Abstract
Objective
Contingency management (CM) reduces cocaine use in methadone patients, but only about 50% of patients respond to CM interventions. This study evaluated whether increasing magnitudes of reinforcement will improve outcomes.
Methods
Cocaine-dependent methadone patients (N = 240) were randomized to one of four 12-week treatment conditions: usual care (UC), UC plus “standard” prize CM in which average expected prize earnings were about $300, UC plus high magnitude prize CM in which average expected prize earnings were about $900, or UC plus voucher CM with an expected maximum of about $900 in vouchers.
Results
All three CM conditions yielded significant reductions in cocaine use relative to UC, with effect sizes (d) ranging from 0.38 to 0.59. No differences were noted between CM conditions, with at least 55% of patients in each CM condition achieving one week or more of cocaine abstinence versus 35% in UC. During the 12 weeks after the intervention ended, CM increased time until relapse relative to UC, but the effects of CM were no longer significant at a 12-month follow-up.
Conclusions
Providing the standard magnitude of $300 in prizes was as effective as larger magnitude CM in cocaine-dependent methadone patients in this study. Given its strong evidence base and relatively low costs, standard magnitude prize CM should be considered for adoption in methadone clinics to encourage cocaine abstinence, but new methods need to be developed to sustain abstinence.
Keywords: contingency management, cocaine, methadone maintenance
Up to 60% of methadone-maintained patients abuse cocaine (Leri, Bruneau, & Stewart, 2003; Sees et al., 2000; Wu et al., 2012). Cocaine use in methadone patients is associated with unemployment, psychiatric disturbances, criminal activity, early attrition from treatment, and spread of HIV and other infectious diseases (Bandettini Di Poggio et al., 2006; DeMaria, Sterling, & Weinstein, 2000; Sees et al., 2000). Although methadone decreases heroin use (Council on Addiction Psychiatry, 1994), it has only marginal effects on reducing non-opioid drug use, including cocaine (Sees et al., 2000). No medication is reliably efficacious in reducing cocaine use (Sofuoglu & Kosten, 2006), necessitating use of psychosocial treatments.
One evidence-based practice for reducing cocaine use is contingency management (CM; Dutra et al., 2008; Lussier, Heil, Mongeon, Badger, & Higgins, 2006). This treatment arranges the environment so that drug use is readily detected and provides tangible reinforcers whenever abstinence occurs (Petry, 2012). CM treatments typically provide vouchers, exchangeable for retail goods, or the chance to win $1 to $100 prizes upon submission of cocaine-negative urine samples. In terms of the former, Silverman et al. (1996) found that significantly more cocaine-dependent methadone patients who were randomized to a voucher CM condition achieved and maintained cocaine abstinence than those assigned to a control condition. Efficacious voucher CM programs in methadone patients typically arrange for about $1000 in vouchers over a 12-week treatment period (e.g., Epstein, Hawkins, Covi, Umbricht, & Preston, 2003; Preston et al. 1998; Rawson et al., 2002; Silverman et al., 1996).
A CM system that provides prizes as reinforcers also reduces cocaine use. In a large, national multicenter study in the United States, Pierce et al. (2006) found that provision of up to an expected average of $400 in prizes significantly reduced cocaine use in methadone patients. Petry and Martin (2002), Petry, Martin, and Simcic (2005) and Petry, Alessi, Hanson, and Sierra (2007) likewise demonstrated the efficacy of prize CM, using expected magnitudes of prizes of $240-$400. The prize CM system integrates important behavioral parameters, including increasing reinforcement for successive abstinence (Roll, Higgins, & Badger, 1996), minimizing delays between behavior and reinforcement delivery (Lussier et al., 2006; Roll, Reilly, & Johanson, 2000), and allowing for personalized reinforcers (Schmitz, Rhoades, & Grabowski, 1994). These features may relate to its efficacy at relatively low costs.
Magnitude of reinforcement, nevertheless, appears to impact outcomes in response to voucher CM interventions in at least some studies. Silverman, Chutuape, Bigelow, and Stitzer (1999) found that cocaine-dependent methadone patients who were “treatment-resistant” at standard voucher amounts of $1000 achieved abstinence if vouchers increased about 3-fold to $3000, and Dallery, Silverman, Chutuape, Bigelow, and Stitzer (2001) noted a non-significant trend between greater voucher amounts and abstinence in treatment-resistant cocaine-dependent methadone patients.
Limited research exists on the impact of reinforcement magnitude with prize CM. Petry et al. (2004) compared prize CM conditions that arranged average maximal expected earnings of $80 and $240 in prizes relative to usual care, and only the $240 prize condition significantly improved outcomes, suggesting prize reinforcement below this level is ineffective. Ghitza et al. (2007) randomized patients to a control treatment or CM conditions that arranged different magnitudes of expected maximal prize earnings, and only those randomized to the larger magnitude condition realized benefits. Thus, higher magnitudes of expected prize earnings may be beneficial, and one purpose of this study was to evaluate if $900 in expected maximal prize earnings improves outcomes relative to $300.
Enhancing magnitudes of reinforcement may be particularly germane to improving outcomes in cocaine-dependent methadone patients. When “standard” magnitudes are in effect (i.e., $240-$400 in prizes or $900-$1200 in vouchers), about 40% of patients fail to achieve more than a week of abstinence (Petry, Alessi, Hanson, & Sierra, 2007; Petry & Martin, 2002; Petry et al., 2005, Silverman et al., 1996). If magnitudes are increased, especially during early phases of abstinence, more patients may respond to CM and achieve initial periods of abstinence. Robles et al. (2000) demonstrated the efficacy of an initial start-up bonus in initiating abstinence, and Katz et al. (2002) found that after responding to the initial start-up bonus, continued reinforcement produced greater rates of sustained abstinence than the initial start-up bonus alone. Silverman et al. (1998), in contrast, found that a CM treatment that provided up to six $50 start-up bonuses for initial abstinence was unable to improve outcomes relative to the standard voucher system. Thus, two of these three studies found benefits of initial start-up bonuses. The present study arranged relatively high reinforcement magnitudes during the initial periods of abstinence (about 60% of expected overall earnings) in each CM condition in attempt to improve the proportion of patients who responded to CM.
This study evaluated the efficacy of three CM interventions relative to usual care. One CM condition provided a similar amount of vouchers as previously found efficacious ($900; Silverman et al., 1996). The other two CM conditions arranged for prize reinforcers, with overall expected magnitudes of $300 and $900, respectively. These amounts were sufficiently distinct to ascertain whether increasing the magnitude of expected prize earnings improves outcomes. The primary hypothesis was that all three CM conditions would improve outcomes relative to usual care. We also examined between-group differences to determine whether tripling the magnitude of prize reinforcement enhanced outcomes and whether prize CM was more beneficial than voucher CM when magnitudes of expected earnings were similar.
