the weight acceptance phase of the gait cycle (24). This greater muscle activation, and corresponding greater muscle metabolic activity, may partly explain the greater Cwkg in OWOB walkers.
During walking, forward acceleration of the center of mass occurs primarily during double-support as the trailing limb pushes away from the ground (13). These step-to-step transitions require substantial mechanical work that has been estimated to account for up to 60 – 70% of the net metabolic rate during walking (9). At a fixed speed, longer double-support time should result in a weaker push-off force (40), causing a smaller forward acceleration of the body, and reduced economy of movement that increases Cwkg per meter of distance walked. In this study there was an inverse relationship between push-off force and Cwkg in the pooled group data that provides preliminary evidence of this association. In OWOB, at PSF, impulse was inversely related to Cwkg suggesting that a dynamic step is a more economical in these subjects. Also, longer periods of double support in OWOB individuals may be related to greater postural instability seen with excess weight (McGraw et al, 2000) that has been shown to be related to a greater Cw (19).
Our hypothesis was supported for the relationships between Cwkg and BMI, percent body fat, waist circumference, and stride frequency, but it was not supported for the relationships between Cwkg and stride width, or double-limb support time, despite the latter being different between groups. There was little evidence of associations between gait parameters and Cwkg at PSF when bivariate correlations were performed on the entire sample. When correlating gait variables to Cwkg for each group, the lack of association persisted for NW, however, in OWOB impulse was inversely associated with Cwkg, and stride frequency was positively associated with Cwkg. The inverse relationship between stride length and Cwkg for OWOB at PSF demonstrated a statistical trend (P = 0.051). Together, these data suggest that the OWOB subjects who were able to take longer strides, and who accelerated mass to a greater extent but less frequently, were able to maintain a lower Cwkg. We speculate that this strategy may take advantage of a better ability to store and return elastic energy in the musculotendinous structures of the lower-limb under more forceful loading. It has been demonstrated that obese subjects walk more erect with 12% less knee flexion and generate 50% lower knee extensor torque relative to mass than NW individuals when walking at the same speed (8). This pattern requires greater utilization of the ankle musculature which may be less suited for the absorption and return of work in the vertical direction.
The metabolic work required to accelerate the limbs as they move forward and backward with each stride has been shown to increase with mass of the limbs (5). Furthermore, Peyrot et al. (31) demonstrated in obese teenagers that the magnitude of the mediolateral displacement of the center of mass was directly related to both percent body fat and to Cwkg. Thus, the metabolic costs of the work required to move the limbs and reposition the center of mass with each step may be exacerbated at faster stride frequencies as these accelerations occur more frequently. This theory is reinforced by the greater Cwkg seen in OWOB at +10% PSF that did not occur in NW. At +10% PSF, weight acceptance peak force was greater than at PSF and was correlated to Cwkg. Presumably the greater weight acceptance forces at +10% PSF, and corresponding muscle activation, would increase the metabolic demand to support greater muscle contraction at faster stride frequencies. For instance, Hortobágyi et al have demonstrated that agonist and antagonist muscle activation during walking explain much of the variance in Cwkg seen between young and older adults (17), and the same may be true between NW and OWOB older persons.
A number of studies have demonstrated that when obese individuals walk they perform a similar amount of external work per kilogram of mass as normal weight (4, 26), yet obese walkers have greater metabolic costs relative to mass (3, 31). Two primary explanations of this paradox exist; reduced mechanical efficiency (discussed above), and reduced metabolic efficiency. Absent in the biomechanical studies’ explanation of greater Cwkg in OWOB are the additional cardiorespiratory costs associated with a greater body mass. In the current study VE was nearly 40% greater in OWOB than NW and was strongly correlated to Cwkg. The greater ventilation is presumably a result of the greater absolute metabolic cost (J m−1) needed to move a larger body mass, but could also partly explain the difference in the mass-specific metabolic cost of walking (J kg−1 m−1).