Methods
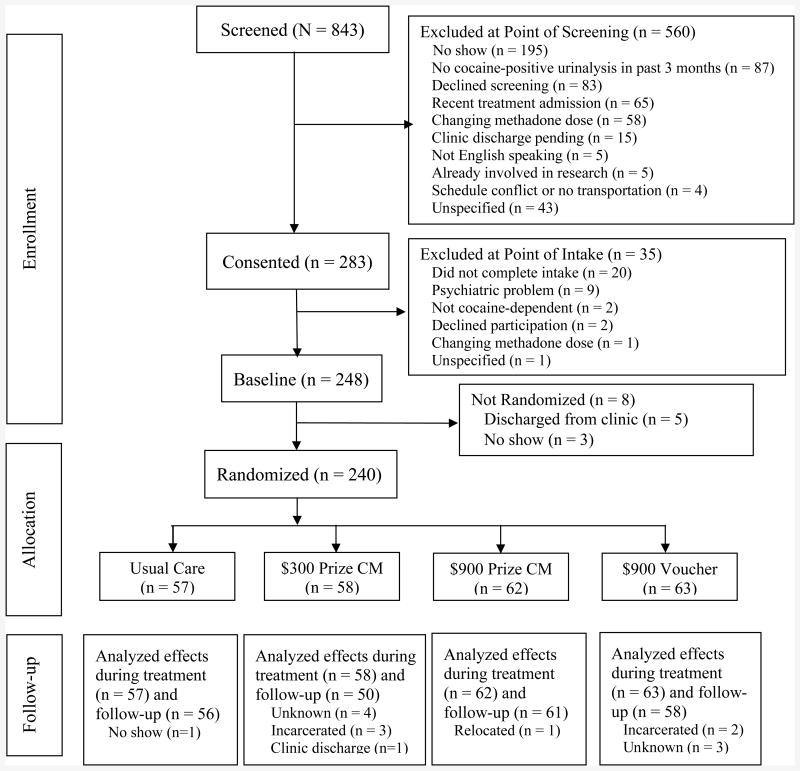
Participants were recruited from three community-based methadone clinics between 2006 and 2011. Inclusion criteria were: age ≥ 18 years; DSM-IV diagnosis of cocaine dependence; at the clinic ≥3 months, and on a stable dose of methadone for ≥1 month and not requesting a dose alteration;≥1 cocaine positive urine sample collected as part of usual treatment in the past 3 months; and English speaking. Patients were excluded if they: had a significant uncontrolled psychiatric illness (e.g., active psychosis or suicide risk); scored< 23 on Mini Mental State Exam (Folstein, Folstein, & McHugh, 1975) or could not pass an informed consent quiz; or were in recovery from pathological gambling due to potential similarities between prize reinforcers and gambling (but see Petry & Alessi, 2010; Petry et al., 2006). Numbers of screened and enrolled participants are shown in Figure 1. Approximately 60 patients were enrolled in each condition, allowing for detection of medium effect sizes between conditions (Cohen, 1988).
Figure 1.
The flow of participants from the point of initial contact through data analysis is presented per Consolidating Standards of Reporting Trials (CONSORT) guidelines.
Assessments
After providing informed consent approved by Institutional Review Boards, participants completed an evaluation which included checklists derived from the Diagnostic and Statistical Manual of Mental Disorders-IV to assess drug use diagnoses (American Psychiatric Association, 2000) and the Addiction Severity Index (McLellan, 1988). Breath samples were screened for alcohol using an Alco-sensor IV Alcometer (Intoximeters, St. Louis, MO) and urine samples for cocaine using OnTrak TestSticks (Varian, Inc., Walnut Creek, CA).
Baseline phase
For 2 weeks after the evaluation, patients provided breath and urine samples 2-3 times per week: Monday-Thursday, Monday-Friday, Tuesday-Friday, or Monday-Wednesday-Friday. Testing schedules varied in part based on staff and facility availability, but one of the four schedules above was always in effect. Staff observed submissions when same-sex staff members were present, and strips were used to assess appropriate temperature of samples.
Randomization
After baseline, a computerized program (Stout, Wirtz, Carbonari, & Del Boca, 1994) randomized participants to a study treatment, balancing groups on whether participants submitted a cocaine-negative sample during the 2-week baseline phase, a variable consistently associated with response to CM (Petry et al., 2004;Petry, Barry, Alessi, Rounsaville, & Carroll, 2012; Preston et al., 1998; Silverman et al., 1999; Stitzer et al., 2007). Due to the nature of the interventions, blinding of patients to conditions was not possible. All patients were aware of the specific parameters of each reinforcement condition below.
Usual care
Patients in usual care received daily methadone doses, at least monthly individual counseling, and weekly group counseling focusing on relapse prevention, coping and life skills training, and AIDS education. Drug abuse counselors, ranging in education from high school to masters' level, led groups. Study patients submitted urine and breath samples using the same procedures as in baseline, and same-sex staff observed submissions when possible. Specifically, at least two samples were scheduled for collection each week, with at least two days between tests; days of scheduled tests could change from week to week within one of the four schedules: Monday-Thursday, Monday-Friday, Tuesday-Thursday, or Monday-Wednesday-Friday. Patients in this condition received $3 in restaurant coupons, bus tokens or other items for every sample submitted, regardless of results.
$300 prize CM
Patients assigned to this condition receive usual care outlined above, including urine/breath sample monitoring. However, rather than a $3 item for each sample, patients earned the opportunity to draw from a bowl and win prizes when they provided samples negative for cocaine and alcohol (<.004 g/dL). Alcohol was targeted along with cocaine due to the high comorbidities (Hartzler, Donovan, & Huang, 2011). Draws increased by one for each successive negative sample, up to a maximum of 8 draws/sample during the Initial Abstinence Phase and a maximum of 5 draws/sample during the Standard Phase (see below). They also received 5 bonus draws each week that they submitted all negative samples. Patients received a reminder slip at each CM session indicating draws earned at that session and draws possible at the next session if they tested negative for cocaine and alcohol.
Initial Abstinence Phase
For the first cocaine/alcohol negative sample, patients drew from an initial abstinence bowl, with a dense probability of reinforcement. Sixty slips of paper were present, and 20 stated “Good Job!” but did not result in prizes; 36 stated “Small” (choice of $1 coupons, food items, bus tokens, etc.), 3 stated “Large” (choice of $20 gift cards, phone cards, watches, etc.), and one stated “Jumbo” (up to $100; choice of stereo, TV, five larges). Cards were replaced after each draw, so chances remained constant; items were purchased in bulk or on sale when possible to reduce costs. Patients drew from the initial abstinence bowl for the first negative sample and continued drawing from this bowl for four weeks, if they tested negative.
Standard Phase
Four weeks after submitting the first negative sample, patients switched to a standard bowl for the remainder of the intervention period. The standard bowl contained 600 slips, 1/3 of which were winning; 184 were small prizes, 15 were large prizes, and one was a jumbo. Thus, the types of prizes in both bowls were identical, but probabilities of winning were lower in this bowl. Patients could earn up to 5 draws/sample (plus 5 bonus draws/week).