During exercise, ventilation is achieved by the diaphragm and by movement of the rib cage through the work of the intercostal, abdominal, neck, and chest wall muscles (1). The mechanical efficiency of breathing in obese individuals has been shown to be approximately half that of NW subjects as a result of the additional mass of the thorax (6). In the current study, the VE/VO2, which is used as an index of ventilatory efficiency, was lower in OWOB and was inversely correlated to Cwkg. This suggests that ventilation is constrained in OWOB older walkers, likely requiring a greater effort of the respiratory muscles that contributes to the greater Cwkg in OWOB. In subjects at rest, Kress et al. (22) demonstrated a 16% reduction in whole-body oxygen uptake when obese subjects were transitioned from spontaneous breathing to mechanical ventilation, whereas NW controls only experienced a 1% decrease in oxygen uptake. Kress’ work showed that a significant portion of resting energy expenditure was dedicated to ventilation in obese individuals, which might be expected to increase during exercise when a greater portion of respiratory work is performed by the muscles of the chest as opposed to diaphragmatic breathing.
A limitation of this study was that it was not sufficiently powered to separate the OWOB group into overweight and obese groups despite their likely being differences in gait and Cw between these groups. The average BMI of OWOB was 30 ± 4 kg m−2 and the differences between groups may have been greater with a purely obese sample that possessed additional comorbidities. Subjects were not habituated to the −10% PSF and +10% PSF conditions, which could have influenced their performance. A limitation of this study, and others that use indirect calorimetry to estimate Cw, is that it cannot measure the anaerobic energy systems’ contribution to Cw, which may underestimate Cw in OWOB individuals. It has been shown that obese women walking at preferred walking speed are at a relative exercise intensity of approximately 50% of maximal aerobic capacity (VO2max), whereas NW women walk near 25% of their VO2max at the same speed (3). It might therefore be expected that since OWOB individuals walk at a higher exercise intensity that they rely more heavily on the glycolytic energy system, whose contribution to Cwkg was not measured.
In conclusion, this study demonstrated that OWOB older adults expend 62% more gross metabolic energy, and 20% more gross metabolic energy per kilogram, during steady-state walking than NW older adults. In OWOB, the energy cost of walking was minimized at PSF and −10% PSF, but was 8% greater at +10% PSF. OWOB subjects who were able to walk with longer, dynamic, but less frequent strides had lower Cwkg. OWOB older walkers also had greater VE and a lower VE/VO2, which suggests that greater effort of the respiratory muscles contributes to the elevated energy cost of walking in this group. The greater energy cost of walking and ventilatory effort are likely to increase perceived exertion, elicit early fatigue, and contribute to the reduced mobility seen in overweight and obese older adults.
Supplementary Material
Supplemental Digital Content 1. Figure showing relationships between minute ventilation and the gross energy cost of walking (A) and between the ventilatory equivalent for oxygen and gross energy cost of walking (B).
Acknowledgments
D.P. LaRoche was supported by the National Center for Advancing Translational Sciences of the National Institutes of Health under award number L30TR000588. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health. N.R. Marques was supported by the Fundação de Âmparo à Pesquisa do Estado de São Paulo (FAPESP) process number: 2011/11639-7
Footnotes
Conflicts of Interest: The authors report no conflicts of interest.
The results of the present study do not constitute endorsement by the American College of Sports Medicine.
DISCLAIMER:
All manuscripts submitted to Medicine & Science in Sports & Exercise® for evaluation are protected by international copyright laws. Reviewers have permission to print this manuscript on paper in their individual effort to provide a thorough critique of the scientific quality of the manuscript to the Editorial Office. Any other use of the material contained in the manuscript or distribution of the manuscript to other individuals is strictly prohibited by federal, state, and international laws governing copyright. Such actions are also in direct conflict with the professional and ethical standards of the American College of Sports Medicine and its official journal, Medicine & Science in Sports & Exercise®.
The Editorial Office staff of MSSE® thanks you for participating in the review process. If you have any problems reviewing this manuscript, or you are unable to meet the deadline given, please write to the Editorial Office at msse@acsm.org.
References
- 1.Aliverti A, Iandelli I, Duranti R, et al. Respiratory muscle dynamics and control during exercise with externally imposed expiratory flow limitation. J Appl Physiol (1985) 2002;92(5):1953–63. doi: 10.1152/japplphysiol.01222.2000. [DOI] [PubMed] [Google Scholar]
- 2.Bouchard DR, McGuire KA, Davidson L, Ross R. Cardiorespiratory fitness, obesity, and functional limitation in older adults. Journal of aging and physical activity. 2011;19(4):336–46. doi: 10.1123/japa.19.4.336. [DOI] [PubMed] [Google Scholar]
- 3.Browning RC, Baker EA, Herron JA, Kram R. Effects of obesity and sex on the energetic cost and preferred speed of walking. J Appl Physiol. 2006;100(2):390–8. doi: 10.1152/japplphysiol.00767.2005. [DOI] [PubMed] [Google Scholar]
- 4.Browning RC, McGowan CP, Kram R. Obesity does not increase external mechanical work per kilogram body mass during walking. J Biomech. 2009;42(14):2273–8. doi: 10.1016/j.jbiomech.2009.06.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Browning RC, Modica JR, Kram R, Goswami A. The effects of adding mass to the legs on the energetics and biomechanics of walking. Med Sci Sports Exerc. 2007;39(3):515–25. doi: 10.1249/mss.0b013e31802b3562. [DOI] [PubMed] [Google Scholar]
- 6.Chlif M, Keochkerian D, Choquet D, Vaidie A, Ahmaidi S. Effects of obesity on breathing pattern, ventilatory neural drive and mechanics. Respiratory physiology & neurobiology. 2009;168(3):198–202. doi: 10.1016/j.resp.2009.06.012. [DOI] [PubMed] [Google Scholar]
- 7.Delextrat A, Matthew D, Cohen DD, Brisswalter J. Effect of stride frequency on the energy cost of walking in obese teenagers. Human movement science. 2011;30(1):115–24. doi: 10.1016/j.humov.2010.10.004. [DOI] [PubMed] [Google Scholar]
- 8.DeVita P, Hortobagyi T. Obesity is not associated with increased knee joint torque and power during level walking. J Biomech. 2003;36(9):1355–62. doi: 10.1016/s0021-9290(03)00119-2. [DOI] [PubMed] [Google Scholar]
- 9.Donelan JM, Kram R, Kuo AD. Mechanical work for step-to-step transitions is a major determinant of the metabolic cost of human walking. J Exp Biol. 2002;205(Pt 23):3717–27. doi: 10.1242/jeb.205.23.3717. [DOI] [PubMed] [Google Scholar]
- 10.Flegal KM, Carroll MD, Ogden CL, Curtin LR. Prevalence and trends in obesity among US adults, 1999–2008. JAMA : the journal of the American Medical Association. 2010;303(3):235–41. doi: 10.1001/jama.2009.2014. [DOI] [PubMed] [Google Scholar]
- 11.Grabowski A, Farley CT, Kram R. Independent metabolic costs of supporting body weight and accelerating body mass during walking. J Appl Physiol (1985) 2005;98(2):579–83. doi: 10.1152/japplphysiol.00734.2004. [DOI] [PubMed] [Google Scholar]
- 12.Guralnik JM, Simonsick EM, Ferrucci L, et al. A short physical performance battery assessing lower extremity function: association with self-reported disability and prediction of mortality and nursing home admission. J Gerontol. 1994;49(2):M85–94. doi: 10.1093/geronj/49.2.m85. [DOI] [PubMed] [Google Scholar]
- 13.Hernandez A, Silder A, Heiderscheit BC, Thelen DG. Effect of age on center of mass motion during human walking. Gait Posture. 2009;30(2):217–22. doi: 10.1016/j.gaitpost.2009.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Heyward VH, Wagner DR. Applied Body Composition Assessment. 2. Champaign (IL): Human Kinetics; 2004. p. 268. [Google Scholar]