If a patient provided a sample positive for cocaine or alcohol, refused to submit a sample, or had an unexcused absence on a testing day (excused absences included court appearances, medical appointments, verified emergencies), the string of abstinence was broken. The patient earned no draws that day, and draws for the next negative sample reset to one and then escalated as before. The maximum number of draws was about 225 (about 75 from the initial bowl and 150 from the standard bowl), which varied slightly depending on the testing frequencies (2-3 times/week). For patients leaving 33 samples throughout 12 weeks, average expected earnings was about $300.
$900 prize CM
These patients received the same usual care and draws for abstinence as patients in the $300 prize CM condition. The only difference between conditions related to probabilities and numbers of slips in the bowls. The Initial Abstinence Phase in this group used a bowl containing 30 slips: 7 were not associated with a prize, 14 were for “Small,” 8 for “Large,” and one for a “Jumbo” prize. The Standard Phase also went into effect four weeks after the first negative sample was submitted. The standard bowl contained 300 slips with 50% winning; 119 were small, 30 were large, and 1 was the jumbo prize. The maximum number of draws was identical, and the same procedures were in effect for resets. Because of increased probabilities of winning, the overall expected magnitude of prizes was about $900 for patients leaving 33 negative samples throughout treatment.
$900 voucher CM
These patients received usual care and sample monitoring as above. Rather than earning draws from a prize bowl, these patients earned vouchers, worth defined monetary amounts. Vouchers could be spent on a variety of items, but patients primarily exchanged vouchers for items in the prize cabinet outlined above. In the Initial Abstinence Phase, patients earned $8 in vouchers for the first negative sample, and amounts increased by $8 for each consecutive negative sample for four weeks, up to a maximum of $64/sample. Patients also received a $30 voucher bonus for a week of negative samples. A reset during this phase resulted in a return to $8 in vouchers for the next negative sample.
Four weeks after the first negative sample, patients switched to the Standard Phase, during which they earned up to a maximum of $10 in vouchers per negative sample, and a $10 bonus for each week of abstinence. If they reset during this phase, vouchers earned for the next negative sample reset to $2, and increased in increments of $2/consecutive negative sample until they returned to the $10 maximum. Patients scheduled to leave 33 samples over 12 weeks could earn up to about $900 in vouchers if all samples tested negative. As in prize conditions, reminder slips were provided at each CM session indicating vouchers possible at the next session.
Follow-up treatment and assessments
After the 12-week intervention phase, patients continued to receive usual care at the methadone clinic. Research assistants collected urine and breath samples once weekly on randomly selected days for the next 12 weeks. All patients received $3 in gift certificates for each sample submitted, regardless of results. A follow-up evaluation with sample testing was scheduled 12 months after randomization. Patients were compensated $35 for completing it, and participation rates appear in Figure 1.
Data analyses
Groups were compared with respect to demographic and baseline drug use characteristics using χ2 for categorical and F-tests for continuous variables. When overall effects were significant, post-hoc tests compared each condition to the others. Data were transformed to normalize distributions when needed.
The primary during-treatment outcomes were longest duration of continuous cocaine and alcohol negative samples (LDA) and proportion of samples submitted negative for cocaine and alcohol. These data were available for 100% of randomized patients. Analysis of variance evaluated group differences in these outcomes. A week of abstinence was a 7-day period during which all scheduled samples tested negative. A refused or missed sample broke a period of abstinence. Because LDA is impacted by missing samples and attrition, we analyzed proportions of negative samples submitted using the actual number of samples submitted in the denominator, a conservative approach that does not presume missed samples are positive. For analyses, samples were considered negative if they tested negative for both cocaine and alcohol. The vast majority of positive samples were for cocaine; only five samples (<0.1%) tested alcohol positive.
Survival analysis, using the Kaplan-Meier-Breslow model, evaluated differences between groups in days until submission of the first cocaine or alcohol positive sample after the intervention ended. Logistic regression identified predictors of a negative sample at month 12. In step 1, clinic, age, gender, and percent negative samples during the 2-week baseline phase were included; gender and clinic were categorical variables, and the others continuous. In step 2, treatment condition and LDA were entered. Analyses were conducted twice—both excluding patients who did not complete the follow-up, and including them as positive. Analyses were performed on SPSS for Windows (Version 15), with two-tailed alphas< 0.05 significant.