- 15.Himes CL, Reynolds SL. Effect of obesity on falls, injury, and disability. Journal of the American Geriatrics Society. 2012;60(1):124–9. doi: 10.1111/j.1532-5415.2011.03767.x. [DOI] [PubMed] [Google Scholar]
- 16.Holt KG, Hamill J, Andres RO. Predicting the minimal energy costs of human walking. Medicine and science in sports and exercise. 1991;23(4):491–8. [PubMed] [Google Scholar]
- 17.Hortobagyi T, Finch A, Solnik S, Rider P, DeVita P. Association between muscle activation and metabolic cost of walking in young and old adults. The journals of gerontology Series A, Biological sciences and medical sciences. 2011;66(5):541–7. doi: 10.1093/gerona/glr008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Huang L, Chen P, Zhuang J, Walt S. Metabolic cost, mechanical work, and efficiency during normal walking in obese and normal-weight children. Res Q Exerc Sport. 2013;84 (Suppl 2):S72–9. doi: 10.1080/02701367.2013.849159. [DOI] [PubMed] [Google Scholar]
- 19.Ijmker T, Houdijk H, Lamoth CJ, Beek PJ, van der Woude LH. Energy cost of balance control during walking decreases with external stabilizer stiffness independent of walking speed. J Biomech. 2013;46(13):2109–14. doi: 10.1016/j.jbiomech.2013.07.005. [DOI] [PubMed] [Google Scholar]
- 20.Jackson AS, Pollock ML. Practical assessment of body composition. The Physician and Sportsmedicine. 1985;13(5):76–80. 2–90. doi: 10.1080/00913847.1985.11708790. [DOI] [PubMed] [Google Scholar]
- 21.Ko SU, Stenholm S, Ferrucci L. Characteristic gait patterns in older adults with obesity--Results from the Baltimore Longitudinal Study of Aging. J Biomech. 2010;43(6):1104–10. doi: 10.1016/j.jbiomech.2009.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kress JP, Pohlman AS, Alverdy J, Hall JB. The impact of morbid obesity on oxygen cost of breathing (VO(2RESP)) at rest. Am J Respir Crit Care Med. 1999;160(3):883–6. doi: 10.1164/ajrccm.160.3.9902058. [DOI] [PubMed] [Google Scholar]
- 23.Lai PP, Leung AK, Li AN, Zhang M. Three-dimensional gait analysis of obese adults. Clin Biomech (Bristol, Avon) 2008;23 (Suppl 1):S2–6. doi: 10.1016/j.clinbiomech.2008.02.004. [DOI] [PubMed] [Google Scholar]
- 24.LaRoche DP, Kralian RJ, Millett ED. Fat mass limits lower-extremity relative strength and maximal walking performance in older women. Journal of Electromyography and Kinesiology. 2011;21(5):754–61. doi: 10.1016/j.jelekin.2011.07.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.LaRoche DP, Millett ED, Kralian RJ. Low strength is related to diminished ground reaction forces and walking performance in older women. Gait & posture. 2011;33(4):668–72. doi: 10.1016/j.gaitpost.2011.02.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Malatesta D, Vismara L, Menegoni F, Galli M, Romei M, Capodaglio P. Mechanical external work and recovery at preferred walking speed in obese subjects. Medicine and science in sports and exercise. 2009;41(2):426–34. doi: 10.1249/MSS.0b013e31818606e7. [DOI] [PubMed] [Google Scholar]
- 27.McGraw B, McClenaghan BA, Williams HG, Dickerson J, Ward DS. Gait and postural stability in obese and nonobese prepubertal boys. Archives of physical medicine and rehabilitation. 2000;81(4):484–9. doi: 10.1053/mr.2000.3782. [DOI] [PubMed] [Google Scholar]
- 28.Mitchell RJ, Lord SR, Harvey LA, Close JC. Associations between obesity and overweight and fall risk, health status and quality of life in older people. Aust N Z J Public Health. 2014;38(1):13–8. doi: 10.1111/1753-6405.12152. [DOI] [PubMed] [Google Scholar]
- 29.Newman AB, Simonsick EM, Naydeck BL, et al. Association of long-distance corridor walk performance with mortality, cardiovascular disease, mobility limitation, and disability. JAMA : the journal of the American Medical Association. 2006;295(17):2018–26. doi: 10.1001/jama.295.17.2018. [DOI] [PubMed] [Google Scholar]
- 30.Peterson DS, Martin PE. Effects of age and walking speed on coactivation and cost of walking in healthy adults. Gait & posture. 2010;31(3):355–9. doi: 10.1016/j.gaitpost.2009.12.005. [DOI] [PubMed] [Google Scholar]
- 31.Peyrot N, Thivel D, Isacco L, Morin JB, Duche P, Belli A. Do mechanical gait parameters explain the higher metabolic cost of walking in obese adolescents? J Appl Physiol (1985) 2009;106(6):1763–70. doi: 10.1152/japplphysiol.91240.2008. [DOI] [PubMed] [Google Scholar]
- 32.Russell EM, Braun B, Hamill J. Does stride length influence metabolic cost and biomechanical risk factors for knee osteoarthritis in obese women? Clin Biomech (Bristol, Avon) 2010;25(5):438–43. doi: 10.1016/j.clinbiomech.2010.01.016. [DOI] [PubMed] [Google Scholar]
- 33.Spyropoulos P, Pisciotta JC, Pavlou KN, Cairns MA, Simon SR. Biomechanical gait analysis in obese men. Arch Phys Med Rehabil. 1991;72(13):1065–70. [PubMed] [Google Scholar]
- 34.Stathokostas L, Jacob-Johnson S, Petrella RJ, Paterson DH. Longitudinal changes in aerobic power in older men and women. J Appl Physiol (1985) 2004;97(2):781–9. doi: 10.1152/japplphysiol.00447.2003. [DOI] [PubMed] [Google Scholar]
- 35.Stenholm S, Sainio P, Rantanen T, Alanen E, Koskinen S. Effect of co-morbidity on the association of high body mass index with walking limitation among men and women aged 55 years and older. Aging Clin Exp Res. 2007;19(4):277–83. doi: 10.1007/BF03324702. [DOI] [PubMed] [Google Scholar]
- 36.Topolski TD, LoGerfo J, Patrick DL, Williams B, Walwick J, Patrick MB. The Rapid Assessment of Physical Activity (RAPA) among older adults. Prev Chronic Dis. 2006;3(4):A118. [PMC free article] [PubMed] [Google Scholar]
- 37.Volpato S, Leveille SG, Blaum C, Fried LP, Guralnik JM. Risk factors for falls in older disabled women with diabetes: the women’s health and aging study. The journals of gerontology Series A, Biological sciences and medical sciences. 2005;60(12):1539–45. doi: 10.1093/gerona/60.12.1539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Weir JB. New methods for calculating metabolic rate with special reference to protein metabolism. The Journal of physiology. 1949;109(1–2):1–9. doi: 10.1113/jphysiol.1949.sp004363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Wert DM, Brach JS, Perera S, VanSwearingen J. The association between energy cost of walking and physical function in older adults. Arch Gerontol Geriatr. 2013;57(2):198–203. doi: 10.1016/j.archger.2013.04.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Winter DA, Patla AE, Frank JS, Walt SE. Biomechanical walking pattern changes in the fit and healthy elderly. Physical therapy. 1990;70(6):340–7. doi: 10.1093/ptj/70.6.340. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental Digital Content 1. Figure showing relationships between minute ventilation and the gross energy cost of walking (A) and between the ventilatory equivalent for oxygen and gross energy cost of walking (B).