Results
Table 1 shows demographic and pre-treatment substance use characteristics of patients assigned to the four conditions. Groups did not differ on any baseline variables.
Table 1. Demographic and baseline characteristics.
| Variable | Usual care | $300 Prize CM |
$900 Prize CM |
$900 Voucher CM |
Significance test, p-value |
|---|---|---|---|---|---|
| N | 57 | 58 | 62 | 63 | |
| Clinic, % (n) | χ2 (6) = 0.93, .99 | ||||
| A | 36.8 (21) | 31.0 (18) | 33.9 (21) | 34.9 (22) | |
| B | 19.3 (11) | 25.9 (15) | 22.6 (14) | 23.8 (15) | |
| C | 43.9 (25) | 43.1 (25) | 43.5 (27) | 41.3 (26) | |
| Age | 40.5 (9.8) | 41.0 (9.9) | 40.6 (9.1) | 39.1 (10.2) | F(3,236)=0.46, .71 |
| Male, % (n) | 52.6 (30) | 43.1 (25) | 43.5 (27) | 61.9 (39) | χ2 (3) = 5.85, .12 |
| Years of education | 11.6 (1.8) | 11.5 (1.6) | 11.9 (2.0) | 11.6 (2.3) | F(3,236)=0.34, .80 |
| Never married, % (n) | 47.4 (27) | 51.7 (30) | 46.8 (29) | 57.1 (36) | χ2 (3) = 1.70, .64 |
| Income | $15,668 (20,505) | $11,080 (9,455) | $17,088 (27,935) | $12,056 (10,692) | F(3,234) =1.40, .25 |
| Race/ethnicity, % (n) | χ2 (9) = 7.69, .57 | ||||
| African American | 19.3 (11) | 32.8 (19) | 27.4 (17) | 20.6 (13) | |
| European American | 54.4 (31) | 41.4 (24) | 46.8 (29) | 44.4 (28) | |
| Hispanic | 24.6 (14) | 25.9 (15) | 24.2 (15) | 34.9 (22) | |
| Other | 1.8 (1) | 0.0 (0) | 1.6 (1) | 0.0 (0) | |
| Days of use in past 30 | |||||
| Cocaine | 13.1 (10.4) | 10.9 (10.2) | 12.1 (10.7) | 13.8 (11.9) | F(3,236)=0.84, .47 |
| Alcohol | 3.2 (6.7) | 1.4 (4.0) | 3.2 (6.8) | 2.7 (5.8) | F(3,236)=1.19, .32 |
| Heroin | 1.2 (4.9) | 0.8 (3.3) | 1.5 (3.6) | 1.7 (5.0) | F(3,236)=0.66, .58 |
| Methadone dose, mg | 80.1 (30.1) | 80.1 (35.1) | 77.0 (30.3) | 85.4 (34.1) | F(3,236)=0.71, .55 |
| Days on current dose | 44.4 (65.0) | 96.9 (210.5) | 66.5 (119.0) | 82.9 (163.4) | F(3,236)=1.32, .27 |
| Months on methadone, median (IQ range) | 10.7 (25.4) | 10.1 (23.2) | 12.9 (20.8) | 8.7 (23.7) | χ2 (3) = 2.68, .44 |
| Addiction Severity | Index Scores | ||||
| Medical | 0.41 (0.42) | 0.37 (0.36) | 0.32 (0.37) | 0.28 (0.34) | F(3,236)=1.34, .26 |
| Employment | 0.60 (0.33) | 0.67 (0.30) | 0.64 (0.33) | 0.66 (0.28) | F(3,236)=0.66, .58 |
| Alcohol | 0.11 (0.15) | 0.08 (0.13) | 0.10 (0.13) | 0.08 (0.10) | F(3,236)=0.74, .53 |
| Drug | 0.20 (0.11) | 0.20 (0.11) | 0.20 (0.10) | 0.22 (0.11) | F(3,236)=0.35, .79 |
| Legal | 0.10 (0.19) | 0.10 (0.20) | 0.06 (0.15) | 0.09 (0.16) | F(3,236)=0.67, .57 |
| Family/social | 0.16 (0.22) | 0.21 (0.21) | 0.18 (0.23) | 0.15 (0.21) | F(3,236)=0.98, .40 |
| Psychiatric | 0.21 (0.21) | 0.21 (0.19) | 0.18 (0.20) | 0.20 (0.19) | F(3,236)=0.44, .72 |
| No. of samples submitted in baseline | 4.7 (1.4) | 4.6 (1.2) | 4.2 (1.2) | 4.3 (1.3) | F(3,236)=1.53, .21 |
| Proportion baseline samples negative | 33.2 (39.3) | 30.8 (40.9) | 34.5 (40.5) | 37.0 (42.0) | F(3,236)=0.25, .86 |
Notes. Values are means (with standard deviations in parentheses) unless noted.
CM = contingency management.
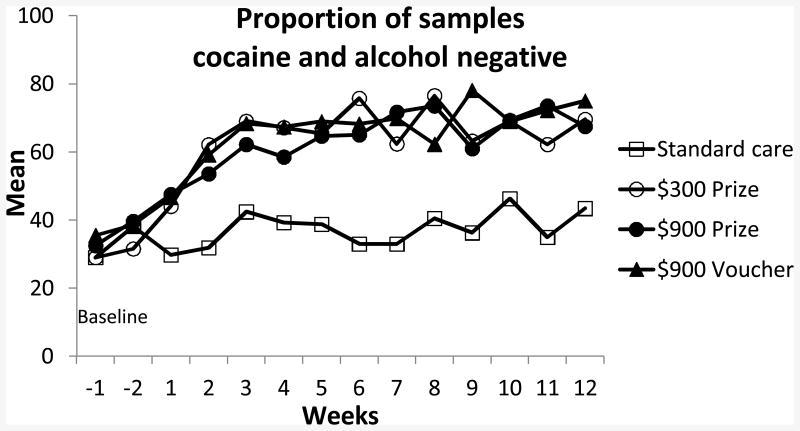
Figure 2 depicts proportions of samples cocaine and alcohol negative during the baseline phase and throughout the 12-week treatment. Mean (SD) samples submitted in the four respective conditions was 19.1 (9.6), 14.9 (10.0), 16.9 (11.0) and 16.9 (10.2), which did not differ significantly across groups but trended toward lower submission rates in the $300 prize condition relative to usual care, p = .08. The longest duration of abstinence and proportion of samples testing negative were significantly greater in each of the three CM conditions relative to usual care (Table 2). Effect sizes comparing each CM condition to usual care ranged from d = 0.38 to 0.59 across outcome measures. The three CM conditions did not differ from one another, ps > .42, and effect sizes between CM conditions were very small, with effect sizes≤0.15 (data not shown).
Figure 2.
Proportion of samples testing cocaine and alcohol negative during baseline and throughout the 12 weeks of treatment.
Table 2. During treatment outcomes.
| Variable | Usual care | $300 Prize | $900 Prize | $900 Voucher | Omnibus | CM relative to usual care | ||
|---|---|---|---|---|---|---|---|---|
| CM | CM | CM | Significance test, p | $300 Prize | $900 Prize | $900 Voucher | ||
| N | 57 | 58 | 62 | 63 | ||||
| Proportion cocaine and alcohol negative | 36.0 a (39.5) | 55.5 b (39.1) | 55.1 b (41.6) | 59.1 b (38.4) | F(3,236)=3.94, .009 | Cohen's d = 0.50 | Cohen's d = 0.48 | Cohen's d =0.59 |
| Clinic A | 46.4 (40.3) | 60.7 (38.4) | 53.3 (40.3) | 47.1 (41.1) | ||||
| Clinic B | 31.0 (40.6) | 62.8 (34.8) | 56.9 (37.4) | 69.3 (35.5) | ||||
| Clinic C | 28.9 (37.8) | 47.6 (41.8) | 55.7 (45.8) | 63.4 (36.5) | ||||
| Longest duration of abstinence (weeks) | 1.7 a (2.7) | 3.1 b (4.0) | 3.7 b (4.0) | 3.4 b (3.7) | F(3,236) =3.39, .02 | Cohen's d =0.38 | Cohen's d = 0.59 | Cohen's d = 0.52 |
| Clinic A | 3.0 (3.7) | 4.5 (4.2) | 4.1 (4.0) | 3.4 (3.8) | ||||
| Clinic B | 0.7 (1.4) | 2.2 (2.9) | 2.6 (3.5) | 3.9 (3.7) | ||||
| Clinic C | 1.0 (1.4) | 2.7 (4.3) | 3.9 (4.4) | 3.1 (3.8) | ||||
Notes. Values are means (with standard deviations in parentheses). Values with different superscripts next to them differ significantly from one another in post-hoc tests. CM = contingency management.
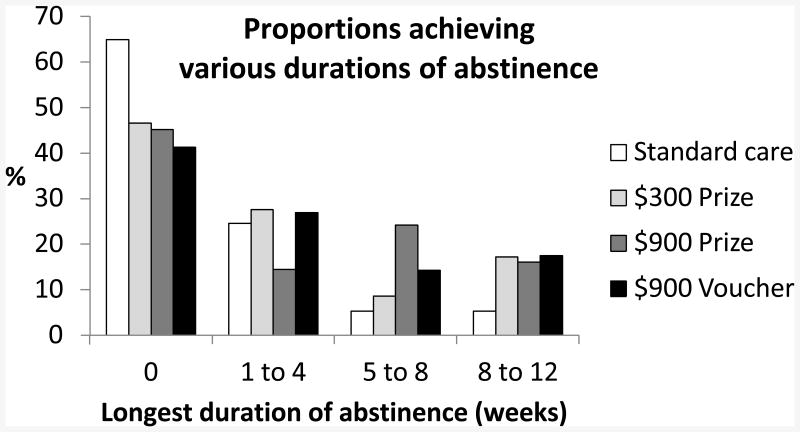
Figure 3 shows proportions of patients in each condition who achieved various durations of abstinence. Nearly two-thirds of patients in usual care did not achieve even a week of abstinence, but over half of patients in each of the CM conditions achieved at least one full week of abstinence. About 16-17% of patients in each CM condition maintained abstinence for at least 8 weeks versus 5% of usual care patients.
Figure 3.
Proportions of patients achieving various durations of abstinence during treatment.
Draws earned did not differ between prize conditions, (p = .57), but median (inter quartile range = IQR) earnings did: $25 ($197) and $233 ($791) for the $300 and $900 prize conditions, respectively, U = 1290.0, p< .01. Patients in the $900 Voucher condition earned a median (IQR) of $184 ($552), which did not differ from earnings in the $900 Prize condition, U = 1802.0, p = .65, but was higher than the $300 prize condition, U = 1371.5, p< .02.
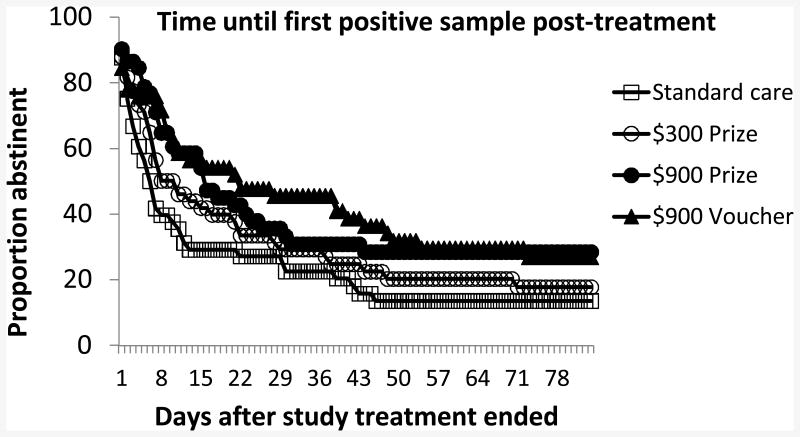
In the 12 weeks after the end of study treatment, participants in the four respective groups submitted an average (SD) of 7.8 (5.0), 7.0 (4.8), 6.6 (4.8), and 7.4 (4.9) samples, with submission rates similar across groups, p = .60. Figure 4 depicts Kaplan-Meier curves showing time until first submission of a positive sample. In usual care, mean time until first positive sample was 20.5 (4.0) days. For the $300 Prize, $900 Prize, and $900 Voucher CM conditions, respectively, it was 26.5 (4.4), 33.0 (4.9), and 36.4 (4.8) days, χ2 (3, N = 240) = 7.85, p< .05. None of the CM conditions differed from one another, ps > .16. However, the two $900 conditions differed significantly from usual care, χ2 (1, N = 119) = 5.50, p< .05 and χ2 (1, N = 120) = 4.42, p< .05 for the $900 Prize and Voucher conditions, respectively, while the $300 Prize condition did not, p>.27.
Figure 4.
Time until submission of first cocaine-positive urine sample after treatment.
At the 12-month follow-up, 113 of 225 (50.2%) patients submitted negative samples. In the logistic regression, Step 1, including clinic, demographic characteristics, and percent negative samples during baseline, predicted submission of a negative sample, χ2 (5) = 40.55, p< .001. Only percent negative samples during baseline was related to post-treatment abstinence, β (SE) = 0.021 (0.004), Wald = 28.09, p< .001, OR (95% CI) = 1.02 (1.01 – 1.03). Inclusion of step 2 improved the fit, χ2 (4) = 10.34, p< .05, and the model was significant, χ2 (9) = 50.89, p< .001. Two variables were associated with abstinence at month 12: baseline percent negative samples and LDA during treatment. The respective βs (SE) were 0.01 (0.01) and 0.17 (0.06), and Walds were 7.31 and 9.22, ps < .01. The ORs (95% CIs) were 1.01 (1.00 – 1.01) for baseline negative samples and 1.19 (1.06 – 1.32) for LDA.
When patients who missed the follow-up (n = 15) were included in the analyses as testing positive, results were similar. The final model was significant, χ2 (9) = 50.74, p< .001, with percent negative samples during baseline and LDA during treatment associated with negative samples at month 12. The βs (SE), Walds, ps and ORs (95% CIs), respectively, were 0.01 (0.00), 4.94, p< .05, and 1.01 (1.00 – 1.02) for percent baseline negative samples, and 0.18 (0.05), 11.79, p< .001, and 1.20 (1.08 – 1.33) for LDA. No study-related adverse events occurred.
Discussion
All CM conditions significantly reduced cocaine use relative to usual care. Effect sizes of CM ranged from 0.38 to 0.59, which are within ranges noted in other studies using prize and voucher CM to reduce cocaine use in methadone patients (Peirce et al., 2006; Petry & Martin, 2002; Rawson et al., 2002).
In all CM conditions, about two-thirds of the reinforcement was directed toward the initial abstinence period. These “start up” reinforcers, however, did not appear effective in improving proportions of patients responding to CM. In prior studies without start-up bonuses (Lussier et al., 2006), effect sizes of CM were similar to those reported herein, and proportions of patients achieving at least brief periods of abstinence with CM were also about 50%. Although this study was not designed to test the efficacy of start-up bonuses, about 40% of CM patients failed to achieve a single week of abstinence, even in CM conditions that arranged over $500 for the initial month of abstinence. Similarly to Silverman et al. (1998), providing initially large reinforcers did not appear advantageous. These data call for the need to evaluate alternate methods for initiating abstinence in cocaine-dependent methadone patients, as these procedures and magnitudes are successful in reducing cocaine use in only about half the patients.
Contrary to expectations, none of the CM conditions differed. The condition that arranged for an average maximum of about $300 in prizes worked as well as the larger magnitude conditions, suggesting little need to provide greater amounts of reinforcement in this population. Although $300 prize CM was as efficacious as $900 CM, data suggest that lowering prize reinforcement below this level is ineffective (Petry et al., 2004). The exact magnitude of reinforcement needed to reliably improve outcomes is not determined, but a variety of studies find that $300-$400 in prize reinforcement over 12 weeks is efficacious (Peirce et al., 2006; Petry, Alessi, Hanson, & Sierra, 2007; Petry & Martin, 2002; Petry et al., 2005). Because lower amounts do not engender benefits, clinicians should be cautioned against instituting CM that provides less dense reinforcement schedules than the $300 prize CM system (Petry, 2012).
It is unclear why larger magnitude reinforcers were not more efficacious than $300 in prizes in this study. Basic laboratory research finds robust effects of reinforcement magnitude on behavior change (Ferster & Skinner, 1957). Although some studies in clinical populations have found that increasing reinforcement magnitude improves outcomes (Higgins et al., 2007; Silverman et al., 1999), others have not (e.g., Carroll, Sinha, Nich, Babuscio, & Rounsaville, 2002; Garcia-Rodriguiz et al., 2009; Vandrey, Bigelow & Stitzer, 2007). Differential effects may relate to severity of dependence and baseline rates of substance use. Substance use is also impacted by a variety of environmental factors that may override effects of reinforcement magnitudes. In any case, available data suggest that a minimal amount of reinforcement is necessary to confer benefits, but increasing magnitudes beyond these levels may not yield substantially greater clinical improvements.
This study carefully monitored relapse following CM treatment. For 12 weeks after tangible reinforcement ended, some benefits of CM remained. However, nine months following cessation of CM, treatment condition was not a significant predictor of point-prevalence rates of abstinence, indicating that long-term sustained effects were not apparent. Novel approaches toward maintaining abstinence are needed. One option includes modifications of reinforcement schedules to maintain behavior change, such as reducing frequencies of monitoring and reinforcement once sustained abstinence has occurred (Andrade, Barry, Litt & Petry, in press). Another possibility is investigating other psychosocial strategies. Cognitive-behavioral therapy (CBT) has been associated with long-term behavior change (Carroll et al., 1994), but to date has yielded limited or inconsistent beneficial effects when applied in conjunction with CM (Epstein et al., 2003; Rawson et al., 2002, 2006). Perhaps altering the timing of CBT delivery in the course of CM treatment may lead to synergistic effects. For example, patients may be more apt to benefit from CBT after CM is underway and abstinence initiated. Typically, the two interventions are delivered concurrently, but introducing CBT after abstinence is initiated may enhance outcomes to a greater extent than providing both treatments at the same time.
A strong and consistent predictor of poor response to CM includes submission of positive samples before CM (Higgins, Badger, & Budney, 2000; Petry et al., 2004, 2012; Preston et al., 1998; Stitzer et al., 2007). Further, the vast majority of patients who respond to CM do so within one month (Weinstock, Rash, & Petry, 2010). An approach to direct limited resources toward those most likely to respond would be to allow persistently using cocaine-dependent methadone patients into CM programs only after they provide their first cocaine-negative sample. In this manner, all such patients could access CM, but only after they demonstrated ability to refrain from cocaine use long enough to earn at least their first reinforcer.
Limitations to the study should be considered in interpreting the results. Due to university purchasing policies, the voucher CM condition did not allow for the range of voucher expenditures as is typical. Voucher expenditures were restricted to state-approved vendors, which resulted in patients typically selecting amongst prize items, as onsite prizes spanned the approved vendors. This practice allowed for instantaneous redemption of vouchers, but it also restricted options, and these issues may impact the effectiveness of CM (Petry, 2012). Further, the effects noted in this sample may not generalize to other populations that differ in types and severity of drug use disorders (e.g., non-methadone patients).
Despite these limitations and considerations, this study included a large sample size, sufficient to detect medium effect sizes between CM interventions, involved three methadone clinics, and closely monitored relapse following removal of reinforcement. Results from this study indicate that providing larger magnitude reinforcement did not yield benefits relative to modest prize reinforcement. This finding ultimately could enhance dissemination efforts of CM as it indicates that arranging a prize reinforcement system can significantly reduce cocaine use in cocaine-dependent methadone patients. In this study, the median cost of prizes was only $25 per patient over 12 weeks in the $300 prize condition; although the median was low because nearly half of the patients did not respond to CM, rates of response were similar across the CM conditions. Widespread and longer-term CM administration will increase costs, and balance must be achieved between costs of implementing CM and benefits derived from individual, program, and societal perspectives. CM interventions result in advantages that extend beyond reducing drug use, such as reducing psychiatric symptoms (Petry, Alessi, & Rash, 2013) and improving quality of life (Petry, Alessi, & Hanson, 2007). Growing evidence is also pointing to their cost-effectiveness (Lott & Jencius, 2009; Olmstead & Petry, 2009). However, methods to improve response to CM and to extend its effects are still needed.
Acknowledgments
We thank the patients and staff at the methadone clinics for their participation in and support of this study. This study and preparation of this report was supported in part by NIH grants R01-DA13444, P30-DA023918, R01-DA027615, P50-DA09241, P60-AA03510, and M01-RR06192.
Footnotes
Public health significance statement: This study shows that even relatively low magnitude reinforcement interventions can reduce cocaine use in methadone patients. Adoption of these interventions in treatment settings may improve outcomes of these patients.
References
- American Psychiatric Association. The Diagnostic and Statistical Manual for Mental Disorders, Revision-IV-TR. Washington, DC: American Psychiatric Association Press; 2000. [Google Scholar]
- Andrade LF, Barry D, Litt MD, Petry NM. Maintaining high ambulatory activity levels of sedentary adults with a reinforcement thinning schedule. Journal of Applied Behavior Analysis. doi: 10.1002/jaba.147. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bandettini Di Poggio A, Fornai F, Paparelli A, Pacini M, Perugi G, Maremmani I. Comparison between heroin and heroin-cocaine polyabusers: A psychopathological study. Annals of the New York Academy of Sciences. 2006;1074:438–445. doi: 10.1196/annals.1369.044. [DOI] [PubMed] [Google Scholar]
- Carroll KM, Rounsaville BJ, Nich C, Gordon LT, Wirtz PW, Gawin F. One-year follow-up of psychotherapy and pharmacotherapy for cocaine dependence. Delayed emergence of psychotherapy effects. Archives of General Psychiatry. 1994;51:989–997. doi: 10.1001/archpsyc.1994.03950120061010. [DOI] [PubMed] [Google Scholar]
- Carroll KM, Sinha R, Nich C, Babuscio T, Rounsaville BJ. Contingency management to enhance naltrexone treatment of opioid dependence: A randomized clinical trial of reinforcement magnitude. Experimental and Clinical Psychopharmacology. 2002;10:54–63. doi: 10.1037//1064-1297.10.1.54. [DOI] [PubMed] [Google Scholar]
- Cohen J. Statistical Power Analysis for the Behavioral Sciences. 2nd. Hillsdale, NJ: Lawrence Erlbaum Associates; 1988. [Google Scholar]
- Council on Addiction Psychiatry. Position statement on methadone maintenance treatment. American Journal of Psychiatry. 1994;151:792–794. doi: 10.1176/ajp.151.5.792. [DOI] [PubMed] [Google Scholar]
- Dallery J, Silverman K, Chutuape MA, Bigelow GE, Stitzer ML. Voucher-based reinforcement of opiate plus cocaine abstinence in treatment-resistant methadone patients: Effects of reinforcer magnitude. Experimental and Clinical Psychopharmacology. 2001;9:317–325. doi: 10.1037//1064-1297.9.3.317. [DOI] [PubMed] [Google Scholar]
- DeMaria PA, Sterling R, Weinstein SP. The effect of stimulant and sedative use on treatment outcome of patients admitted to methadone maintenance treatment. American Journal on Addictions. 2000;9:145–153. doi: 10.1080/10550490050173217. [DOI] [PubMed] [Google Scholar]
- Dutra L, Stathopoulou G, Basden SL, Leyro TM, Powers MB, Otto MW. Ameta-analytic review of psychosocial interventions for substance use disorders. The American Journal of Psychiatry. 2008;165:179–187. doi: 10.1176/appi.ajp.2007.06111851. [DOI] [PubMed] [Google Scholar]
- Epstein DH, Hawkins WE, Covi L, Umbricht A, Preston KL. Cognitive-behavioral therapy plus contingency management for cocaine use: Findings during treatment and across 12-month follow-up. Psychology of Addictive Behaviors. 2003;17:73–82. doi: 10.1037/0893-164X.17.1.73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferster CB, Skinner BF. Schedules of reinforcement. New York, NY: Appleton-Century Crofts; 1957. [Google Scholar]
- Folstein MF, Folstein SE, McHugh PR. “Mini-mental state”: A practical method for grading the cognitive state of patients for the clinician”. Journal of Psychiatric Research. 1975;12:189–198. doi: 10.1016/0022-3956(75)90026-6. [DOI] [PubMed] [Google Scholar]
- Garcia-Rodriguez O, Secades-Villa R, Higgins ST, Fernandez-Hermida JR, Carballo JL, Errasti Perez JM, Diaz SAH. Effects of voucher-based intervention on abstinence and retention in an outpatient treatment for cocaine addiction: A randomized controlled trial. Experimental and Clinical Psychopharmacology. 2009;17:131–138. doi: 10.1037/a0015963. [DOI] [PubMed] [Google Scholar]
- Ghitza UE, Epstein DH, Schmittner J, Vahabzadeh M, Lin JL, Preston KL. Randomized trial of prize-based reinforcement density for simultaneous abstinence from cocaine and heroin. Journal of Consulting and Clinical Psychology. 2007;75:765–774. doi: 10.1037/0022-006X.75.5.765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hartzler B, Donovan DM, Huang Z. Rates and influences of alcohol use disorder comorbidity among primary stimulant misusing treatment-seekers: Meta-analytic findings across eight NIDA CTN trials. American Journal of Drug and Alcohol Abuse. 2011;37:460–471. doi: 10.3109/00952990.2011.602995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Higgins ST, Badger GJ, Budney AJ. Initial abstinence and success in achieving longer term cocaine abstinence. Experimental and Clinical Psychopharmacology. 2000;8:377–386. doi: 10.1037//1064-1297.8.3.377. [DOI] [PubMed] [Google Scholar]
- Higgins ST, Heil SH, Dantona R, Donham R, Matthews M, Badger GJ. Effects of varying the monetary value of voucher-based incentives on abstinence achieved during and following treatment among cocaine-dependent outpatients. Addiction. 2007;102:271–281. doi: 10.1111/j.1360-0443.2006.01664.x. [DOI] [PubMed] [Google Scholar]
- Katz EC, Robles-Sotelo E, Correia CJ, Silverman K, Stitzer ML, Bigelow G. The brief abstinence test: Effects of continued incentive availability on cocaine abstinence. Experimental and Clinical Psychopharmacology. 2002;10:10–17. doi: 10.1037//1064-1297.10.1.10. [DOI] [PubMed] [Google Scholar]
- Leri F, Bruneau J, Stewart J. Understanding polydrug use: Review of heroin and cocaine co-use. Addiction. 2003;98:7–22. doi: 10.1046/j.1360-0443.2003.00236.x. [DOI] [PubMed] [Google Scholar]
- Lott DC, Jencius S. Effectiveness of very low-cost contingency management in a community adolescent treatment program. Drug and Alcohol Dependence. 2009;102:162–165. doi: 10.1016/j.drugalcdep.2009.01.010. [DOI] [PubMed] [Google Scholar]
- Lussier JP, Heil SH, Mongeon JA, Badger GJ, Higgins ST. A meta-analysis of voucher-based reinforcement therapy for substance use disorders. Addiction. 2006;101:192–203. doi: 10.1111/j.1360-0443.2006.01311.x. [DOI] [PubMed] [Google Scholar]
- McLellan AT. Guide to the Addiction Severity Index: Background, Administration, and Field Testing Results. Rockville, MD: U.S Dept of Health and Human Services, Public Health Service, Alcohol, Drug Abuse, and Mental Health Administration, National Institute on Drug Abuse; 1988. [Google Scholar]
- Olmstead TA, Petry NM. The cost-effectiveness of prize-based and voucher-based contingency management in a population of cocaine- or opioid-dependent outpatients. Drug and Alcohol Dependence. 2009;102:108–115. doi: 10.1016/j.drugalcdep.2009.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peirce JM, Petry NM, Stitzer ML, Blaine J, Kellogg S, Satterfield F, Li R. Effects of lower-cost incentives on stimulant abstinence in methadone maintenance treatment: A National Drug Abuse Treatment Clinical Trials Network study. Archives of General Psychiatry. 2006;63:201–208. doi: 10.1001/archpsyc.63.2.201. [DOI] [PubMed] [Google Scholar]
- Petry NM. Contingency Management for Substance Abuse Treatment: A Guide to Implementing this Evidence-based Practice. New York: Routledge/Taylor & Francis; 2012. [Google Scholar]
- Petry NM, Alessi SM. Prize-based contingency management is efficacious in cocaine-abusing patients with and without recent gambling participation. Journal of Substance Abuse Treatment. 2010;39:282–288. doi: 10.1016/j.jsat.2010.06.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petry NM, Alessi SM, Hanson T. Contingency management improves abstinence and quality of life in substance abusers. Journal of Consulting and Clinical Psychology. 2007;75:307–315. doi: 10.1037/0022-006X.75.2.307. [DOI] [PubMed] [Google Scholar]
- Petry NM, Alessi SM, Hanson T, Sierra S. Randomized trial of contingent prizes versus vouchers in cocaine-using methadone patients. Journal of Consulting and Clinical Psychology. 2007;75:983–991. doi: 10.1037/0022-006X.75.6.983. [DOI] [PubMed] [Google Scholar]
- Petry NM, Alessi SM, Rash CJ. Contingency management treatments decrease psychiatric symptoms. Journal of Consulting and Clinical Psychology. 2013;81:926–931. doi: 10.1037/a0032499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petry NM, Barry D, Alessi SM, Rounsaville BJ, Carroll KM. A randomized trial adapting contingency management targets based on initial abstinence status of cocaine-dependent patients. Journal of Consulting and Clinical Psychology. 2012;80:276–285. doi: 10.1037/a0026883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petry NM, Kolodner KB, Li R, Peirce JM, Roll JM, Stitzer ML, Hamilton JA. Prize-based contingency management does not increase gambling. Drug and Alcohol Dependence. 2006;83:269–273. doi: 10.1016/j.drugalcdep.2005.11.023. [DOI] [PubMed] [Google Scholar]
- Petry NM, Martin B. Low-cost contingency management for treating cocaine and opioid-abusing methadone patients. Journal of Consulting and Clinical Psychology. 2002;70:398–405. doi: 10.1037//0022-006x.70.2.398. [DOI] [PubMed] [Google Scholar]
- Petry NM, Martin B, Simcic F. Prize reinforcement contingency management for cocaine dependence: Integration with group therapy in a methadone clinic. Journal of Consulting and Clinical Psychology. 2005;73:354–359. doi: 10.1037/0022-006X.73.2.354. [DOI] [PubMed] [Google Scholar]
- Petry NM, Tedford J, Austin M, Nich C, Carroll KM, Rounsaville BJ. Prize reinforcement contingency management for treatment of cocaine abusers:How low can we go, and with whom? Addiction. 2004;99:349–360. doi: 10.1111/j.1360-0443.2003.00642.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Preston KL, Silverman K, Higgins ST, Brooner RK, Montoya I, Schuster CR, Cone EJ. Cocaine use early in treatment predicts outcome in a behavioral treatment program. Journal of Consulting and Clinical Psychology. 1998;66:691–696. doi: 10.1037//0022-006x.66.4.691. [DOI] [PubMed] [Google Scholar]
- Rawson RA, Huber A, McCann MJ, Shoptaw S, Farabee D, Reiber C, Ling W. A comparison of contingency management and cognitive-behavioral approaches during methadone maintenance treatment for cocaine dependence. Archives of General Psychiatry. 2002;59:817–824. doi: 10.1001/archpsyc.59.9.817. [DOI] [PubMed] [Google Scholar]
- Rawson RA, McCann MJ, Flammino F, Shoptaw S, Miotto K, Reiber C, Ling W. A comparison of contingency management and cognitive-behavioral approaches for stimulant-dependent individuals. Addiction. 2006;101:267–274. doi: 10.1111/j.1360-0443.2006.01312.x. [DOI] [PubMed] [Google Scholar]
- Robles E, Silverman K, Preston KL, Cone EJ, Katz E, Bigelow GE, Stitzer ML. The brief abstinence test: Voucher based reinforcement of cocaine abstinence. Drug and Alcohol Dependence. 2000;58:205–212. doi: 10.1016/s0376-8716(99)00090-3. [DOI] [PubMed] [Google Scholar]
- Roll JM, Higgins ST, Badger GJ. An experimental comparison of three different schedules of reinforcement of drug abstinence using cigarette smoking as an exemplar. Journal of Applied Behavioral Analysis. 1996;29:495–505. doi: 10.1901/jaba.1996.29-495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roll JM, Reilly MP, Johanson CE. The influence of exchange delays on cigarette versus money choice: A laboratory analog of voucher-based reinforcement therapy. Experimental & Clinical Psychopharmacology. 2000;8:366–370. doi: 10.1037//1064-1297.8.3.366. [DOI] [PubMed] [Google Scholar]
- Schmitz JM, Rhoades H, Grabowski J. A menu of potential reinforcers in a methadone maintenance program. Journal of Substance Abuse Treatment. 1994;11:425–431. doi: 10.1016/0740-5472(94)90095-7. [DOI] [PubMed] [Google Scholar]
- Sees KL, Delucchi KL, Masson C, Rosen A, Clark HW, Robillard H, Hall SM. Methadone maintenance vs. 180-day psychosocially enriched detoxification for treatment of opioid dependence: A randomized controlled trial. Journal of the American Medical Association. 2000;283:1303–1310. doi: 10.1001/jama.283.10.1303. [DOI] [PubMed] [Google Scholar]
- Silverman K, Chutuape M, Bigelow G, Stitzer ML. Voucher-based reinforcement of cocaine abstinence in treatment-resistant methadone patients: Effects of reinforcement magnitude. Psychopharmacology. 1999;146:128–138. doi: 10.1007/s002130051098. [DOI] [PubMed] [Google Scholar]
- Silverman K, Higgins ST, Brooner RK, Montoya ID, Cone EJ, Schuster CR, Preston KL. Sustained cocaine abstinence in methadone maintenance patients through voucher-based reinforcement therapy. Archives of General Psychiatry. 1996;53:409–415. doi: 10.1001/archpsyc.1996.01830050045007. [DOI] [PubMed] [Google Scholar]
- Silverman K, Wong CJ, Umbricht-Schneiter A, Montoya ID, Schuster CR, Preston KL. Broad beneficial effects of cocaine abstinence reinforcement among methadone patients. Journal of Consulting and Clinical Psychology. 1998;66:811–824. doi: 10.1037//0022-006x.66.5.811. [DOI] [PubMed] [Google Scholar]
- Sofuoglu M, Kosten TR. Emerging pharmacological strategies in the fight against cocaine addiction. Expert Opinion on Emerging Drugs. 2006;11:91–98. doi: 10.1517/14728214.11.1.91. [DOI] [PubMed] [Google Scholar]
- Stitzer ML, Peirce J, Petry NM, Kirby K, Roll J, Krasnansky J, Li R. Abstinence-based incentives in methadone maintenance: Interaction with intake stimulant test results. Experimental and Clinical Psychopharmacology. 2007;15:344–350. doi: 10.1037/1064-1297.15.4.344. [DOI] [PubMed] [Google Scholar]
- Stout RL, Wirtz PW, Carbonari JP, Del Boca FK. Ensuring balanced distribution of prognostic factors in treatment outcome research. Journal of Studies on Alcohol. 1994;12(Suppl):70–75. doi: 10.15288/jsas.1994.s12.70. [DOI] [PubMed] [Google Scholar]
- Vandrey R, Bigelow GE, Stitzer ML. Contingency management in cocaine abusers: A dose-effect comparison of goods-based versus cash-based incentives. Experimental and Clinical Psychopharmacology. 2007;15:338–343. doi: 10.1037/1064-1297.15.4.338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weinstock J, Rash CJ, Petry NM. Contingency management for cocaine use in methadone maintenance patients: When does abstinence happen? Psychology of Addictive Behaviors. 2010;24:282–291. doi: 10.1037/a0017542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu LT, Blazer DG, Woody GE, Burchett B, Yang C, Pan JJ, Ling WJ. Alcohol and drug dependence symptom items as brief screeners for substance use disorders: Results from the Clinical Trials Network. Journal of Psychiatric Research. 2012;46:360–369. doi: 10.1016/j.jpsychires.2011.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]